Functionalized Gold Nanoparticles for Facile Pattern-Controlled Surface Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. B-AuNP Synthesis and Characterization
2.2. Adhesive AuNP Activation and Controllable Surface Coating
2.3. Image Process and Statistical Analysis
3. Results and Discussion
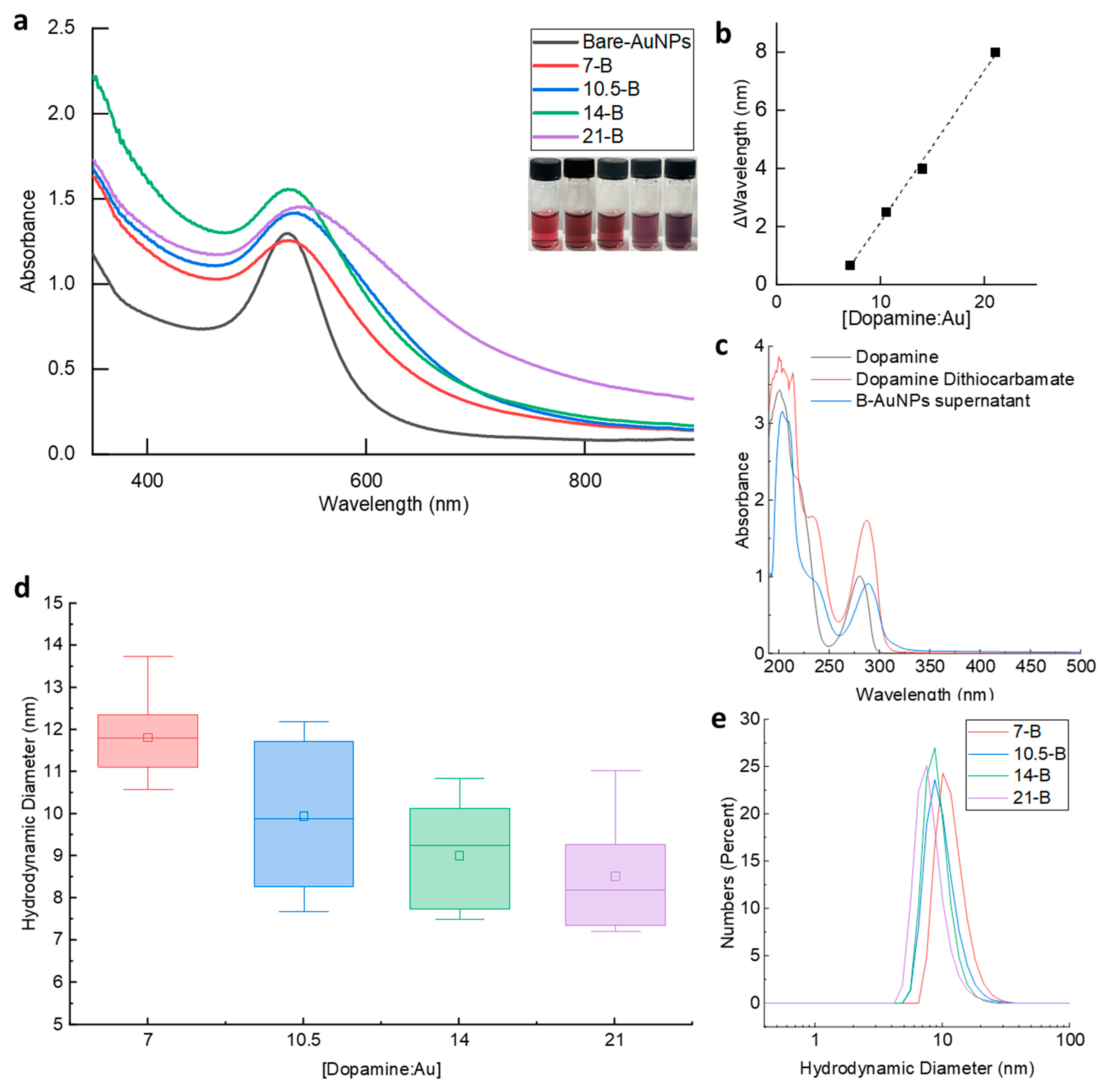
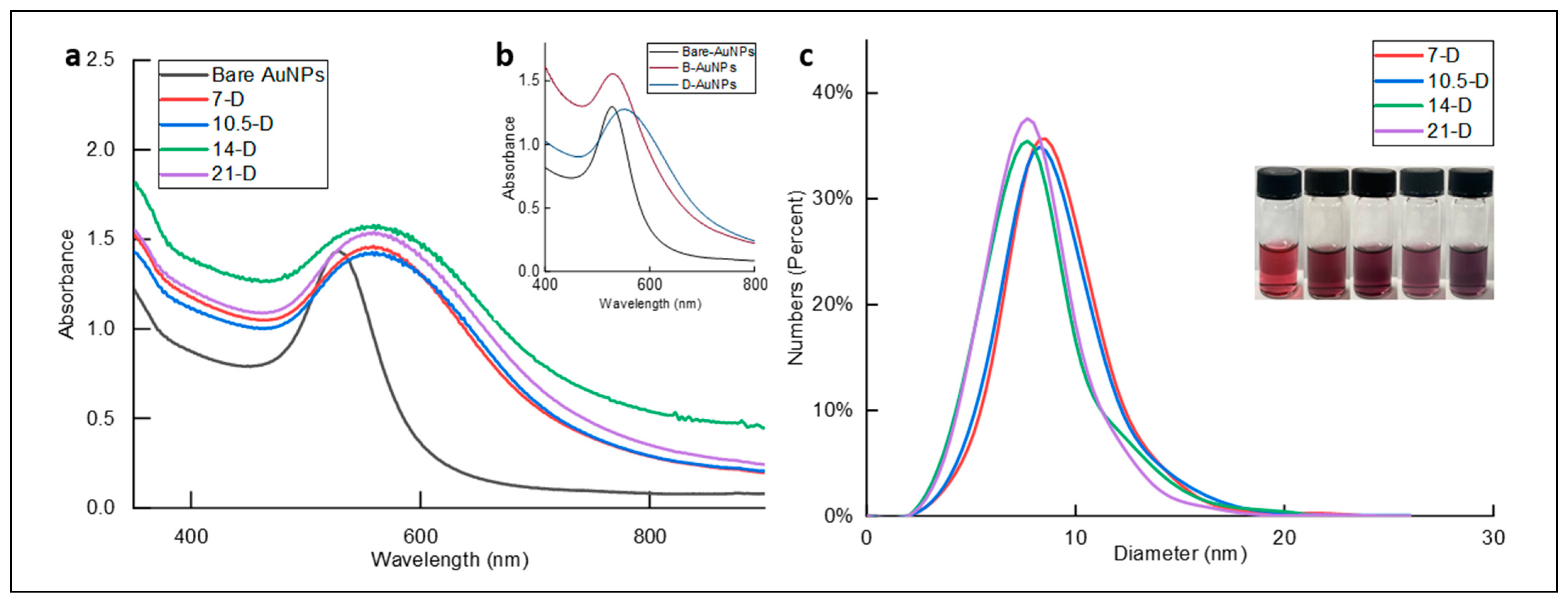
3.1. Synthesis of Dopamine-Grafted AuNPs with Varied Dopamine Densities
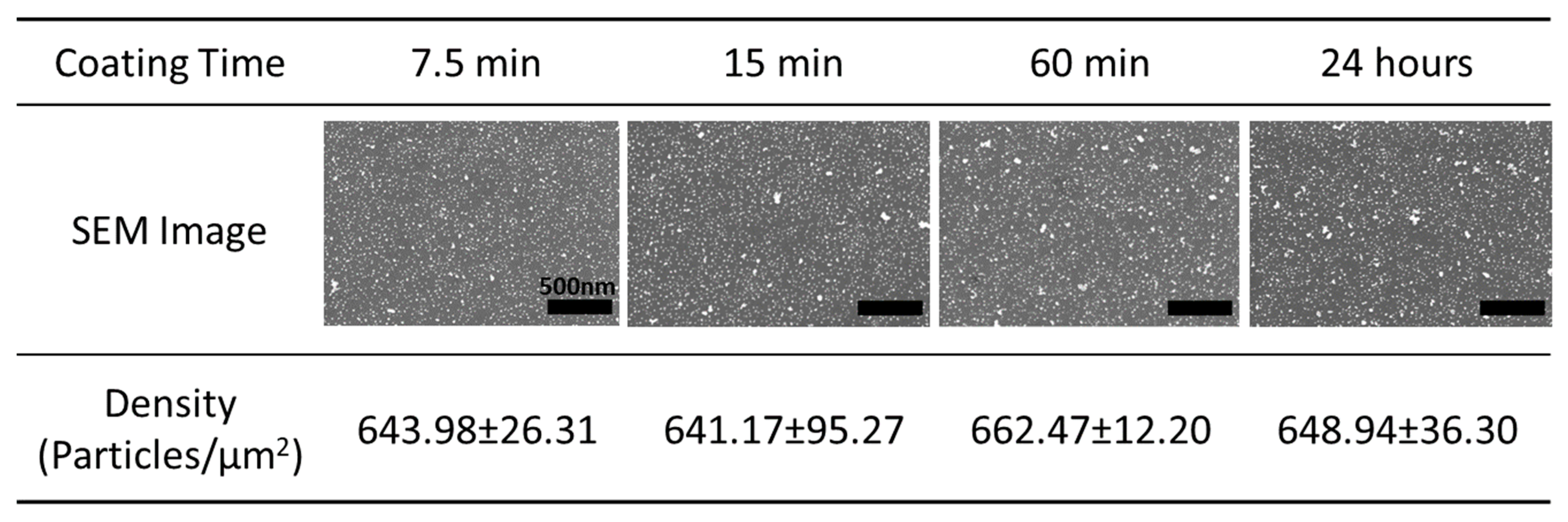
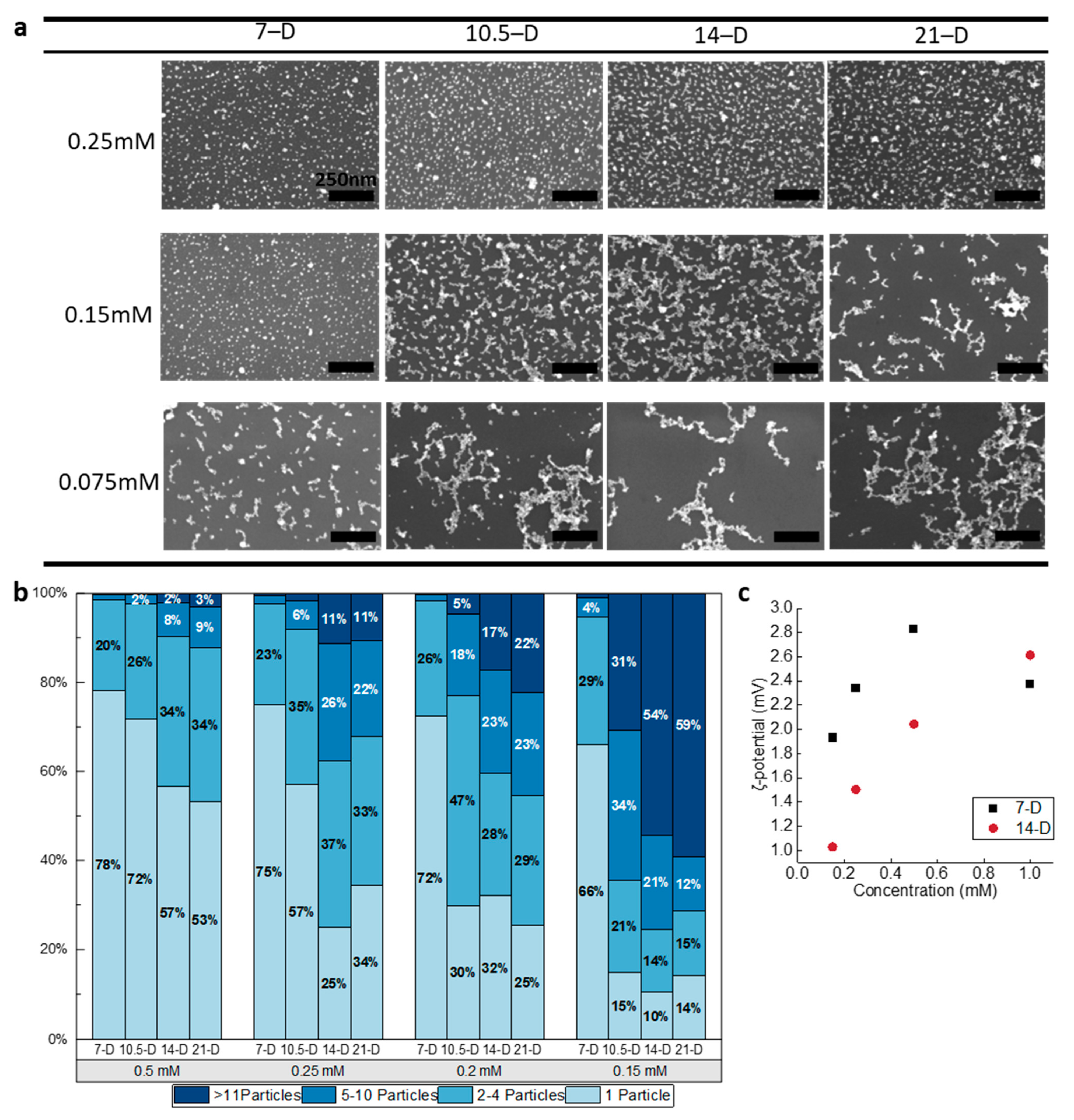
3.2. Surface Coatings Using D-AuNPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fleischer, M.; Zhang, D.; Braun, K.; Jager, S.; Ehlich, R.; Haffner, M.; Stanciu, C.; Horber, J.K.; Meixner, A.J.; Kern, D.P. Tailoring gold nanostructures for near-field optical applications. Nanotechnology 2010, 21, 065301. [Google Scholar] [CrossRef]
- Abdelhalim, M.A.K.; Mady, M.M.; Ghannam, M.M. Physical properties of different gold nanoparticles: Ultraviolet-visible and fluorescence measurements. J. Nanomed. Nanotechnol. 2012, 3, 178–194. [Google Scholar] [CrossRef]
- Amina, S.J.; Guo, B. A Review on the Synthesis and Functionalization of Gold Nanoparticles as a Drug Delivery Vehicle. Int. J. Nanomed. 2020, 15, 9823–9857. [Google Scholar] [CrossRef]
- Wozniak, A.; Malankowska, A.; Nowaczyk, G.; Grzeskowiak, B.F.; Tusnio, K.; Slomski, R.; Zaleska-Medynska, A.; Jurga, S. Size and shape-dependent cytotoxicity profile of gold nanoparticles for biomedical applications. J. Mater. Sci. Mater. Med. 2017, 28, 92. [Google Scholar] [CrossRef]
