Effects of CO2 Enrichment on Carbon Assimilation, Yield and Quality of Oriental Melon Cultivated in a Solar Greenhouse
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
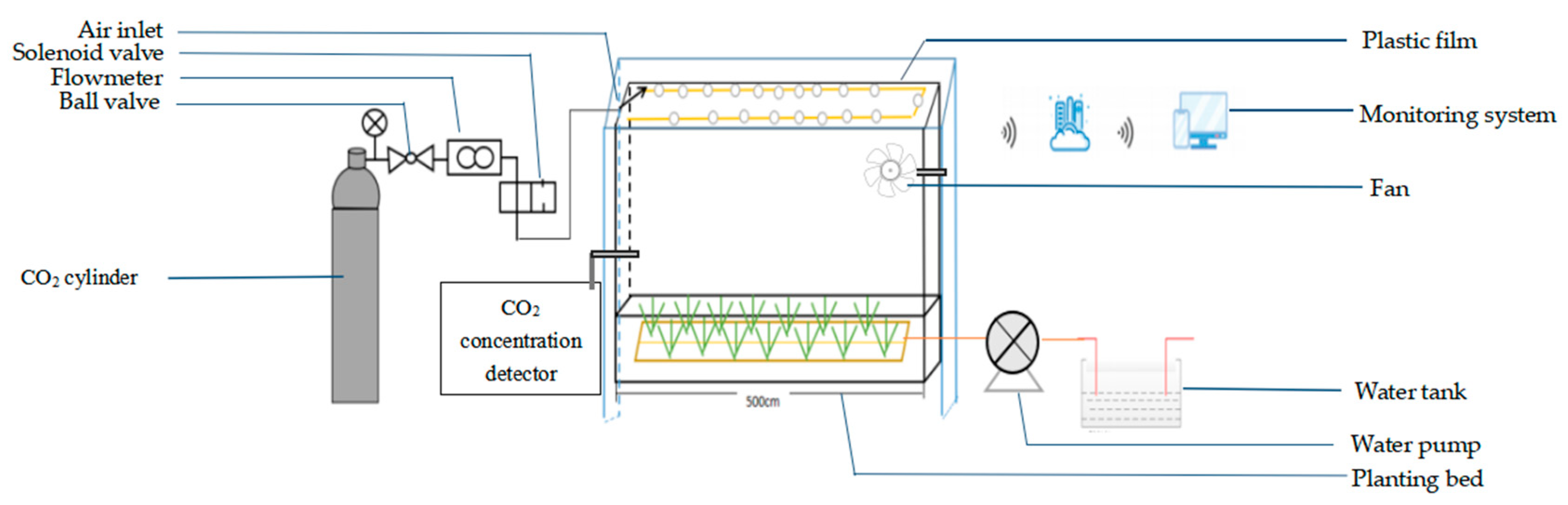
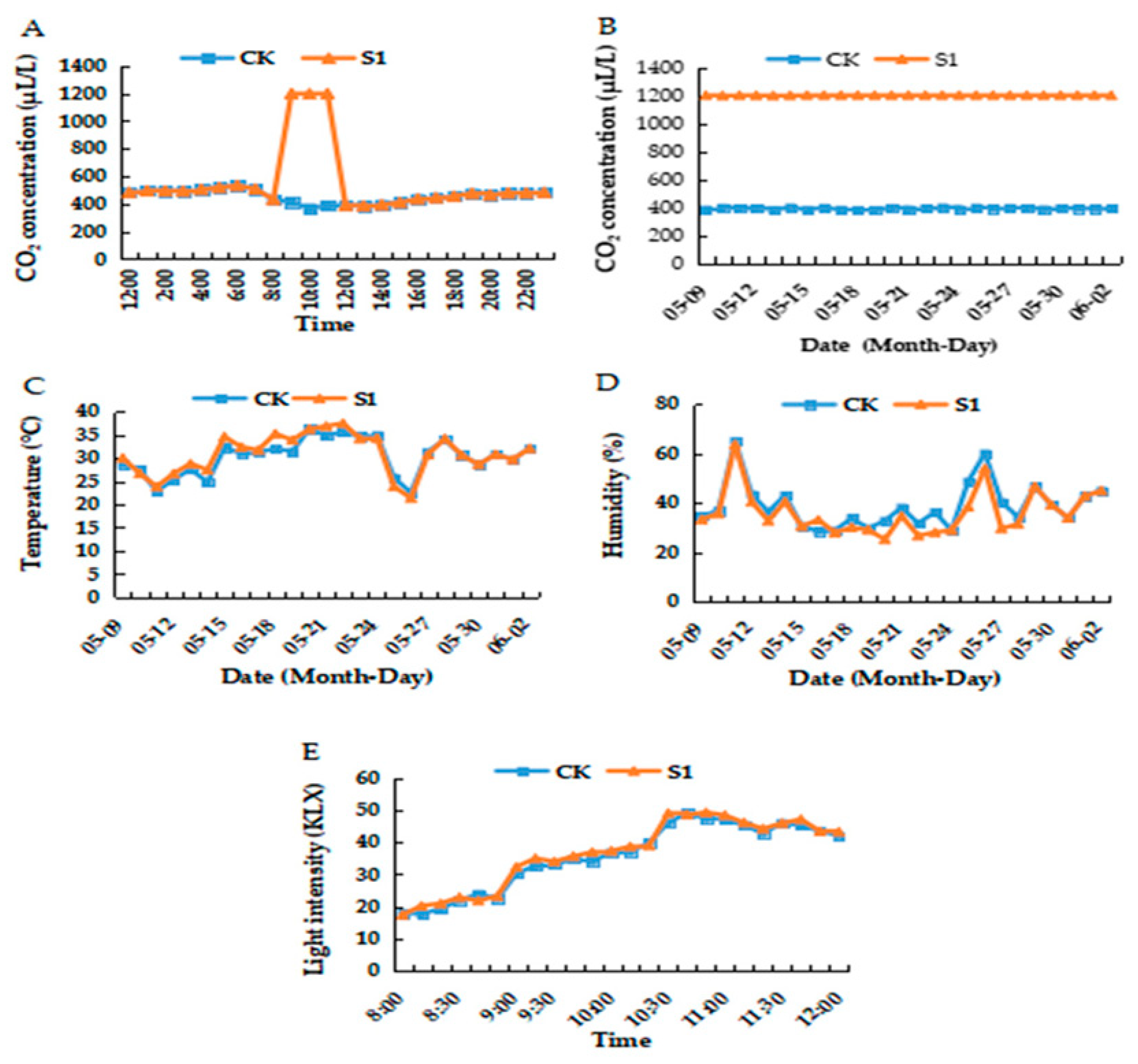
2.2. Environmental Variables within the Greenhouse
2.3. Measurement Items and Methods
2.3.1. Measurement of Growth Indexes
2.3.2. Measurement of Photosynthetic Parameters and Photosynthetic Pigments
2.3.3. Measurement of Soluble Sugar in the Leaves
2.3.4. Measurement of Fruit-Related Indexes
2.3.5. Measurement of Carbon-Assimilation-Related Enzyme Activity and Gene Expression
2.4. Statistical Analysis
3. Results
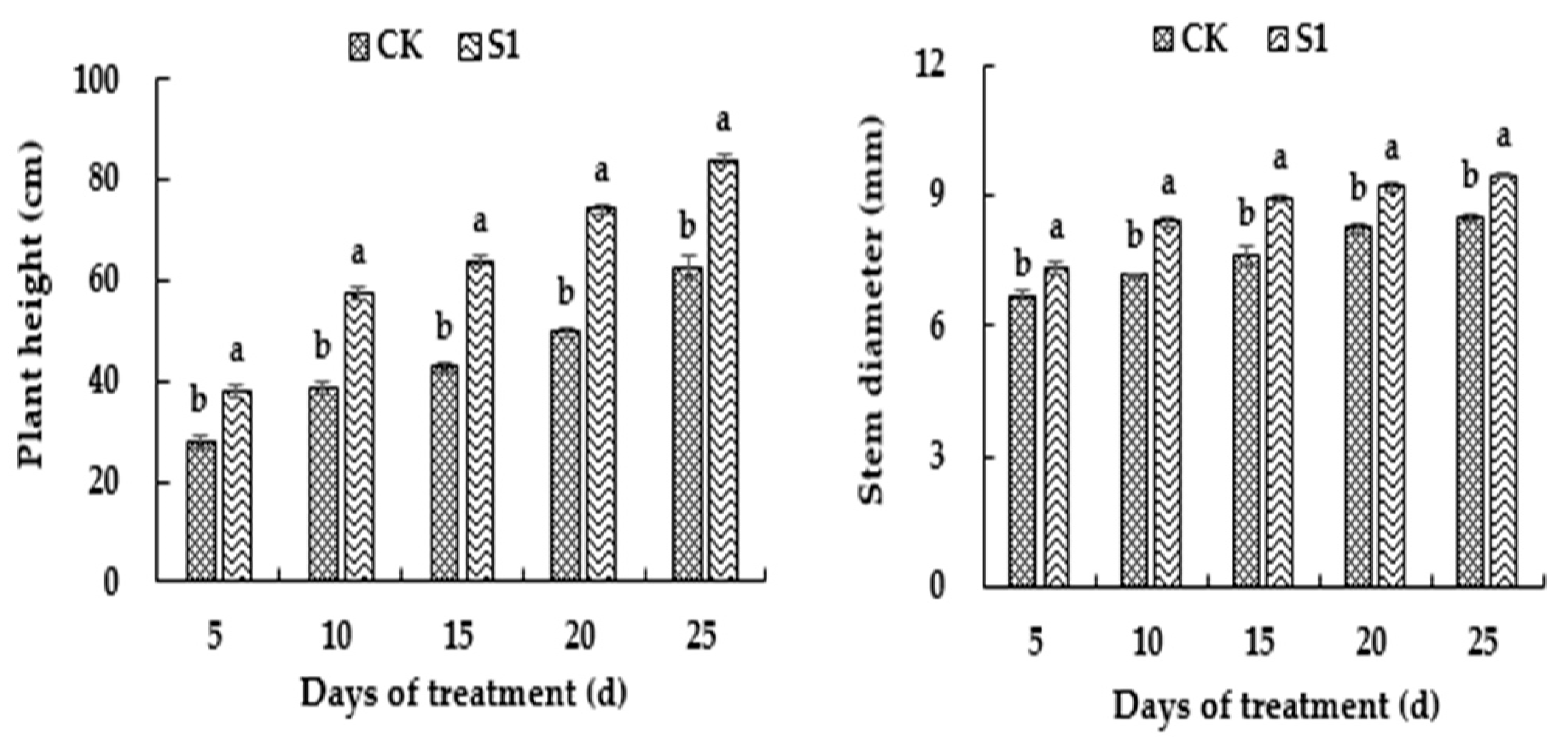
3.1. Effects of CO2 Enrichment on Growth of the Oriental Melon Plants in the Solar Greenhouse
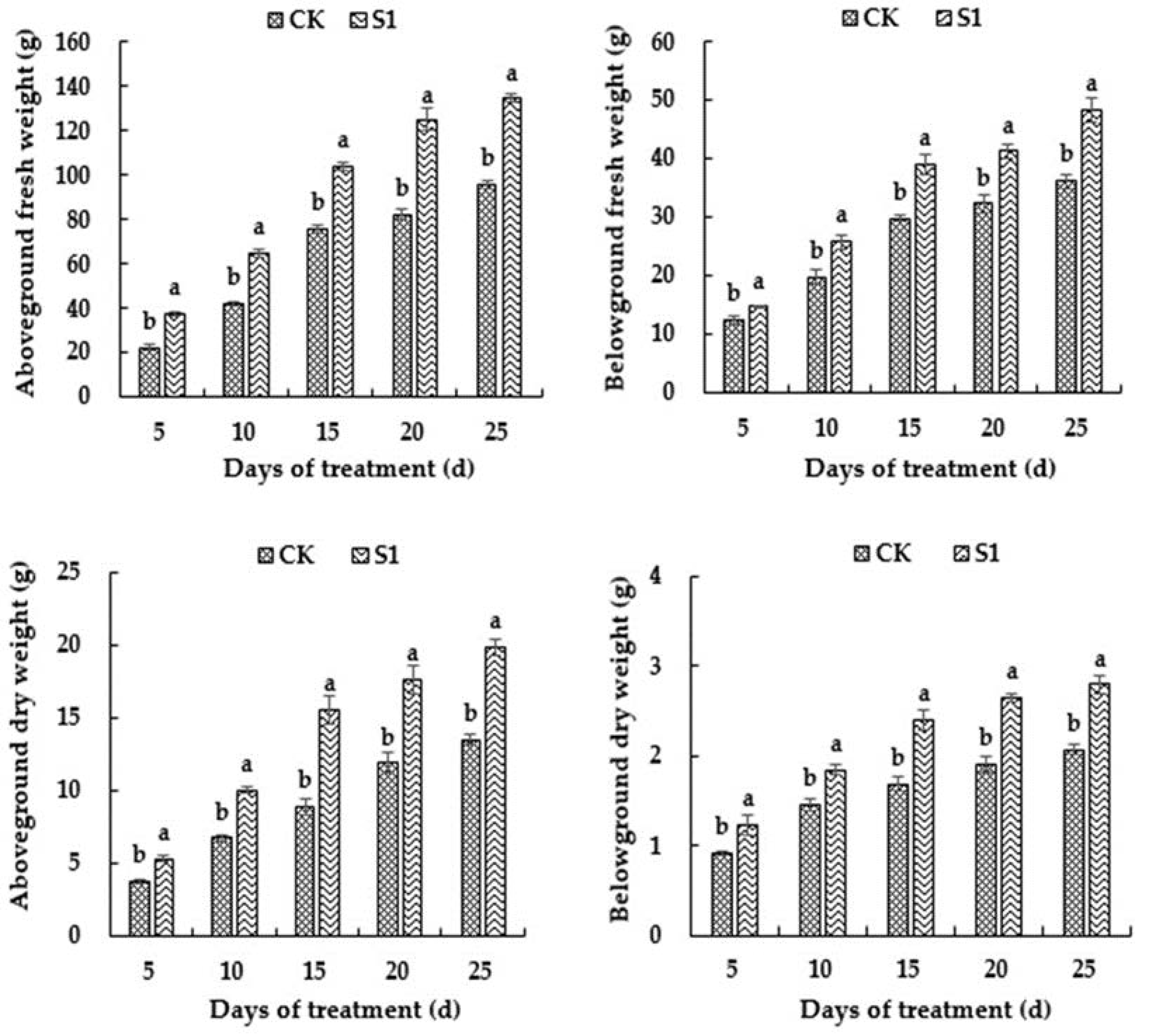
3.2. Effects of CO2 Enrichment on Biomass of the Oriental Melon Plants in the Solar Greenhouse
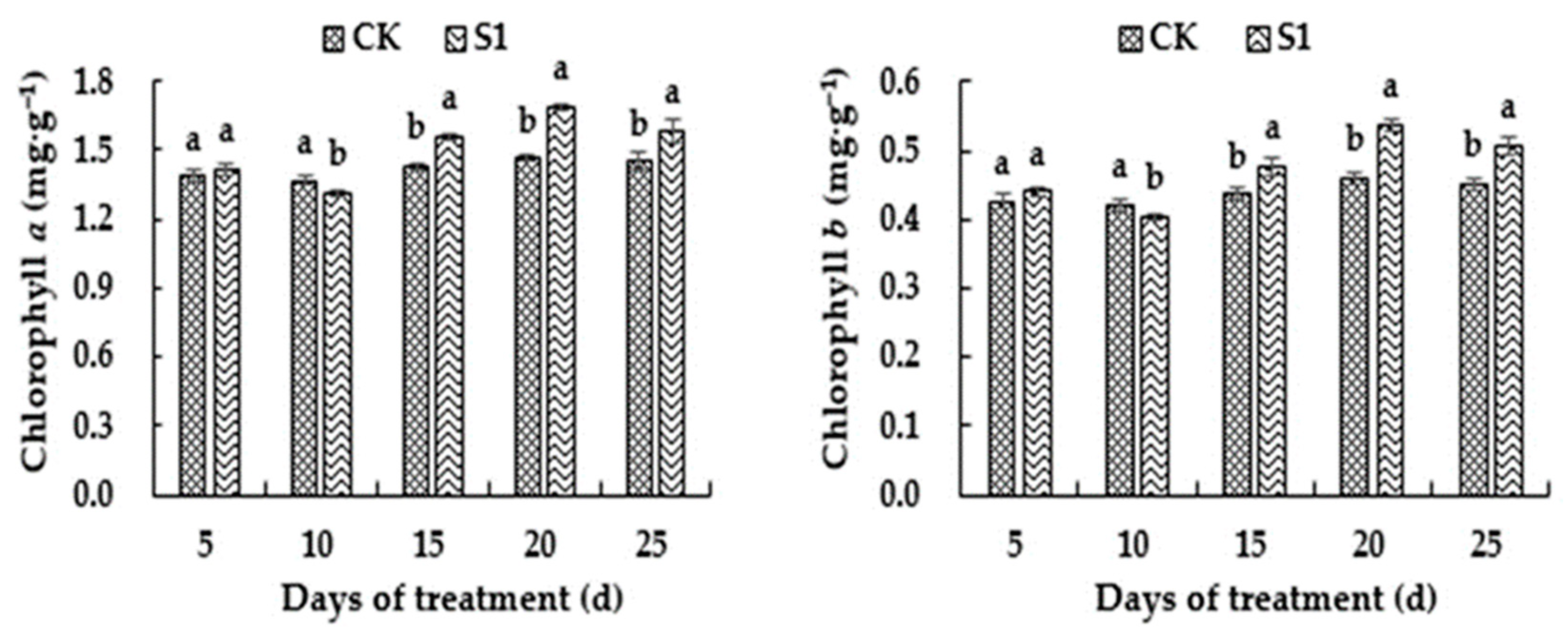
3.3. Effects of CO2 Enrichment on Photosynthetic Pigment Content of the Oriental Melon Leaves in the Solar Greenhouse
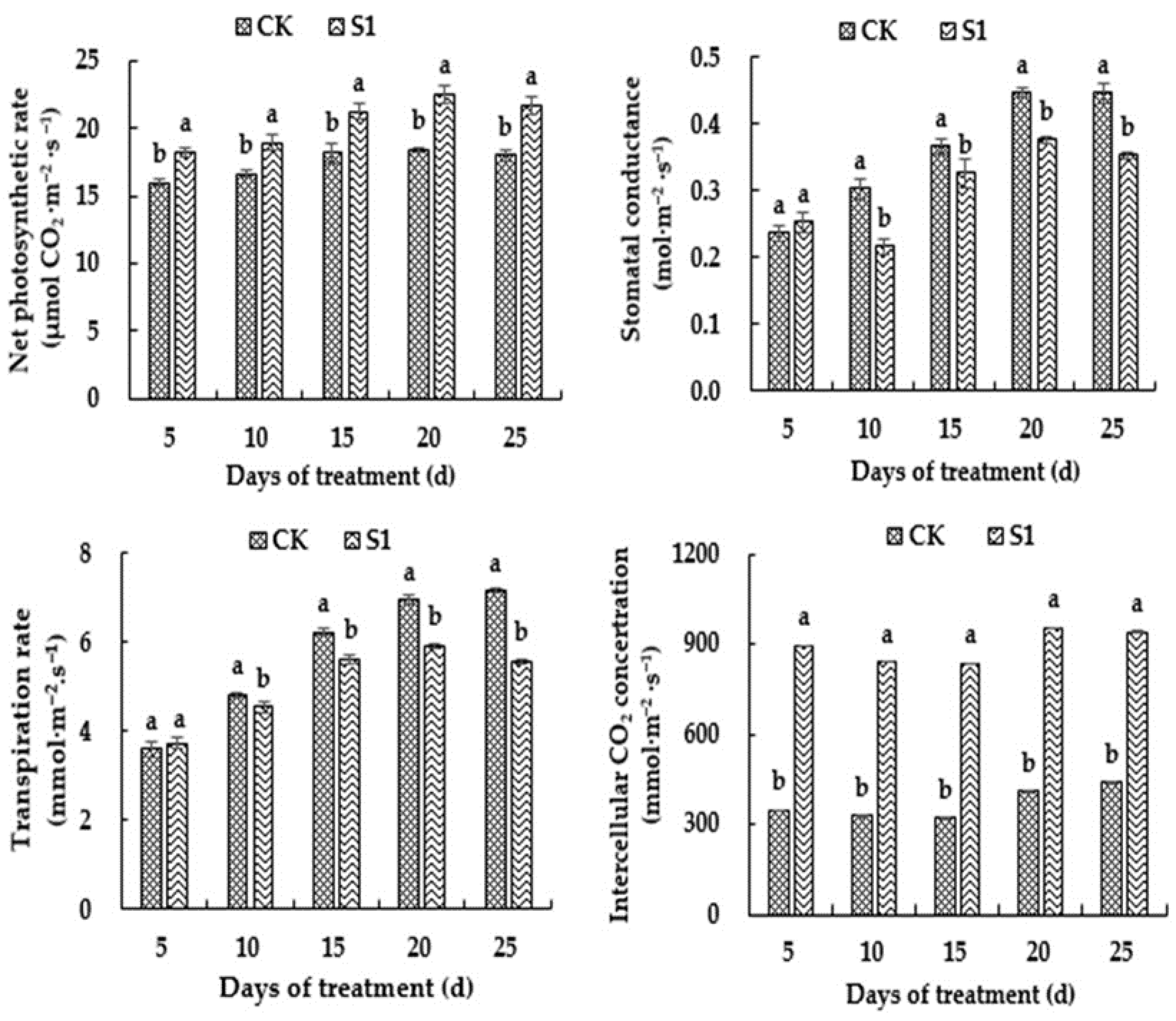
3.4. Effects of CO2 Enrichment on Photosynthetic Parameters of the Oriental Melon Leaves in the Solar Greenhouse
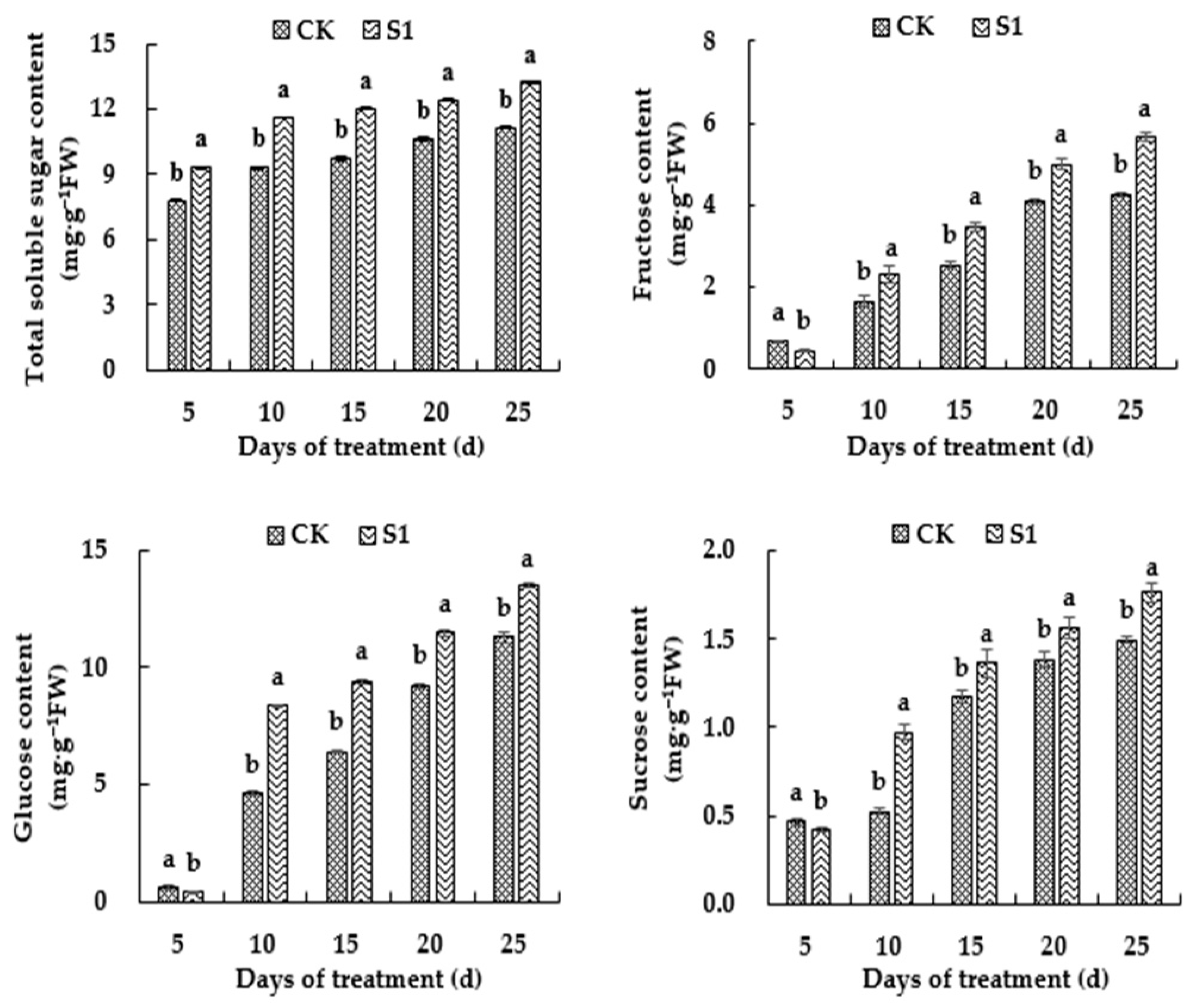
3.5. Effects of CO2 Enrichment on the Soluble Sugar Content of the Oriental Melon Leaves in the Solar Greenhouse
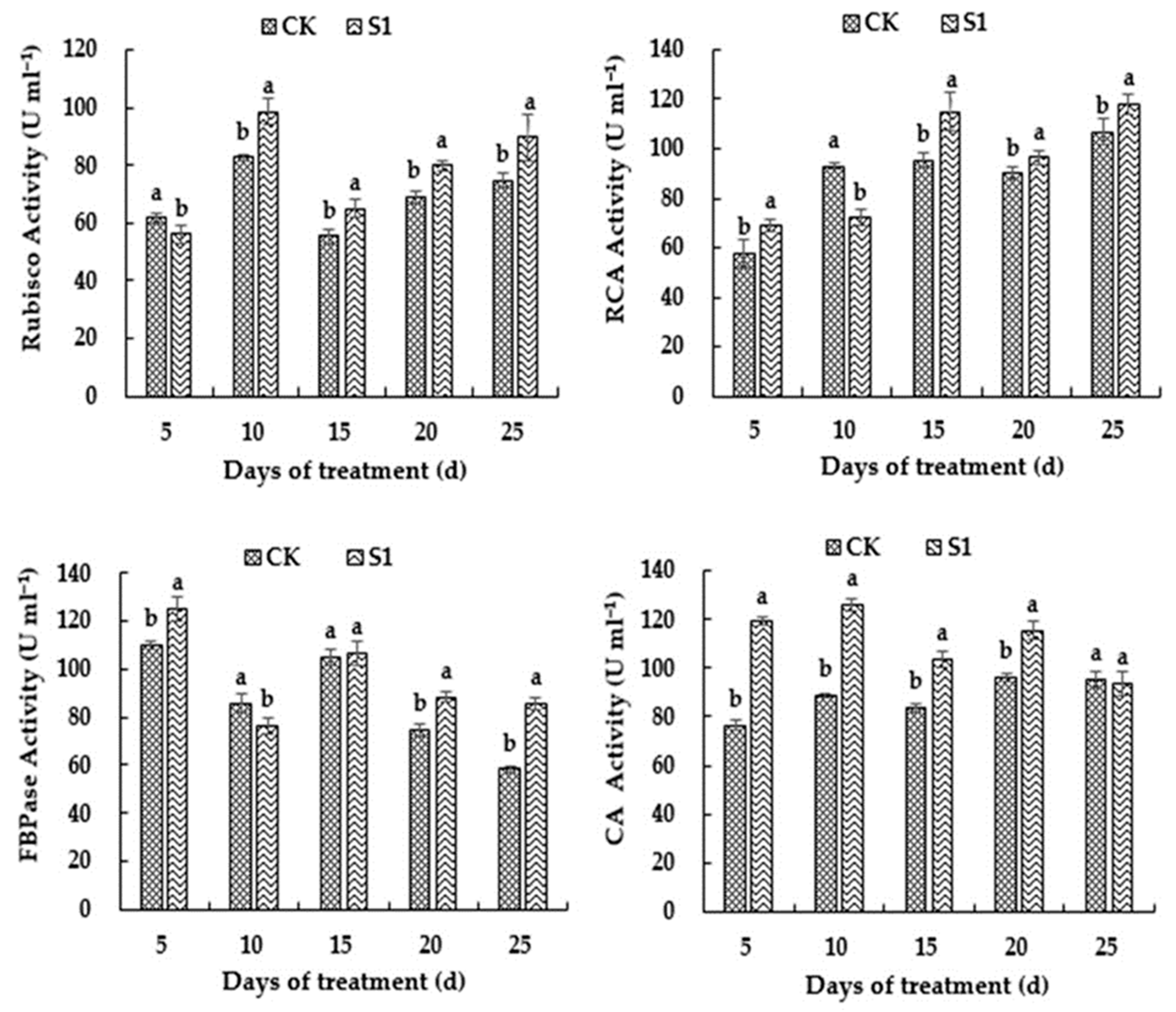
3.6. Effects of CO2 Enrichment on Activity of Carbon-Assimilation-Related Enzymes of the Oriental Melon Leaves in the Solar Greenhouse
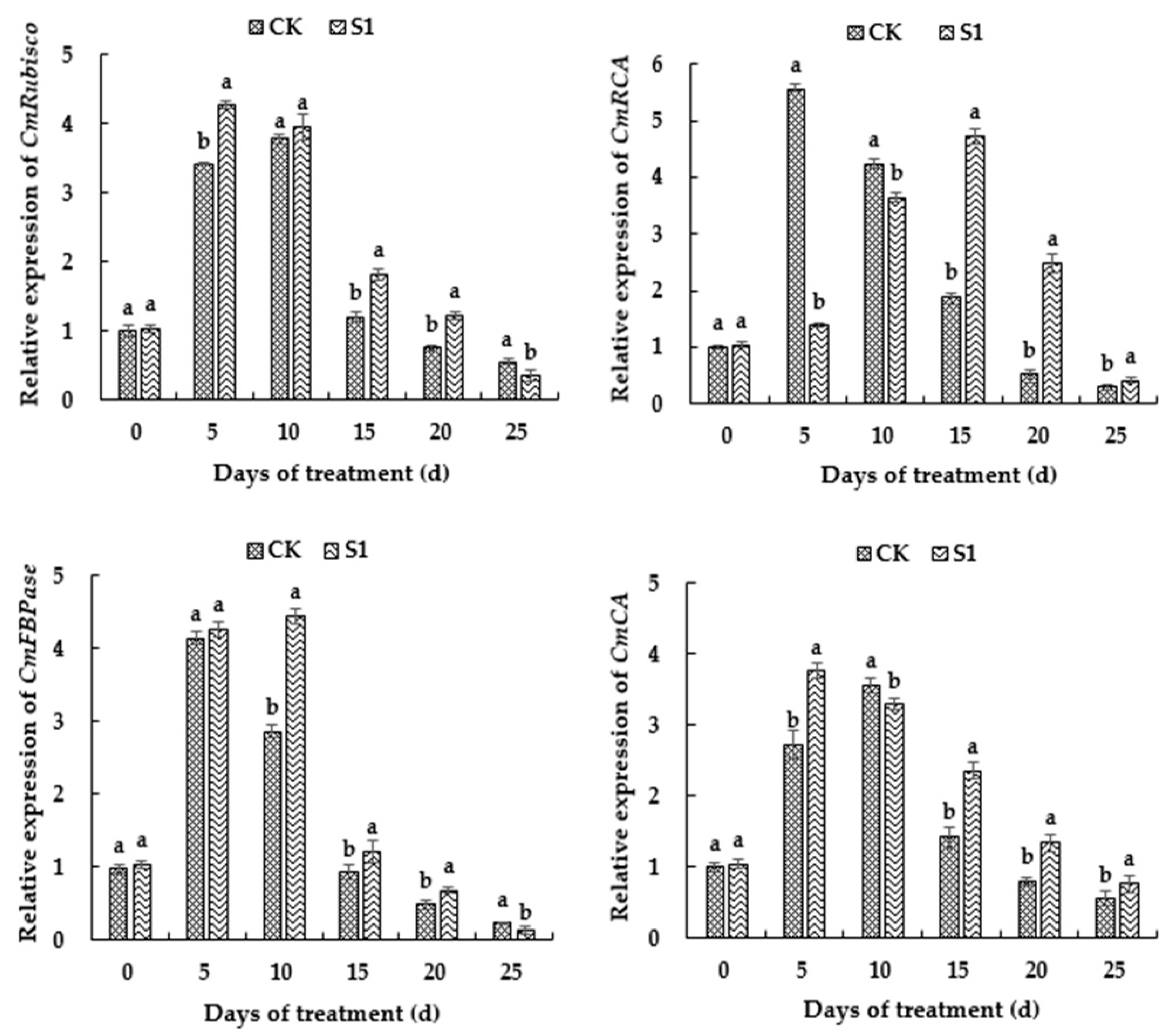
3.7. Effects of CO2 Enrichment on Gene Expression of Carbon-Assimilation-Related Enzymes of the Oriental Melon Leaves in the Solar Greenhouse
3.8. Effects of CO2 Enrichment on Yield and Quality of the Oriental Melon Fruit in the Solar Greenhouse
4. Discussion
4.1. Effects of CO2 Enrichment on Growth and Photosynthesis-Related Indexes of the Oriental Melon Plants in the Solar Greenhouse
4.2. Effects of CO2 Enrichment on Activity of Carbon-Assimilation-Related Enzymes and Associated Gene Expression of the Oriental Melon Leaves in the Solar Greenhouse
4.3. Effects of CO2 Enrichment on the Yield and Quality of the Oriental Melon in the Solar Greenhouse
