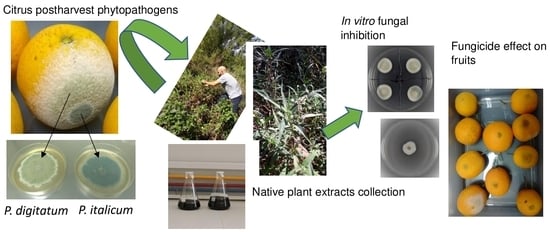
Natural Products Obtained from Argentinean Native Plants Are Fungicidal against Citrus Postharvest Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction Process
2.3. Fungal Strains
2.4. Molecular Characterization
2.5. In Vitro Susceptibility Test
2.6. Fungicidal Assays on Wounded Oranges Using S. pilcomayense Methanolic Extract
2.7. Statistical Analysis
3. Results
3.1. Plant Extract Yields
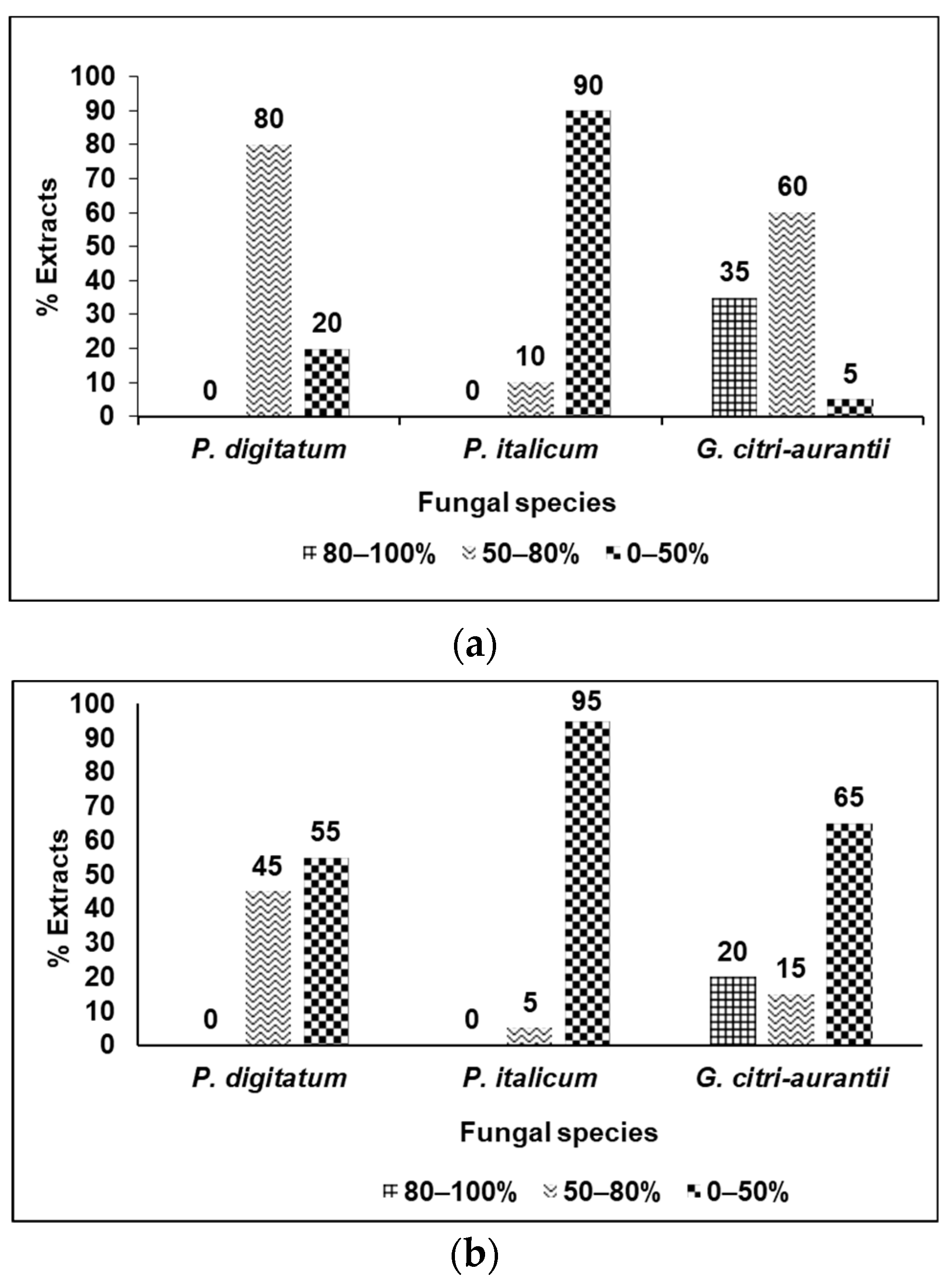
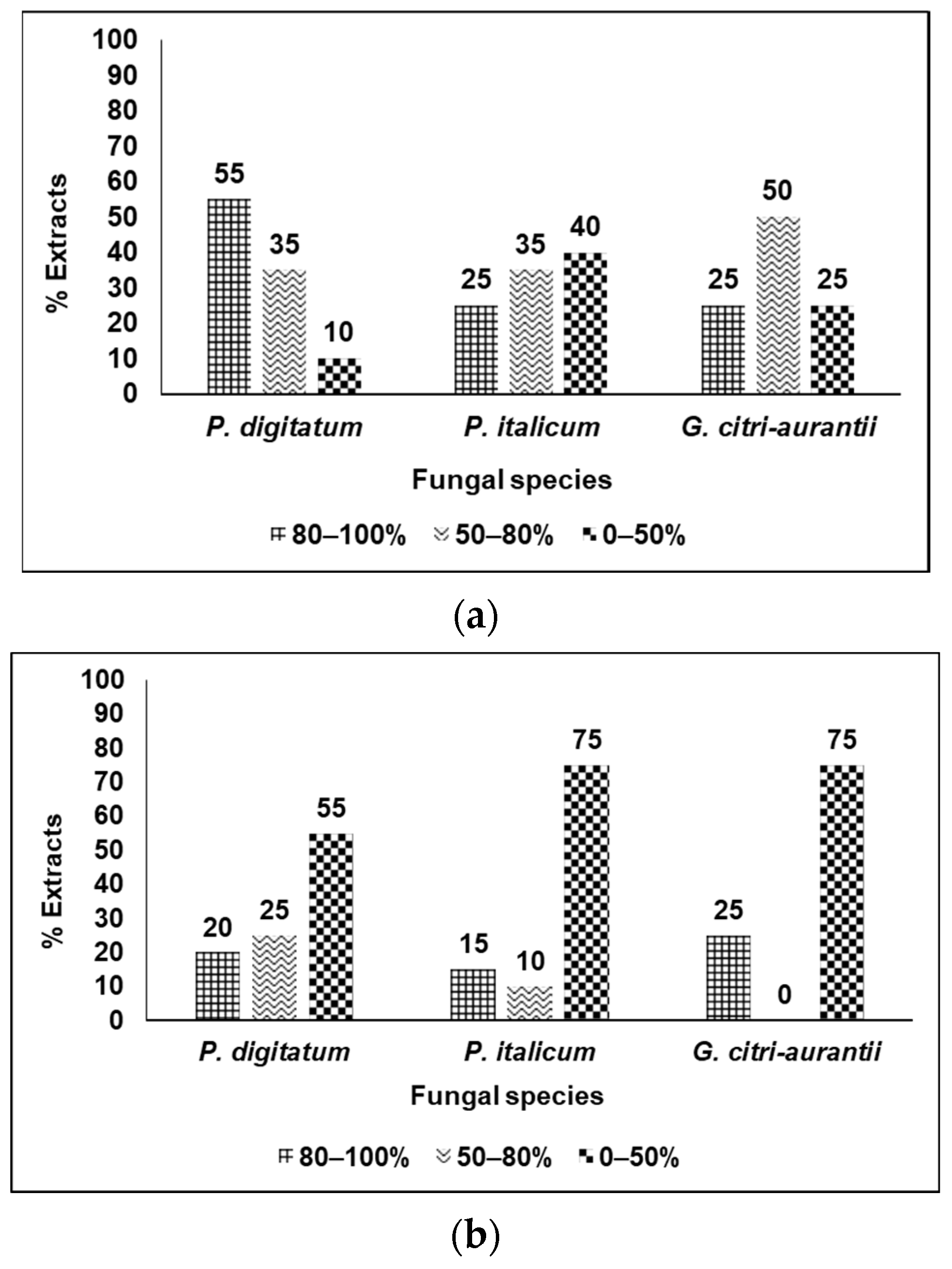
3.2. Fungicidal Evaluation of the Selected Plant Extracts against P. digitatum, P. italicum, and G. citri-aurantii
3.3. Analysis of the Results by Categories of Fungicidal Action
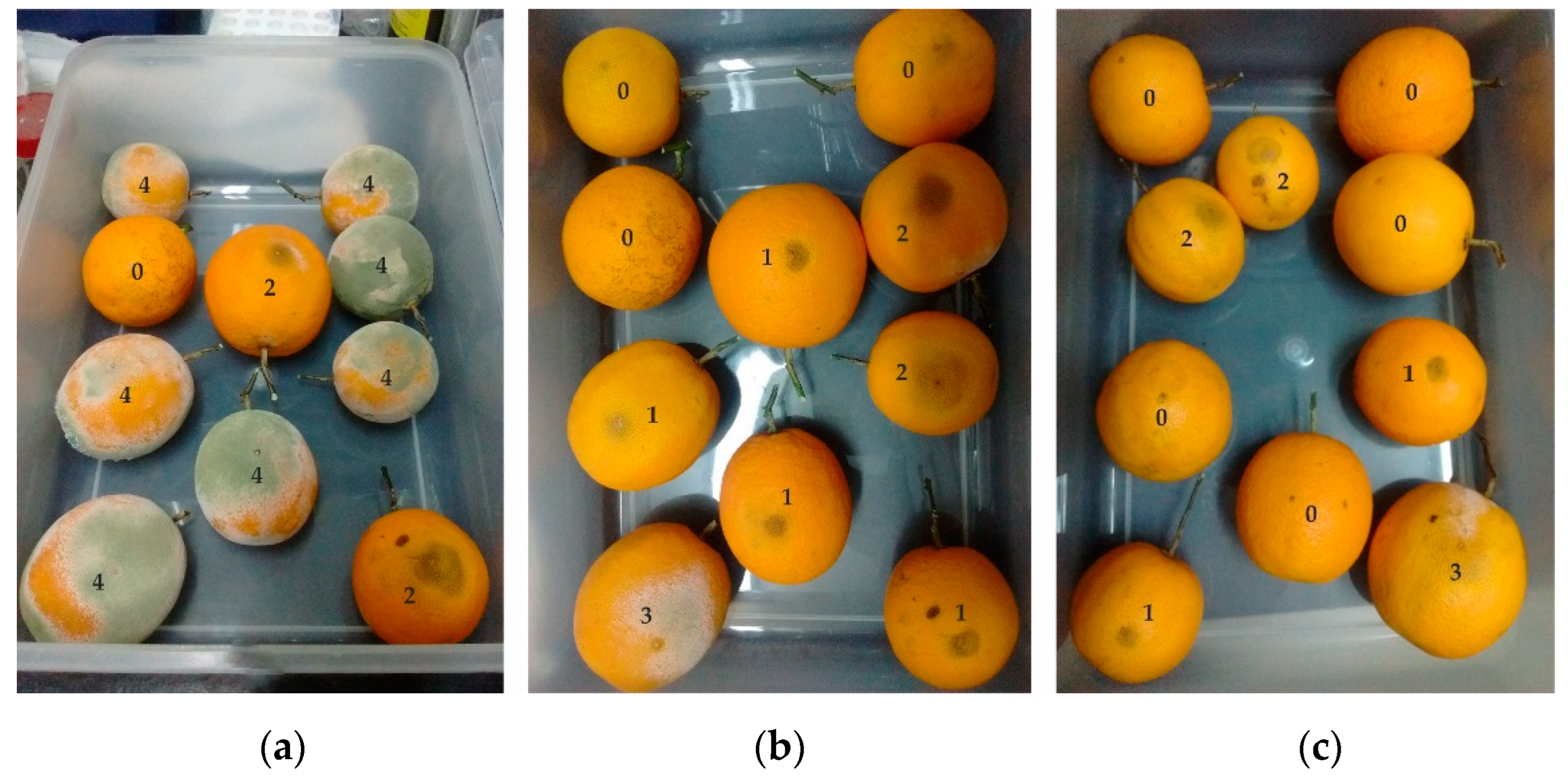
3.4. Curative Effect of S. pilcomayense Methanolic Extract over Oranges Infected with P. digitatum
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palou, L. Penicillium digitatum, Penicillium italicum (green mold, blue mold). In Postharvest Decay, Control Strategies; Bautista-Baños, S., Ed.; Academic Press: Valencia, Spain, 2014; pp. 45–102. [Google Scholar] [CrossRef]
- La Actividad Citrícola. Available online: https://www.federcitrus.org/wp-content/uploads/2022/07/La-Actividad-Citricola-2022.pdf (accessed on 12 March 2023).
- Eckert, J.W.; Eaks, I.L. Postharvest disorders and diseases of citrus fruits. In The Citrus Industry: Division of Agriculture and Natural Resources; Reuter, W., Calavan, E.C., Carman, G.E., Eds.; California Press: Los Angeles, CA, USA, 1989; pp. 179–260. [Google Scholar]
- Ogawa, J.M.; Zehr, E.; Bird, G.W.; Ritchie, D.F.; Uriu, K.; Uyemoto, J.K. Compendium of Stone Fruit Diseases; American Phytopathological Society: Saint Paul, MN, USA, 1995; pp. 1–168. [Google Scholar]
- Smilanick, J.L.; Mansour, M.F.; Gabler, F.M.; Sorenson, D. Control of citrus postharvest green mold and sour rot by potassium sorbate combined with heat and fungicides. Postharvest Biol. Technol. 2008, 47, 226–238. [Google Scholar] [CrossRef]
- Ismail, M.; Zhang, J. Post-harvest citrus diseases and their control. Outlooks Pest Manag. 2004, 15, 29–35. [Google Scholar] [CrossRef]
- Stegmayer, M.I.; Álvarez, N.H.; Favaro, M.A.; Fernandez, L.N.; Carrizo, M.E.; Reutemann, A.G.; Derita, M.G. Argentinian wild plants as controllers of fruits phytopathogenic fungi: Trends and perspectives. In Wild Plants: The Treasure of Natural Healers; Rai, M., Bhattarai, S., Feitosa, C., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 121–137. [Google Scholar]
- Di Liberto, M.G.; Stegmayer, M.I.; Svetaz, L.A.; Derita, M.G. Evaluation of Argentinean medicinal plants and isolation of their bioactive compounds as an alternative for the control of postharvest fruits phytopathogenic fungi. Rev. Bras. Farmacogn. 2019, 29, 686–688. [Google Scholar] [CrossRef]
- Moura, V.S.; Moretto, R.K.; Machado, B.I.; Kupper, K.C. Alternativas de controle de doenças de pós-colheita em citros. Citrus Res. Technol. 2019, 40, e1044. [Google Scholar] [CrossRef]
