Abstract
Wood vinegar (WV) by-product of charcoal production is considered one of the most promising alternatives to synthetic pesticide and fertilizer applications, especially for organic production. Our goal in this study is to evaluate the efficacy of guava (Psidium guajava) WV to control Colletotrichum coccodes, which causes black dot disease, and how it influences potato plant development and yield. This study tested the efficacy of guava WV against the pathogen both in vitro and under greenhouse conditions. Different guava WV concentrations were tested on pathogen growth development, including 0, 0.25%, 0.50%, 1%, 2%, and 3% (v/v). Data revealed that the pathogen’s mycelial growth was significantly inhibited at all the concentrations, and the highest inhibition (100%) was obtained at 3% guava WV. In greenhouse trials conducted for two seasons (2021 and 2022), guava WV applied as a foliar spray at the concentration of 2% and 3% considerably reduced the potato black dot severity evaluated as stem colonization (average of 22.9% for 2021, average of 22.5% for 2022), root covering with sclerotia (average of 21.7% for 2021, average of 18.3% for 2022) and wilted plants percentage (average of 27.8% for 2021, average of 33.3% for 2022). Overall, guava WV also showed a positive effect on plant growth by increasing plant height, stem diameter, and tuber yield per plant of treated potato in both seasons. Gas chromatography-mass spectrometry (GC-MS) analyses revealed the presence in guava WV of phenols, esters, organic acids, antioxidants, and alcohols. In conclusion, guava WV could represent a viable alternative for potato black dot disease management and for plant growth promotion.
1. Introduction
Black dot is one of the common potato (Solanum tuberosum L.) diseases caused by Colletotrichum coccodes (Wallr.) S. Hughes [1]. C. coccodes can infect any part of a potato plant, such as leaves, tubers, stolons, roots, and basal stems [1,2,3,4,5,6]. Early reports of the disease in potatoes and tomatoes date back to the early 19th century and are described in detail by Dickson [7]. In Egypt, the disease was first reported in Salhiya and Abo Swair areas during the 2009–2010 seasons [8]. During storage, the pathogen causes a reduction in tuber weight and quality [9].
Wood vinegar (WV), also known as pyroligneous acid, is a clear brown liquid formed by the condensation of smoke from the charcoal-making process [10]. More than 200 chemical substances, including organic acids, phenols, acetic acid, dimethyl phenol, trimethyl phenol, esters, and nitrogen pyrimidines, are found in WV [11,12]. It is low-cost [13], with a price that is only one-third that of synthetic fungicides. According to Zulkarami et al. [14], WV contains a number of essential elements. These elements perform important roles in plant life cycles and promote photosynthesis. Moreover, the acids, phenol, and other organic compounds in WV have antifungal properties that inhibit the growth of fungi at high concentrations [15].
WV has also been shown to improve soil physicochemical parameters [16] and microbiome, including plant-growth-promoting rhizobacteria [17]. Additionally, WV is able to protect vegetable and horticultural crops from fungal diseases, especially root rots. Sugars, carboxylic acids, hydroxy aldehydes, hydroxy ketones, and phenolic acids are among the major groups of substances in oak, poplar, pine, pruning litter, and forest waste WV [11,18]. It is a low-cost, all-natural product that has no negative effects on living organisms or the environment [19]. It has been demonstrated to suppress a number of soil-borne plant pathogens [20]. The mycelial growth of Plasmopara viticola (Berk. and M.A.Curtis) Berl. and De Toni, Verticillium dahliae (Klebahn), Phytophthora capsici (Leonian), and Fusarium graminearum (Schwabe) have been slowed down by WV made from apricot trees [21]. WV causes complete inhibition of the growth of Alternaria mali (Roberts), the causal agent of apple Alternaria blight, when applied at a 1:32 dilution [22].
WV ester compounds can increase chlorophyll, photosynthesis, sugar, and amino acids production and stimulate plants’ resistance to diseases and pests [23]. Cryptomeria japonica (Linnaeus) WV demonstrated potent antifungal activity against Pythium splendens (H.Braun), Phytopthora capsici, and Ralstonica solanacearum (Smith and Yabuuchi) [24]. In a related study, Velmurugan et al. [25] showed that WV of bamboo significantly reduced the growth of the Ophiostoma species that cause wood rot in forest trees. Several studies have conclusively demonstrated that the phenolic compounds in WV are responsible for their antifungal properties [26,27,28]. Additionally, WV increases the abiotic stress tolerance [29], growth, production, and quality of a wide range of crops [30]. WV, when used as a foliar application, increases yields in cucumber, lettuce, and cole, and jasmine rice [31,32]. The objectives of this study were to evaluate the efficacy of guava wood vinegar against Colletotrichum coccodes which causes black dot disease and investigate its effect on potato plant growth as well. Determine and identify the bioactive components that are most prevalent in guava WV using the GC-MS technique.
2. Materials and Methods
2.1. Fungal Isolation and Identification
Colletotrichum coccodes virulent strain was recovered from diseased potato tubers with a black dot collected from potato fields in the Nubaria region (El-Beheira Governorate, Egypt; 30° 91125″ N, 29° 97119″ E). Small sections of the infected tubers were cut up and surface treated for 1 min with 1% sodium hypochlorite (commercial bleach) before being rinsed in sterile water and dried with sterile filter paper. The surface-sterilized samples were then plated onto a PDA medium and incubated at 25 °C in the dark. The developed colonies were then purified using the hyphal tip method and identified according to the cultural features and morphological and microscopical characteristics stated by Sutton [33]. Pure cultures were sub-cultured on PDA slants and stored at 4 °C until use.