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cao, K.; Xu, H.J.; Zhang, R.; Xu, D.W.; Yan, L.L. Renewable and sustainable strategies for improving the thermal environment of Chinese solar greenhouses. Energy Build. 2019, 202, 109414. [Google Scholar] [CrossRef]
- Jiang, Y.Q.; Li, T.; Zhang, M.; Sha, S.; Ji, Y.H. WSN-based control system of CO2 concentration in greenhouse. Intell. Autom. Soft Comput. 2015, 21, 285–294. [Google Scholar] [CrossRef]
- Curtis, P.S.; Wang, X. A meta-analysis of elevated CO2 effects on woody plant mass, form, and physiology. Oecologia 1998, 113, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Drake, B.G.; González-Meler, M.A.; Long, S.P. More efficient plants: A consequence of rising atmospheric CO2. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 609–639. [Google Scholar] [CrossRef]
- Jacotot, A.; Marchand, C.; Gensous, S.; Allenbach, M. Effects of elevated atmospheric CO2 and increased tidal flooding on leaf gas-exchange parameters of two common mangrove species: Avicennia marina and Rhizophora stylosa. Photosynth. Res. 2018, 138, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, Z.; Li, J.; Chang, Y.; Du, Q.; Pan, T. Regulation of vapor pressure deficit by greenhouse micro-fog systems improved growth and productivity of tomato via enhancing photosynthesis during summer season. PLoS ONE 2015, 10, e0133919. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef]
- Leakey, A.D.B.; Ainsworth, E.A.; Bernacchi, C.J.; Rogers, A.; Long, S.P.; Ort, D.R. Elevated CO2 effects on plant carbon, nitrogen, and water relations: Six important lessons from FACE. J. Exp. Bot. 2009, 60, 2859–2876. [Google Scholar] [CrossRef]
- Singh, S.K.; Reddy, V.R. Response of carbon assimilation and chlorophyll fluorescence to soybean leaf phosphorus across CO2: Alternative electron sink, nutrient efficiency and critical concentration. J. Photochem. Photobiol. B 2015, 151, 276–284. [Google Scholar] [CrossRef]
- Cohen, I.; Rapaport, T.; Berger, R.T.; Rachmilevitch, S. The effects of elevated CO2 and nitrogen nutrition on root dynamics. Plant Sci. 2018, 272, 294–300. [Google Scholar] [CrossRef]
- Li, J.; Zhou, J.M.; Duan, Z.Q.; Du, C.W.; Wang, H.Y. Effect of CO2 enrichment on the growth and nutrient uptake of tomato seedlings. Pedosphere 2007, 17, 343–351. [Google Scholar] [CrossRef]
- Boondum, S.; Chulaka, P.; Kaewsorn, P.; Nukaya, T.; Takagaki, M.; Yamori, W. Carbon dioxide (CO2) enrichment in greenhouse enhanced growth and productivity of tomato (Solanum lycopersicum L.) during winter. Acta Hortic. 2019, 1245, 61–64. [Google Scholar] [CrossRef]