- Carmona-Hernandez, S.; Reyes-Pérez, J.J.; Chiquito Contreras, R.G.; Rincon-Enriquez, G.; Cerdan-Cabrera, C.R.; Hernandez-Montiel, L.G. Biocontrol of postharvest fruit fungal diseases by bacterial antagonists: A review. Agronomy 2019, 9, 121. [Google Scholar] [CrossRef]
- Stegmayer, M.I.; Fernández, L.; Álvarez, N.; Olivella, L.; Gutiérrez, H.; Favaro, M.A.; Derita, M. Aceites esenciales provenientes de plantas nativas para el control de hongos fitopatógenos que afectan a frutales. Rev. FAVE Cienc. Agrar. 2021, 20, 317–329. [Google Scholar] [CrossRef]
- Rodriguez, A.; Derita, M.; Borkosky, S.; Socolsky, C.; Bardón, A.; Hernández, M. Bioactive farina of Notholaena sulphurea (Pteridaceae): Morphology and histochemistry of glandular trichomes. Flora 2018, 240, 144–151. [Google Scholar] [CrossRef]
- Stegmayer, M.I.; Fernández, L.N.; Álvarez, N.H.; Seimandi, G.M.; Reutemann, A.G.; Derita, M.G. In vitro antifungal screening of Argentine native or naturalized plants against the phytopathogen Monilinia fructicola. Comb. Chem. High Throughput Screen. 2022, 25, 1158–1166. [Google Scholar] [CrossRef]
- Svetaz, L.; Zuljan, F.; Derita, M.; Petenatti, E.; Tamayo, G.; Cáceres, A.; Cechinel Filho, V.; Giménez, A.; Pinzón, R.; Zacchino, S.A.; et al. Value of the ethnomedical information for the discovery of plants with antifungal properties. A survey among seven Latin American countries. J. Ethnopharmacol. 2010, 127, 137–158. [Google Scholar] [CrossRef]
- Gupta, V.K.; Tuohy, M.G.; Gaur, R. Methods for high-quality DNA extraction from fungi. In Laboratory Protocols in Fungal Biology; Springer: New York, NY, USA, 2012; pp. 403–406. [Google Scholar]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA Genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press: Cambridge, MA, USA, 1990; pp. 315–322. [Google Scholar]
- Boratyn, G.M.; Camacho, C.; Cooper, P.S.; Coulouris, G.; Fong, A.; Ma, N.; Zaretskaya, I. BLAST: A more efficient report with usability improvements. Nucleic Acids Res. 2013, 41, 29–33. [Google Scholar] [CrossRef]
- CLSI (Clinical and Laboratory Standards Institute). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi, 3rd ed.; CLSI Standard M38; CLSI: Berwyn, PA, USA, 2017; pp. 1–35. [Google Scholar]
- Rasband, W.S. Available online: https://imagej.nih.gov/ij/ (accessed on 7 February 2020).
- Di Liberto, M.G.; Seimandi, G.M.; Fernández, L.N.; Ruiz, V.E.; Svetaz, L.A.; Derita, M.G. Botanical control of citrus green mold and peach brown rot on fruits assays using a Persicaria acuminata phytochemically characterized extract. Plants 2021, 10, 425. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Montiel, L.G.; Holguín-Peña, R.J.; Latisnere-Barragan, H. First report of sour rot caused by Geotrichum citri-aurantii on Key Lime (Citrus aurantifolia) in Colima State, Mexico. Plant Dis. 2010, 94, 488. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Kong, S.; Wang, F.; Liu, Y.; Liao, I.; Lu, Y.; Zhang, F.; Wu, J.; Wang, L.; Li, X. Identification of a new Bacillus sonorensis strain KLBC GS-3 as a biocontrol agent for postharvest green mold in grapefruit. Biol. Control 2020, 151, 104393. [Google Scholar] [CrossRef]
