Superhydrophobicity, Photocatalytic Self-Cleaning and Biocidal Activity Combined in a Siloxane-ZnO Composite for the Protection of Limestone
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Coating Preparation and Deposition
2.3. Characterisation and Sample Treatments
3. Results and Discussion
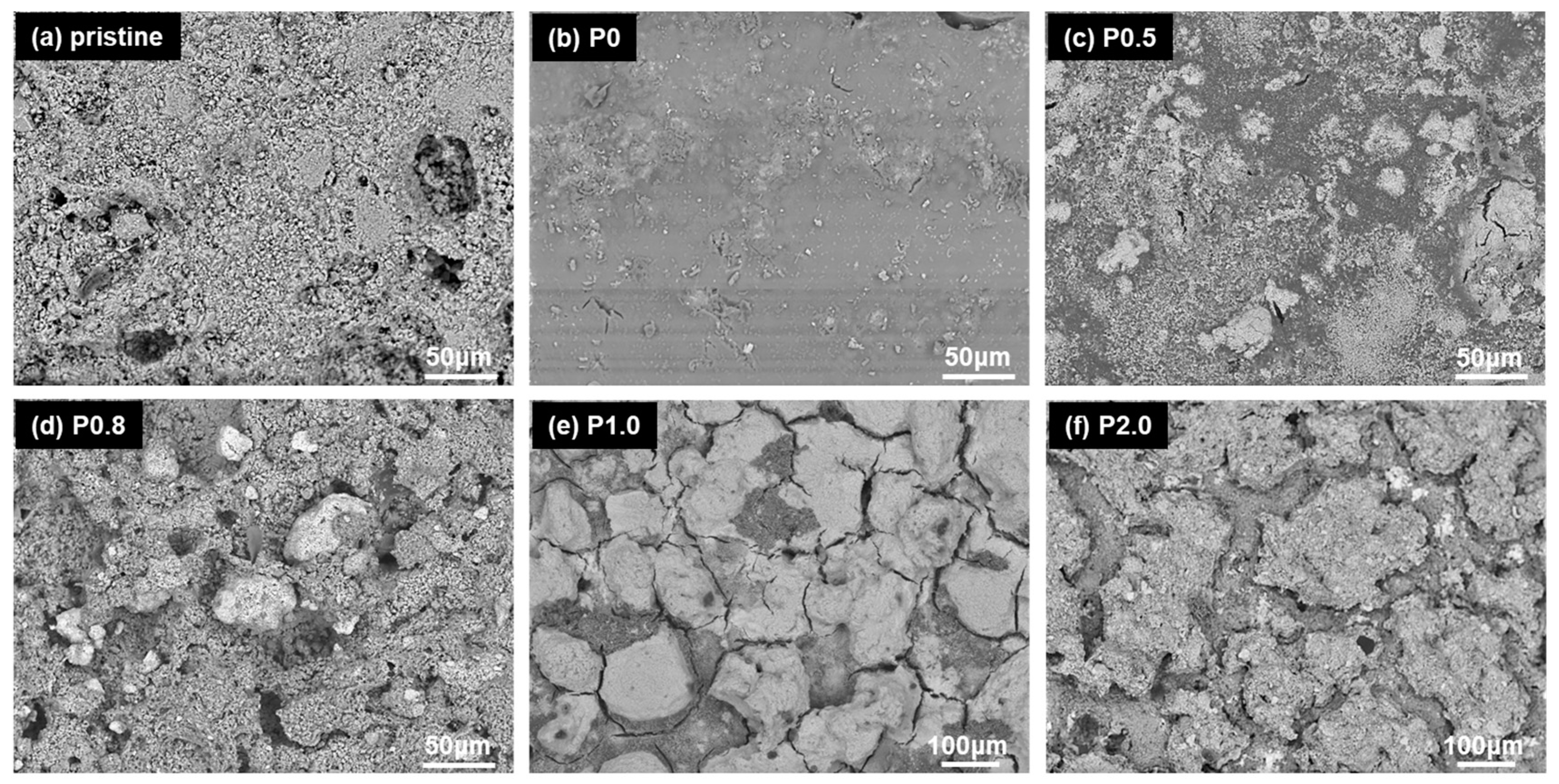
3.1. Surface Morphology and Wetting Properties vs. NP Concentration
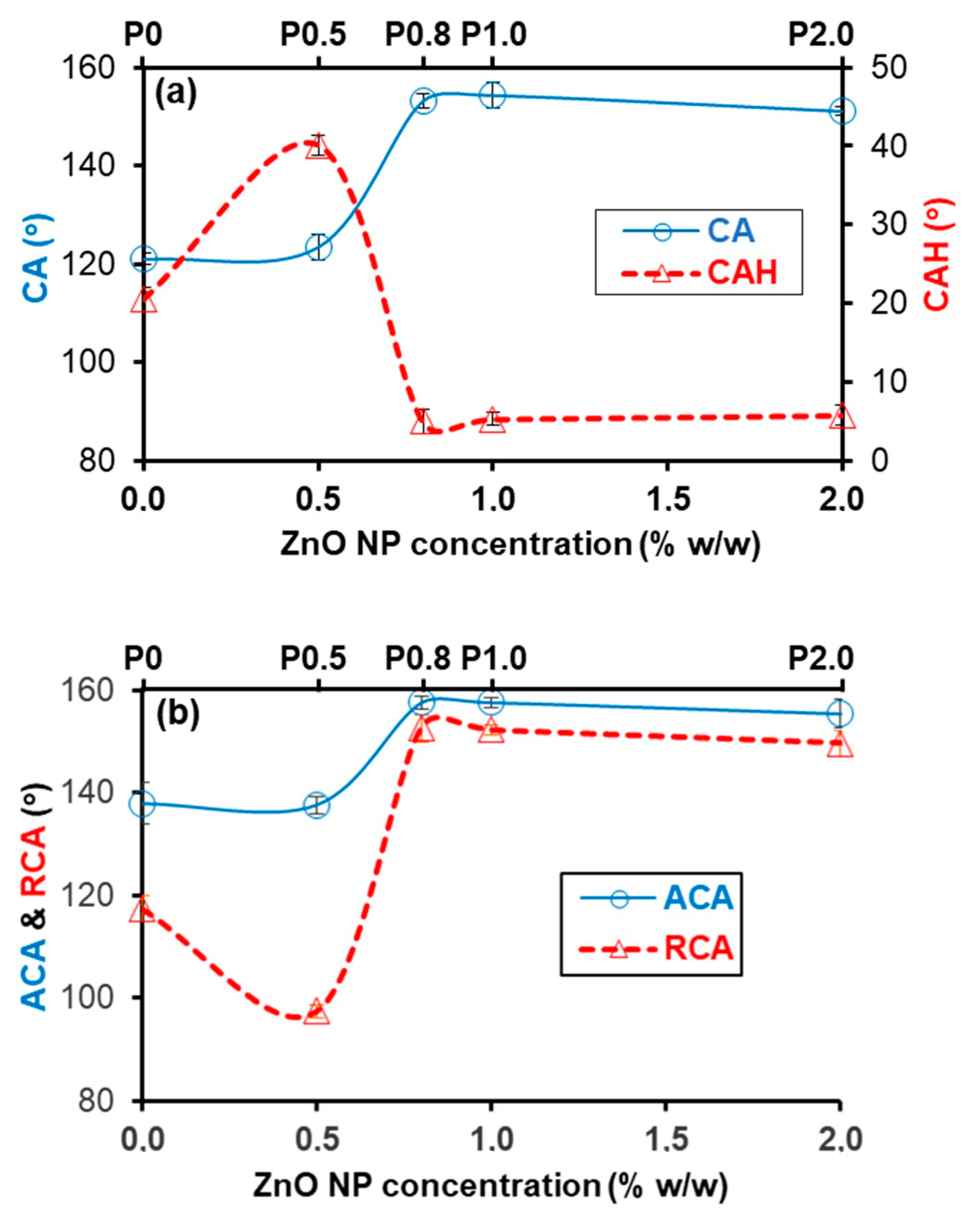
3.2. Selection of NP Concentration
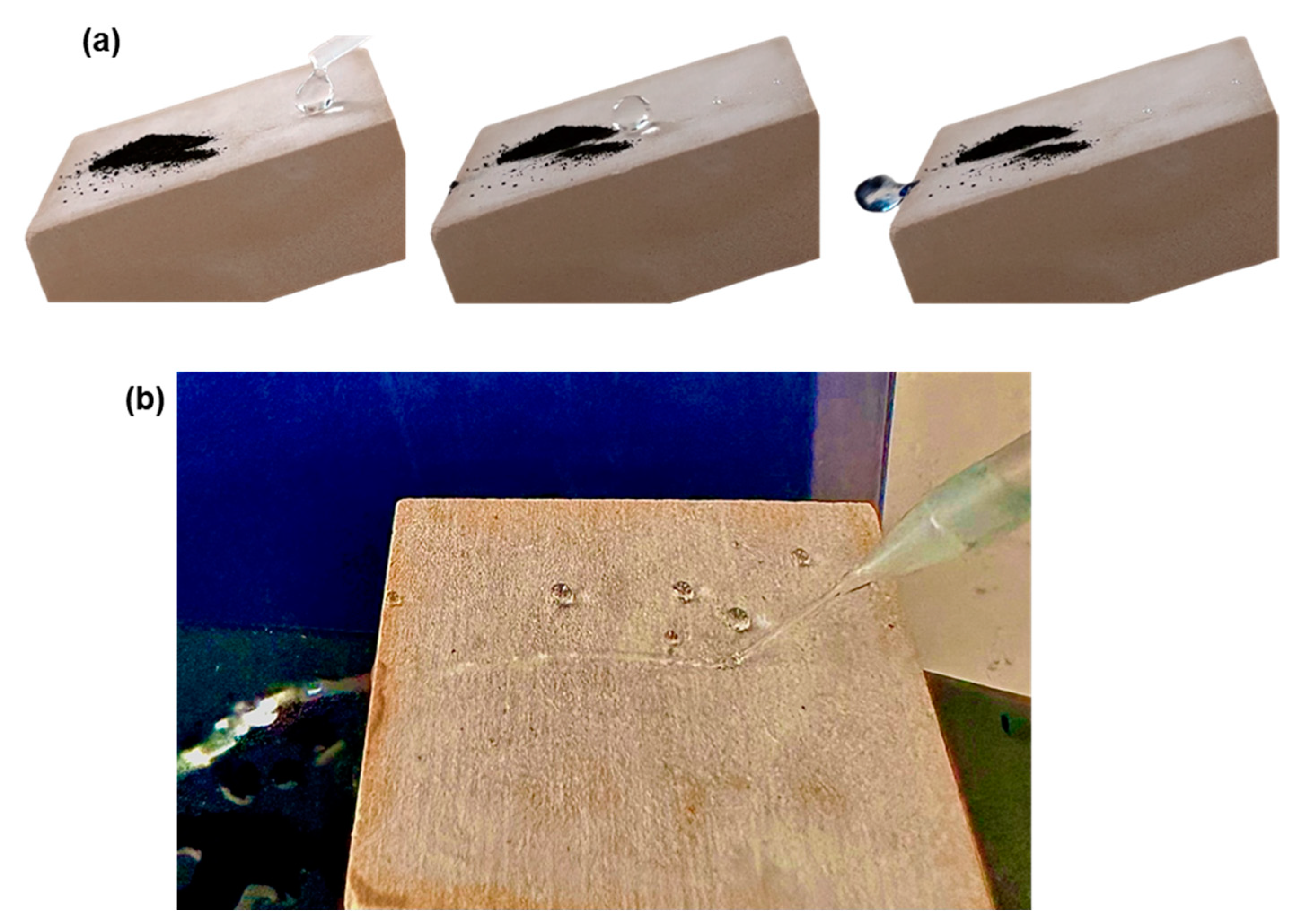
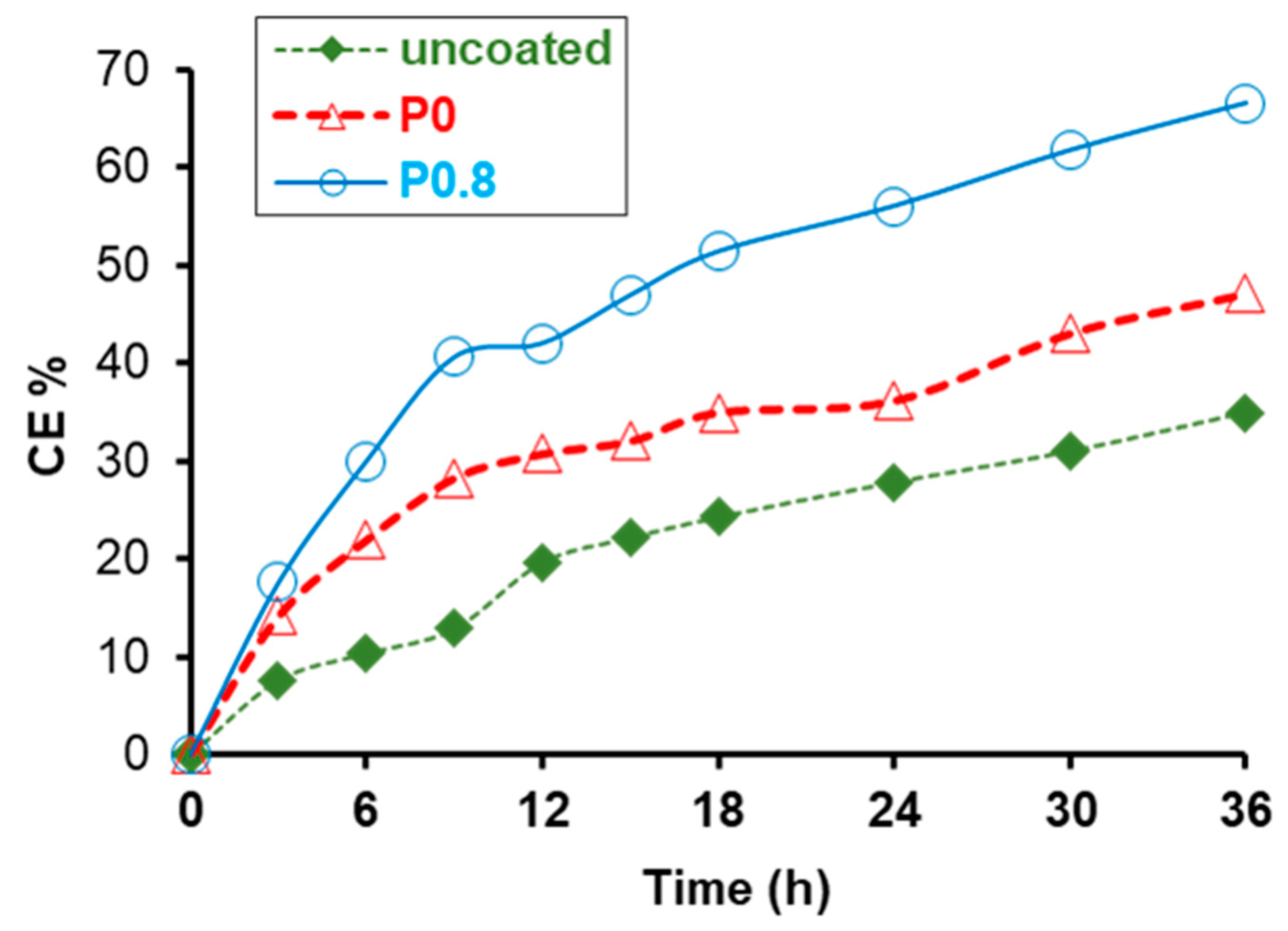
3.3. Chemical (Photocatalytic) Self-Cleaning and Biocidal Activity
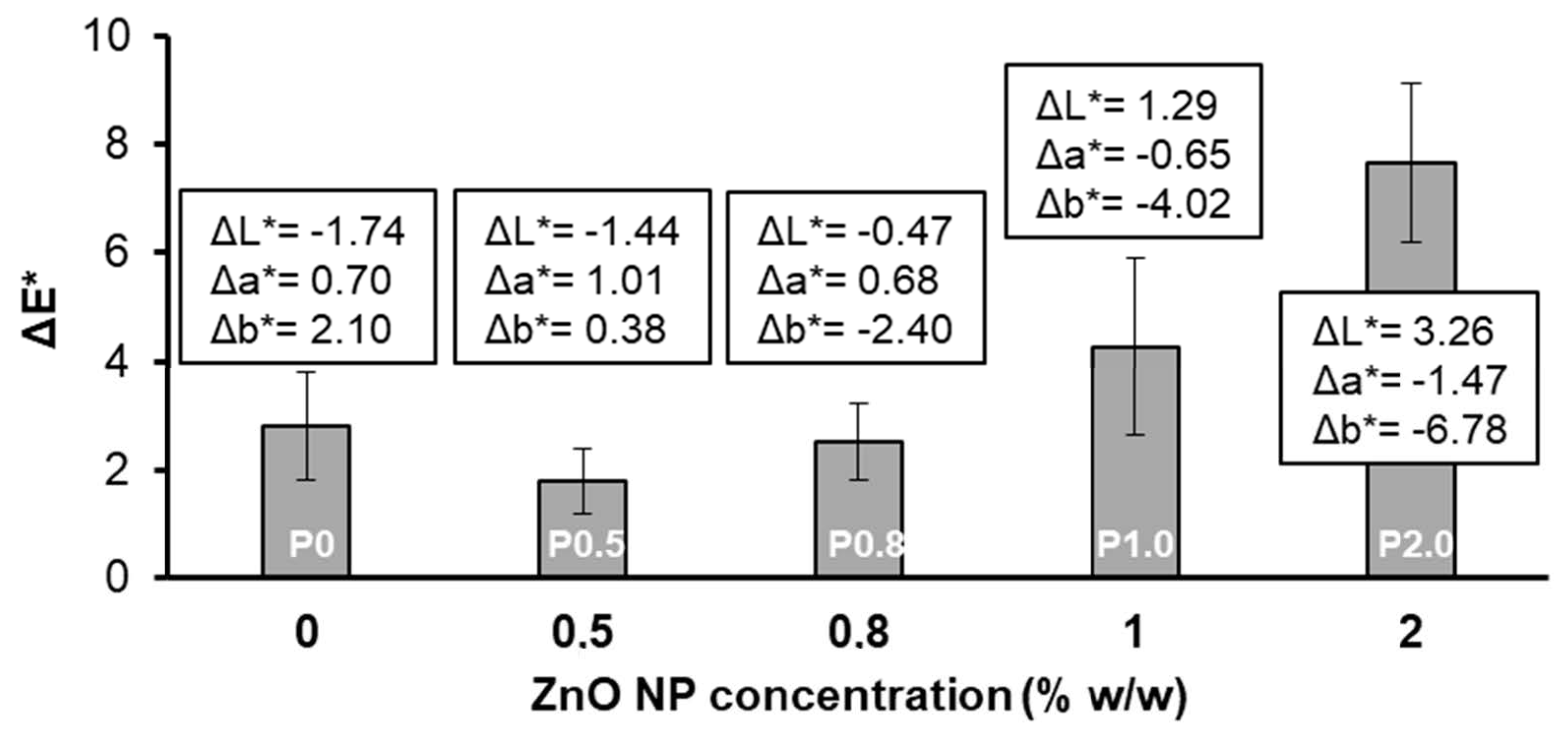
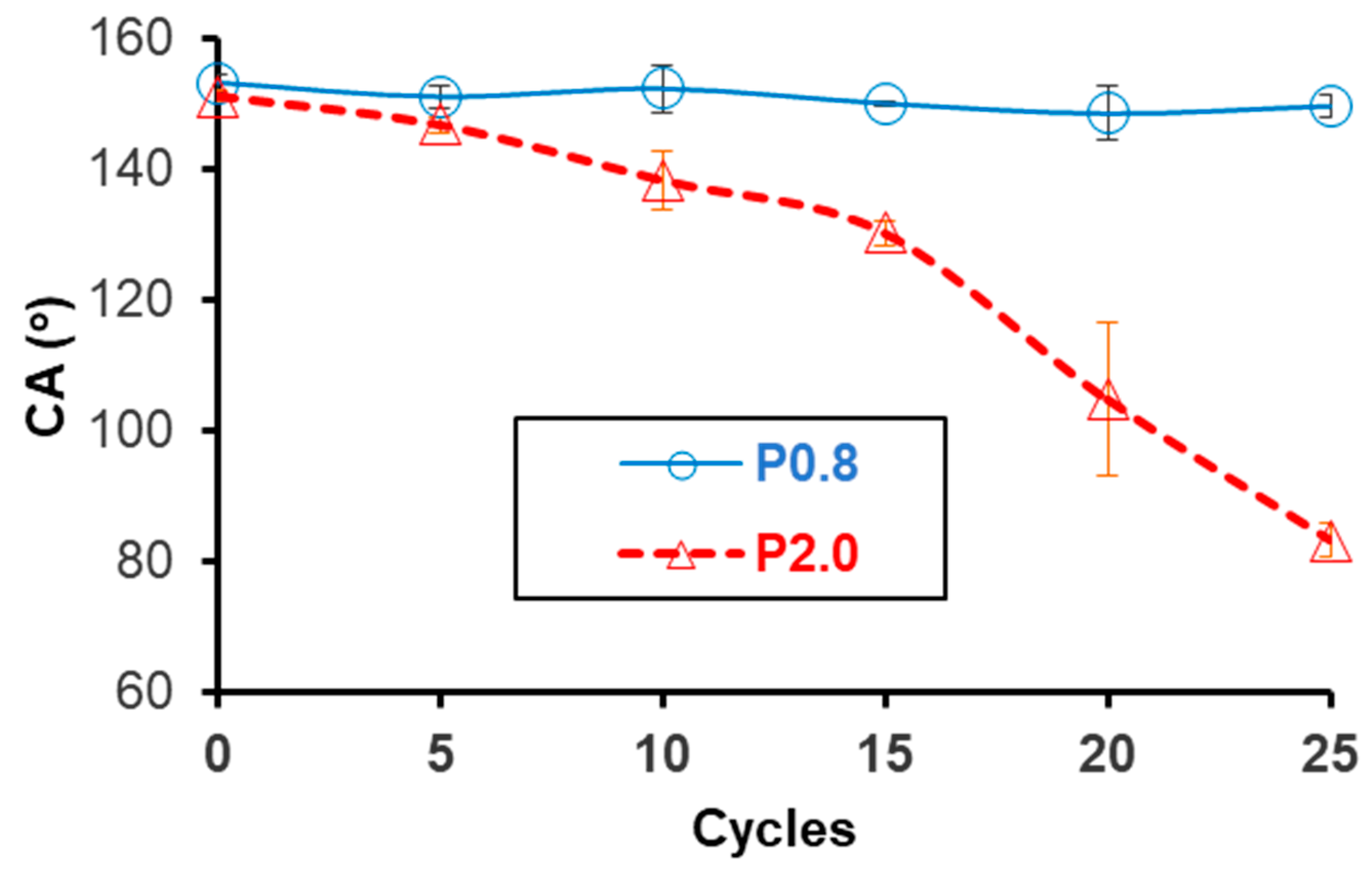
3.4. Other Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gomollón-Bel, F. IUPAC top ten emerging technologies in chemistry 2021: Breakthroughs for a circular, climate-neutral future. Chem. Int. 2021, 43, 13–20. [Google Scholar] [CrossRef]
- Khan, M.Z.; Militky, J.; Petru, M.; Tomková, B.; Ali, A.; Tören, E.; Perveen, S. Recent advances in superhydrophobic surfaces for practical applications: A review. Eur. Polym. J. 2022, 178, 111481. [Google Scholar] [CrossRef]
- Mei, J.; Guo, R.; Sun, Z. Tunable wettability on metal oxide surfaces for future applications. Coord. Chem. Rev. 2024, 510, 215843. [Google Scholar] [CrossRef]
