Arbuscular Mycorrhizae Contribute to Growth, Nutrient Uptake, and Ornamental Characteristics of Statice (Limonium sinuatum [L.] Mill.) Subject to Appropriate Inoculum and Optimal Phosphorus
Abstract
1. Introduction
2. Materials and Methods
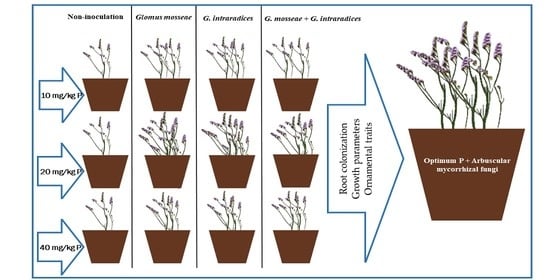
2.1. Experimental Design
2.2. Mycorrhizal Inoculation and Plant Growth
2.3. Evaluation of Vegetative and Ornamental Characteristics
2.4. Assessment of Root Mycorrhizal Colonization
2.5. Measurement of Nutrients
2.6. Statistical Analyses
3. Results
3.1. Growth and Ornamental Traits
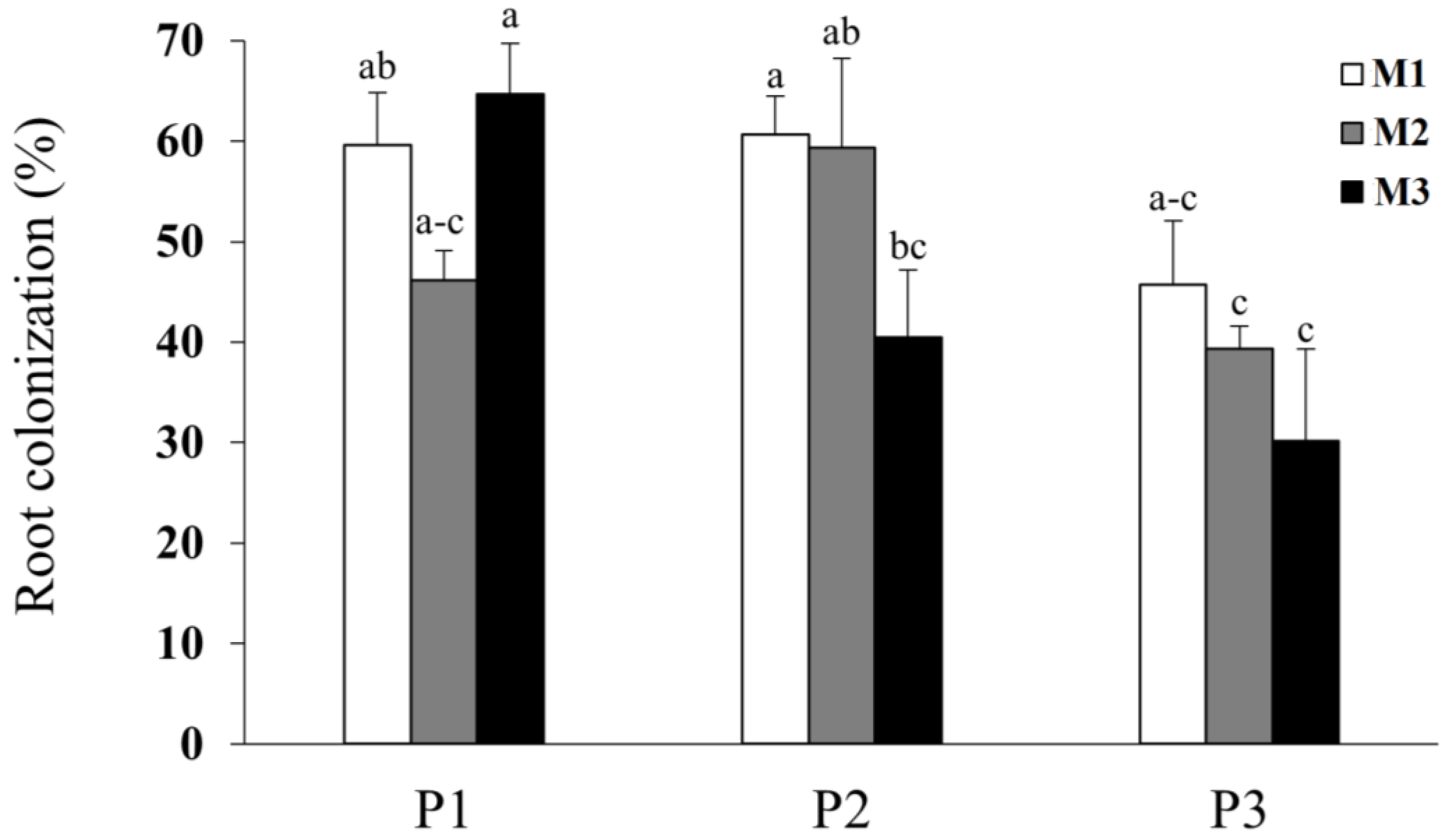
3.2. Root Colonization
3.3. Shoot and Root Nutrients
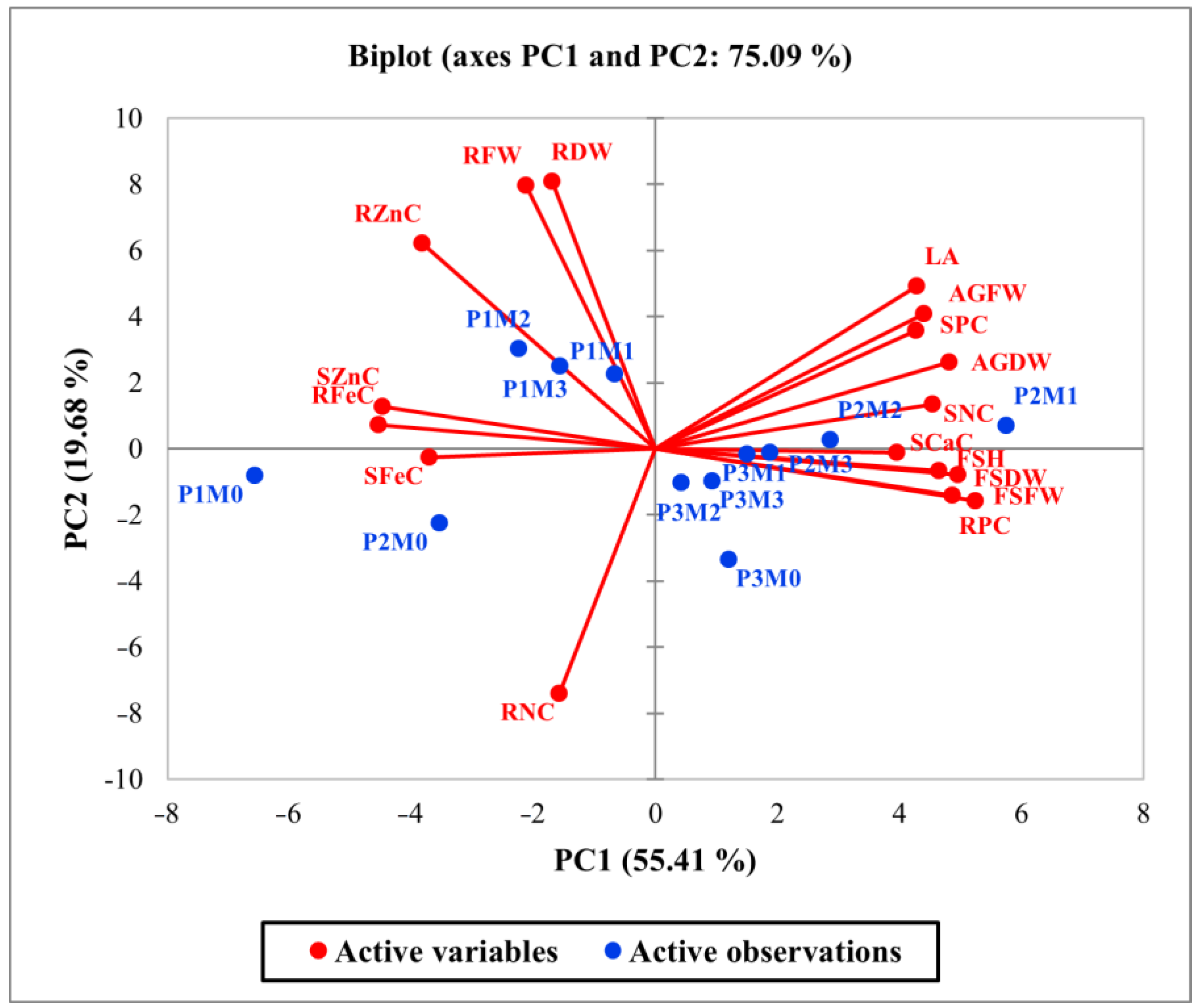
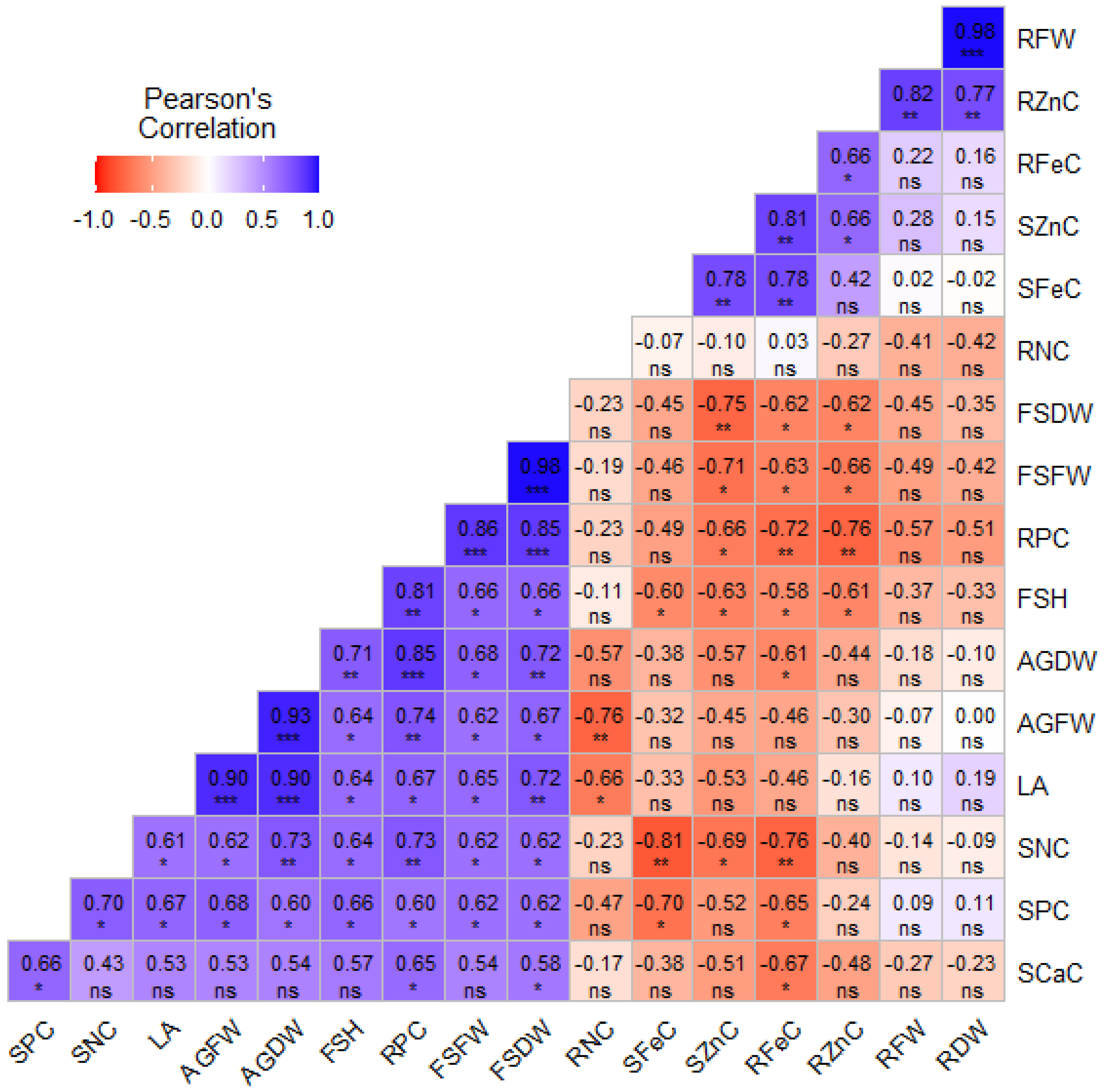
3.4. Principal Component Analysis and Correlation
4. Discussion
4.1. Growth and Ornamental Parameters
4.2. Root Colonization
4.3. Root and Shoot Nutrient Contents
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmed, J.U.; Linda, I.J.; Majid, M.A. Royal FloraHolland: Strategic Supply Chain of Cut Flowers Business. In Royal FloraHolland: Strategic Supply Chain of Cut Flowers Business; SAGE Business Cases Originals; SAGE Publications: Washington, DC, USA, 2018; ISBN 1526461919. [Google Scholar]
- Morgan, E.; Funnell, K. Limonium. Ornam. Crop. 2018, 11, 513–527. [Google Scholar]
- Blainski, A.; Gionco, B.; Oliveira, A.G.; Andrade, G.; Scarminio, I.S.; Silva, D.B.; Lopes, N.P.; Mello, J.C.P. Antibacterial Activity of Limonium brasiliense (Baicuru) against Multidrug-Resistant Bacteria Using a Statistical Mixture Design. J. Ethnopharmacol. 2017, 198, 313–323. [Google Scholar] [CrossRef]
- Medini, F.; Legault, J.; Pichette, A.; Abdelly, C.; Ksouri, R. Antiviral Efficacy of Limonium densiflorum against HSV-1 and Influenza Viruses. S. Afr. J. Bot. 2014, 92, 65–72. [Google Scholar] [CrossRef]
- Medini, F.; Bourgou, S.; Lalancette, K.; Snoussi, M.; Mkadmini, K.; Coté, I.; Abdelly, C.; Legault, J.; Ksouri, R. Phytochemical Analysis, Antioxidant, Anti-Inflammatory, and Anticancer Activities of the Halophyte Limonium densiflorum Extracts on Human Cell Lines and Murine Macrophages. S. Afr. J. Bot. 2015, 99, 158–164. [Google Scholar] [CrossRef]
- González-Orenga, S.; Grigore, M.-N.; Boscaiu, M.; Vicente, O. Constitutive and Induced Salt Tolerance Mechanisms and Potential Uses of Limonium Mill. Species. Agronomy 2021, 11, 413. [Google Scholar] [CrossRef]
- Gancedo, N.C.; Isolani, R.; de Oliveira, N.C.; Nakamura, C.V.; de Medeiros Araújo, D.C.; Sanches, A.C.C.; Tonin, F.S.; Fernandez-Llimos, F.; Chierrito, D.; de Mello, J.C.P. Chemical Constituents, Anticancer and Anti-Proliferative Potential of Limonium Species: A Systematic Review. Pharmaceuticals 2023, 16, 293. [Google Scholar] [CrossRef]
- Xu, D.-P.; Zheng, J.; Zhou, Y.; Li, Y.; Li, S.; Li, H.-B. Ultrasound-Assisted Extraction of Natural Antioxidants from the Flower of Limonium sinuatum: Optimization and Comparison with Conventional Methods. Food Chem. 2017, 217, 552–559. [Google Scholar] [CrossRef]
- Sheikh-Assadi, M.; Khandan-Mirkohi, A.; Alemardan, A.; Moreno-Jiménez, E. Mycorrhizal Limonium sinuatum (L.) Mill. Enhances Accumulation of Lead and Cadmium. Int. J. Phytoremediat. 2015, 17, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Grieve, C.M.; Poss, J.A.; Grattan, S.R.; Shouse, P.J.; Lieth, J.H.; Zeng, L. Productivity and Mineral Nutrition of Limonium Species Irrigated with Saline Wastewaters. HortScience 2005, 40, 654–658. [Google Scholar] [CrossRef]
- Whipker, B.E.; Hammer, P.A. Growth and Yield Characteristics of Field-Grown Limonium sinuatum (L.). HortScience 1994, 29, 638–640. [Google Scholar] [CrossRef]
- Verlinden, S.; McDonald, L. Productivity and Quality of Statice (Limonium sinuatum Cv. Soiree Mix) and Cockscomb (Celosia argentea Cv. Chief Mix) under Organic and Inorganic Fertilization Regiments. Sci. Hortic. 2007, 114, 199–206. [Google Scholar] [CrossRef]