- AbdElgawad, H.; Farfan-Vignolo, E.R.; Vos, D.D.; Asard, H. Elevated CO2 mitigates drought and temperature-induced oxidative stress differently in grasses and legumes. Plant Sci. 2015, 231, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mamatha, H.; Srinivasa Rao, N.K.; Laxman, R.H.; Shivashankara, K.S.; Bhatt, R.M.; Pavithra, K.C. Impact of elevated CO2 on growth, physiology, yield, and quality of tomato (Lycopersicon esculentum Mill) cv. Arka Ashish. Photosynthetica 2014, 52, 519–528. [Google Scholar] [CrossRef]
- Helyes, L.; Lugasi, A.; Peli, E.; Pek, Z. Effect of elevated CO2 on lycopene content of tomato (Lycopersicon lycopersicum L. Karsten) fruits. Acta Aliment. 2011, 40, 80–86. [Google Scholar] [CrossRef]
- Wei, Z.; Du, T.; Li, X.; Fang, L.; Liu, F. Interactive effects of CO2 concentration elevation and nitrogen fertilization on water and nitrogen use efficiency of tomato grown under reduced irrigation regimes. Agric. Water Manag. 2018, 202, 174–182. [Google Scholar] [CrossRef]
- Islam, M.A.; Kuwar, G.; Clarke, J.L. Artificial light from light emitting diodes (LEDs) with a high portion of blue light results in shorter poinsettias compared to high pressure sodium (HPS) lamps. Sci. Hortic. 2012, 147, 136–143. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, L.; Zhang, M.; Zhang, Y.; Wang, Q. Effect of carbon dioxide enrichment on health-promoting compounds and organoleptic properties of tomato fruits grown in greenhouse. Food Chem. 2014, 153, 157–163. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.; Shang, C.; Wang, Y.; Pan, Z.; Wang, L. Effects of CO2 Enrichment on yield, soluble solid content and downy mildew of greenhouse grape. Agric. Biotechnol. 2018, 7, 71–73. [Google Scholar]
- Parry, M.A.J.; Madgwick, P.J.; Carvahlo, J.F.C.; Andralojc, P.J. Prospects for increasing photosynthesis by overcoming the limitations of Rubisco. J. Agric. Sci. 2007, 145, 31–43. [Google Scholar] [CrossRef]
- Sawada, S.; Usuda, H.; Tsukui, T. Participation of inorganic orthophosphate in regulation of the ribulose-1,5-bisphosphate carboxylase activity in response to changes in the photosynthetic source-sink balance. Plant Cell Physiol. 1992, 33, 943–949. [Google Scholar]
- Bernacchi, C.J.; Singsaas, E.L.; Pimentel, C.; Portis, A.R.; Long, S.P. Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant Cell Environ. 2001, 24, 253–259. [Google Scholar] [CrossRef]