- Pan, M.; Shao, H.; Fan, Y.; Yang, J.; Liu, J.; Deng, Z.; Liu, Z.; Chen, Z.; Zhang, J.; Yi, K.; et al. Superhydrophobic surface-assisted preparation of microspheres and supraparticles and their applications. Nano-Micro Lett. 2024, 16, 68. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Wang, Y.; Zhang, H.; An, K. Recent advances and strategies in mechanical stability of superhydrophobic surfaces. Prog. Org. Coat. 2024, 194, 108595. [Google Scholar] [CrossRef]
- Karapanagiotis, I.; Manoudis, P.N. Superhydrophobic and superamphiphobic materials for the conservation of natural stone: An overview. Constr. Build. Mater. 2022, 320, 126175. [Google Scholar] [CrossRef]
- Wheeler, G. Alkoxysilanes and the Consolidation of Stone; The Getty Conservation Institute: Los Angeles, CA, USA, 2005. [Google Scholar]
- Manoudis, P.N.; Karapanagiotis, I.; Tsakalof, A.; Zuburtikudis, I.; Panayiotou, C. Superhydrophobic composite films produced on various substrates. Langmuir 2008, 24, 11225–11232. [Google Scholar] [CrossRef] [PubMed]
- Karapanagiotis, I.; Manoudis, P. Superhydrophobic and Water Repellent Polymer-Nanoparticle Composite films. In Industrial Applications for Intelligent Polymers and Coatings; Hosseini, M., Makhlouf, A.S.H., Eds.; Springer: Cham, Switzerland, 2016; pp. 205–221. [Google Scholar]
- Facio, D.S.; Carrascosa, L.A.M.; Mosquera, M.J. Producing lasting amphiphobic building surfaces with self-cleaning properties. Nanotechnology 2017, 28, 265601. [Google Scholar] [CrossRef]
- Zarzuela, R.; Carbú, M.; Gil, M.L.A.; Cantoral, J.M.; Mosquera, M.J. Ormosils loaded with SiO2 nanoparticles functionalized with Ag as multifunctional superhydrophobic/biocidal/consolidant treatments for buildings conservation. Nanotechnology 2019, 30, 345701. [Google Scholar] [CrossRef]