- Khan, I.H.; Javaid, A. Molecular characterization of Penicillium italicum causing blue mold on lemon in Pakistan. J. Plant Pathol. 2022, 104, 845–846. [Google Scholar] [CrossRef]
- Derita, M.; Leiva, M.; Zacchino, S. Influence of plant part, season of collection and content of the main active constituent, on the antifungal properties of Polygonum acuminatum Kunth. J. Ethnopharmacol. 2009, 124, 377–383. [Google Scholar] [CrossRef]
- Paye, I.A.; Pensiero, J.F.; Grenón, D.A.; Exner, E. Irupé: Sitio web de imágenes de la flora nativa de Argentina asociado al herbário SF. Rev. FAVE Cienc. Agrar. 2021, 20, 19–31. [Google Scholar] [CrossRef]
- Mohammed, A.E.; Abdul-Hameed, Z.H.; Alotaibi, M.O.; Bawakid, N.O.; Sobahi, T.R.; Abdel-Lateff, A.; Alarif, W.M. Chemical diversity and bioactivities of monoterpene indole alkaloids (MIAs) from six Apocynaceae genera. Molecules 2021, 26, 488. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, I.L.; Guadarrama Olivera, M.A.; Ortiz Guadarrama, M.; Labastida Astudillo, M.; Jiménez Pérez, N.D.C. Funastrum clausum (Jacq.) Schltr.: El Bejuco De Leche. Kuxulkab 2021, 27, 59–61. [Google Scholar] [CrossRef]
- Morcelle, S.R.; Barberis, S.; Priolo, N.; Caffini, N.O.; Clapés, P. Comparative behavior of proteinases from the latex of Carica papaya and Funastrum clausum as catalysts for the synthesis of Z-Ala-Phe-OMe. J. Mol. Catal. B Enzym. 2006, 41, 117–124. [Google Scholar] [CrossRef]
- Zabala, J.M.; Exner, E.; Cerino, C.; Buyatti, M.; Cuffia, C.; Marinoni, L.; Kern, V.; Pensiero, J.F. Recursos fitogenéticos forestales, forrajeros, de interés apícola y paisajístico nativos de la provincia de Santa Fe (Argentina). Rev. FAVE Cienc. Agrar. 2021, 20, 99–131. [Google Scholar] [CrossRef]
- He, Y.; Wang, Q.; Ye, Y.; Liu, Z.; Sun, H. The ethnopharmacology, phytochemistry, pharmacology and toxicology of genus Albizia: A review. J. Ethnopharmacol. 2020, 257, 112677. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.M. A short review on phytoconstituents from genus Albizzia and Erythrina. Bangladesh Pharmacol. J. 2018, 21, 160–172. [Google Scholar] [CrossRef]
- Zhang, H.P.; Samadi, A.K.; Rao, K.V.; Cohen, M.S.; Timmermann, B.N. Cytotoxic oleanane-type saponins from Albizia inundata. J. Nat. Prod. 2011, 74, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Shetu, H.J. Phytochemical and Biological Evaluation of Albizia richadiana (Benth. Fabaceae Family). Bachelor’s Thesis, BRAC University, Dhaka, Bangladesh, 2015. [Google Scholar]
- Luz, D.A.; Pinheiro, A.M.; Silva, M.L.; Monteiro, M.C.; Prediger, R.D.; Maia, C.S.F.; Fontes-Junior, E.A. Ethnobotany, phytochemistry and neuropharmacological effects of Petiveria alliacea L. (Phytolaccaceae): A review. J. Ethnopharmacol. 2016, 185, 182–201. [Google Scholar] [CrossRef]
