Effect of Temperature and Storage Time on Some Biochemical Compounds from the Kernel of Some Walnut Cultivars Grown in Romania
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Sampling
2.2. Chemicals and Reagents
2.3. Extraction Procedures and Biochemical Determinations
2.4. Statistical Analysis
3. Results and Discussions
3.1. Determination of Some Biochemical Parameters
3.2. Analysis of TPC Variation Depending on Temperature Treatment and the Storage Period
3.2.1. Short-Term Storage
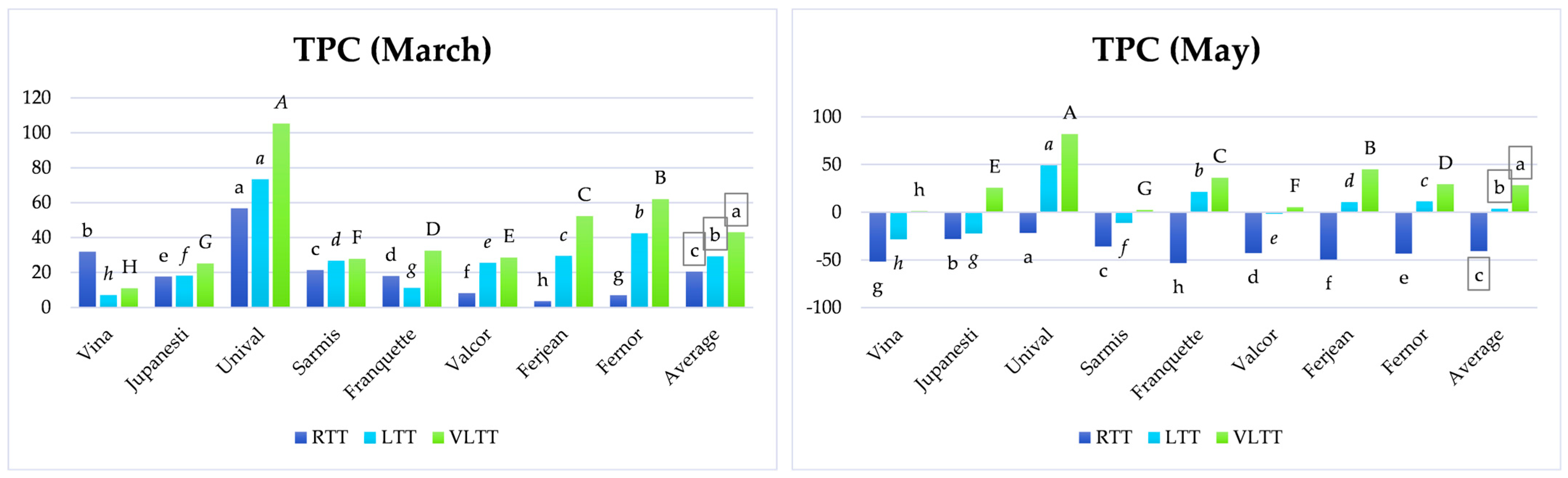

3.2.2. Medium-Term Storage
3.2.3. Long-Term Storage
3.3. Analysis of TFC Variation Depending on Temperature Treatment and the Storage Period
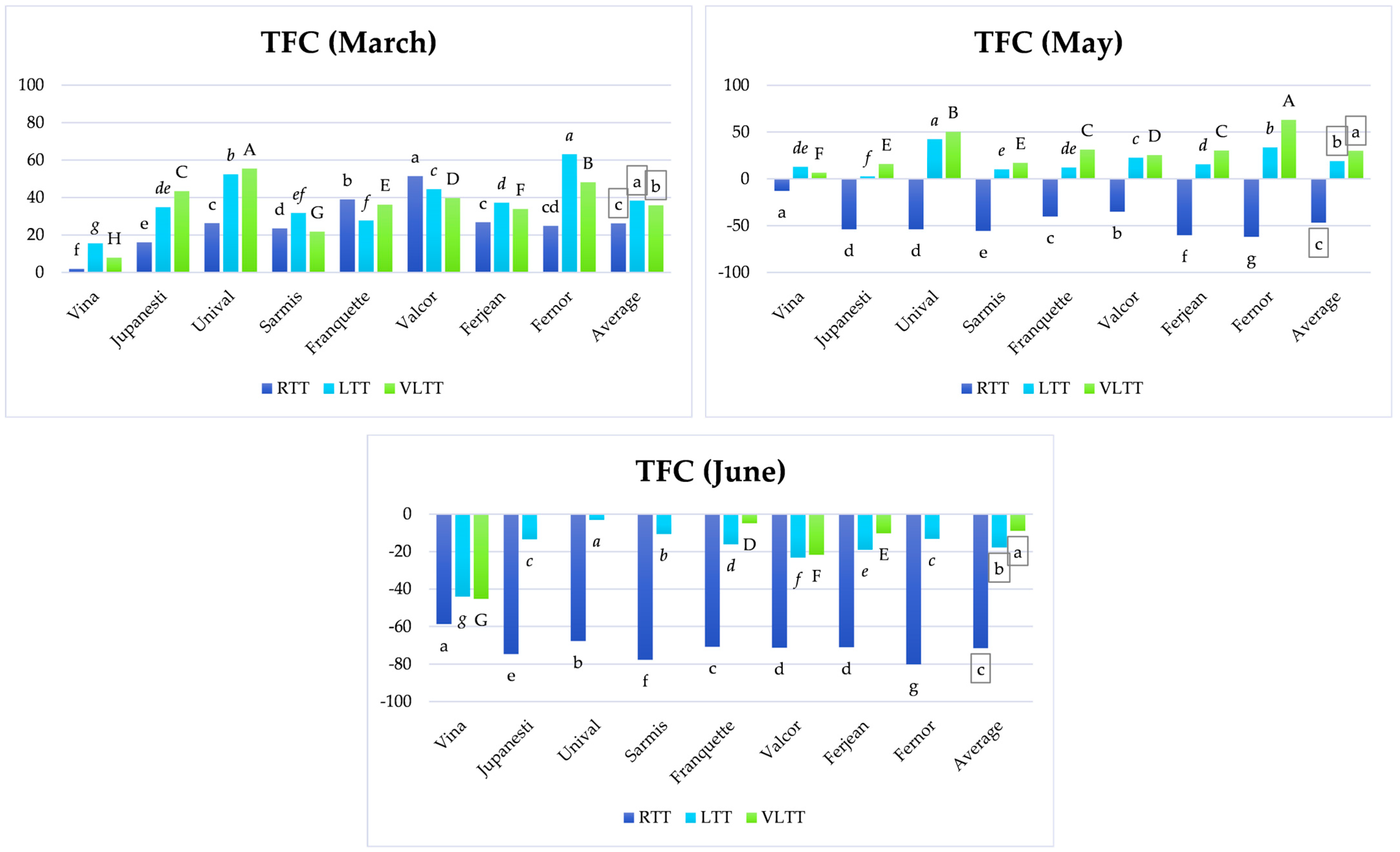
3.3.1. Short-Term Storage
3.3.2. Medium-Term Storage
3.3.3. Long-Term Storage
3.4. Analysis of TTC Variation Depending on Temperature Treatment and the Storage Period

3.4.1. Short-Term Storage
3.4.2. Medium-Term Storage
3.4.3. Long-Term Storage
3.5. Analysis of the Variation of Lycopene and β-Carotene Content Depending on Temperature Treatment and the Storage Period
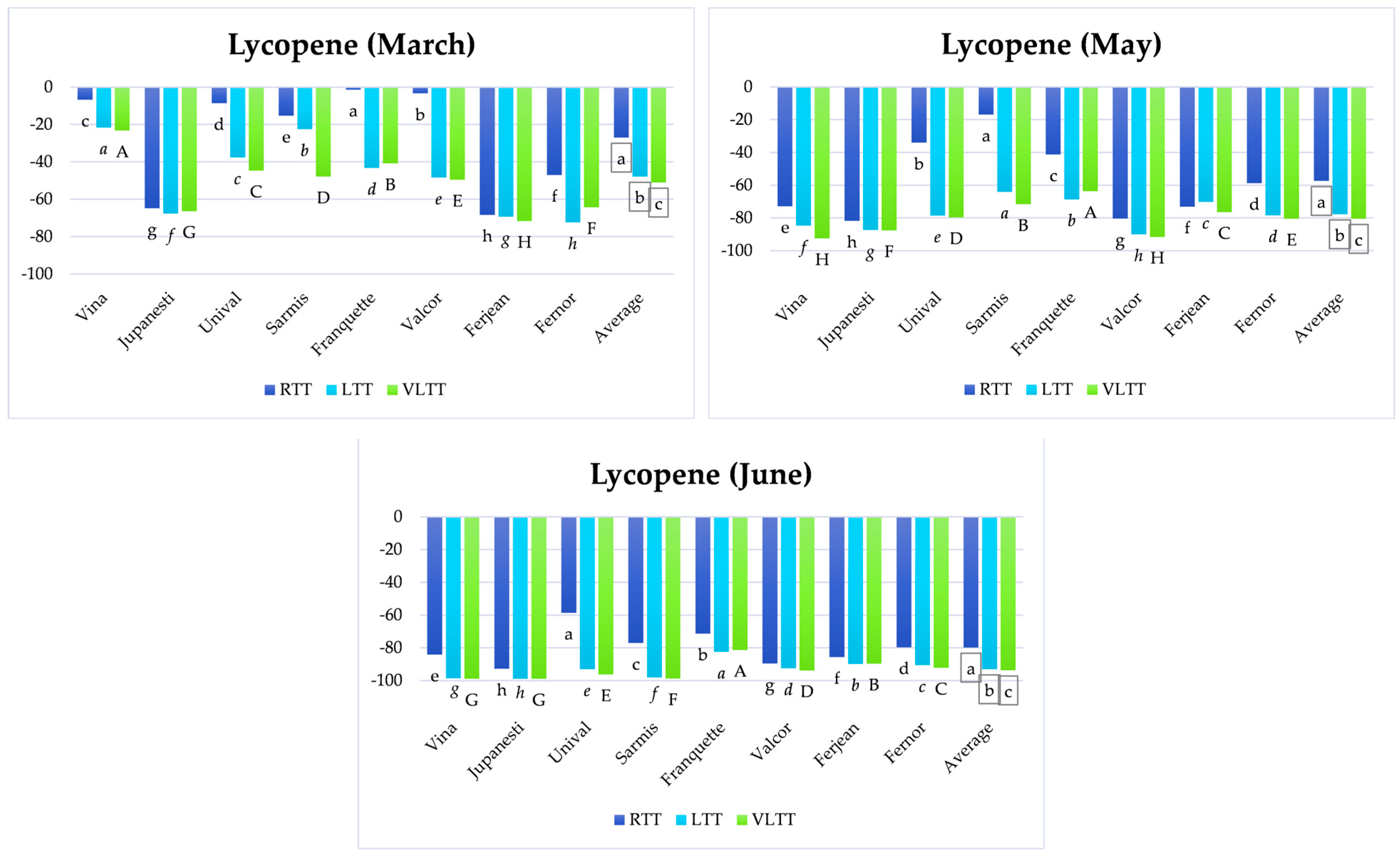
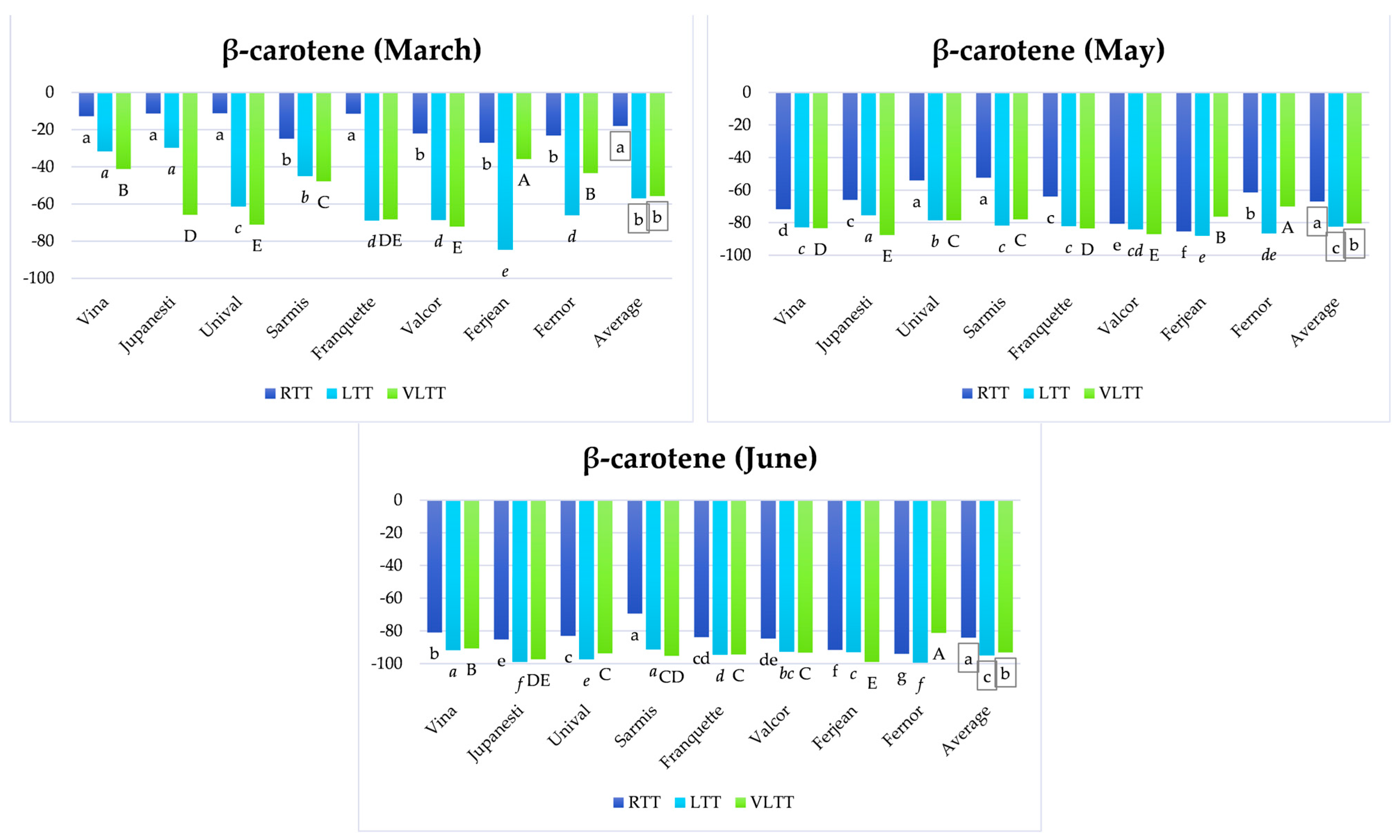
3.5.1. Short-Term Storage
