A Simple and Fast Manual Centrifuge to Spin Solutions in 96-Well PCR Plates
Abstract
1. Introduction
2. Experimental Design
2.1. Materials
2.2. Making the Manual Centrifuge for Spinning down Solutions in 96-Well PCR Plates (Time for Completion: ~30 min)
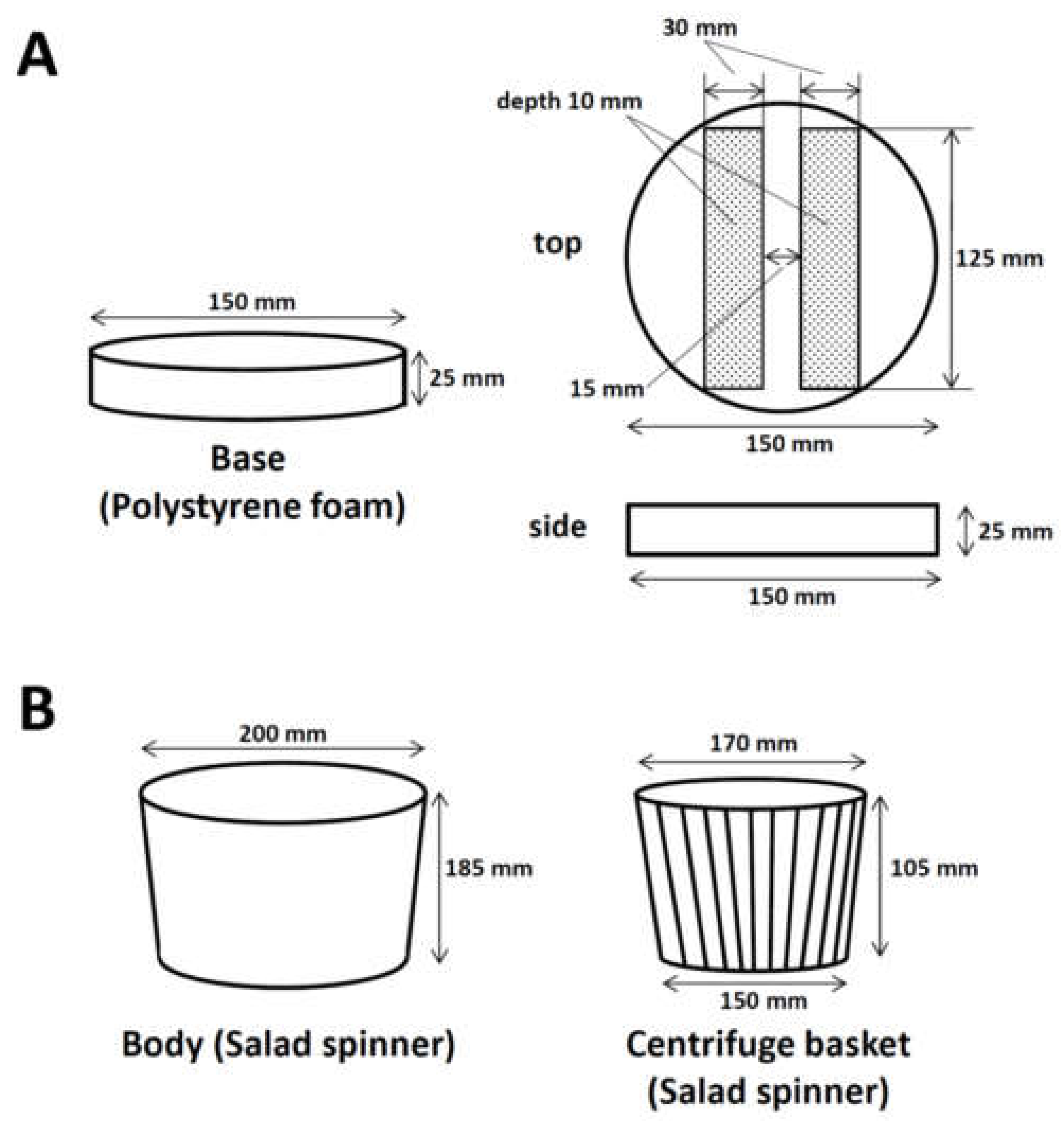
2.2.1. Processing Polystyrene Foam for the Base to Fix Two 96-Well PCR Plates