- Blainski, A.; Piccolo, V.K.; Mello, J.C.P.; de Oliveira, R.M. Dual effects of crude extracts obtained from Petiveria alliacea L. (Phytolaccaceae) on experimental anxiety in mice. J. Ethnopharmacol. 2010, 128, 541–544. [Google Scholar] [CrossRef]
- Rosado-Aguilar, J.A.; Aguilar-Caballero, A.; Rodriguez-Vivas, R.I.; Borges-Argaez, R.; Garcia-Vazquez, Z.; Mendez-Gonzalez, M. Acaricidal activity of extracts from Petiveria alliacea (Phytolaccaceae) against the cattle tick, Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Vet. Parasitol. 2010, 168, 299–303. [Google Scholar] [CrossRef]
- Silva do Nascimento, S.; Okabe, D.; Pinto, A.; de Oliveira, F.; da Paixão, T.; Souza Siqueira, M.L.; Baetas, A.C.; de Andrade, M. Antimicrobial and anticancer potential of Petiveria alliacea L. (Herb to “Tame the Master”): A review. Pharmacogn. Rev. 2018, 12, 85–93. [Google Scholar] [CrossRef]
- Ramos, M.A.; Machado, L.P. Potencial antifúngico de tipi (Petiveria alliacea L.) em fungos de Aspergillus flavus. Rev. Cient. FAESA 2020, 16, 32–41. [Google Scholar]
- Zavala-Ocampo, L.M.; Aguirre-Hernández, E.; Pérez-Hernández, N.; Rivera, G.; Marchat, L.A.; Ramírez-Moreno, E. Antiamoebic activity of Petiveria alliacea leaves and their main component, isoarborinol. J. Microbiol. Biotechnol. 2017, 27, 1401–1408. [Google Scholar] [CrossRef]
- Palchetti, M.V.; Cantero, J.J.; Barboza, G.E. Solanaceae diversity in South America and its distribution in Argentina. Anais Acad. Brasil. Cienc. 2020, 92, 1–17. [Google Scholar] [CrossRef]
- Muelas-Serrano, S.; Nogal, J.J.; Martınez-Dıaz, R.A.; Escario, J.A.; Martınez-Fernández, A.R.; Gómez-Barrio, A. In vitro screening of American plant extracts on Trypanosoma cruzi and Trichomonas vaginalis. J. Ethnopharmacol. 2000, 71, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Diep, T.; Pook, C.; Yoo, M. Phenolic and anthocyanin compounds and antioxidant activity of tamarillo (Solanum betaceum Cav.). Antioxidants 2020, 9, 169. [Google Scholar] [CrossRef] [PubMed]
- Vaz, N.P.; Costa, E.V.; Santos, E.L.; Mikich, S.B.; Marques, F.A.; Braga, R.M.; Delarmelina, C.; Duarte, M.; Ruiz, A.N.; Souza, V.H.; et al. Caavuranamide, a novel steroidal alkaloid from the ripe fruits of Solanum caavurana Vell. (Solanaceae). J. Braz. Chem. Soc. 2012, 23, 361–366. Available online: https://www.scielo.br/pdf/jbchs/v23n2/a25v23n2.pd (accessed on 26 May 2022). [CrossRef]
- Aparecida Pereira, A.P.; Figueiredo Angolini, C.F.; Paulino, B.N.; Chatagnier Lauretti, L.B.; Orlando, E.A.; Siqueira Silva, J.G.; Neri-Numa, I.A.; Pimentel Souza, J.D.; Lima Pallone, J.A.; Nogueira Eberlin, M.; et al. A comprehensive characterization of Solanum lycocarpum St. Hill and Solanum oocarpum Sendtn: Chemical composition and antioxidant properties. Food Res. Int. 2019, 124, 61–69. [Google Scholar] [CrossRef] [PubMed]

| Family | Plant Scientific Name | V. specimen | Collection Data and Place of Acquisition |