3.5.2. Medium-Term Storage
3.5.3. Long-Term Storage
3.6. Three Factor Analysis of Walnut Kernel Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guiné, R.P.F.; Almeida, C.F.F.; Correia, P.M.R. Influence of storage conditions and type of package on some properties of walnuts. Millenium 2015, 48, 185–193. [Google Scholar]
- Banel, D.K.; Hu, F.B. Effects of walnut consumption on blood lipids and other cardiovascular risk factors: A meta-analysis and systematic review. Am. J. Clin. Nutr. 2009, 90, 56–63. [Google Scholar] [CrossRef]
- Tapia, M.I.; Sánchez-Morgado, J.R.; García-Parra, J.; Ramírez, R.; Hernández, T.; González-Gómez, D. Comparative study of the nutritional and bioactive compounds content of four walnut (Juglans regia L.) cultivars. J. Food Compost. Anal. 2013, 31, 232–237. [Google Scholar] [CrossRef]
- Mazilu, I.; Vîjan, L.E.; Giura, S.; Botu, M. Evolution of polyphenols and flavonoids levels in leaves, mesocarp and kernel of three Juglans regia L. cultivars. Curr. Trends Nat. Sci. 2021, 10, 270–281. [Google Scholar] [CrossRef]
- Cabanillas, B.; Maleki, S.J.; Rodríguez, J.; Cheng, H.; Teuber, S.S.; Wallowitz, M.L.; Muzquiz, M.; Pedrosa, M.M.; Linacero, R.; Burbano, C.; et al. Allergenic properties and differential response of walnut subjected to processing treatments. Food Chem. 2014, 157, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Chatrabnous, N.; Yazdani, N.; Vahdati, K. Determination of nutritional value and oxidative stability of fresh walnut. J. Nuts 2018, 9, 11–20. [Google Scholar] [CrossRef]
- Ampofo, J.; Grilo, F.S.; Langstaff, S.; Wang, S.C. Oxidative stability of walnut kernel and oil: Chemical compositions and sensory aroma compounds. Foods 2022, 11, 3151. [Google Scholar] [CrossRef]
- Topală, C.M.; Vîjan, L.E.; Giura, S.; Botu, M. Attenuated total reflection Fourier transform infrared (ATR-FTIR): A method for the biochemical study of walnut leaves. Curr. Trends Nat. Sci. 2020, 9, 266–272. [Google Scholar] [CrossRef]
- Nicolescu, V.-N.; Rédei, K.; Vor, T.; Bastien, J.-C.; Brus, R.; Benčať, T.; Đodan, M.; Cvjetkovic, B.; Andrašev, S.; La Porta, N.; et al. A review of black walnut (Juglans nigra L.) ecology and management in Europe. Trees 2020, 34, 1087–1112. [Google Scholar] [CrossRef]