- Jiang, D.A.; Lu, Q.; Weng, X.Y.; Zhen, B.S.; Xi, H.F. Regulation of Rubisco carboxylation activity and photosynthetic rate by Rubisco activase during leaf senesce in rice. J. Zhejiang Univ. Agric. Life Sci. 2000, 26, 119–124. [Google Scholar]
- Martínez-Barajas, E.; Molina-Galán, J.; De Jimenez, E.S. Regulation of Rubisco activity during grain-fill in maize: Possible role of Rubisco activase. J. Agric. Sci. 1997, 128, 155–161. [Google Scholar] [CrossRef]
- Raines, C.A. The Calvin cycle revisited. Photosynth. Res. 2003, 75, 1–10. [Google Scholar] [CrossRef]
- Hewett-Emmett, D.; Tashian, R.E. Functional diversity, conservation, and convergence in the evolution of the α-, β-, and γ-carbonic anhydrase gene families. Mol. Phylogenet. Evol. 1996, 5, 50–77. [Google Scholar] [CrossRef] [PubMed]
- Kaul, T.; Reddy, P.S.; Mahanty, S.; Thirulogachandar, V.; Reddy, R.A. Biochemical and molecular characterization of stress-induced β-carbonic anhydrase from a C4 plant, Pennisetum glaucum. J. Plant Physiol. 2011, 168, 601–610. [Google Scholar] [CrossRef]
- Diamantopoulos, P.D.; Aivalakis, G.; Flemetakis, E.; Katinakis, P. Expression of three β-type carbonic anhydrases in tomato fruits. Mol. Biol. Rep. 2013, 40, 4189–4196. [Google Scholar] [CrossRef]
- Momayyezi, M.; McKown, A.D.; Bell, S.C.S.; Guy, R.D. Emerging roles for carbonic anhydrase in mesophyll conductance and photosynthesis. Plant J. 2020, 101, 831–844. [Google Scholar] [CrossRef]
- Studer, A.J.; Gandin, A.; Kolbe, A.R.; Wang, L.; Cousins, A.B.; Brutnell, T.P. A limited role for carbonic anhydrase in C4 photosynthesis as revealed by a ca1ca2 double mutant in maize. Plant Physiol. 2014, 165, 608–617. [Google Scholar] [CrossRef]
- Xu, X.; Wu, P.; Song, H.; Zhang, J.; Zhang, S.; Xing, G. Identification of candidate genes associated with photosynthesis in eggplant under elevated CO2. Biotechnol. Biotechnol. Equip. 2020, 34, 1166–1175. [Google Scholar] [CrossRef]
- Lin, Y.; Shen, C.; Lin, E.; Hao, X.; Han, X. Transcriptome response of wheat Norin 10 to long-term elevated CO2 under high yield field condition. J. Integr. Agric. 2016, 15, 2142–2152. [Google Scholar] [CrossRef]
- Wei, M.; Xing, Y.; Wang, X.; Ma, H. Variation of CO2 concentration in solar greenhouse in Northern China. J. Appl. Ecol. 2003, 14, 354–358. [Google Scholar]