- Nandwani, D. Organic Farming for Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2016; Volume 9, ISBN 3319268031. [Google Scholar]
- Thompson, G. International Consumer Demand for Organic Foods. Horttechnology 2000, 10, 663–674. [Google Scholar] [CrossRef]
- Wahid, F.; Fahad, S.; Danish, S.; Adnan, M.; Yue, Z.; Saud, S.; Siddiqui, M.H.; Brtnicky, M.; Hammerschmiedt, T.; Datta, R. Sustainable Management with Mycorrhizae and Phosphate Solubilizing Bacteria for Enhanced Phosphorus Uptake in Calcareous Soils. Agriculture 2020, 10, 334. [Google Scholar] [CrossRef]
- Caspersen, S.; Bergstrand, K.-J. Phosphorus Restriction Influences P Efficiency and Ornamental Quality of Poinsettia and Chrysanthemum. Sci. Hortic. 2020, 267, 109316. [Google Scholar] [CrossRef]
- Igiehon, N.O.; Babalola, O.O. Biofertilizers and Sustainable Agriculture: Exploring Arbuscular Mycorrhizal Fungi. Appl. Microbiol. Biotechnol. 2017, 101, 4871–4881. [Google Scholar] [CrossRef]
- Thomas, L.; Singh, I. Microbial Biofertilizers: Types and Applications. Biofertil. Sustain. Agric. Environ. 2019, 55, 1–19. [Google Scholar]
- Daniel, A.I.; Fadaka, A.O.; Gokul, A.; Bakare, O.O.; Aina, O.; Fisher, S.; Burt, A.F.; Mavumengwana, V.; Keyster, M.; Klein, A. Biofertilizer: The Future of Food Security and Food Safety. Microorganisms 2022, 10, 1220. [Google Scholar] [CrossRef]
- Bashan, Y.; Holguin, G. Azospirillum–Plant Relationships: Environmental and Physiological Advances (1990–1996). Can. J. Microbiol. 1997, 43, 103–121. [Google Scholar] [CrossRef]
- Goel, A.K.; Laura, R.D.; Pathak, D.V.; Goel, A. Use of Biofertilizers: Potential, Constraints and Future Strategies-a Review. Int. J. Trop. Agric. 1999, 17, 1–18. [Google Scholar]
- Sahu, P.K.; Brahmaprakash, G.P. Formulations of Biofertilizers–Approaches and Advances. Microb. Inoculants Sustain. Agric. Product. Funct. Appl. 2016, 2, 179–198. [Google Scholar]
- Lee, E.-H.; Eo, J.-K.; Ka, K.-H.; Eom, A.-H. Diversity of Arbuscular Mycorrhizal Fungi and Their Roles in Ecosystems. Mycobiology 2013, 41, 121–125. [Google Scholar] [CrossRef]
- Morton, J.B.; Benny, G.L. Revised Classification of Arbuscular Mycorrhizal Fungi (Zygomycetes): A New Order, Glomales, Two New Suborders, Glomineae and Gigasporineae, and Two New Families, Acaulosporaceae and Gigasporaceae, with an Emendation of Glomaceae. Mycotaxon 1990, 37, 471–491. [Google Scholar]
- Morton, J.B.; Bentivenga, S.P. Levels of Diversity in Endomycorrhizal Fungi (Glomales, Zygomycetes) and Their Role in Defining Taxonomic and Non-Taxonomic Groups. Plant Soil 1994, 159, 47–59. [Google Scholar] [CrossRef]
- Simon, L.; Bousquet, J.; Lévesque, R.C.; Lalonde, M. Origin and Diversification of Endomycorrhizal Fungi and Coincidence with Vascular Land Plants. Nature 1993, 363, 67–69. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, L.; Zhou, J.; George, T.S.; Feng, G. Arbuscular Mycorrhizal Fungi Enhance Mineralisation of Organic Phosphorus by Carrying Bacteria along Their Extraradical Hyphae. New Phytol. 2021, 230, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.S. A Critical Review on the Role of Mycorrhizal Fungi in the Uptake of Phosphorus by Plants. Plant Soil 1991, 134, 189–207. [Google Scholar] [CrossRef]
- Fernández, F.; Vicente-Sánchez, J.; Maestre-Valero, J.F.; Bernabé, A.J.; Nicolás, E.; Pedrero, F.; Alarcón, J.J. Physiological and Growth Responses of Young Tomato Seedlings to Drip-Irrigation Containing Two Low Doses of the Arbuscular Mycorrhizal Fungus Glomus Iranicum Var. Tenuihypharum Sp. Nova. J. Hortic. Sci. Biotechnol. 2014, 89, 679–685. [Google Scholar] [CrossRef]