- Pirayesh, H.; Khazaeian, A.; Tabarsa, T. The potential for using walnut (Juglans regia L.) shell as a raw material for wood-based particleboard manufacturing. Compos. B Eng. 2012, 43, 3276–3280. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Ostadrahimi, A.; Tabibiazar, M.; Amarowicz, R. A comprehensive review on the chemical constituents and functional uses of walnut (Juglans spp.) husk. Int. J. Mol. Sci. 2019, 20, 3920. [Google Scholar] [CrossRef]
- Giura, S.; Botu, M.; Vulpe, M.; Vîjan, L.E.; Mitrea, R. Evolution of the polyphenols, flavonoids, and tannins content in walnut leaves and green walnut husk during growing season. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 1264–1271. [Google Scholar] [CrossRef]
- Cosmulescu, S.; Botu, M.; Achim, G.; Baciu, A.; Gruia, M.; Trandafir, I. Polyphenol content in walnut (Juglans regia L.) mature leaves. Acta Hortic. 2014, 1050, 205–212. [Google Scholar] [CrossRef]
- Cosmulescu, S.N.; Trandafir, I.; Achim, G.; Baciu, A. Juglone content in leaf and green husk of five walnut (Juglans regia L.) cultivars. Not. Bot. Horti Agrobot. Cluj-Napoca 2011, 39, 237. [Google Scholar] [CrossRef]
- Cosmulescu, S.; Trandafir, I.; Nour, V.; Ionica, M.; Tutulescu, F. Phenolics content, antioxidant activity and color of green walnut extracts for preparing walnut liquor. Not. Bot. Horti Agrobot. Cluj-Napoca 2014, 42, 551–555. [Google Scholar] [CrossRef]