|---|---|---|---|
| Apocynaceae | Araujia brachystephana (Griseb.) Fontella & Goyder | Pensiero et Zabala 13800 | 13/08/2019, San Javier, Santa Fe, 30°03′23.2″ S 59°51′34.8″ W |
| Forsteronia glabrescens Müll. Arg. | Pensiero et Zabala 13804 | 13/08/2019, Gral. Obligado, Santa Fe, 28°42′54.8″ S 59°23′13.7″ W | |
| Orthosia virgata (Poir.) E. Fourn. | Pensiero et Zabala 13802 | 13/08/2019, San Javier, Santa Fe, 30°03′23.2″ S 59°51′34.8″ W | |
| Orthosia virgata (Poir.) E. Fourn. | Pensiero et Zabala 13805 | 13/08/2019, Gral. Obligado, Santa Fe, 28°42′54.8″ S 59°23′13.7″ W | |
| Funastrum clausum (Jacq.) Schltr. | Pensiero et Zabala 13797 | 13/08/2019, San Javier, Santa Fe, 30°03′23.2″ S 59°51′34.8″ W | |
| Bromeliaceae | Tillandsia tricholepis Baker | Pensiero et Zabala 13798 | 13/08/2019, San Javier, Santa Fe, 30°03′23.2″ S 59°51′34.8″ W |
| Fabaceae | Albizia inundata (Mart.) Barneby & J.W. Grimes | Pensiero et Zabala 13816 | 14/08/2019, Gral. Obligado, Santa Fe, 28°12′53.9″ S 59°08′58.3″ W |
| Vachellia caven (Molina) Seigler & Ebinger | Derita M. 39 | 17/03/2019, Las Colonias, Santa Fe, 31°26′30.3″ S 60°56′24.7″ W | |
| Phytolaccaceae | Petiveria alliacea L. | Pensiero et Zabala 13975 | 13/08/2019, San Javier, Santa Fe, 30°03′23.2″ S 59°51′34.8″ W |
| Polygonaceae | Polygonum stelligerum Cham. | Derita M. 55 | 16/03/2019, San Pedro, Bs As, 33°40′11.6″ S 59°39′38.4″ W |
| Polygonum lapathifolium L. | Derita M. 54 | 16/03/2019, San Pedro, Bs As, 33°40′11.6″ S 59°39′38.4″ W | |
| Ranunculaceae | Clematis montevidensis Spreng. | Pensiero et Zabala 13824 | 17/08/2019, Vera, Santa Fe, 29°28′17.6″ S 60°05′27.7″ W |
| Nictaginaceae | Pisonia zapallo Griseb | Pensiero et Zabala 13808 | 13/08/2019, Gral. Obligado, Santa Fe, 28°42′54.8″ S 59°23′13.7″ W |
| Solanaceae | Solanum argentinum Bitter & Lillo | Pensiero et Zabala 13820 | 16/08/2019, Tapenagá, Chaco, 27°53′17.9″ S 59°51′39.4″ W |
| Solanum argentinum Bitter & Lillo | Pensiero et Zabala 13817 | 16/08/2019, Lib. San Martín, Chaco, 26°07′13.9″ S 59°56′25.1″ W | |
| Solanum granulosum-leprosum Dunal | Pensiero et Zabala 13806 | 13/08/2019, Gral. Obligado, Santa Fe, 28°42′54.8″ S 59°23′13.7″ W | |
| Solanum pilcomayense Morong | Pensiero et Zabala 13815 | 14/08/2019, Gral. Obligado, Santa Fe, 28°13′42.2″ S 59°06′52.6″ W | |
| Solanum pilcomayense Morong | Pensiero et Zabala 13801 | 13/08/2019, San Javier, Santa Fe, 30°03′23.2″ S 59°51′34.8″ W | |
| Solanum caavurana Vell. | Pensiero et Zabala 13813 | 14/08/2019, Gral. Obligado, Santa Fe, 28°13′42.2″ S 59°06′52.6″ W | |
| Verbenaceae | Lantana megapotamica (Spreng.) Tronc. | Pensiero et Zabala 13796 | 13/08/2019, San Javier, Santa Fe, 30°03′23.2″ S 59°51′34.8″ W |
| Plant Scientific Name | Part Used | DCM Extract Yield (%) | MeOH Extract Yield (%) |
|---|---|---|---|
| A. brachystephana | Aerial parts | 2.4 | 10.7 |
| F. glabrescens | Aerial parts | 1.9 | 7.3 |
| O. virgata | Aerial parts | 2.4 | 4.1 |