- Meena, R.S.; Vijayakumar, V.; Yadav, G.S.; Mitran, T. Response and Interaction of Bradyrhizobium japonicum and Arbuscular Mycorrhizal Fungi in the Soybean Rhizosphere. Plant Growth Regul. 2018, 84, 207–223. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Cambridge, MA, USA, 2010; ISBN 0080559344. [Google Scholar]
- Nunes, C.E.P.; Stancato, G.C.; Da Silveira, A.P.D. Anthurium Growth Responses to Phosphate Fertilisation and Inoculation with an Arbuscular Mycorrhizal Fungus. J. Hortic. Sci. Biotechnol. 2014, 89, 261–267. [Google Scholar] [CrossRef]
- Kaeppler, S.M.; Parke, J.L.; Mueller, S.M.; Senior, L.; Stuber, C.; Tracy, W.F. Variation among Maize Inbred Lines and Detection of Quantitative Trait Loci for Growth at Low Phosphorus and Responsiveness to Arbuscular Mycorrhizal Fungi. Crop. Sci. 2000, 40, 358–364. [Google Scholar] [CrossRef]
- Watts-Williams, S.J.; Cavagnaro, T.R. Arbuscular Mycorrhizas Modify Tomato Responses to Soil Zinc and Phosphorus Addition. Biol. Fertil. Soils 2012, 48, 285–294. [Google Scholar] [CrossRef]
- Etesami, H.; Jeong, B.R.; Glick, B.R. Contribution of Arbuscular Mycorrhizal Fungi, Phosphate–Solubilizing Bacteria, and Silicon to P Uptake by Plant. Front. Plant Sci. 2021, 12, 1355. [Google Scholar] [CrossRef]
- Ortas, I.; Rafique, M.; Ahmed, I.A.M. Application of Arbuscular Mycorrhizal Fungi into Agriculture. In Arbuscular Mycorrhizas and Stress Tolerance of Plants; Springer: Berlin/Heidelberg, Germany, 2017; pp. 305–327. ISBN 9789811041150. [Google Scholar]
- Santander, C.; Ruiz, A.; García, S.; Aroca, R.; Cumming, J.; Cornejo, P. Efficiency of Two Arbuscular Mycorrhizal Fungal Inocula to Improve Saline Stress Tolerance in Lettuce Plants by Changes of Antioxidant Defense Mechanisms. J. Sci. Food Agric. 2020, 100, 1577–1587. [Google Scholar] [CrossRef]
- Plenchette, C.; Fortin, J.A.; Furlan, V. Growth Responses of Several Plant Species to Mycorrhizae in a Soil of Moderate P-Fertility—I. Mycorrhizal Dependency under Field Conditions. Plant Soil 1983, 70, 199–209. [Google Scholar] [CrossRef]
- Al-Yahya’ei, M.N.; Oehl, F.; Vallino, M.; Lumini, E.; Redecker, D.; Wiemken, A.; Bonfante, P. Unique Arbuscular Mycorrhizal Fungal Communities Uncovered in Date Palm Plantations and Surrounding Desert Habitats of Southern Arabia. Mycorrhiza 2011, 21, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Tennant, D. A Test of a Modified Line Intersect Method of Estimating Root Length. J. Ecol. 1975, 63, 995. [Google Scholar] [CrossRef]
- Koske, R.E.; Gemma, J.N. A Modified Procedure for Staining Roots to Detect VA Mycorrhizas. Mycol. Res. 1989, 92, 486–488. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An Evaluation of Techniques for Measuring Vesicular Arbuscular Mycorrhizal Infection in Roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Determination of Total Nitrogen in Plant Material 1. Agron. J. 1973, 65, 109–112. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis: Advanced Course; UW-Madison Libraries Parallel Press: Madison, WI, USA, 2005; ISBN 1893311473. [Google Scholar]
- Turjaman, M.; Tamai, Y.; Santoso, E.; Osaki, M.; Tawaraya, K. Arbuscular Mycorrhizal Fungi Increased Early Growth of Two Nontimber Forest Product Species Dyera polyphylla and Aquilaria filaria under Greenhouse Conditions. Mycorrhiza 2006, 16, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, L.; Wei, S.; Xiao, X.; Su, C.; Jiang, P.; Song, Z.; Wang, T.; Yu, Z. Effects of Arbuscular Mycorrhizal Fungi on the Growth, Nutrient Uptake and Glycyrrhizin Production of Licorice (Glycyrrhiza uralensis Fisch). Plant Growth Regul. 2007, 52, 29–39. [Google Scholar] [CrossRef]
- Vosnjak, M.; Likar, M.; Osterc, G. The Effect of Mycorrhizal Inoculum and Phosphorus Treatment on Growth and Flowering of Ajania (Ajania pacifica (Nakai) Bremer et Humphries) Plant. Horticulturae 2021, 7, 178. [Google Scholar] [CrossRef]