- Miłek, M.; Marcinčáková, D.; Kolesárová, M.; Legáthová, D.; Dżugan, M. The effect of adding spices to green walnut tinctures on their polyphenolic profile, antioxidant capacity and action on renal cells. Appl. Sci. 2022, 12, 3669. [Google Scholar] [CrossRef]
- Chamorro, F.; Carpena, M.; Lourenço-Lopes, C.; Taofiq, O.; Otero, P.; Cao, H.; Xiao, J.; Simal-Gandara, J.; Prieto, M.A. By-products of walnut (Juglans regia) as a source of bioactive compounds for the formulation of nutraceuticals and functional foods. Biol. Life Sci. Forum 2022, 12, 35. [Google Scholar] [CrossRef]
- Petrović, M.; Pastor, F.; Đurović, S.; Veljović, S.; Gorjanović, S.; Sredojević, M.; Vukosavljević, P. Evaluation of novel green walnut liqueur as a source of antioxidants: Multi-method approach. J. Food Sci. Technol. 2021, 58, 2160–2169. [Google Scholar] [CrossRef]
- Pardaev, G.Y.; Normakhmatov, R. Jam from green walnuts is an important source of iron, iodine, chromium, and a number of other elements. Int. J. Future Gener. Commun. Netw. 2020, 13, 4015–4022. [Google Scholar]