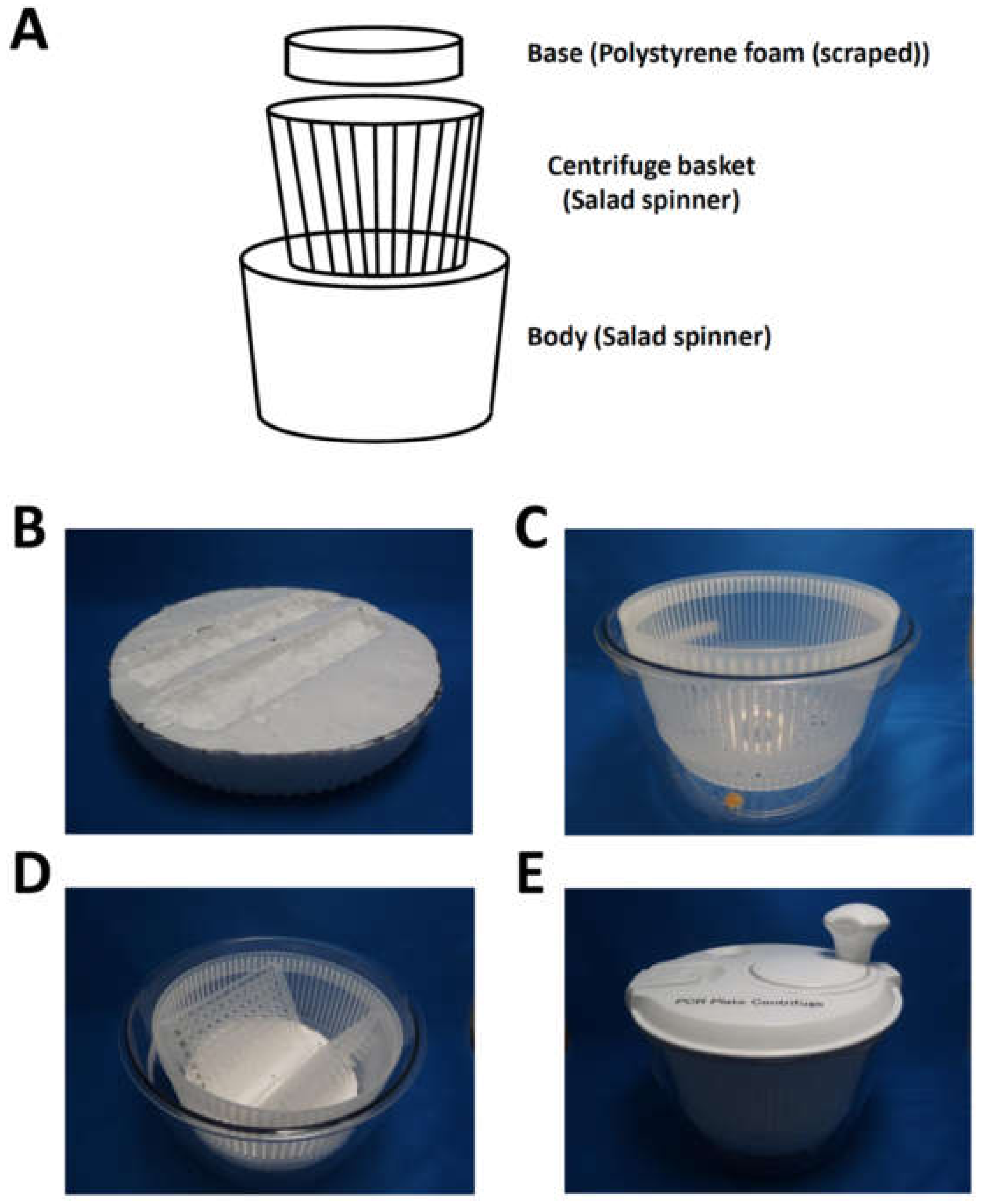
2.2.2. Assembly of the Manual Centrifuge
2.3. Equipment
3. Procedure
Centrifuging Solutions in 96-Well PCR Plates before DNA Agarose Gel Electrophoresis (Time for Completion: ~5 min)
- Add 2 µL of EZ-Vision One to all PCR samples (10 µL) in 96-well PCR plates.