- Aslanidou, D.; Karapanagiotis, I.; Lampakis, D. Waterborne superhydrophobic and superoleophobic coatings for the protection of marble and sandstone. Materials 2018, 11, 585. [Google Scholar] [CrossRef]
- Mosquera, M.J.; Carrascosa, L.A.M.; Badreldin, N. Producing superhydrophobic/oleophobic coatings on cultural heritage building materials. Pure Appl. Chem. 2018, 90, 551–561. [Google Scholar] [CrossRef]
- Elhaddad, F.; Luna, M.; Gemelli, G.M.C.; Gil, M.L.A.; Mosquera, M.J. Effectiveness and durability assessment, under extreme environmental conditions, of a superhydrophobic coating applied onto sandstone from Carteia roman archaeological site. Chem. Eng. Sci. 2023, 265, 118236. [Google Scholar] [CrossRef]
- Guo, L.; Wang, L.; Zhao, X.; Peng, M. Non-whitening superhydrophobic coating for heritage protection. Colloids Surf. A 2023, 676, 132294. [Google Scholar] [CrossRef]
- Wang, G.; Chai, Y.; Li, Y.; Luo, H.; Zhang, B.; Zhu, J. Sandstone protection by using nanocomposite coating of silica. Appl. Surf. Sci. 2023, 615, 156193. [Google Scholar] [CrossRef]
- Chatzigrigoriou, A.; Karapanagiotis, I.; Poulios, I. Superhydrophobic coatings based on siloxane resin and calcium hydroxide nanoparticles for marble protection. Coatings 2020, 10, 334. [Google Scholar] [CrossRef]