- Binici, H.İ.; Şat, İ.G.; Aoudeh, E. Nutritional composition and health benefits of walnut and its products. Atatürk Univ. J. Agric. Fac. 2021, 52, 224–230. [Google Scholar] [CrossRef]
- Soto-Maldonado, C.; Caballero-Valdés, E.; Santis-Bernal, J.; Jara-Quezada, J.; Fuentes-Viveros, L.; Zúñiga-Hansen, M.E. Potential of solid wastes from the walnut industry: Extraction conditions to evaluate the antioxidant and bioherbicidal activities. Electron. J. Biotechnol. 2022, 58, 25–36. [Google Scholar] [CrossRef]
- Masoodi, L.; Gull, A.; Masoodi, F.A.; Gani, A.; Nissar, J.; Ahad, T.; Nayik, G.A.; Mukarram, S.A.; Kovács, B.; Prokisch, J.; et al. An overview on traditional vs. green technology of extraction methods for producing high quality walnut oil. Agronomy 2022, 12, 2258. [Google Scholar] [CrossRef]
- Rébufa, C.; Artaud, J.; Le Dréau, Y. Walnut (Juglans regia L.) oil chemical composition depending on variety, locality, extraction process and storage conditions: A comprehensive review. J. Food Compost. Anal. 2022, 110, 104534. [Google Scholar] [CrossRef]
- FAO Stat Database. Available online: https://www.fao.org/home/en/ (accessed on 10 March 2023).
- UNECE. Dry and Dried Produce—Standards. Available online: https://unece.org/trade/wp7/DDP-Standards (accessed on 10 March 2023).