- Set two 96-well PCR plates on the base of the manual centrifuge (Figure 2D).
CRITICAL STEP: When centrifuging one 96-well PCR plate, another should be placed on the opposite side for balance.
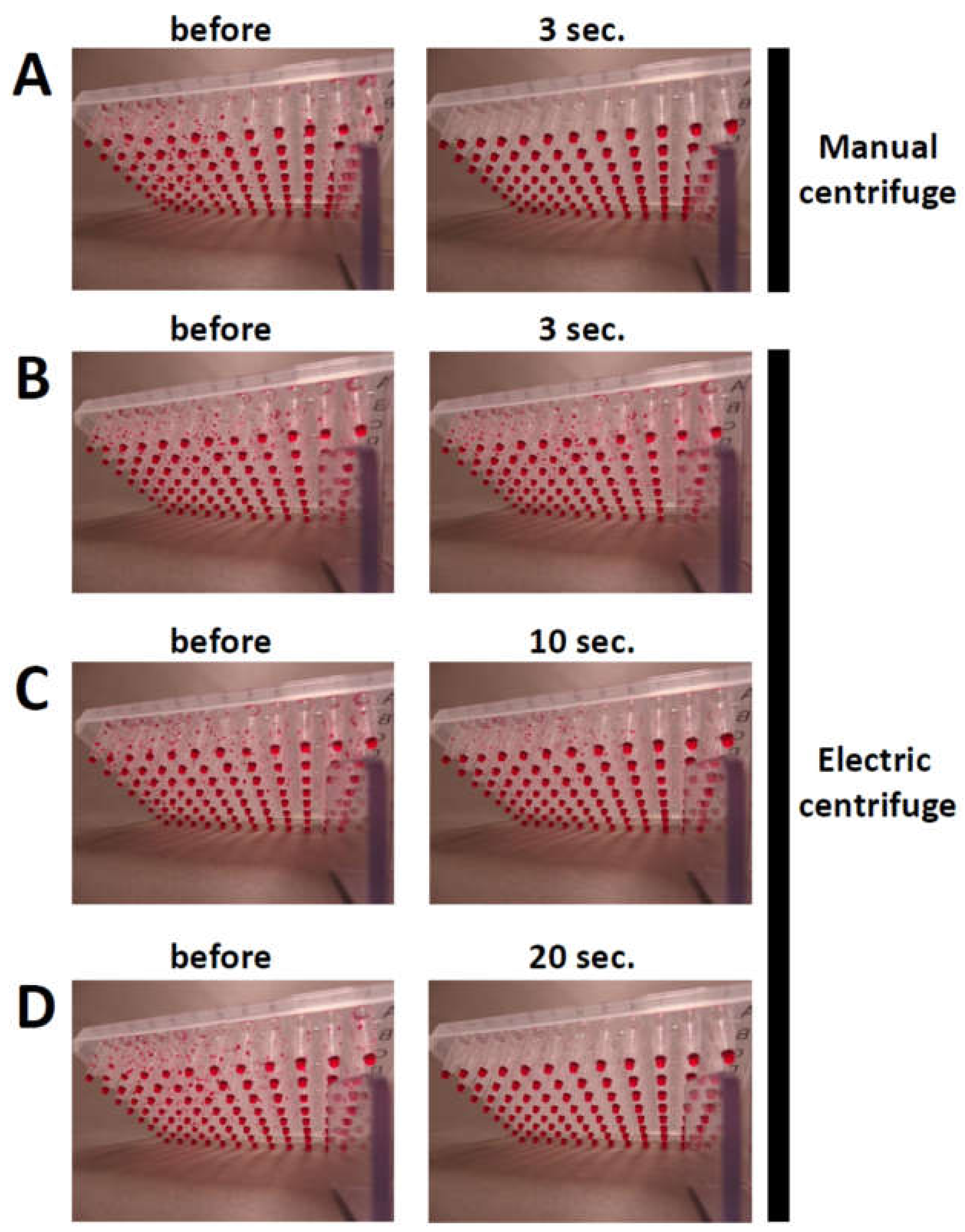
- Rotate the handle of the salad spinner for 3 s.