- Gkrava, E.; Tsiridis, V.; Manoudis, P.; Zorba, T.; Pavlidou, E.; Konstantinidis, A.; Karapantsios, T.D.; Spathis, P.K.; Karapanagiotis, I. A robust superhydrophobic coating of siloxane resin and hydrophobic calcium carbonate nanoparticles for limestone protection. Mater. Today Commun. 2024, 38, 108393. [Google Scholar] [CrossRef]
- Helmi, F.M.; Hefni, Y.K. Using nanocomposites in the consolidation and protection of sandstone. Int. J. Conserv. Sci. 2016, 7, 29–40. [Google Scholar]
- Hefni, Y.K. Hydrophobic zinc oxide nanocomposites for consolidation and protection of quartzite sculptures: A case study. J. Nano Res. 2020, 63, 64–75. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Kapridaki, C.; Maravelaki-Kalaitzaki, P. TiO2–SiO2–PDMS nano-composite hydrophobic coating with self-cleaning properties for marble protection. Prog. Org. Coat. 2013, 76, 400–410. [Google Scholar] [CrossRef]
- La Russa, M.F.; Rovella, N.; De Buergo, M.A.; Belfiore, C.M.; Pezzino, A.; Crisci, G.M.; Ruffolo, S.A. Nano-TiO2 coatings for cultural heritage protection: The role of the binder on hydrophobic and self-cleaning efficacy. Prog. Org. Coat. 2016, 91, 1–8. [Google Scholar] [CrossRef]
- Crupi, V.; Fazio, B.; Gessini, A.; Kis, Z.; La Russa, M.F.; Majolino, D.; Masciovecchio, C.; Ricca, M.; Rossi, B.; Ruffolo, S.A.; et al. TiO2–SiO2–PDMS nanocomposite coating with self-cleaning effect for stone material: Finding the optimal amount of TiO2. Constr. Build. Mater. 2018, 166, 464–471. [Google Scholar] [CrossRef]