| O. virgata | Aerial parts | 2.3 | 5.0 |
| F. clausum | Aerial parts | 3.7 | 7.2 |
| T. tricholepis | Whole plant | 3.4 | 4.4 |
| A. inundata | Aerial parts | 1.1 | 3.4 |
| V. caven | Flowers | 1.3 | 7.3 |
| P. alliacea | Aerial parts | 0.4 | 3.0 |
| P. stelligerum | Leaves | 0.7 | 2.8 |
| P. lapathifolium | Leaves | 0.6 | 3.0 |
| C. montevidensis | Aerial parts | 1.0 | 10.2 |
| P. zapallo | Aerial parts | 1.3 | 7.7 |
| S. argentinum | Aerial parts | 0.7 | 7.0 |
| S. argentinum | Aerial parts | 3.5 | 7.7 |
| S. granulosum-leprosum | Aerial parts | 3.2 | 5.0 |
| S. pilcomayense | Aerial parts | 1.3 | 7.6 |
| S. pilcomayense | Aerial parts | 0.5 | 3.2 |
| S. caavurana | Aerial parts | 1.4 | 10.3 |
| L. megapotamica | Aerial parts | 0.4 | 4.5 |
| Plant Scientific Name | P. digitatum | P. italicum | G. citri-aurantii | Controls | ||||
|---|---|---|---|---|---|---|---|---|
| DCM | MeOH | DCM | MeOH | DCM | MeOH | +(DMSO) | −(IMZ) | |
| A. brachystephana | 61.0 ± 5.2 c | 44.3 ± 6.2 a | 30.9 ± 6.5 a | 30.5 ± 5.3 a | 65.4 ± 5.0 c | 66.6 ± 1.8 c | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| F. glabrescens | 52.9 ± 7.1 c | 75.1 ± 1.5 c | 45.8 ± 6.9 a | 54.1 ± 5.4 c | 71.0 ± 0.4 c | 70.1 ± 2.8 c | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| O. virgata | 55.3 ± 2.9 c | 50.9 ± 5.9 c | 33.2 ± 6.5 a | 48.4 ± 6.5 a | 88.0 ± 4.9 b | 50.8 ± 1.9 c | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| O. virgata | 70.6 ± 2.0 c | 86.3 ± 3.8 b | 31.6 ± 4.9 a | 48.5 ± 4.7 a | 99.2 ± 0.7 b | 100.0 ± 0.0 b | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| F. clausum | 62.4 ± 3.8 c | 81.5 ± 1.5 b | 40.8 ± 4.4 a | 43.1 ± 6.9 a | 100.0 ± 0.0 b | 33.0 ± 5.2 a | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| T. tricholepis | 80.0 ± 2.2 b c | 85.9 ± 0.7 b | 60.8 ± 7.0 c | 67.1 ± 3.6 c | 87.8 ± 1.5 b | 54.9 ± 1.0 c | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| A. inundata | 79.3 ± 2.1 b c | 100.0 ± 0.0 b | 35.7 ± 5.2 a | 100.0 ± 0.0 b | 79.3 ± 7.0 b c | 97.3 ± 0.5 b | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| V. caven | 59.5 ± 4.0 c | 76.8 ± 3.5 c | 36.2 ± 3.8 a | 58.1 ± 6.5 c | 56.5 ± 5.1 c | 32.3 ± 4.2 a | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| P. alliacea | 72.5 ± 1.7 c | 83.3 ± 3.8 b | 53.2 ± 2.8 c | 81.3 ± 2.2 b | 100.0 ± 0.0 b | 62.9 ± 3.9 c | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| P. stelligerum | 75.2 ± 2.3 c | 80.1 ± 2.8 b | 32.8 ± 6.5 a | 57.0 ± 5.3 c | 76.0 ± 2.1 c | 38.4 ± 4.1 a | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| P. lapathifolium | 64.2 ± 6.4 c | 80.7 ± 0.9 b c | 33.0 ± 3.2 a | 43.8 ± 5.2 a | 82.1 ± 3.5 b | 40.2 ± 3.9 a | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| C. montevidensis | 49.4 ± 5.2 a | 56.4 ± 5.1 c | 24.6 ± 3.2 a | 45.8 ± 2.7 a | 65.5 ± 2.8 c | 52.7 ± 4.6 c | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| P. zapallo | 73.2 ± 4.0 c | 81.7 ± 2.2 b | 37.5 ± 4.7 a | 51.8 ± 4.7 c | 90.6 ± 7.9 b | 70.0 ± 4.2 c | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| S. argentinum | 45.2 ± 6.3 a | 62.3 ± 5.3 c | 29.0 ± 3.3 a | 62.7 ± 5.1 c | 63.8 ± 0.9 c | 73.3 ± 1.9 c | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| S. argentinum | 73.3 ± 4.0 c | 70.0 ± 5.6 c | 27.9 ± 4.0 a | 51.6 ± 4.8 c | 75.0 ± 6.8 c | 48.6 ± 4.9 a | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| S. granulosum-leprosum | 54.7 ± 6.0 c | 49.5 ± 4.5 a | 44.6 ± 4.7 a | 40.0 ± 4.3 a | 41.3 ± 5.4 a | 61.5 ± 5.1 c | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| S. pilcomayense | 44.5 ± 4.9 a | 100.0 ± 0.0 b | 15.3 ± 3.4 a | 100.0 ± 0.0 b | 74.4 ± 2.4 c | 100.0 ± 0.0 b | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| S. pilcomayense | 46.4 ± 4.7 a | 100.0 ± 0.0 b | 41.9 ± 5.3 a | 100.0 ± 0.0 b | 71.0 ± 1.9 c | 100.0 ± 0.0 b | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| S. caavurana | 60.0 ± 5.6 c | 94.6 ± 1.5 b | 35.5 ± 3.5 a | 87.8 ± 1.3 b | 79.8 ± 4.5 c | 100.0 ± 0.0 b | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| L. megapotamica | 75.0 ± 2.2 c | 56.3 ± 4.4 c | 34.9 ± 4.8 a | 41.6 ± 3.4 a | 70.3 ± 1.2 c | 62.9 ± 5.1 c | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| Plant Scientific Name | P. digitatum | P. italicum | G. citri-aurantii | Controls | ||||
|---|---|---|---|---|---|---|---|---|
| DCM | MeOH | DCM | MeOH | DCM | MeOH | +(DMSO) | −(IMZ) | |
| A. brachystephana | 40.8 ± 2.5 a | 18.4 ± 3.4 a | 0.0 ± 0.0 a | 7.2 ± 2.3 a | 13.7 ± 3.2 a | 39.5 ± 4.3 a | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| F. glabrescens | 38.0 ± 3.8 a | 38.8 ± 4.3 a | 30.6 ± 4.6 a | 10.3 ± 2.1 a | 26.1 ± 3.7 a | 37.2 ± 4.9 a | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| O. virgata | 37.9 ± 5.2 a | 33.7 ± 4.3 a | 7.7 ± 2.1 a | 33.8 ± 4.6 a | 80.0 ± 4.4 b c | 29.7 ± 3.2 a | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| O. virgata | 45.9 ± 3.9 a | 57.0 ± 3.6 c | 0.0 ± 0.0 a | 16.9 ± 3.2 a | 90.4 ± 6.0 b | 100.0 ± 0.0 b | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| F. clausum | 50.3 ± 4.5 a c | 53.6 ± 3.7 c | 15.1 ± 3.6 a | 31.2 ± 4.6 a | 100.0 ± 0.0 b | 16.0 ± 1.2 a | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| T. tricholepis | 76.8 ± 2.7 c | 69.1 ± 1.8 c | 55.0 ± 2.7 c | 39.1 ± 6.5 a | 26.7 ± 1.7 a | 46.9 ± 2.8 a | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| A. inundata | 60.3 ± 2.7 c | 100.0 ± 0.0 b | 34.3 ± 6.2 a | 100.0 ± 0.0 b | 71.5 ± 5.3 c | 92.5 ± 0.2 b | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| V. caven | 42.3 ± 3.7 a | 33.0 ± 6.1 a | 30.0 ± 5.8 a | 11.0 ± 3.0 a | 0.0 ± 0.0 a | 22.0 ± 5.1 a | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| P. alliacea | 67.2 ± 2.9 c | 58.8 ± 6.7 c | 41.0 ± 2.0 a | 57.8 ± 3.3 c | 100.0 ± 0.0 b | 34.4 ± 5.8 a | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| P. stelligerum | 50.2 ± 1.5 a c | 42.3 ± 4.3 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 59.1 ± 4.1 c | 21.5 ± 1.6 a | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| P. lapathifolium | 37.8 ± 6.5 a | 33.0 ± 5.3 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 22.1 ± 3.6 a | 20.5 ± 1.2 a | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| C. montevidensis | 41.4 ± 6.2 a | 10.4 ± 2.1 a | 0.0 ± 0.0 a | 11.6 ± 4.5 a | 0.0 ± 0.0 a | 31.6 ± 4.3 a | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| P. zapallo | 52.9 ± 5.9 c | 39.5 ± 4.4 a | 18.3 ± 4.4 a | 0.0 ± 0.0 a | 10.3 ± 2.7 a | 44.6 ± 5.3 a | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| S. argentinum | 29.5 ± 4.3 a | 40.2 ± 6.7 a | 0.0 ± 0.0 a | 13.8 ± 2.3 a | 0.0 ± 0.0 a | 37.5 ± 5.8 a | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| S. argentinum | 50.1 ± 6.3 a c | 50.1 ± 5.2 a c | 0.0 ± 0.0 a | 27.8 ± 3.5 a | 39.3 ± 4.5 a | 33.1 ± 2.8 a | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| S. granulosum-leprosum | 38.4 ± 4.3 a | 10.4 ± 3.7 a | 29.9 ± 4.7 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 37.4 ± 3.6 a | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| S. pilcomayense | 32.4 ± 4.7 a | 100.0 ± 0.0 b | 0.0 ± 0.0 a | 100.0 ± 0.0 b | 17.8 ± 4.7 a | 100.0 ± 0.0 b | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| S. pilcomayense | 32.8 ± 4.8 a | 100.0 ± 0.0 b | 27.4 ± 3.7 a | 100.0 ± 0.0 b | 0.0 ± 0.0 a | 100.0 ± 0.0 b | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| S. caavurana | 50.4 ± 5.5 a c | 88.3 ± 2.2 b | 18.2 ± 3.2 a | 55.7 ± 4.3 c | 71.0 ± 3.9 c | 100.0 ± 0.0 b | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
| L. megapotamica | 62.9 ± 5.4 c | 23.6 ± 5.3 a | 34.6 ± 4.2 a | 22.5 ± 4.4 a | 0.0 ± 0.0 a | 34.6 ± 3.4 a | 0.0 ± 0.0 a | 100.0 ± 0.0 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarez, N.H.; Stegmayer, M.I.; Seimandi, G.M.; Pensiero, J.F.; Zabala, J.M.; Favaro, M.A.; Derita, M.G. Natural Products Obtained from Argentinean Native Plants Are Fungicidal against Citrus Postharvest Diseases. Horticulturae 2023, 9, 562. https://doi.org/10.3390/horticulturae9050562
Alvarez NH, Stegmayer MI, Seimandi GM, Pensiero JF, Zabala JM, Favaro MA, Derita MG. Natural Products Obtained from Argentinean Native Plants Are Fungicidal against Citrus Postharvest Diseases. Horticulturae. 2023; 9(5):562. https://doi.org/10.3390/horticulturae9050562
Chicago/Turabian StyleAlvarez, Norma Hortensia, María Inés Stegmayer, Gisela Marisol Seimandi, José Francisco Pensiero, Juan Marcelo Zabala, María Alejandra Favaro, and Marcos Gabriel Derita. 2023. "Natural Products Obtained from Argentinean Native Plants Are Fungicidal against Citrus Postharvest Diseases" Horticulturae 9, no. 5: 562. https://doi.org/10.3390/horticulturae9050562
APA StyleAlvarez, N. H., Stegmayer, M. I., Seimandi, G. M., Pensiero, J. F., Zabala, J. M., Favaro, M. A., & Derita, M. G. (2023). Natural Products Obtained from Argentinean Native Plants Are Fungicidal against Citrus Postharvest Diseases. Horticulturae, 9(5), 562. https://doi.org/10.3390/horticulturae9050562