- Jakobsen, I. Carbon Metabolism in Mycorrhiza. In Methods in Microbiology; Elsevier: Amsterdam, The Netherlands, 1991; Volume 23, pp. 149–180. ISBN 0580-9517. [Google Scholar]
- Rousseau, J.V.D.; Reid, C.P.P. Effects of Phosphorus and Ectomycorrhizas on the Carbon Balance of Loblolly Pine Seedlings. For. Sci. 1990, 36, 101–112. [Google Scholar]
- Sánchez-Díaz, M.; Pardo, M.; Antolín, M.; Peña, J.; Aguirreolea, J. Effect of Water Stress on Photosynthetic Activity in the Medicago-Rhizobium-Glomus Symbiosis. Plant Sci. 1990, 71, 215–221. [Google Scholar] [CrossRef]
- Allen, M.F.; Moore, T.S., Jr.; Christensen, M. Phytohormone Changes in Bouteloua gracilis Infected by Vesicular–Arbuscular Mycorrhizae: I. Cytokinin Increases in the Host Plant. Can. J. Bot. 1980, 58, 371–374. [Google Scholar] [CrossRef]
- Johnson, C.R. Phosphorus Nutrition on Mycorrhizal Colonization, Photosynthesis, Growth and Nutrient Composition of Citrus aurantium. Plant Soil 1984, 80, 35–42. [Google Scholar] [CrossRef]
- Roth, R.; Paszkowski, U. Plant Carbon Nourishment of Arbuscular Mycorrhizal Fungi. Curr. Opin. Plant Biol. 2017, 39, 50–56. [Google Scholar] [CrossRef]
- Qi, S.; Wang, J.; Wan, L.; Dai, Z.; da Silva Matos, D.M.; Du, D.; Egan, S.; Bonser, S.P.; Thomas, T.; Moles, A.T. Arbuscular Mycorrhizal Fungi Contribute to Phosphorous Uptake and Allocation Strategies of Solidago Canadensis in a Phosphorous-Deficient Environment. Front. Plant Sci. 2022, 13, 1–11. [Google Scholar] [CrossRef]
- Chandrasekaran, M. Arbuscular Mycorrhizal Fungi Mediated Enhanced Biomass, Root Morphological Traits and Nutrient Uptake under Drought Stress: A Meta-Analysis. J. Fungi 2022, 8, 660. [Google Scholar] [CrossRef]
- Feng, G.; Zhang, F.S.; Li, X.L.; Tian, C.Y.; Tang, C.; Rengel, Z. Improved Tolerance of Maize Plants to Salt Stress by Arbuscular Mycorrhiza Is Related to Higher Accumulation of Soluble Sugars in Roots. Mycorrhiza 2002, 12, 185–190. [Google Scholar] [CrossRef]
- Jin, H.R.; Liu, J.; Liu, J.; Huang, X.W. Forms of Nitrogen Uptake, Translocation, and Transfer via Arbuscular Mycorrhizal Fungi: A Review. Sci. China Life Sci. 2012, 55, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, L.; Palla, M.; Agnolucci, M.; Avio, L.; Sbrana, C.; Turrini, A.; Giovannetti, M. Arbuscular Mycorrhizal Fungi and Associated Microbiota as Plant Biostimulants: Research Strategies for the Selection of the Best Performing Inocula. Agronomy 2020, 10, 106. [Google Scholar] [CrossRef]
- Jeffries, P.; Gianinazzi, S.; Perotto, S.; Turnau, K.; Barea, J.M. The Contribution of Arbuscular Mycorrhizal Fungi in Sustainable Maintenance of Plant Health and Soil Fertility. Biol. Fertil. Soils 2003, 37, 1–16. [Google Scholar] [CrossRef]
- Basyal, B.; Emery, S.M. An Arbuscular Mycorrhizal Fungus Alters Switchgrass Growth, Root Architecture, and Cell Wall Chemistry across a Soil Moisture Gradient. Mycorrhiza 2021, 31, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.F.; Moore, T.S., Jr.; Christensen, M. Phytohormone Changes in Bouteloua Gracilis Infected by Vesicular–Arbuscular Mycorrhizae. II. Altered Levels of Gibberellin-like Substances and Abscisic Acid in the Host Plant. Can. J. Bot. 1982, 60, 468–471. [Google Scholar] [CrossRef]
- Srivastava, P.; Saxena, B.; Giri, B. Arbuscular Mycorrhizal Fungi: Green Approach/Technology for Sustainable Agriculture and Environment. In Mycorrhiza—Nutrient Uptake, Biocontrol, Ecorestoration: Fourth Edition; Springer: Berlin/Heidelberg, Germany, 2018; pp. 355–386. ISBN 9783319688671. [Google Scholar]
- Linderman, R.G.; Davis, E.A. Varied Response of Marigold (Tagetes spp.) Genotypes to Inoculation with Different Arbuscular Mycorrhizal Fungi. Sci. Hortic. 2004, 99, 67–78. [Google Scholar] [CrossRef]
- Hart, M.M.; Forsythe, J.A. Using Arbuscular Mycorrhizal Fungi to Improve the Nutrient Quality of Crops; Nutritional Benefits in Addition to Phosphorus. Sci. Hortic. 2012, 148, 206–214. [Google Scholar] [CrossRef]