2.2. Pathogenicity Test
2.2.1. Plant Material
Cara cv. potato tubers were sterilized with sodium hypochlorite 1% for 2 min and placed in the dark for 3 weeks at 18–25 °C for sprouting [34]. Tubers were cut into pieces and left for 48 h before planting in vitro at room temperature to allow for partial wound healing. One piece of potato tuber with three eyes of almost the same size was planted in each plastic pot of 30 cm diameter (2.4 kg soil), filled with a sterilized mixture of clay and sand soil (4:1 w/w). Sowing was done on 1st February in season 2020. The plants were fertilized with NPK (Dotra Fert: 20/20/20) from the Egyptian Dotra Company at a concentration of 2 g/L water. The pots were kept under careful observation in greenhouse conditions in natural light at the Agricultural Botany Dept., Agric., Al-Azhar University, Assiut Branch, and were irrigated when it was needed.
2.2.2. Inoculum Preparation
Colletotrichum coccodes isolate was grown on a PDA medium in Petri dishes for 7 days at 25 °C in the dark. Conidia were harvested from the agar surface with sterile distilled water using a scraper, then filtered through several layers of sterilized cheesecloth. A hemacytometer was used to adjust the conidia suspension to a final concentration of 2 × 106 conidia/mL [35].
2.2.3. Plants Inoculation
Six weeks after sowing, potato stems were inoculated with the pathogen. Using a sterile scalpel, one wound was made into the base of the stem (above the soil’s surface). An Agar disc (6 mm) containing the causal pathogen was placed in each wound. On the wounds of the control plants, only agar discs without pathogens were applied. In addition, each pot received 15 mL of the spore suspension (2 × 106 conidia/mL) by hand sprayer, as El-Marzoky [8] explained; in case of the control plant, it was sprayed with 15 mL sterilized distilled water. Three plants were used for each treatment as replicates, and the experiment was repeated twice. After inoculation, all plants were transferred to a greenhouse. To maintain a high humidity level, inoculated plants were covered with transparent plastic bags for 24 h. The plants were then examined every day to check for symptoms. After three days, every inoculated stem exhibited necrosis, but control stems exhibited no signs [36].
2.2.4. Disease Assessment
The disease severity of the black dot on roots was assessed visually using a 0–3 scale based on the percentage of roots covered with sclerotia as follows: 0 = no sclerotia, 1 = 1–30%, 2 = 31–60%, and 3 = ˃60% [35]. Additionally, the percentage of wilted plants to the total number of inoculated plants was calculated. The disease severity index (DSI) on the aboveground stem was calculated by multiplying the colonization outcome (0 or 1) by the height aboveground from which the segment was removed. The stem segments (1 cm) were cut off at 2, 6, 10, and 14 cm above ground level. The segments were cultured on a PDA medium after being sterilized and incubated at 25 °C in the dark [36]. The fungal colonization of stems was recorded as a binary outcome with 1 = colonized, and 0 = non-colonized. The disease severity index percentage (DSI%) was calculated according to the following equation [37].
DSI (%) = 100 × [2 × (0 or 1) + 6 × (0 or 1) + 10 × (0 or 1) + 14 × (0 or 1)]/32
2.3. Antifungal Activity
2.3.1. Guava Wood Vinegar Production
WV is a secondary product produced when smoke from charcoal production is cooled by outside air while passing through a chimney or flue pipe. The cooling effect causes condensation of WV, particularly when the temperature of the smoke produced by carbonization ranges between 80 °C and 180 °C [10]. The guava WV used in this study was derived from the charcoal production of guava trees wood (Psidium guajava) in Egypt’s El-Kalyobia governorate. The obtained guava WV was stored at room temperature for further studies.
2.3.2. Analysis of the Chemical Composition of Guava Wood Vinegar by GC–MS
A gas chromatography-mass spectrometry (GC-MS) system (GC Trace 1300 Thermo Scientific) was used to analyze the components of guava WV at Assiut University’s Faculty of Science Department of Chemical. A single quadruple mass spectrometer (ISQ 7000 Thermo Scientific) was used. The carrier (He, 99.999%) flow was 1 mL min−1, split 10:1, and injected volumes 2 µL. The column temperature was maintained initially at 110 °C and held for 5 min at a rate of 10 °C/min, the temperature increased up to 200 °C and held for 5 min, then the temperature increased up to 250 °C at a rate of 5 °C/min and held for 5 min. The injector temperature was 250 °C, and this temperature was held constant during the analysis. The electron impact energy was 70 eV, and the ion source temperature was set at 250 °C. Electron impact (EI) mass scan (m/s) was recorded in the 40–650 amu range [38]. These components were identified using the following parameters: retention time (RT), molecular weight, molecular formula, and area %.
2.3.3. Plate Assays
The inhibition of C. coccodes mycelial radial growth by the guava WV was tested at concentrations of 0.25%, 0.50%, 1.00%, 2.00%, and 3.00%. The guava WV was added directly to the autoclaved (20 min at 121 °C) PDA medium cooled to 45 °C, poured into sterile Petri dishes [39], and allowed to set. The center of each test plate was subsequently inoculated with a 6 mm size plug of 15-day-old C. coccodes culture and incubated at 27 °C in the dark. As a control, the fungus was cultivated on a PDA medium without guava WV. For each treatment, three replications were performed, and the experiment was repeated twice. When maximum growth was observed in the control plates, the colony’s radial growth was measured, and the percentage of growth inhibition (R) was calculated using the formula.
whereas: C = radial growth in control and B = radial growth in treatment.
R = [(C − B)/C] × 100
2.3.4. Greenhouse Experiments
During the growing seasons of 2021 and 2022, greenhouse experiments were conducted at the farm of the Faculty of Agriculture, Al-Azhar University, Assiut Branch, Assiut, Egypt, to study the effect of guava WV on the disease. On 1st February in seasons 2021 and 2022, one piece of potato tubers was planted in pots (30 cm in diameter). The seedlings were kept in a greenhouse with regular irrigation and fertilization. The plants were inoculated with the pathogen as described in the pathogenicity test, as previously stated. Following the appearance of the first symptoms of disease, the plants were sprayed with guava WV at 2% and 3% based on laboratory studies. Two sprays were applied to each treatment (10 days between each one). As a control, the fungicide Amistar (Azoxystrobin 25%) from Syngenta Company, Basel, Switzerland, was applied at a concentration of 0.50 cm3/L water. Three plants were used for each treatment as replicates, and control plants were only sprayed with distilled water. The plants were then examined every day to check for symptoms. Plant height, stem diameter, and tuber yield of potato per pot were measured.