- Xie, X.; Liao, J.; Shao, X.; Li, Q.; Lin, Y. The Effect of shape on Cellular Uptake of Gold Nanoparticles in the forms of Stars, Rods, and Triangles. Sci. Rep. 2017, 7, 3827. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Bansal, V.; Chaudhary, M.; Basu, A.; Bhonde, R.R.; Sastry, M.J.L. Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: A microscopic overview. Langmuir 2005, 21, 10644–10654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shareena Dasari, T.P.; Deng, H.; Yu, H. Antimicrobial Activity of Gold Nanoparticles and Ionic Gold. J. Environ. Sci. Health Part C 2015, 33, 286–327. [Google Scholar] [CrossRef]
- Abdalla, S.S.I.; Katas, H.; Azmi, F.; Busra, M.F.M. Antibacterial and Anti-Biofilm Biosynthesised Silver and Gold Nanoparticles for Medical Applications: Mechanism of Action, Toxicity and Current Status. Curr. Drug Deliv. 2020, 17, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Surendran, S.P.; Moon, M.J.; Park, R.; Jeong, Y.Y. Bioactive Nanoparticles for Cancer Immunotherapy. Int. J. Mol. Sci. 2018, 19, 3877. [Google Scholar] [CrossRef]
- Panahi, Y.; Mohammadhosseini, M.; Nejati-Koshki, K.; Abadi, A.J.; Moafi, H.F.; Akbarzadeh, A.; Farshbaf, M. Preparation, Surface Properties, and Therapeutic Applications of Gold Nanoparticles in Biomedicine. Drug Res. 2017, 67, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Falahati, M.; Attar, F.; Sharifi, M.; Saboury, A.A.; Salihi, A.; Aziz, F.M.; Kostova, I.; Burda, C.; Priecel, P.; Lopez-Sanchez, J.A.; et al. Gold nanomaterials as key suppliers in biological and chemical sensing, catalysis, and medicine. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2020, 1864, 129435. [Google Scholar] [CrossRef]