- Schmidt, B.; Domonkos, M.; Sumalan, R.; Biro, B. Suppression of Arbuscular Mycorrhiza’s Development by High Concentrations of Phosphorous at Tagetes patula L. Res. J. Agric. Sci. 2010, 42, 156–162. [Google Scholar]
- Sohn, B.K.; Kim, K.Y.; Chung, S.J.; Kim, W.S.; Park, S.M.; Kang, J.G.; Rim, Y.S.; Cho, J.S.; Kim, T.H.; Lee, J.H. Effect of the Different Timing of AMF Inoculation on Plant Growth and Flower Quality of Chrysanthemum. Sci. Hortic. 2003, 98, 173–183. [Google Scholar] [CrossRef]
- Prasad, K.; Aggarwal, A.; Yadav, K.; Tanwar, A. Impact of Different Levels of Superphosphate Using Arbuscular Mycorrhizal Fungi and Pseudomonas Fluorescens on Chrysanthemum indicum L. J. Soil Sci. Plant Nutr. 2012, 12, 451–462. [Google Scholar] [CrossRef]
- Liang, J.F.; An, J.; Gao, J.Q.; Zhang, X.Y.; Yu, F.H. Effects of Arbuscular Mycorrhizal Fungi and Soil Nutrient Addition on the Growth of Phragmites australis under Different Drying-Rewetting Cycles. PLoS ONE 2018, 13, e0191999. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, N.O.; Atayese, M.O.; Olubode, A.A.; Akan, M.E. Effect of Commercial Arbuscular Mycorrhizal Fungi Inoculant on Growth and Yield of Soybean under Controlled and Natural Field Conditions. J. Plant Nutr. 2020, 43, 487–499. [Google Scholar] [CrossRef]
- Schroeder, M.S.; Janos, D.P. Plant Growth, Phosphorus Nutrition, and Root Morphological Responses to Arbuscular Mycorrhizas, Phosphorus Fertilization, and Intraspecific Density. Mycorrhiza 2005, 15, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Wittenmayer, L.; Merbach, W. Plant Responses to Drought and Phosphorus Deficiency: Contribution of Phytohormones in Root-Related Processes. J. Plant Nutr. Soil Sci. 2005, 168, 531–540. [Google Scholar] [CrossRef]
- Augé, R.M.; Schekel, K.A.; Wample, R.L. Greater Leaf Conductance of Well-Watered Va Mycorrhizal Rose Plants Is Not Related To Phosphorus Nutrition. New Phytol. 1986, 103, 107–116. [Google Scholar] [CrossRef]
- Gaur, A.; Adholeya, A. Diverse Response of Five Ornamental Plant Species to Mixed Indigenous and Single Isolate Arbuscular-Mycorrhizal Inocula in Marginal Soil Amended with Organic Matter. J. Plant Nutr. 2005, 28, 707–723. [Google Scholar] [CrossRef]
- Long, L.K.; Yao, Q.; Huang, Y.H.; Yang, R.H.; Guo, J.; Zhu, H.H. Effects of Arbuscular Mycorrhizal Fungi on Zinnia and the Different Colonization between Gigaspora and Glomus. World J. Microbiol. Biotechnol. 2010, 26, 1527–1531. [Google Scholar] [CrossRef]
- Aboul-Nasr, A. Effects of Vesicular-Arbuscular Mycorrhiza on Tagetes erecta and Zinnia elegans. Mycorrhiza 1995, 6, 61–64. [Google Scholar] [CrossRef]
- Popescu, G.C.; Popescu, M. Role of Combined Inoculation with Arbuscular Mycorrhizal Fungi, as a Sustainable Tool, for Stimulating the Growth, Physiological Processes, and Flowering Performance of Lavender. Sustainability 2022, 14, 951. [Google Scholar] [CrossRef]
- Rashidi, S.; Yousefi, A.R.; Pouryousef, M.; Goicoechea, N. Total Phenol, Anthocyanin, and Terpenoid Content, Photosynthetic Rate, and Nutrient Uptake of Solanum nigrum L. and Digitaria sanguinalis L. as Affected by Arbuscular Mycorrhizal Fungi Inoculation. Weed Biol. Manag. 2020, 20, 95–108. [Google Scholar] [CrossRef]
- Perner, H.; Schwarz, D.; Bruns, C.; Mäder, P.; George, E. Effect of Arbuscular Mycorrhizal Colonization and Two Levels of Compost Supply on Nutrient Uptake and Flowering of Pelargonium Plants. Mycorrhiza 2007, 17, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.J.; Li, W.; Zhai, Y.L.; Hu, X.Y.; Guo, S.X. Arbuscular Mycorrhizal Fungi Promote Early Flowering and Prolong Flowering in Antirrhinum majus L. by Regulating Endogenous Hormone Balance under Field-Planting Conditions. Not. Bot. Horti Agrobot. Cluj-Napoca 2022, 50, 12503. [Google Scholar] [CrossRef]