- Colangiuli, D.; Lettieri, M.; Masieri, M.; Calia, A. Field study in an urban environment of simultaneous self-cleaning and hydrophobic nanosized TiO2-based coatings on stone for the protection of building surface. Sci. Total Environ. 2019, 650, 2919–2930. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Salvini, A.; Camaiti, M. Multi-functional TiO2-based nanocomposite coating with durable superhydrophobicity and enhanced photocatalytic and antimicrobial properties for the sustainable maintenance of building stones. Constr. Build. Mater. 2023, 404, 133139. [Google Scholar] [CrossRef]
- O’Neill, S.; Robertson, J.M.C.; Héquet, V.; Chazarenc, F.; Pang, X.; Ralphs, K.; Skillen, N.; Robertson, P.K.J. Comparison of titanium dioxide and zinc oxide photocatalysts for the inactivation of Escherichia coli in water using slurry and rotating-disk photocatalytic reactors. Ind. Eng. Chem. Res. 2023, 62, 18952–18959. [Google Scholar] [CrossRef]
- Tokarský, J.; Martinec, P.; Mamulová Kutláková, K.; Ovčačiková, H.; Študentová, S.; Ščučka, J. Photoactive and hydrophobic nano-ZnO/poly (alkyl siloxane) coating for the protection of sandstone. Constr. Build. Mater. 2019, 199, 549–559. [Google Scholar] [CrossRef]
- Speziale, A.; González-Sánchez, J.F.; Taşcı, B.; Pastor, A.; Sánchez, L.; Fernández-Acevedo, C.; Oroz-Mateo, T.; Salazar, C.; Navarro-Blasco, I.; Fernández, J.M.; et al. Development of multifunctional coatings for protecting stones and lime mortars of the architectural heritage. Int. J. Archit. Herit. 2020, 14, 1008–1029. [Google Scholar] [CrossRef]
- Weththimuni, M.L.; Chobba, M.B.; Sacchi, D.; Messaoud, M.; Licchelli, M. Durable polymer coatings: A comparative study of PDMS-based nanocomposites as protective coatings for stone materials. Chemistry 2022, 4, 60–76. [Google Scholar] [CrossRef]