- Lou-Franco, J.; Das, B.; Elliott, C.; Cao, C. Gold Nanozymes: From Concept to Biomedical Applications. Nano-Micro Lett. 2020, 13, 10. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Y.; Chen, Y.; Liu, L.; Mo, A.; Peng, Q. Nanomaterials-based photothermal therapy and its potentials in antibacterial treatment. J. Control. Release 2020, 328, 251–262. [Google Scholar] [CrossRef]
- Joshi, A.S.; Singh, P.; Mijakovic, I. Interactions of Gold and Silver Nanoparticles with Bacterial Biofilms: Molecular Interactions behind Inhibition and Resistance. Int. J. Mol. Sci. 2020, 21, 7658. [Google Scholar] [CrossRef]
- Fang, P.-P.; Chen, S.; Deng, H.; Scanlon, M.D.; Gumy, F.; Lee, H.J.; Momotenko, D.; Amstutz, V.; Cortés-Salazar, F.; Pereira, C.M. Conductive gold nanoparticle mirrors at liquid/liquid interfaces. ACS Nano 2013, 7, 9241–9248. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef]
- Jawad, M.; Khan, A.F.; Waseem, A.; Kamboh, A.H.; Mohsin, M.; Shahzad, S.A.; Shah, S.H.; Mathur, S.; Shaikh, A.J. Effect of gold nanoparticles on transmittance and conductance of graphene oxide thin films and efficiency of perovskite solar cells. Appl. Nanosci. 2019, 10, 485–497. [Google Scholar] [CrossRef]
- Driskell, J.D.; Lipert, R.J.; Porter, M.D. Labeled gold nanoparticles immobilized at smooth metallic substrates: Systematic investigation of surface plasmon resonance and surface-enhanced Raman scattering. J. Phys. Chem. B 2006, 110, 17444–17451. [Google Scholar] [CrossRef] [PubMed]
- Moirangthem, R.S.; Chang, Y.-C.; Wei, P.-K. Ellipsometry study on gold-nanoparticle-coated gold thin film for biosensing application. Biomed. Opt. Express 2011, 2, 2569–2576. [Google Scholar] [CrossRef] [PubMed]