- Allen, M.F.; Smith, W.K.; Moore, T.S.; Christensen, M. Comparative Water Relations and Photosynthesis of Mycorrhizal and Non-Mycorrhizal Bouteloua gracilis H.B.K. Lag Ex Steud. New Phytol. 1981, 88, 683–693. [Google Scholar] [CrossRef]
- Mosse, B. Plant Growth Responses to Vesicular-Arbuscular Mycorrhiza. New Phytol. 1973, 72, 127–136. [Google Scholar] [CrossRef]
- Zhang, C.; Simpson, R.J.; Kim, C.M.; Warthmann, N.; Delhaize, E.; Dolan, L.; Byrne, M.E.; Wu, Y.; Ryan, P.R. Do Longer Root Hairs Improve Phosphorus Uptake? Testing the Hypothesis with Transgenic Brachypodium distachyon Lines Overexpressing Endogenous RSL Genes. New Phytol. 2018, 217, 1654–1666. [Google Scholar] [CrossRef]
- Bei, S.; Xu, M.; Lyu, X.; Chen, C.; Li, A.; Qiao, X. Arbuscular Mycorrhizal Fungi Enhanced Coix Responses to Phosphorous Forms but Not for Faba Bean in Intercropping Systems, under Controlled Environment. Agron. J. 2021, 113, 2578–2590. [Google Scholar] [CrossRef]
- Li, M.; Cai, L. Biochar and Arbuscular Mycorrhizal Fungi Play Different Roles in Enabling Maize to Uptake Phosphorus. Sustainability 2021, 13, 3244. [Google Scholar] [CrossRef]
- Jansa, J.; Mozafar, A.; Frossard, E. Long-Distance Transport of P and Zn through the Hyphae of an Arbuscular Mycorrhizal Fungus in Symbiosis with Maize. Agronomie 2003, 23, 481–488. [Google Scholar] [CrossRef]
- Cardoso, I.M.; Kuyper, T.W. Mycorrhizas and Tropical Soil Fertility. Agric. Ecosyst. Environ. 2006, 116, 72–84. [Google Scholar] [CrossRef]
- Ngo, H.T.T.; Watts-Williams, S.J.; Cavagnaro, T.R. Mycorrhizal Growth and Phosphorus Responses of Tomato Differ with Source but Not Application Rate of Phosphorus Fertilisers. Appl. Soil Ecol. 2021, 166, 104089. [Google Scholar] [CrossRef]
- Hu, J.; Lin, X.; Wang, J.; Dai, J.; Cui, X.; Chen, R.; Zhang, J. Arbuscular Mycorrhizal Fungus Enhances Crop Yield and P-Uptake of Maize (Zea mays L.): A Field Case Study on a Sandy Loam Soil as Affected by Long-Term P-Deficiency Fertilization. Soil Biol. Biochem. 2009, 41, 2460–2465. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A.; Jakobsen, I. Functional Diversity in Arbuscular Mycorrhizal (AM) Symbioses: The Contribution of the Mycorrhizal P Uptake Pathway Is Not Correlated with Mycorrhizal Responses in Growth or Total P Uptake. New Phytol. 2004, 162, 511–524. [Google Scholar] [CrossRef]
- Cavagnaro, T.R. The Role of Arbuscular Mycorrhizas in Improving Plant Zinc Nutrition under Low Soil Zinc Concentrations: A Review. Plant Soil 2008, 304, 315–325. [Google Scholar] [CrossRef]
- Ames, R.N.; Reid, C.P.P.; Porter, L.K.; Cambardella, C. Hyphal Uptake and Transport of Nitrogen from Two 15N-Labelled Sources By Glomus mosseae, a Vesicular-Arbuscular Mycorrhizal Fungus. New Phytol. 1983, 95, 381–396. [Google Scholar] [CrossRef]
- Johansen, A.; Finlay, R.D.; Olsson, P.A. Nitrogen Metabolism of External Hyphae of the Arbuscular Mycorrhizal Fungus Glomus intraradices. New Phytol. 1996, 133, 705–712. [Google Scholar] [CrossRef]
- Hodge, A.; Campbell, C.D.; Fitter, A.H. An Arbuscular Mycorrhizal Fungus Accelerates Decomposition and Acquires Nitrogen Directly from Organic Material. Nature 2001, 413, 297–299. [Google Scholar] [CrossRef]
- Malcová, R.; Albrechtová, J.; Vosátka, M. The Role of the Extraradical Mycelium Network of Arbuscular Mycorrhizal Fungi on the Establishment and Growth of Calamagrostis epigejos in Industrial Waste Substrates. Appl. Soil Ecol. 2001, 18, 129–142. [Google Scholar] [CrossRef]
- Marschner, H.; Dell, B. Nutrient Uptake in Mycorrhizal Symbiosis. Plant Soil 1994, 159, 89–102. [Google Scholar] [CrossRef]
| Trait | Value | |
|---|---|---|
| Texture | Loamy clay | |
| pH | [1:2.5 soil: water (w/v)] | 7.63 |
| N (%) | 0.181 | |
| P (mg kg−1) | Sodium bicarbonate—extractable | 9.89 |