- Rangaswamy, T.C.; Sridhara, S.; Manoj, K.N.; Gopakkali, P.; Ramesh, N.; Shokralla, S. Impact of Elevated CO2 and Temperature on Growth, Development and Nutrient Uptake of Tomato. Horticulturae 2021, 7, 509. [Google Scholar] [CrossRef]
- Song, H.; Li, Y.; Xu, X.; Zhang, J.; Zheng, S.W. Analysis of genes related to chlorophyll metabolism under elevated CO2 in cucumber (Cucumis sativus L.). Sci. Horti. 2019, 261, 108988. [Google Scholar] [CrossRef]
- Xu, W.X.; Liang, L.; Zhu, M.J. Determination of sugars in molasses by HPLC following solid-phase extraction. Int. J. Food Prop. 2014, 18, 547–557. [Google Scholar] [CrossRef]
- Laurentin, A.; Edwards, C.A. A microtiter modification of the anthrone-sulfuric acid colorimetric assay for glucose-based carbohydrates. Anal. Biochem. 2003, 315, 143–145. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Shah, K.; Singh, M.; Rai, A.C. Bioactive compounds of tomato fruits from transgenic plants tolerant to drought. LWT—Food Sci. Technol. 2015, 61, 609–614. [Google Scholar] [CrossRef]
- Stamenković, S.; Marković, M.; Jovanović, V.S.; Mitić, V.; Ilić, M. Total content of organic acids in plants collected second year after the wild fire. Safety Eng. 2016, 6, 1. [Google Scholar] [CrossRef]
- Bencze, S.; Keresztényi, I.; Varga, B.; Balla, K.; Gémesné-Juhász, A.; Veisz, O. Effect of CO2 enrichment on canopy photosynthesis, water use efficiency and early development of tomato and pepper hybrids. Acta Agron. Hung. 2011, 59, 275–284. [Google Scholar] [CrossRef]
- Lopes, A.; Ferreira, A.B.; Pantoja, P.O.; Parolin, P. Combined effect of elevated CO2 level and temperature on germination and initial growth of Montrichardia arborescens (L.) Schott (Araceae): A microcosm experiment. Hydrobiologia 2018, 814, 19–30. [Google Scholar] [CrossRef]
- Singh, H.; Poudel, M.R.; Dunn, B.L.; Fontanier, C.; Kakani, G. Effect of greenhouse CO2 supplementation on yield and mineral element concentrations of leafy greens grown using nutrient film technique. Agronomy 2020, 10, 323. [Google Scholar] [CrossRef]
- Rogers, H.H.; Runion, G.B.; Krupa, S.V. Plant responses to atmospheric CO2 enrichment with emphasis on roots and the rhizosphere. Environ. Pollut. 1994, 83, 155–189. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Huang, J.; Yang, H.; Dong, G.; Liu, G. Seasonal changes in the effects of free-air CO2 enrichment (FACE) on dry matter production and distribution of rice (Oryza sativa L.). Field Crops Res. 2006, 98, 12–19. [Google Scholar] [CrossRef]