2.4. Statistical Analysis
The MSTAT-C software version 2.1 was used to conduct a data analysis of variance (ANOVA) [40]. All experiments were carried out in three replicates, and data are presented as mean ± standard deviation (SD). The treatment means were compared using the least significant difference (LSD) (p < 0.05), according to Gomez and Gomez [41].
3. Results
3.1. Isolation and Identification of the Pathogen
Colletotrichum coccodes were obtained from tubers of infected potatoes with symptoms of black dot disease (Figure 1A). Grown on PDA medium, C. coccodes formed a circular colony at first white, then grey (Figure 1B) with age. Conidiomata (Figure 1C) produced aseptate, hyaline, smooth-walled, and straight conidia (Figure 1D).
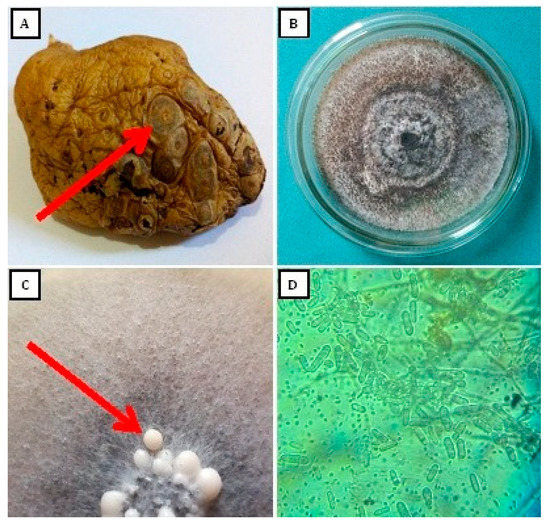
Figure 1.
Colletotrichum coccodes symptoms of black dot disease on naturally infected potato tuber (A), 15-day-old colony (B), conidiomata (C), and conidia at 40× magnification (D) on Potato Dextrose Agar medium.
3.2. Pathogenicity of C. coccodes Isolate on Potato Plants
The symptoms of the disease were observed on potato plants inoculated with the pathogen. Every injected stem showed necrosis after three days, but control stems showed no symptoms. The black dot disease symptoms on inoculated plants were recorded as stem colonization (SC), root covering with sclerotia (RCS), and wilted plant percentages. Koch’s hypotheses were confirmed when the causal pathogen was successfully isolated from inoculated plants.
3.3. Chromatographic Analysis of Guava WV
Data in Figure 2 show that the chromatogram obtained shows the major peaks corresponding to the more abundant compounds present in the sample. The most abundant compound (16.12%) was identified as 2,6-Dimethoxyphenol, which was associated with peak 3, followed by guaiacol (12.82%) associated with peak 1 (Figure 2), and 1,2,4-trimethoxybenzene (6.39%) associated with peak 4. Table 1 describes the overall chemical composition, retention times of each compound, and percentages of each compound present in guava WV. The presence of several bioactive compounds in guava WV, including phenols, esters, alcohols, antioxidants, and organic acids (Table 1 and Figure 3). GC-MS analyses revealed the presence in guava WV of phenols, esters, organic acids, antioxidants, and alcohols.
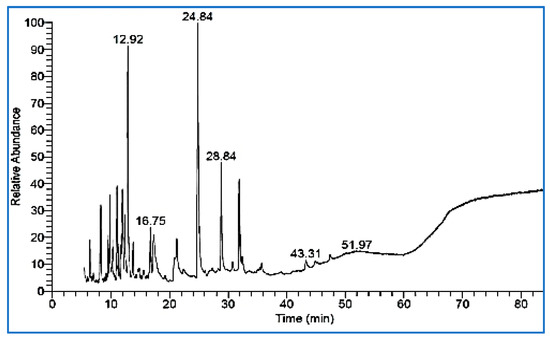
Figure 2.
GC-MS chromatogram analysis of guava wood vinegar.

Table 1.
The main chemical constituents of the guava WV were analyzed by GC-MS.
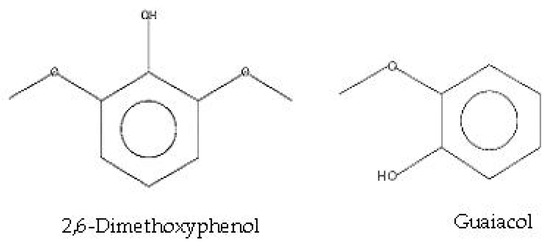
Figure 3.
The chemical structures of the major components of the guava WV.
3.4. Plate Assays
The data in Figure 4 and Figure 5 demonstrate that guava WV treatment significantly (p ≤ 0.05) reduced C. coccodes mycelial growth at all tested concentrations when compared to the control. The percentage of mycelial growth inhibition increased along with increasing concentration. The highest WV concentration (3%) showed a complete inhibition of the pathogen’s growth (100%). Furthermore, the data revealed that there were significant differences in the percentage of pathogen growth inhibition among the tested concentrations.
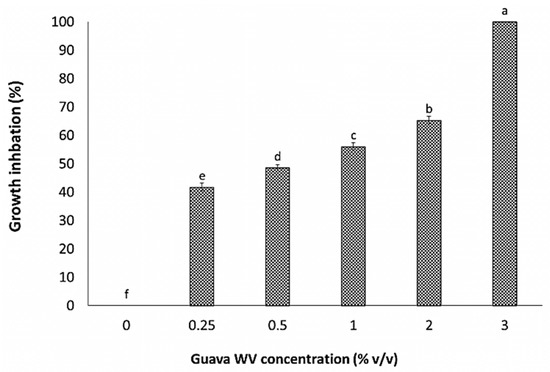
Figure 4.