- Indrasekara, A.S.; Meyers, S.; Shubeita, S.; Feldman, L.C.; Gustafsson, T.; Fabris, L. Gold nanostar substrates for SERS-based chemical sensing in the femtomolar regime. Nanoscale 2014, 6, 8891–8899. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Agrawal, M.; Uhlmann, P.; Simon, F.; Oertel, U.; Stamm, M.J.M. Gold nanoparticles immobilized on stimuli responsive polymer brushes as nanosensors. Macromolecules 2008, 41, 8152–8158. [Google Scholar] [CrossRef]
- Cheng, H.-W.; Huan, S.-Y.; Wu, H.-L.; Shen, G.-L.; Yu, R.-Q. Surface-enhanced Raman spectroscopic detection of a bacteria biomarker using gold nanoparticle immobilized substrates. Anal. Chem. 2009, 81, 9902–9912. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Ma, H.; Xiao, S.; Shen, M.; Guo, R.; Cao, X.; Shi, X. Facile immobilization of gold nanoparticles into electrospun polyethyleneimine/polyvinyl alcohol nanofibers for catalytic applications. J. Mater. Chem. 2011, 21, 4493–4501. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, K.; Bai, Y.; Yang, W.; Sun, C. Glucose oxidase/colloidal gold nanoparticles immobilized in Nafion film on glassy carbon electrode: Direct electron transfer and electrocatalysis. Bioelectrochemistry 2006, 69, 158–163. [Google Scholar] [CrossRef]
- Villa, J.E.L.; Santos, D.P.D.; Poppi, R.J. Fabrication of gold nanoparticle-coated paper and its use as a sensitive substrate for quantitative SERS analysis. Microchim. Acta 2016, 183, 2745–2752. [Google Scholar] [CrossRef]
- Vossmeyer, T.; Stolte, C.; Ijeh, M.; Kornowski, A.; Weller, H. Networked Gold-Nanoparticle Coatings on Polyethylene: Charge Transport and Strain Sensitivity. Adv. Funct. Mater. 2008, 18, 1611–1616. [Google Scholar] [CrossRef]