- Ainsworth, E.A. Rice production in a changing climate: A meta-analysis of responses to elevated carbon dioxide and elevated ozone concentration. Glob. Chang. Biol. 2008, 14, 1642–1650. [Google Scholar] [CrossRef]
- Reddy, A.R.; Rasineni, G.K.; Raghavendra, A.S. The impact of global elevated CO₂ concentration on photosynthesis and plant productivity. Curr. Sci. India 2010, 99, 46–57. [Google Scholar]
- Pang, J.; Zhu, J.-G.; Xie, Z.-B.; Liu, G.; Zhang, Y.-L.; Chen, G.-P.; Zeng, Q.; Cheng, L. A new explanation of the N concentration decrease in tissues of rice (Oryza sativa L.) exposed to elevated atmospheric pCO2. Environ. Exp. Bot. 2006, 57, 98–105. [Google Scholar] [CrossRef]
- Pan, T.; Ding, J.; Qin, G.; Wang, Y.; Xi, L. Interaction of supplementary light and CO2 enrichment improves growth, photosynthesis, yield, and quality of tomato in autumn through spring greenhouse production. HortScience 2019, 54, 246–252. [Google Scholar] [CrossRef]
- Moynul Haque, M.; Hamid, A.; Khanam, M.; Biswas, D.K.; Karim, M.A. The effect of elevated CO2 concentration on leaf chlorophyll and nitrogen contents in rice during post-flowering phases. Biol. Plant. 2006, 50, 69–73. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, X.; Ma, Y. Effect of nitrogen application and elevated CO2 on photosynthetic gas exchange and electron transport in wheat leaves. Photosynthetica 2013, 51, 593–602. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Sharkey, T.D. Stomatal conductance and photosynthesis. Ann. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Bunce, J.A. Short-and Long-term inhibition of respiratory carbon dioxide efflux by elevated carbon dioxide. Ann. Bot. 1990, 65, 637–642. [Google Scholar] [CrossRef]
- Beerling, D.J.; Chaloner, W.G. Stomatal density responses of Egyptian Olea europaea L. leaves to CO2 change since 1327 BC. Ann. Bot. 1993, 71, 431–435. [Google Scholar] [CrossRef]
- Paolet, E.; Geluni, R. Stomatal density variation in beech and holm oak leaves collected over 200 years. Acta Oecol. 1993, 14, 173–178. [Google Scholar]
- Luomala, E.M.; Laitinen, K.; Kellomäki, S.; Vapaavuori, E. Variable photosynthetic acclimation in consecutive cohorts of Scots pine needles during 3 years of growth at elevated CO2 and elevated temperature. Plant Cell Environ. 2003, 26, 645–660. [Google Scholar] [CrossRef]