Effect of different guava WV concentrations on in vitro Colletotrichum coccodes radial growth inhibition. Values are the mean of three replicates ± SD. In each column, data followed by the same letter do not differ significantly as determined by the LSD test p = 0.05.
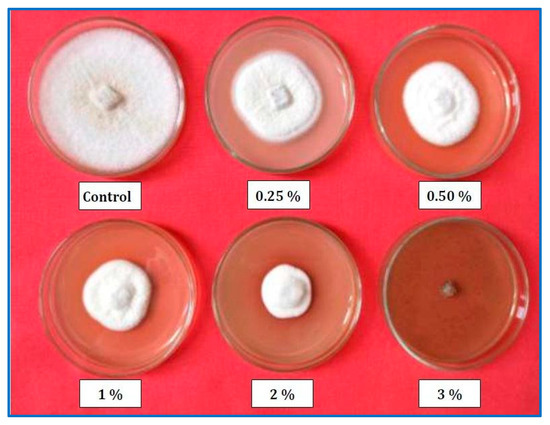
Figure 5.
Mycelial growth of Colletotrichum coccodes on Potato Dextrose Agar medium alone (control) or amended with different guava wood vinegar concentrations.
3.5. Greenhouse Experiments
The application of guava WV as a foliar treatment on infected potato plants significantly decreased the disease severity of black dot in all disease parameters, including stem colonization, root covering with sclerotia, and wilt percent percentages (Table 2). Both of the tested concentrations (2% and 3% v/v) had an effect on the disease in greenhouse conditions, but the 3% concentration was more successful in suppressing the disease than the 2% concentration. The fungicide Amistar recorded the best percent of disease inhibition in both seasons of 2021 and 2022. The Amistar 25% treatment showed lower disease severity index values compared to the inoculated and untreated control, indicating its effectiveness in reducing disease severity. The treatment with a concentration of 3% guava WV recorded the greatest reduction in disease severity of all disease parameters, i.e., stem colonization (20.16% and 21.91%), root covering with sclerotia (20 and 23.33), and wilt (22.22% and 33.33%) in seasons 2021 and 2022, respectively. There are significant differences in the effects of all the treatments on the disease, though there are no significant differences in wilting percentage among treatments in the growing season 2022.

Table 2.
Disease severity index (%) referred to stem colonization, root covered with sclerotia, and wilt of potato plants inoculated with Colletotrichum coccodes and exposed to different treatments under greenhouse conditions during the 2021 and 2022 growing seasons.
Overall, the results presented in this table suggest that the guava treatments, particularly guava WV 2%, had a positive impact in reducing disease severity (SC, RCS, and Wilt) in comparison to the uninoculated and untreated control.
3.6. Effects of Guava Wood Vinegar on Plant Growth and Tuber Yield of Potato
The data presented in Table 3 show that foliar application of guava WV improved plant growth parameters, such as plant height, stem diameter, and tuber yield per pot of potato, in both seasons. Guava WV at 3% gave the highest values of plant height (38.67 and 38.00 cm), stem diameter (11.00 and 11.33 mm), and tuber yield (274.54 and 281.72 g) in seasons 2021 and 2022, respectively (Figure 6). Furthermore, data also showed that using guava WV increased potato tuber yield per pot when compared to untreated plants.

Table 3.
Effect of different treatments on plant height, stem diameter, and tuber yield/pot of potato plants cropped under greenhouse conditions during 2021 and 2022 growing seasons.
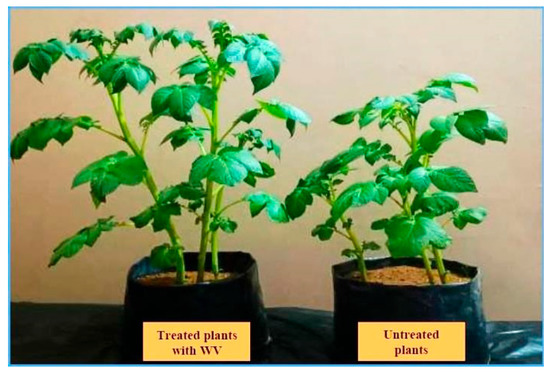
Figure 6.
Effect of the guava WV treatment on the vegetative growth of potato plants grown in greenhouses.
The Amistar 25% treatment also showed positive effects on plant height, stem diameter, and tuber yield, although it was generally outperformed by the guava WV 3% treatment. Overall, the results of this table indicate that the guava WV 3% treatment had the most significant positive impact on plant growth and tuber yield among the treatments evaluated in both growing seasons.
4. Discussion
Charcoal production from wood under anaerobic conditions is a very good way to preserve the environment, and at the same time, WV is the result of this process. WV is rich in many phenolic compounds, organic acids, antioxidants [11], and some nutrients necessary for plant growth, such as K, Ca, Fe, P, Zn, and Mo [14]. In this study, the GC-MS analysis of guava WV revealed the presence of several bioactive compounds, including phenols, esters, alcohols, antioxidants, and organic acids. The compound with the highest area percentage of the chromatogram (16.12%) was 2,6-Dimethoxyphenol, followed by guaiacol (12.82%) and 1,2,4-trimethoxybenzene (6.39%). According to the findings of this study, phenols were the most prevalent of the detected compounds in the analysis of the guava WV sample. The findings of our study are fully consistent with those of Li et al., [42] found that 2,6-dimethoxyphenol is the most potent antioxidant in WV. Additionally, 3-methyl-1, 2-cyclopentanedione and 2-methoxyphenol have an important impact on the antioxidant activity of WV. Numerous studies suggest that the phenolic compounds in wood vinegar are what give it its antifungal properties [26,27,28]. In a related study, Ikergami et al. [43] demonstrated that guaiacol, 4-ethyl, 2, methoxy phenol, 6-2, dimethoxy phenol, and ethyl acetate are the most important phenolic compounds in WV with antifungal properties. Yang et al. [44] found that the strongest antioxidant and antibacterial activity of Litchi chinensis WV was due to its highly phenolic composition.