- Tiwari, P.M.; Vig, K.; Dennis, V.A.; Singh, S.R. Functionalized Gold Nanoparticles and Their Biomedical Applications. Nanomaterials 2011, 1, 31–63. [Google Scholar] [CrossRef]
- Zeng, S.; Yong, K.-T.; Roy, I.; Dinh, X.-Q.; Yu, X.; Luan, F. A Review on Functionalized Gold Nanoparticles for Biosensing Applications. Plasmonics 2011, 6, 491–506. [Google Scholar] [CrossRef]
- Chen, Y.; Xianyu, Y.; Jiang, X. Surface Modification of Gold Nanoparticles with Small Molecules for Biochemical Analysis. Acc. Chem. Res. 2017, 50, 310–319. [Google Scholar] [CrossRef]
- Khongkow, M.; Yata, T.; Boonrungsiman, S.; Ruktanonchai, U.R.; Graham, D.; Namdee, K. Surface modification of gold nanoparticles with neuron-targeted exosome for enhanced blood-brain barrier penetration. Sci. Rep. 2019, 9, 8278. [Google Scholar] [CrossRef]
- Wang, S.; Yan, C.; Zhang, X.; Shi, D.; Chi, L.; Luo, G.; Deng, J. Antimicrobial peptide modification enhances the gene delivery and bactericidal efficiency of gold nanoparticles for accelerating diabetic wound healing. Biomater. Sci. 2018, 6, 2757–2772. [Google Scholar] [CrossRef]
- Mahato, K.; Nagpal, S.; Shah, M.A.; Srivastava, A.; Maurya, P.K.; Roy, S.; Jaiswal, A.; Singh, R.; Chandra, P. Gold nanoparticle surface engineering strategies and their applications in biomedicine and diagnostics. 3 Biotech 2019, 9, 57. [Google Scholar] [CrossRef]
- Filbrun, S.L.; Filbrun, A.B.; Lovato, F.L.; Oh, S.H.; Driskell, E.A.; Driskell, J.D. Chemical modification of antibodies enables the formation of stable antibody-gold nanoparticle conjugates for biosensing. Analyst 2017, 142, 4456–4467. [Google Scholar] [CrossRef]