- Dieleman, W.I.J.; Vicca, S.; Dijkstra, F.A.; Hagedorn, F.; Hovenden, M.J. Simple additive effects are rare: A quantitative review of plant biomass and soil process responses to combined manipulations of CO2 and temperature. Glob. Chang. Biol. 2012, 18, 2681–2693. [Google Scholar] [CrossRef]
- Zhu, C.; Zhu, J.; Cao, J.; Jiang, Q.; Liu, G.; Ziska, L.H. Biochemical and molecular characteristics of leaf photosynthesis and relative seed yield of two contrasting rice cultivars in response to elevated [CO2]. J. Exp. Bot. 2014, 65, 6049–6056. [Google Scholar] [CrossRef]
- Hong, F.; Zhou, J.; Liu, C.; Yang, F.; Wu, C.; Zheng, L.; Yang, P. Effect of nano-TiO2 on photochemical reaction of chloroplasts of spinach. Biol. Trace Elem. Res. 2005, 105, 269–279. [Google Scholar] [CrossRef]
- Ferreira, F.J.; Guo, C.; Coleman, J.R. Reduction of plastid-localized carbonic anhydrase activity results in reduced Arabidopsis seedling survivorship. Plant Physiol. 2008, 147, 585–594. [Google Scholar] [CrossRef]
- Dos Santos, B.M.; Balbuena, T.S. Carbon assimilation in Eucalyptus urophylla grown under high atmospheric CO2 concentrations: A proteomics perspective. J. Proteom. 2017, 150, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kang, S.; Li, F.; Zhang, X.; Huo, Z. Light supplement and carbon dioxide enrichment affect yield and quality of off-season pepper. Agron. J. 2017, 109, 2107–2118. [Google Scholar] [CrossRef]
- Dong, J.; Li, X.; Chu, W.; Duan, Z. High nitrate supply promotes nitrate assimilation and alleviates photosynthetic acclimation of cucumber plants under elevated CO2. Sci. Horti. 2017, 218, 275–283. [Google Scholar] [CrossRef]
- Hao, X.; Gao, J.; Han, X.; Ma, Z.; Merchart, A.; Ju, H. Effects of open-air elevated atmospheric CO2 concentration on yield quality of soybean (Glycine max (L.) Merr). Agric. Ecosyst. Environ. 2014, 192, 80–84. [Google Scholar] [CrossRef]
- Mortensen, L.M. Review, CO2 enrichment in greenhouse crop responses. Sci. Hortic. 1987, 33, 1–25. [Google Scholar] [CrossRef]
- Huang, B.; Xu, Y. Cellular and molecular mechanisms for elevated CO2–regulation of plant growth and stress adaptation. Crop Sci. 2015, 55, 1405–1424. [Google Scholar] [CrossRef]
- Xu, G.; Singh, S.K.; Reddy, V.R.; Barnaby, J.Y.; Sicher, R.C.; Li, T. Soybean grown under elevated CO2 benefits more under low temperature than high temperature stress: Varying response of photosynthetic limitations, leaf metabolites, growth, and seed yield. J. Plant Physiol. 2016, 205, 20–32. [Google Scholar] [CrossRef]