- Tena-Santafé, V.M.; Fernández, J.M.; Fernández-Acevedo, C.; Oroz-Mateo, T.; Navarro-Blasco, Í.; Álvarez, J.I. Development of photocatalytic coatings for building materials with Bi2O3-ZnO nanoparticles. Catalysts 2023, 13, 1412. [Google Scholar] [CrossRef]
- De la Rosa-García, S.; Sierra-Fernández, A.; Solís, C.G.; García, N.S.; Quintana, P.; Gómez-Cornelio, S.; Fort, R. Fungal community dynamics on limestone at the Chichén Itzá archaeological site in Mexico driven by protective treatments. Sci. Total Environ. 2024, 906, 167563. [Google Scholar] [CrossRef]
- Izzi, M.; Sportelli, M.C.; Picca, R.A.; Cioffi, N. Electrochemical synthesis and analytical characterization of hybrid zinc/calcium antimicrobial nano-oxides for cultural heritage applications. ChemElectroChem 2023, 10, e202201132. [Google Scholar] [CrossRef]
- Lázaro-Mass, S.; De la Rosa-García, S.; García-Solis, C.; Reyes-Trujeque, J.; Soria-Castro, M.; Fuentes, A.F.; Quintana, P.; Gómez-Cornelio, S. Controlling growth of phototrophic biofilms on limestone using CaZn2(OH)6·2H2O and ZnO nanoparticles. J. Chem. Technol. Biotechnol. 2022, 97, 3011–3023. [Google Scholar] [CrossRef]
- Schifano, E.; Cavallini, D.; De Bellis, G.; Bracciale, M.P.; Felici, A.C.; Santarelli, M.L.; Sarto, M.S.; Uccelletti, D. Antibacterial effect of zinc oxide-based nanomaterials on environmental biodeteriogens affecting historical building. Nanomaterials 2020, 10, 335. [Google Scholar] [CrossRef]