In laboratory tests, guava WV had the ability to inhibit growth and stop the development of the pathogen on the PDA medium at the tested concentrations. It has been shown to inhibit several fungal plant pathogens [20]. Higher concentrations of WV (2% and 3%) effectively inhibited the growth of the pathogen in the medium. The most effective inhibitor, 3% guava WV, completely inhibited Colletotrichum coccoides growth on the medium (100%). Our findings from this study are consistent with those of Qiaozhi et al. [21], who noted that the apricot tree WV has inhibited the mycelial growth of Plasmopara viticola, Verticillium dahliae, Phytophthora capsici, and Fusarium graminearum. Several studies have found that WV can inhibit the growth of many microbes, including E. coli [45], Bacillus subtilis [46], Staphylococcus aureus [47], and Listeria monocytogenes [48]. In a recent study, both Pestalotiopsis and Curvularia species were suppressed in vitro by high concentrations of WV [15]. Previous studies have reported that bamboo WV reduced the growth of the causal agent of wood rot caused by Ophiostoma spp. in forest trees [25]. Furthermore, Chukeatirote and Jenjai [49] revealed that the longan WV had antibacterial activity against all bacterial strains tested. On the other hand, the WV only showed inhibitory activity against the yeast Candida albicans (C.P. Robin) Berkhout. In a recent study, Desvita et al. [50] showed that WV made from cocoa pod shells inhibited the diameter growth of Candida albicans and Aspergillus niger. Oramahi et al. [51] discovered that WV from Vitex pubescens Vahl could inhibit the growth of Fomitopsis palustris (Berk. and Curtis) Gilb and Ryvarden. According to Kadota and Niimi [27], the antifungal properties of WV depend on its chemical composition and phenolic compound content. In this study, we believe that the inhibitory effect of guava WV against the pathogen is due to the phenolic substances present in it. The phenolic compounds present in WV are toxic to microbes when used in high concentrations [52]. WV produced from cocoa pod shells inhibits the growth of C. albicans and Aspergillus niger Tiegh. With increasing WV concentration, the diameter of the zone inhibiting microbial growth grows [50]. These findings suggest that WV made from cocoa pod shells has antimicrobial properties.
Applying guava, WV significantly decreased the disease severity of black dot in all disease parameters, including stem colonization, root covering with sclerotia, and wilted plant percentage. Both concentrations (2% and 3% v/v) had an effect on the disease in greenhouse conditions, but the 3% concentration was more successful in suppressing the disease than the 2% concentration when compared to untreated plants. The results of our study are in agreement with those obtained by Chuaboon et al. [23], who demonstrated that WV decreased the occurrence of the diseases brown spot and dirty panicles in rice under greenhouse conditions compared to untreated controls. When applied at a 1:32 dilution, WV completely inhibited Alternaria mali growth, the causal agent of apple Alternaria blight [22]. Studies have revealed that WV is capable of effectively inhibiting Pythium aphanidermatum, Penicillium griseofulvum, Rhizobium sp., Sclerotinia sclerotiorum, and Fusarium graminearum [21,53]. According to Yuan et al. [54], the phenol content in WV is thought to have antifungal properties that work by inhibiting fungi enzymes. Saberi et al. [55] investigated the impact of WV on cucumber damping-off and found that P. aphanidermatum and Phytophthora drechsleri Tucker mycelial growth significantly decreased. In comparison to untreated control plants, the severity of root and crown rot diseases in greenhouse-cultivated cucumber was significantly decreased at the tested concentrations of WV [29]. The main ways that WV inhibits fungi from growing are by slowing down cell division, rupturing cell membranes, leaking electrolytes, and preventing protein synthesis [56,57].
The foliar application of guava WV improved plant growth characteristics of potato plants, such as plant height, stem diameter, and tuber yield per plant in both seasons (2021 and 2022). In general, the concentration of 3% was the most effective on all studied traits. The results of this study are in agreement with those obtained by Chuaboon et al. [23], who showed that WV treatment improved germination, seedling vigor, shoot height, root length, and fresh weight in rice plants compared to untreated controls. WV increased yield in cucumber, lettuce, and cole when used as a foliar fertilizer [31], and in jasmine rice [32]. Charcoal and WV improve the growth, branching, and survival rate of zinnia when used as a mix in planting materials [27]. In addition to enhancing photosynthesis and increasing chlorophyll, WV esters compounds that may help produce sugar and amino acids [23]. WV has also been demonstrated to enhance the development, yield, and quality of a variety of crops [30]. On the other hand, it can be used as a soil fertilizer in the proper concentration. It has been reported that charcoal and smoke stimulate the soil microbial community [52]. A study on tomatoes found that WV increases various enzyme activities, auxin, gibberellin, while also promoting plant growth and nitrogen uptake [58]. WV applications, according to Wang et al. [59], may simultaneously activate several plant-growth-promoting mechanisms: (1) an accumulation of proteins, adaptation to stress and carbohydrate metabolism; (2) antioxidant enzyme accumulation; and (3) reduced reactive oxygen species (ROS) and the presence of malonaldehydes in the root. At low concentrations, the phenolic compounds in wood vinegar, particularly polyphenols and dihydric phenols, can also significantly promote plant growth [60,61]. In a recent study, Fedeli et al. [62] showed that the application of wood distillate at 0.2% significantly increased the content of soluble sugars, starch, and total carbohydrates in treated potato tubers. Wood distillate (wood vinegar) is an environmentally safe bio-based product stimulating plant growth and yield [63].