| K (mg kg−1) | Ammonium acetate—extractable | 490 |
| P 1 | M 2 | Flowering Stem Fresh Weight (g) | Flowering Stem Dry Weight (g) | Flowering Stem Height (cm) | Leaf Area (mm2 plant−1) |
| P1 | M0 | 42.75 ± 13.35 d | 6.78 ± 2.05 e | 52.00 ± 4.62 f | 216,295 ± 30,778 ef |
| M1 | 88.42 ± 10.19 bc | 15.26 ± 1.62 bc | 69.00 ± 6.66 ef | 313,967 ± 64,359 c–e | |
| M2 | 84.90 ± 15.08 bc | 13.70 ± 2.92 c | 76.33 ± 8.76 c–e | 360,591 ± 70,461 bc | |
| M3 | 45.52 ± 5.94 d | 7.66 ± 0.99 de | 88.33 ± 0.88 b–e | 328,889 ± 9964 cd | |
| P2 | M0 | 75.77 ± 10.29 c | 12.92 ± 1.71 cd | 74.67 ± 9.60 d–f | 203,345 ± 5875 f |
| M1 | 121.92 ± 12.38 a | 23.53 ± 2.49 a | 113.00 ± 2.65 a | 474,748 ± 14,030 a | |
| M2 | 124.22 ± 10.58 a | 22.85 ± 2.40 a | 107.67 ± 3.38 ab | 440,725 ± 23,532 ab | |
| M3 | 112.83 ± 6.63 ab | 20.98 ± 1.23 ab | 77.33 ± 13.92 c–e | 374,688 ± 37,098 bc | |
| P3 | M0 | 103.02 ± 8.99 a–c | 16.61 ± 0.39 bc | 94.67 ± 14.44 a–d | 240,351 ± 19,567 d–f |
| M1 | 105.58 ± 4.53 a–c | 18.22 ± 1.44 a–c | 82.67 ± 6.12 c–e | 338,014 ± 26,136 cd | |
| M2 | 92.06 ± 8.75 a–c | 14.94 ± 1.86 c | 100.00 ± 5.77 a–c | 302,769 ± 13,271 c–e | |
| M3 | 93.65 ± 4.24 a–c | 15.25 ± 0.22 bc | 85.33 ± 8.45 b–e | 300,219 ± 39,104 c–e | |
| Sig. 3 | P | *** | *** | *** | ** |
| M | ** | *** | ** | *** | |
| P × M | * | * | ** | * | |
| P | M | Above-Ground Fresh Weight (g) | Above-Ground Dry Weight (g) | Root Fresh Weight (g) | Root Dry Weight (g) |
| P1 | M0 | 133.18 ± 4.85 e | 12.80 ± 1.24 de | 17.92 ± 0.35 b | 1.71 ± 0.06 c |
| M1 | 219.70 ± 42.45 cd | 15.08 ± 3.75 c–e | 24.50 ± 0.19 a | 2.49 ± 0.19 a | |
| M2 | 220.37 ± 23.11 cd | 16.22 ± 1.27 b–d | 23.92 ± 0.53 a | 2.21 ± 0.04 b | |
| M3 | 235.67 ± 7.71 b–d | 16.77 ± 0.99 b–d | 24.14 ± 0.61 a | 2.33 ± 0.02 ab | |
| P2 | M0 | 127.36 ± 7.59 e | 10.33 ± 0.96 e | 12.84 ± 1.12 de | 1.00 ± 0.17 e |
| M1 | 356.87 ± 7.92 a | 23.70 ± 0.49 a | 14.29 ± 0.66 cd | 1.41 ± 0.01 d | |
| M2 | 302.26 ± 16.94 ab | 20.69 ± 0.81 ab | 15.49 ± 0.74 c | 1.49 ± 0.07 cd | |
| M3 | 259.02 ± 12.43 bc | 18.31 ± 0.79 bc | 14.21 ± 0.71 c–e | 1.33 ± 0.02 d | |
| P3 | M0 | 177.67 ± 25.19 de | 14.94 ± 1.20 c–e | 12.21 ± 0.53 e | 0.93 ± 0.14 e |
| M1 | 288.01 ± 20.61 bc | 20.02 ± 1.92 a–c | 12.83 ± 0.76 de | 1.02 ± 0.15 e | |
| M2 | 246.89 ± 21.55 bc | 17.17 ± 1.98 b–d | 12.45 ± 0.56 de | 0.93 ± 0.10 e | |
| M3 | 270.87 ± 30.45 bc | 18.59 ± 0.93 bc | 12.73 ± 0.74 de | 0.97 ± 0.10 e | |
| Sig. | P | ** | * | *** | *** |
| M | *** | *** | *** | *** | |
| P × M | * | * | *** | ** |
| Treatments | Number of Flowering Stems/Plant | Days to Flowering | Root Length (cm/plant) | Shoot K (%) | Root K (%) | Root Ca (%) | |
|---|---|---|---|---|---|---|---|
| P 1 | P1 | 9.02 ± 0.39 a | 122.17 ± 2.39 a | 9648 ± 817.54 a | 1.33 ± 0.04 b | 0.69 ± 0.01 a | 0.60 ± 0.02 b |
| P2 | 11.65 ± 0.95 a | 117.42 ± 2.10 a | 5859 ± 498.61 b | 1.51 ± 0.06 a | 0.70 ± 0.01 a | 0.67 ± 0.04 a | |
| P3 | 10.82 ± 0.74 ab | 114.50 ± 3.60 a | 4787 ± 347.66 b | 1.45 ± 0.05 a | 0.69 ± 0.01 a | 0.65 ± 0.03 ab | |
| M 2 | M0 | 9.80 ± 0.73 a | 110.89 ± 3.64 b | 5191 ± 433.26 b | 1.45 ± 0.07 a | 0.70 ± 0.01 a | 0.64 ± 0.03 a |
| M1 | 11.66 ± 0.96 a | 123.33 ± 2.45 a | 7698 ± 1112.30 a | 1.43 ± 0.07 a | 0.70 ± 0.01 a | 0.66 0.05 a | |
| M2 | 11.06 ± 0.89 a | 118.22 ± 3.16 ab | 7322 ± 895.46 a | 1.41 ± 0.07 a | 0.69 ± 0.01 a | 0.64 ± 0.03 a | |
| M3 | 9.46 ± 0.93 a | 119.67 ± 2.81 ab | 6847 ± 1180.00 a | 1.42 ± 0.07 a | 0.69 0.01 a | 0.63 ± 0.03 a | |
| Sig. 3 | P | ns | ns | *** | *** | ns | * |
| M | ns | * | ** | ns | ns | ns | |
| P × M | ns | ns | ns | ns | ns | ns | |
| Shoot | ||||||
| P 1 | M 2 | P (%) | N (%) | Ca (%) | Zn (mg kg−1) | Fe (mg kg−1) |
| P1 | M0 | 3.48 ± 0.07 f | 2.71 ± 0.06 d | 0.55 ± 0.12 e | 27.52 ± 2.29 ab | 213.67 ± 18.67 a |
| M1 | 5.80 ± 0.07 b | 3.15 ± 0.11 b | 0.65 ± 0.08 d | 19.25 ± 1.39 cd | 165.67 ± 13.57 bc | |