- Becerra, J.; Ortiz, P.; Zaderenko, A.P.; Karapanagiotis, I. Assessment of nanoparticles/nanocomposites to inhibit micro-algal fouling on limestone façades. Build. Res. Inf. 2020, 48, 180–190. [Google Scholar] [CrossRef]
- Ditaranto, N.; Van der Werf, I.D.; Picca, R.A.; Sportelli, M.C.; Giannossa, L.C.; Bonerba, E.; Tantillo, G.; Sabbatini, L. Characterization and behaviour of ZnO-based nanocomposites designed for the control of biodeterioration of patrimonial stone works. New J. Chem. 2015, 39, 6836–6843. [Google Scholar] [CrossRef]
- Sierra-Fernandez, A.; De la Rosa-García, S.C.; Gomez-Villalba, L.S.; Gómez-Cornelio, S.; Rabanal, M.E.; Fort, R.; Quintana, P. Synthesis, photocatalytic, and antifungal properties of MgO, ZnO and Zn/Mg oxide nanoparticles for the protection of calcareous stone heritage. ACS Appl. Mater. Interfaces 2017, 9, 24873–24886. [Google Scholar] [CrossRef]
- Aldosari, M.A.; Darwish, S.S.; Adam, M.A.; Elmarzugi, N.A.; Ahmed, S.M. Using ZnO nanoparticles in fungal inhibition and self-protection of exposed marble columns in historic sites. Archaeol. Anthr. Sci. 2019, 11, 3407–3422. [Google Scholar] [CrossRef]
- Ruffolo, S.A.; La Russa, M.F.; Malagodi, M.; Oliviero Rossi, C.; Palermo, A.M.; Crisci, G.M. ZnO and ZnTiO3 nanopowders for antimicrobial stone coating. Appl. Phys. A 2010, 100, 829–834. [Google Scholar] [CrossRef]
- ASTM D3359-97; Standard Test Methods for Measuring Adhesion by Tape Test. ASTM International: West Conshohocken, PA, USA, 1997.