In conclusion, the present study, WV gave amazing results, whether in its effect on the disease or on the growth of potato plants. WV is a potent organic agricultural product that is eco-friendly and also stops the spread of fungal infection in plants. Therefore, it is natural content and reasonable price make it a good alternative to pesticides and chemical fertilizers. In future investigations may shed more light on the possibility of separating some components of guava WV and evaluating their effectiveness in controlling some plant diseases. Overall, the use of WV provides a promising approach to control the black dot disease of potato plants, contributing to the development of sustainable and environmentally friendly agricultural practices.
Author Contributions
M.M.E.-F.: Conceptualization, methodology, writing—original draft, writing—review and editing; K.A.M.A.-E.: conceptualization, investigation, supervision, writing—review and editing, resources; N.M.A.S.: data curation, software, visualization, investigation; R.M.I.E.-S.: project administration, writing—review and editing, investigation; Y.E.I.: formal analysis, funding acquisition, resources, validation, data curation. All authors have read and agreed to the published version of the manuscript.
Funding
This research work was funded by Institutional Fund Project under grant no “IFPIP: 256-155-1443”. The authors acknowledge the technical and financial support provided by the Ministry of Education and King Abdulaziz University, DSR, Jeddah, Saudi Arabia.
Data Availability Statement
Not applicable.
Acknowledgments
This research work was Funded by Institutional Fund Project under grant no “IFPIP: 256-155-1443”. The authors gratefully acknowledge the technical and financial support provided by the Ministry of Education and King Abdulaziz University, DSR, Jeddah, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lees, A.K.; Hilton, A.J. Rewiev, black dot (Colletotrichum coccodes): An increasingly important disease of potato. Plant Pathol. 2003, 52, 3–12. [Google Scholar] [CrossRef]
- Mohan, S.K.; Davis, J.R.; Sorensen, L.H.; Schneider, A.T. Infection of aerial parts of potato plants by Colletotrichum coccodes and its effects on premature vine death and yield. Am. Potato J. 1992, 69, 547–559. [Google Scholar] [CrossRef]
- Johnson, D.A.; Miliczky, E.R. Effects of wounding and wetting duration on infection of potato foliage by Colletotrichum coccodes. Plant Dis. 1993, 77, 13–17. [Google Scholar] [CrossRef]
- Johnson, D.A. Effect of foliar infection caused by Colletotrichum coccodes on yield of Russett Burbank potato. Plant Dis. 1994, 78, 1075–1078. [Google Scholar] [CrossRef]
- Andrivon, D.; Lucas, J.M.; Guerin, C.; Jouan, B. Colonization of roots, stolons, tubers and stems of various potato (Solanum tuberosum) cultivars by the black dot fungus Colletotrichum coccodes. Plant Pathol. 1998, 47, 440–445. [Google Scholar] [CrossRef]
- Andrivon, D.; Ramage, K.; Guerin, C.; Lucan, M.; Jouan, B. Distribution and fungicide sensitivity of Colletotrichum coccodes in French potato-producing areas. Plant Pathol. 1997, 46, 722–728. [Google Scholar] [CrossRef]
- Dickson, B.T. The black dot disease of potato. Phytopathology 1926, 16, 23–40. [Google Scholar]
- El-Marzoky, H.A. First record of black dot disease caused by Colletotrichum coccodes on potato plants in Egypt. Egypt. J. Phytopathol. 2013, 41, 69–84. [Google Scholar] [CrossRef]
- Griffiths, H.M.; Zitter, T.A.; Loeffler, K.; De Jong, W.S.; Menasha, S. First report in North America of atypical symptoms caused by Colletotrichum coccodes on field-grown potato tubers during storage. Plant Health Prog. 2010, 11, 46. [Google Scholar] [CrossRef]
- Anonim. Wood vinegar. In Forest Energy Forum, 9; FAO of United Nations: Rome, Italy, 2001. [Google Scholar]
- Guillen, M.D.; Manzanos, M.J. Study of the volatile composition of an aqueous oak smoke preparation. Food Chem. 2002, 79, 283–292. [Google Scholar] [CrossRef]
- Aguirre, J.L.; Baena, J.; Martin, M.T.; Nozal, L.; Gonzalez, S.; Manjon, J.L.; Peinado, M. Composition, ageing and herbicidal properties of wood vinegar obtained through fast biomass pyrolysis. Energies 2020, 13, 2418. [Google Scholar] [CrossRef]
- Grewal, A.; Abbey, L.; Gunupuru, L.R. Production, prospects and potential application of pyroligneous acid in agriculture. J. Anal. Appl. Pyrolysis 2018, 135, 152–159. [Google Scholar] [CrossRef]
- Zulkarami, B.; Ashrafuzzaman, M.; HusniIsmail, M.R. Effect of pyroligneous acid on growth, yield and quality improvement of rock melon in soilless culture. Aust. J. Crop. Sci. 2011, 5, 1508–1514. [Google Scholar]
- Obeng, J.; Agyei-Dwarko, D.; Teinor, P.; Danso, I.; Lutuf, H.; Lekete-Lawson, E.; Ablormeti, F.K.; Eddy-Doh, M.A. Bioactivity of an organic farming aid with possible fungistatic properties against some oil palm seedling foliar pathogens. Sci. Rep. 2023, 13, 1280. [Google Scholar] [CrossRef] [PubMed]