- Su, H.; Sun, B.; Chen, L.; Xu, Z.; Ai, S. Colorimetric sensing of dopamine based on the aggregation of gold nanoparticles induced by copper ions. Anal. Methods 2012, 4, 3981–3986. [Google Scholar] [CrossRef]
- Tang, J.; Wu, P.; Hou, X.; Xu, K. Modification-free and N-acetyl-L-cysteine-induced colorimetric response of AuNPs: A mechanistic study and sensitive Hg(2+) detection. Talanta 2016, 159, 87–92. [Google Scholar] [CrossRef]
- Li, X.Y.; Feng, F.Y.; Zhou, X.D.; Hu, J.M. Rational design of an anchoring peptide for high-efficiency and quantitative modification of peptides and DNA strands on gold nanoparticles. Nanoscale 2018, 10, 11491–11497. [Google Scholar] [CrossRef]
- Garcia Calavia, P.; Bruce, G.; Perez-Garcia, L.; Russell, D.A. Photosensitiser-gold nanoparticle conjugates for photodynamic therapy of cancer. Photochem. Photobiol. Sci. 2018, 17, 1534–1552. [Google Scholar] [CrossRef] [PubMed]
- Waite, J.H. Nature’s underwater adhesive specialist. Int. J. Adhes. Adhes. 1987, 7, 9–14. [Google Scholar] [CrossRef]
- Waite, J.H.; Tanzer, M.L.J.S. Polyphenolic substance of Mytilus edulis: Novel adhesive containing L-dopa and hydroxyproline. Science 1981, 212, 1038–1040. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.A.; Park, E.; Lee, H. Polydopamine and Its Derivative Surface Chemistry in Material Science: A Focused Review for Studies at KAIST. Adv. Mater. 2020, 32, e1907505. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine Surface Chemistry: A Decade of Discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Pérez-Segarra, W.; Shi, Q.; Wei, A. Dithiocarbamate assembly on gold. J. Am. Chem. Soc. 2005, 127, 7328–7329. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Yang, F.; Wang, X.; Liang, J.F. Adhesive Gold Nanoparticles for Easy and Controlled Surface Coating. Langmuir 2019, 35, 2728–2737. [Google Scholar] [CrossRef] [PubMed]
- Englebienne, P.; Van Hoonacker, A.; Verhas, M.J.S. Surface plasmon resonance: Principles, methods and applications in biomedical sciences. Spectroscopy 2003, 17, 255–273. [Google Scholar] [CrossRef]
- Jans, H.; Liu, X.; Austin, L.; Maes, G.; Huo, Q. Dynamic light scattering as a powerful tool for gold nanoparticle bioconjugation and biomolecular binding studies. Anal. Chem. 2009, 81, 9425–9432. [Google Scholar] [CrossRef]
- Vigil, G.; Xu, Z.; Steinberg, S.; Israelachvili, J. Interactions of silica surfaces. J. Colloid Interface Sci. 1994, 165, 367–385. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Liang, J. Functionalized Gold Nanoparticles for Facile Pattern-Controlled Surface Coatings. Biomimetics 2024, 9, 146. https://doi.org/10.3390/biomimetics9030146
Wang J, Liang J. Functionalized Gold Nanoparticles for Facile Pattern-Controlled Surface Coatings. Biomimetics. 2024; 9(3):146. https://doi.org/10.3390/biomimetics9030146
Chicago/Turabian StyleWang, Jue, and Junfeng Liang. 2024. "Functionalized Gold Nanoparticles for Facile Pattern-Controlled Surface Coatings" Biomimetics 9, no. 3: 146. https://doi.org/10.3390/biomimetics9030146
APA StyleWang, J., & Liang, J. (2024). Functionalized Gold Nanoparticles for Facile Pattern-Controlled Surface Coatings. Biomimetics, 9(3), 146. https://doi.org/10.3390/biomimetics9030146