- Check that the PCR mixtures have settled to the bottom of the wells in the 96-well PCR plate and load samples for agarose gel electrophoresis [19]. OPTIONAL STEP: The centrifuge can also be used to spin the PCR reaction mixtures down in 96-well PCR plates before PCR.
4. Expected Results
Funding
Acknowledgments
Conflicts of Interest
References
- Thomson, D.; Henry, R. Single-Step Protocol for Preparation of Plant Tissue for Analysis by PCR. Biotechniques 1995, 19, 394–397. [Google Scholar] [PubMed]
- Strizhov, N.; Li, Y.; Rosso, M.G.; Viehoever, P.; Dekker, K.A.; Weisshaar, B. High-throughput generation of sequence indexes from T-DNA mutagenized arabidopsis thaliana lines. Biotechniques 2003, 35, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Xin, Z.; Velten, J.P.; Oliver, M.J.; Burke, J.J. High-throughput DNA extraction method suitable for PCR. Biotechniques 2003, 34, 820–824. [Google Scholar] [CrossRef] [PubMed]
- Varshney, G.K.; Pei, W.; LaFave, M.C.; Idol, J.; Xu, L.; Gallardo, V.; Carrington, B.; Bishop, K.; Jones, M.; Li, M.; et al. High-throughput gene targeting and phenotyping in zebrafish using Crispr/Cas9. Genome Res. 2015, 25, 1030–1042. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Chen, J.; Dai, X.; Zhang, D.; Zhao, Y. An effective strategy for reliably isolating heritable and Cas9-free arabidopsis mutants generated by crispr/Cas9-mediated genome editing. Plant Physiol. 2016, 171, 1794–1800. [Google Scholar] [CrossRef] [PubMed]
- Kweon, J.; Kim, Y. High-throughput genetic screens using crispr-Cas9 System. Arch. Pharm. Res. 2018, 41, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, K. Rapid screening of crispr/cas9-induced mutants using the ACT-PCR method. Methods Mol. Biol. 2019, 1917, 27–32. [Google Scholar] [PubMed]
- Woodman, M.E.; Savage, C.R.; Arnold, W.K.; Stevenson, B. Direct PCR of Intact Bacteria (Colony PCR). Curr. Protoc. Microbiol. 2016, 42, A3D1–A3D7. [Google Scholar] [CrossRef] [PubMed]
- Okegawa, Y.; Koshino, M.; Okushima, T.; Motohashi, K. Application of preparative disk gel electrophoresis for antigen purification from inclusion bodies. Protein Expr. Purif. 2016, 118, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, F.; Pereira, H.; Johansson, B. Colony PCR. Methods Mol. Biol. 2017, 1620, 129–139. [Google Scholar] [PubMed]
- Jamal, M.A.; Sharma, S.P.; Chung, H.J.; Kim, H.J.; Hong, S.T.; Lee, S. Ultra-high efficient colony PCR for high throughput screening of bacterial genes. Indian J. Microbiol. 2017, 57, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Ahrberg, C.D.; Manz, A.; Chung, B.G. Polymerase chain reaction in microfluidic devices. Lab Chip 2016, 16, 3866–3884. [Google Scholar] [CrossRef] [PubMed]
- Voytas, D. Agarose gel electrophoresis. Curr. Protoc. Mol. Biol. 2000, 51, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.Y.; Costumbrado, J.; Hsu, C.Y.; Kim, Y.H. Agarose gel electrophoresis for the separation of DNA fragments. J. Vis. Exp. 2012, 20, e3923. [Google Scholar] [CrossRef] [PubMed]