- Najafi-Ghiri, M.; Boostani, H.R.; Hardie, A.G. Investigation of biochars application on potassium forms and dynamics in a calcareous soil under different moisture conditions. Arch. Agron. Soil Sci. 2022, 68, 325–339. [Google Scholar] [CrossRef]
- Sivaram, A.K.; Panneerselvan, L.; Mukunthan, K.; Megharaj, M. Effect of pyroligneous acid on the microbial community composition and Plant Growth-Promoting Bacteria (PGPB) in soils. Soil Syst. 2022, 6, 10. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood: Chemistry, Ultrastructure, Reactions; Walter de Gruyter: Berlin, Germany, 1983. [Google Scholar]
- Yatagai, M.; Nishimoto, M.; Hori, K.; Ohira, T.; Shibata, A. Termiticidal activity of wood vinegar, its components and their homologues. J. Wood Sci. 2002, 48, 338–342. [Google Scholar] [CrossRef]
- Yodthong, B.; Jirasak, T.; Nuethip, D.; Yaowalak, S.; Nuanchai, K. Utilization of wood vinegars as sustainable coagulating and antifungal agents in the production of natural rubber sheets. J. Environ. Sci. Tech. 2008, 1, 157–163. [Google Scholar] [CrossRef]
- Mao, Q.; Zhao, Z.; Ma, X. Preparation, toxicity and components analysis of apricot branch wood vinegar. J. Northwest A F Univ.-Nat. Sci. Ed. 2009, 37, 91–96. [Google Scholar]
- Jung, K.H. Growth inhibition effect of pyroligneous acid on pathogenic fungus, Alternaria mali, the agent of Alternaria blotch of apple. Biotechnol. Bioproc. Eng. 2007, 12, 318–322. [Google Scholar] [CrossRef]
- Chuaboon, W.; Ponghirantanachoke, N.; Athinuwat, D. Application of wood vinegar for fungal disease controls in paddy rice. Appl. Environ. Res. 2016, 38, 77–85. [Google Scholar] [CrossRef]
- Hwang, Y.; Matsushita, Y.; Sugamoto, K.; Matsui, T. Antimicrobial effect of the wood vinegar from Crytomeria japonica sapwood on plant pathogenic microorganisms. J. Microbiol. Biotechnol. 2005, 15, 1106–1109. [Google Scholar]
- Velmurugan, N.; Chun, S.S.; Han, S.S.; Lee, Y.S. Characterization of Chikusaku-eki and Mokusaku-eki and its inhibitory effect on sapstaining fungal growth in laboratory scale. Int. J. Environ. Sci. Technol. 2009, 6, 13–22. [Google Scholar] [CrossRef]
- Bridgewater, A.V. Biomass fast pyrolysis. Thermal Sci. 2004, 8, 21–50. [Google Scholar] [CrossRef]
- Kadota, M.; Niimi, Y. Effects of charcoal with pyroligneous acid and barnyard manure on bedding plants. Sci. Hortic. 2004, 101, 327–332. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Saberi, M.; Askary, H.; Sarpeleh, A. Integrated effects of wood vinegar and tea compost on root rot and vine decline and charcoal root rot diseases of muskmelon. Biocontrol Plant Protect. 2013, 1, 91–101. [Google Scholar] [CrossRef]
- Nakayama, F.S.; Vinyard, S.H.; Chow, P.; Bajwa, D.S.; Youngquist, J.A.; Muehl, J.H.; Krzysik, A.M. Guayule as a wood preservative. Ind. Crop. Prod. 2001, 14, 105–111. [Google Scholar] [CrossRef]
- Mu, J.; Yu, Z.M.; Wu, W.Q.; Wu, Q.L. Preliminary study of application effect of bamboo vinegar on vegetable growth. For. Study China 2006, 8, 43–47. [Google Scholar] [CrossRef]
- Jothityangkoon, D.; Ruamtakhu, C.; Tipparak, S.; Wanapat, S.; Polthanee, A. Using wood vinegar in increasing rice productivity. In Proceedings of the 2nd International Conference on Rice for the Future, Bangkok, Thailand, 5–9 November 2007; pp. 28–34. [Google Scholar]
- Sutton, B.C. The genus Glomerella and its anamorph Colletotrichum. In Colletotrichum: Biology, Pathology and Control; Bailey, J.A., Jeger, M.J., Eds.; CAB International: Wallingford, UK, 1992; pp. 1–26. [Google Scholar]
- Rehman, F.; Lee, S.K.; Kim, H.S.; Jeon, J.H.; Park, J.; Joung, H. Dormancy breaking and effects on tuber yield of potato subjected to various chemicals and growth regulators under greenhouse conditions. J. Biol. Sci. 2001, 1, 818–820. [Google Scholar] [CrossRef]
- Abdel-Hafez, S.I.I.; Abo-Elyousr, K.A.M.; Abdel-Rahim, I.R. Fungicidal activity of extracellular products of cyanobacteria against Alternaria porri. Eur. J. Phycol. 2015, 50, 239–245. [Google Scholar] [CrossRef]
- Perez-Mora, J.L.; Cota-Rodriguez, D.A.; Rodriguez-Palafox, E.E.; Garcia-Leon, E.; Beltran-Pena, H.; Lima, N.B.; Tovar-Pedraza, J.M. First confirmed report of Colletotrichum coccodes causing black dot on potato in Mexico. J. Plant Dis. Prot. 2020, 127, 269–273. [Google Scholar] [CrossRef]
- Nitzan, N.; Evans, M.A.; Cummings, T.F.; Johnson, D.A.; Batchelor, D.L.; Olsen, C.; Haynes, K.G.; Brown, C.R. Field resistance to potato stem colonization by the black dot pathogen Colletotrichum coccodes. Plant Dis. 2009, 93, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, R.; Manivannan, R.; Balasubramanian, A.; Rajkapoor, B. Antioxidant and hepatoprotective activity of ethanol extract of Indigofera trita Linn. On CCl4 induced hepatoxicity in rats. J. Pharmacol. Toxicol. 2008, 3, 344–350. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, Y.; Li, J. Inhibitory effect of wood vinegar produced from apricot shell on Aspergillus fumigatus. Agric. Biotechnol. 2018, 7, 112–115. [Google Scholar]