| M2 | 5.63 ± 0.08 b–d | 3.03 ± 0.14 bc | 0.67 ± 0.12 cd | 29.33 ± 1.35 a | 198.33 ± 30.33 ab | |
| M3 | 5.67 ± 0.07 bc | 3.03 ± 0.10 bc | 0.79 ± 0.07 ab | 25.07 ± 3.16 a–c | 180.33 ± 23.38 bc | |
| P2 | M0 | 5.04 ± 0.04 e | 2.75 ± 0.06 cd | 0.71 ± 0.7 b–d | 25.65 ± 2.10 a–c | 195.00 ± 24.33 a–c |
| M1 | 6.08 ± 0.03 a | 3.57 ± 0.11 a | 0.80 ± 0.04 ab | 14.35 ± 0.75 d | 162.00 ± 24.79 c | |
| M2 | 5.60 ± 0.13 b–d | 2.96 ± 0.15 b–d | 0.76 ± 0.5 bc | 18.51 ± 1.08 cd | 188.67 ± 17.70 a–c | |
| M3 | 5.54 ± 0.10 b–d | 3.08 ± 0.12 b | 0.87 ± 0.02 a | 20.91 ± 1.71 b–d | 192.00 ± 43.84 a–c | |
| P3 | M0 | 5.38 ± 0.07 cd | 3.24 ± 0.06 b | 0.75 ± 0.07 b–d | 18.29 ± 1.45 cd | 163.33 ± 22.60 c |
| M1 | 5.52 ± 0.07 b–d | 3.27 ± 0.13 b | 0.71 ± 0.07 b–d | 23.68 ± 3.39 a–c | 184.67 ± 27.57 a–c | |
| M2 | 5.33 ± 0.03 d | 3.13 ± 0.13 b | 0.67 ± 0.07 cd | 24.43 ± 3.65 a–c | 182.33 ± 17.84 a–c | |
| M3 | 5.45 ± 0.03 cd | 2.99 ± 0.06 b–d | 0.77 ± 0.09 b | 24.00 ± 2.33 a–c | 192.67 ± 28.06 a–c | |
| Sig. 3 | P | *** | * | ** | ** | ns |
| M | *** | *** | ns | * | ns | |
| P × M | *** | ** | * | * | * | |
| Root | ||||||
| P | M | P (%) | N (%) | Zn (mg kg−1) | Fe (mg kg−1) | |
| P1 | M0 | 3.42 ± 0.06 f | 2.44 ± 0.11 b | 43.73 ± 5.27 ab | 1206 ± 22.42 a | |
| M1 | 4.05 ± 0.16 d | 2.05 ± 0.04 fg | 41.93 ± 3.19 bc | 836 ± 105.01 b–d | ||
| M2 | 4.17 ± 0.03 d | 1.92 ± 0.9 h | 48.93 ± 4.60 a | 1092 ± 132.82 a–c | ||
| M3 | 4.20 ± 0.07 d | 2.09 ± 0.09 ef | 44.00 ± 3.92 ab | 932 ± 111.20 a–d | ||
| P2 | M0 | 3.76 ± 0.15 e | 2.54 ± 0.09 b | 39.67 ± 4.93 b–d | 1176 ± 254.32 ab | |
| M1 | 5.84 ± 0.04 a | 2.12 ± 0.11 de | 34.27 ± 5.32 ed | 698 ± 61.20 d | ||
| M2 | 5.48 ± 0.10 b | 2.10 ± 0.10 d–f | 34.33 ± 5.02 de | 921 ± 156.10 a–d | ||
| M3 | 5.29 ± 0.04 bc | 2.09 ± 0.07 ef | 36.80 ± 3.44 c–e | 801 ± 65.12 cd | ||
| P3 | M0 | 5.14 ± 0.03 c | 2.79 ± 0.09 a | 31.53 ± 3.60 e | 675 ± 61.48 d | |
| M1 | 5.42 ± 0.05 b | 2.01 ± 0.11 g | 36.67 ± 4.36 c–e | 936 ± 194.30 a–d | ||
| M2 | 5.23 ± 0.04 bc | 2.16 ± 0.08 d | 35.67 ± 4.16 de | 996 ± 187.62 a–d | ||
| M3 | 5.12 ± 0.04 c | 2.06 ± 0.09 e–g | 31.87 ± 4.78 e | 791 ± 36.67 cd | ||
| Sig. | P | *** | *** | *** | ns | |
| M | *** | *** | ns | ns | ||
| P × M | *** | *** | * | * | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheikh-Assadi, M.; Khandan-Mirkohi, A.; Taheri, M.R.; Babalar, M.; Sheikhi, H.; Nicola, S. Arbuscular Mycorrhizae Contribute to Growth, Nutrient Uptake, and Ornamental Characteristics of Statice (Limonium sinuatum [L.] Mill.) Subject to Appropriate Inoculum and Optimal Phosphorus. Horticulturae 2023, 9, 564. https://doi.org/10.3390/horticulturae9050564
Sheikh-Assadi M, Khandan-Mirkohi A, Taheri MR, Babalar M, Sheikhi H, Nicola S. Arbuscular Mycorrhizae Contribute to Growth, Nutrient Uptake, and Ornamental Characteristics of Statice (Limonium sinuatum [L.] Mill.) Subject to Appropriate Inoculum and Optimal Phosphorus. Horticulturae. 2023; 9(5):564. https://doi.org/10.3390/horticulturae9050564
Chicago/Turabian StyleSheikh-Assadi, Morteza, Azizollah Khandan-Mirkohi, Mohammad Reza Taheri, Mesbah Babalar, Hossein Sheikhi, and Silvana Nicola. 2023. "Arbuscular Mycorrhizae Contribute to Growth, Nutrient Uptake, and Ornamental Characteristics of Statice (Limonium sinuatum [L.] Mill.) Subject to Appropriate Inoculum and Optimal Phosphorus" Horticulturae 9, no. 5: 564. https://doi.org/10.3390/horticulturae9050564
APA StyleSheikh-Assadi, M., Khandan-Mirkohi, A., Taheri, M. R., Babalar, M., Sheikhi, H., & Nicola, S. (2023). Arbuscular Mycorrhizae Contribute to Growth, Nutrient Uptake, and Ornamental Characteristics of Statice (Limonium sinuatum [L.] Mill.) Subject to Appropriate Inoculum and Optimal Phosphorus. Horticulturae, 9(5), 564. https://doi.org/10.3390/horticulturae9050564