- Green, M.R.; Sambrook, J. Analysis of DNA by agarose gel electrophoresis. Cold Spring Harb. Protoc. 2019, 2019, pdb–top100388. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Theis, L.; Kerr, L.; Zakhidova, N.; O’Connor, K.; Uthman, M.; Oden, Z.M.; Richards-Kortum, R. A hand-powered, portable, low-cost centrifuge for diagnosing anemia in low-resource settings. Am. J. Trop. Med. Hyg. 2011, 85, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Marcos, R.; Santos, M.; Marrinhas, C.; Correia-Gomes, C.; Caniatti, M. Cytocentrifuge preparation in veterinary cytology: A quick, simple, and affordable manual method to concentrate low cellularity fluids. Vet. Clin. Pathol. 2016, 45, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Bhamla, M.S.; Benson, B.; Chai, C.; Katsikis, G.; Johri, A.; Prakash, M. Hand-powered ultralow-cost paper centrifuge. Nat. Biomed. Eng. 2017, 1, 1–7. [Google Scholar] [CrossRef]
- Motohashi, K. Development of highly sensitive and low-cost DNA agarose gel electrophoresis detection systems, and evaluation of non-mutagenic and loading dye-type DNA-staining reagents. PLoS ONE 2019, 14, e0222209. [Google Scholar] [CrossRef] [PubMed]
- Motohahi, K. Evaluation of the efficiency and utility of recombinant enzyme-free seamless DNA cloning methods. Biochem. Biophys. Rep. 2017, 9, 310–315. [Google Scholar]
| Manual Centrifuge for the 96-Well PCR Plates | Manual Centrifuge for the Blood Sample | Paper Centrifuge for the Blood Sample | |
|---|---|---|---|
| Application | Spin-down for PCR solution | Separation of plasma for whole blood | Separation of plasma for whole blood |
| Sample Capacity | 96 samples × 2 | 30 samples | 8 samples |
| Materials | Salad spinner | Salad spinner | Paper and string |
| Maximum Speed | 760 rpm (25× g) | 600 rpm (24× g) | 125,000 rpm (30,000× g) |
| Price | USD 18 | USD 35 | USD 0.2 |
| Weight | 0.59 kg | 1.5 kg | 2 g |
| Reference | This study | [16] | [18] |
| Manual Centrifuge (Salad Spinner) | Electric Centrifuge (Labnet MPS1000) | |
|---|---|---|
| Time of Centrifugation | 3 s | 20 s |
| Sample Capacity | Two 96-well PCR plates | Two 96-well PCR plates |
| Maximum Speed | 760 rpm (25× g) | 2500 rpm (500× g) |
| Price | USD 18 | USD 710 |
| Power Supply | None | ~120 V, 50/60 Hz |
| Weight | 0.59 kg | 1.5 kg |
| Installation Area | 205 × 200 mm (mobile) | 190 × 210 mm (immobile) |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motohashi, K. A Simple and Fast Manual Centrifuge to Spin Solutions in 96-Well PCR Plates. Methods Protoc. 2020, 3, 41. https://doi.org/10.3390/mps3020041
Motohashi K. A Simple and Fast Manual Centrifuge to Spin Solutions in 96-Well PCR Plates. Methods and Protocols. 2020; 3(2):41. https://doi.org/10.3390/mps3020041
Chicago/Turabian StyleMotohashi, Ken. 2020. "A Simple and Fast Manual Centrifuge to Spin Solutions in 96-Well PCR Plates" Methods and Protocols 3, no. 2: 41. https://doi.org/10.3390/mps3020041
APA StyleMotohashi, K. (2020). A Simple and Fast Manual Centrifuge to Spin Solutions in 96-Well PCR Plates. Methods and Protocols, 3(2), 41. https://doi.org/10.3390/mps3020041