- MSTAT-C. A Software Program for the Design, Management and Analysis of Agronomic Research Experiments; Michigan State University: East Lansing, MI, USA, 1991; p. 400. [Google Scholar]
- Gomez, K.A.; Gomez, A.A. Comparison between treatment means. In Statistical Procedures for Agricultural Research; Lviley, A., Ed.; Interscience Publication: New York, NY, USA, 1984; pp. 187–680. [Google Scholar]
- Li, Z.; Zhang, Z.; Wu, L.; Zhang, H.; Wang, Z. Characterization of five kinds of wood vinegar obtained from agricultural and forestry wastes and identification of major antioxidants in wood vinegar. Chem. Res. Chin. Univ. 2019, 35, 12–20. [Google Scholar] [CrossRef]
- Ikergami, F.; Sekin, T.; Fuji, Y. Antidemaptophyte activity of phenolic compounds in Mokusaku-eki. Yakugaku Zasshi 1992, 118, 27–30. [Google Scholar] [CrossRef]
- Yang, J.F.; Yang, C.H.; Liang, M.T.; Gao, Z.J.; Wu, Y.W.; Chuang, L.Y. Chemical composition, antioxidant, and antibacterial activity of wood vinegar from Litchi chinensis. Molecules 2016, 21, 1150. [Google Scholar] [CrossRef]
- Soares, J.M.; da Silva, P.F.; Puton, B.M.S.; Brustolin, A.P.; Cansian, R.L.; Dallago, R.M.; Valduga, E. Antimicrobial and antioxidant activity of liquid smoke and its potential application to bacon. Innov. Food Sci. Emerg. Technol. 2016, 38, 189–197. [Google Scholar] [CrossRef]
- Saloko, S.; Darmadji, P.; Setiaji, B.; Pranoto, Y. Antioxidative and antimicrobial activities of liquid smoke nanocapsules using chitosan and maltodextrin and its application on tuna fish preservation. Food Biosci. 2014, 7, 71–79. [Google Scholar] [CrossRef]
- Desvita, H.; Faisal, M.; Mahidin, M.; Suhendrayatna, S. Preliminary study on the antibacterial activity of liquid smoke from cacao pod shells (Theobroma cacao L.). IOP Conf. Ser. Mater. Sci. Eng. 2021, 1098, 022004. [Google Scholar] [CrossRef]
- Morey, A.; Bratcher, C.L.; Singh, M.; dan McKee, S.R. Effect of liquid smoke as an ingredient in frankfurters on Listeria monocytogenes and quality attributes. Poult. Sci. J. 2012, 91, 2341–2350. [Google Scholar] [CrossRef]
- Chukeatirote, E.; Jenjai, N. Antimicrobial activity of wood vinegar from dimocarpus longan. Environ. Asia 2018, 11, 161–169. [Google Scholar] [CrossRef]
- Desvita, H.; Faisal, M.; Mahidin, M.; Suhendrayatna, S. Antimicrobial potential of wood vinegar from cocoa pod shells (Theobroma cacao L.) against Candida albicans and Aspergillus niger. Mater. Today Proc. 2022, 63, S210–S213. [Google Scholar] [CrossRef]
- Oramahi, H.A.; Yoshimura, T. Antifungal and antitermitic activities of wood vinegar from Vitex pubescens Vahl. J. Wood Sci. 2013, 59, 344–350. [Google Scholar] [CrossRef]
- Steiner, C.; Das, K.C.; Garcia, M.; Forster, B.; Zech, W. Charchoal and smoke extract stimulate the soil microbial community in highly weathered xanthic Ferralsol. Pedobiologia 2007, 51, 359–366. [Google Scholar] [CrossRef]
- Quan, S. Application of wood vinegar to control diseases. J. Agric. Sci. Yanbian Univ. 1994, 2, 113–116. [Google Scholar]
- Yuan, F.; Zhang, C.; Shen, Q.R. Allevating effect of phenol compounds on cucumber Fusarium wilt and mechanism. Agric. Sci. China 2003, 2, 647–652. [Google Scholar]
- Saberi, M.; Sarpeleh, A.; Askary, H. Management of damping-off and increasing of dome growth traits of cucumber in greenhouse culture using citrus wood vinegar. Appl. Res. Plant Prot. 2015, 4, 99–111. [Google Scholar]
- Wei, Q.; Ma, X.; Zhao, Z.; Zhang, S.; Liu, S. Antioxidant activities and chemical profiles of pyroligneous acids from walnut shell. J. Anal. Appl. Pyrolysis 2010, 88, 149–154. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.D.; Gong, W. Chemical constituents and antibacterial activity of Jujube kernel vinegar. Food Sci. 2016, 37, 123–127. [Google Scholar]
- Zhu, K.; Gu, S.; Liu, J.; Luo, T.; Khan, Z.; Zhang, K.; Hu, L. Wood vinegar as a complex growth regulator promotes the growth, yield, and quality of rapeseed. Agronomy 2021, 11, 510. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, L.; Song, Q.; Wang, S.; Wang, Y.; Ge, Y. Root proteomics reveals the effects of wood vinegar on wheat growth and subsequent tolerance to drought stress. Int. J. Mol. Sci. 2019, 20, 943. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Ye, F.; Lin, Y.; Li, Q. Research progress on the mechanism of phenolic substances on soil and plants. J. Eco-Agric. 2010, 5, 1130–1137. [Google Scholar]
- Yan, Y.; Lu, X.; Li, L.; Zheng, J.; Pan, G. The composition of straw pyrolysis wood vinegar and its effect on the growth and quality of pepper. J. Nanjing Agric. Univ. 2011, 5, 58–62. [Google Scholar]
- Fedeli, R.; Vannini, A.; Grattacaso, M.; Loppi, S. Wood distillate (pyroligneous acid) boosts nutritional traits of potato tubers. Ann. Appl. Biol. 2023, 1–6. [Google Scholar] [CrossRef]
- Fanfarillo, E.; Fedeli, R.; Fiaschi, T.; de Simone, L.; Vannini, A.; Angiolini, C.; Loppi, S.; Maccherini, S. Effects of wood distillate on seedling emergence and first-stage growth in five threatened arable plants. Diversity 2022, 14, 669. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).