- Basu, B.J.; Hariprakash, V.; Aruna, S.T.; Lakshmi, R.V.; Manasa, J.; Shruthi, B.S. Effect of microstructure and surface roughness on the wettability of superhydrophobic sol–gel nanocomposite coatings. J. Sol-Gel Sci. Technol. 2010, 56, 278–286. [Google Scholar] [CrossRef]
- Baba, E.M.; Cansoy, C.E.; Zayim, E.O. Investigation of wettability and optical properties of superhydrophobic polystyrene-SiO2 composite surfaces. Prog. Org. Coat. 2016, 99, 378–385. [Google Scholar] [CrossRef]
- Furmidge, C.G.L. Studies at phase interfaces I. The sliding of liquid drops on solid surfaces and a theory for spray retention. J. Colloid Sci. 1962, 17, 309–324. [Google Scholar] [CrossRef]
- Chen, W.; Fadeev, A.Y.; Hsieh, M.C.; Oner, D.; Youngblood, J.; McCarthy, T.J. Ultrahydrophobic and ultralyophobic surfaces: Some comments and examples. Langmuir 1999, 15, 3395–3399. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Evaluation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Wang, H.; Luo, G.; Chen, L.; Song, Y.; Liu, C.; Wu, L. Preparation of a bionic lotus leaf microstructured surface and its drag reduction performance. RSC Adv. 2022, 12, 16723–16731. [Google Scholar] [CrossRef]
- Wang, P.; Li, C.; Zhang, D. Recent advances in chemical durability and mechanical stability of superhydrophobic materials: Multi-strategy design and strengthening. J. Mater. Sci. Technol. 2022, 129, 40–69. [Google Scholar] [CrossRef]
- Rodrigues, J.D.; Grossi, A. Indicators and ratings for the compatibility assessment of conservation actions. J. Cult. Herit. 2007, 8, 32–43. [Google Scholar] [CrossRef]
- Lettieri, M.; Masieri, M.; Frigione, M. Novel nano-filled coatings for the protection of built heritage stone surfaces. Nanomaterials 2021, 11, 301. [Google Scholar] [CrossRef]
- Lettieri, M.; Masieri, M.; Aquaro, M.; Dilorenzo, D.; Frigione, M. Eco-friendly protective coating to extend the life of art-works and structures made in porous stone materials. Coatings 2021, 11, 1270. [Google Scholar] [CrossRef]
- Manoudis, P.N.; Zuburtikudis, I.; Khalifeh, H.A.; Karapanagiotis, I. Robust and transparent smooth amphi-repellent coating with physical and chemical self-cleaning properties. Surf. Interfaces 2024, 52, 104928. [Google Scholar] [CrossRef]
- Elhaddad, F.; Carrascosa, L.A.M.; Mosquera, M.J. Long-term effectiveness, under a coastal environment, of a novel conservation nanomaterial applied on sandstone from a Roman archaeological site. J. Cult. Herit. 2018, 34, 208–217. [Google Scholar] [CrossRef]



| Liquid | (mJ m−2) | (mJ m−2) | (mJ m−2) | CA (°) |
|---|---|---|---|---|
| Water | 21.8 | 51.0 | 72.8 | 153.2 ± 1.4 |
| Diiodomethane | 49.5 | 1.3 | 50.8 | 129.5 ± 2.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manoudis, P.N.; Zuburtikudis, I.; Konstantopoulos, G.; Khalifeh, H.A.; Kottaridi, C.; Karapanagiotis, I. Superhydrophobicity, Photocatalytic Self-Cleaning and Biocidal Activity Combined in a Siloxane-ZnO Composite for the Protection of Limestone. Biomimetics 2024, 9, 573. https://doi.org/10.3390/biomimetics9090573
Manoudis PN, Zuburtikudis I, Konstantopoulos G, Khalifeh HA, Kottaridi C, Karapanagiotis I. Superhydrophobicity, Photocatalytic Self-Cleaning and Biocidal Activity Combined in a Siloxane-ZnO Composite for the Protection of Limestone. Biomimetics. 2024; 9(9):573. https://doi.org/10.3390/biomimetics9090573
Chicago/Turabian StyleManoudis, Panagiotis N., Ioannis Zuburtikudis, Georgios Konstantopoulos, Hadil Abu Khalifeh, Christine Kottaridi, and Ioannis Karapanagiotis. 2024. "Superhydrophobicity, Photocatalytic Self-Cleaning and Biocidal Activity Combined in a Siloxane-ZnO Composite for the Protection of Limestone" Biomimetics 9, no. 9: 573. https://doi.org/10.3390/biomimetics9090573
APA StyleManoudis, P. N., Zuburtikudis, I., Konstantopoulos, G., Khalifeh, H. A., Kottaridi, C., & Karapanagiotis, I. (2024). Superhydrophobicity, Photocatalytic Self-Cleaning and Biocidal Activity Combined in a Siloxane-ZnO Composite for the Protection of Limestone. Biomimetics, 9(9), 573. https://doi.org/10.3390/biomimetics9090573