- Kimball, B.A. Crop responses to elevated CO2 and interactions with H2O, N, and temperature. Curr. Opin. Plant Biol. 2016, 31, 36–43. [Google Scholar] [CrossRef]
| Gene | Primer Sequences 5’-3’ | Accession Number |
|---|---|---|
| Actin | (F)AAGGCAAACAGGGAGAAGATGA | |
| (R)AGCAAGGTCGAGACGTAGGATA | ||
| CmRCA | (F)CAACGATGTGGAGGGTTTTTAC | MELO3C008231.2 |
| (R)TATGTCTGCTGCTTCACGGTAC | ||
| CmCA | (F)CCTCTATTTTCGCTTTCTCTCTTT | MELO3C009476.2 |
| (R)TTTCAGGTCCATGTGAACCCT | ||
| CmFBPase | (F)TCTCGTCGCTTCTCCCTTCA | MELO3C018610.2 |
| (R)GCCATCACAGCAACTTTTCCA | ||
| CmRubisco | (F)TCGCAAGAACAACGACATCAC | MELO3C012252.2 |
| (R)TCACGGTAAACGAATCCACTG |
| Treatment | Yield Per Plant (g) | Single Fruit Weight (g) | Soluble Solids (%) | Vitamin C (mg/g) | Soluble Protein (mg/g) | Soluble Sugars (%) | Organic Acids (%) | Sugar Acid Ratio |
|---|---|---|---|---|---|---|---|---|
| CK | 1043 ± 6.08 b | 496 ± 8.08 b | 11.46 ± 0.15 b | 25.72 ± 0.41 b | 3.58 ± 0.04 b | 12.46 ± 0.20 b | 0.16 ± 0.05 b | 68.23 ± 2.31 b |
| S1 | 1295 ± 7.63 a | 638 ± 4.50 a | 14.43 ± 0.20 a | 28.06 ± 0.26 a | 4.54 ± 0.12 a | 17.83 ± 0.81 a | 0.23 ± 0.03 a | 90.82 ± 3.47 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Sun, Y.; Chen, J.; Wang, Z.; Qi, H.; Liu, Y.; Liu, Y. Effects of CO2 Enrichment on Carbon Assimilation, Yield and Quality of Oriental Melon Cultivated in a Solar Greenhouse. Horticulturae 2023, 9, 561. https://doi.org/10.3390/horticulturae9050561
Han X, Sun Y, Chen J, Wang Z, Qi H, Liu Y, Liu Y. Effects of CO2 Enrichment on Carbon Assimilation, Yield and Quality of Oriental Melon Cultivated in a Solar Greenhouse. Horticulturae. 2023; 9(5):561. https://doi.org/10.3390/horticulturae9050561
Chicago/Turabian StyleHan, Xintong, Yue Sun, Junqin Chen, Zicong Wang, Hongyan Qi, Yufeng Liu, and Yiling Liu. 2023. "Effects of CO2 Enrichment on Carbon Assimilation, Yield and Quality of Oriental Melon Cultivated in a Solar Greenhouse" Horticulturae 9, no. 5: 561. https://doi.org/10.3390/horticulturae9050561
APA StyleHan, X., Sun, Y., Chen, J., Wang, Z., Qi, H., Liu, Y., & Liu, Y. (2023). Effects of CO2 Enrichment on Carbon Assimilation, Yield and Quality of Oriental Melon Cultivated in a Solar Greenhouse. Horticulturae, 9(5), 561. https://doi.org/10.3390/horticulturae9050561