- Labavitch, J. Pistachios. In The Commercial Storage of Fruits, Vegetables, and Florist and Nursery Stocks. USDA Agricultural Handbook; Gross, K.C., Wang, C.Y., Saltveit, M., Eds.; USDA: Washington, DC, USA, 2004; p. 66. [Google Scholar]
- Chen, C.; Pan, Z. Postharvest processing of tree nuts: Current status and future prospects—A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1702–1731. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.N.; Sørensen, G.; Brockhoff, P.; Bertelsen, G. Investigation of packaging systems for shelled walnuts based on oxygen absorbers. J. Agric. Food Chem. 2003, 51, 4941–4947. [Google Scholar] [CrossRef]
- Bakkalbaşı, E.; Yilmaz, Ö.M.; Javidipour, I.; Artık, N. Effects of packaging materials, storage conditions and variety on oxidative stability of shelled walnuts. LWT—Food Sci. Technol. 2012, 46, 203–209. [Google Scholar] [CrossRef]
- Mexis, S.F.; Badeka, A.V.; Riganakos, K.A.; Karakostas, K.X.; Kontominas, M.G. Effect of packaging and storage conditions on quality of shelled walnuts. Food Control 2009, 20, 743–751. [Google Scholar] [CrossRef]
- Maté, J.I.; Saltveit, M.E.; Krochta, J.M. Peanut and walnut rancidity: Effects of oxygen concentration and relative humidity. J. Food Sci. 1996, 61, 465–469. [Google Scholar] [CrossRef]
- Rizzolo, A.; Senesi, E.; Colombo, C. Studies on the storage of shelled and in-shell almonds. Acta Hortic. 1994, 373, 259–264. [Google Scholar] [CrossRef]
- Zacheo, G.; Cappello, M.S.; Gallo, A.; Santino, A.; Cappello, A.R. Changes associated with post-harvest ageing in almond seeds. LWT—Food Sci. Technol. 2000, 33, 415–423. [Google Scholar] [CrossRef]
- Faruk Gamlı, Ö.; Hayoğlu, İ. The effect of the different packaging and storage conditions on the quality of pistachio nut paste. J. Food Eng. 2007, 78, 443–448. [Google Scholar] [CrossRef]
- Habibi, A.; Yazdani, N.; Koushesh Saba, M.; Chatrabnous, N.; Molassiotis, A.; Sarikhani, S.; Vahdati, K. Natural preservation and improving lipid oxidation inhibition of fresh walnut. Hortic. Environ. Biotechnol. 2023, 64, 133–142. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Abeyrathne, E.D.N.S.; Nam, K.; Ahn, D.U. Analytical methods for lipid oxidation and antioxidant capacity in food systems. Antioxidants 2021, 10, 1587. [Google Scholar] [CrossRef]
- Addis, P.B. Occurrence of lipid oxidation products in foods. Food Chem. Toxicol. 1986, 24, 1021–1030. [Google Scholar] [CrossRef]
- Sattar, A.; Khan, D.; Jan, M.; Ahmad, A.; Khan, L. Effect of gamma irradiation on peroxidation of dry nut oils and fats. Sarhad J. Agric. 1987, 3, 61–66. [Google Scholar]
- Sattar, A.; Mohammad, J.; Saleem, A.; Jan, M.; Ahmad, A. Effect of fluorescent light gamma radiation and packages on oxidative deterioration of dry nuts. Sarhad J. Agric. 1990, 6, 235–240. [Google Scholar]
- Gou, P.; Diaz, I.; Guerrero, L.; Valero, A.; Arnau, J.; Romero, A. Physico-chemical and sensory property changes in almonds of Desmayo Largueta variety during toasting. Food Sci. Technol. Int. 2000, 6, 1–7. [Google Scholar] [CrossRef]
- Velasco, J.; Dobarganes, C.; Màrquez-Ruiz, G. Oxidative rancidity in foods and food quality. In Chemical Deterioration and Physical Instability of Food and Beverages; Skibsted, R.L., Risbo, J., Andersen, M.L., Eds.; Woodhead Publishing: Cambridge, UK, 2010; pp. 3–32. [Google Scholar]
- Fourie, P.C.; Basson, D.S. Predicting rancidity in stored nuts by means of chemical analyses. LWT—Food Sci. Technol. 1989, 22, 251–253. [Google Scholar]
- Jensen, P.N.; Sørensen, G.; Engelsen, S.B.; Bertelsen, G. Evaluation of quality changes in walnut kernels (Juglans regia L.) by Vis/NIR Spectroscopy. J. Agric. Food Chem. 2001, 49, 5790–5796. [Google Scholar] [CrossRef]
- Shahidi, F.; John, J.A. Oxidation in foods and beverages and antioxidant applications. In Management in Different Industry Sectors; Woodhead Publishing: Cambridge, UK, 2010; pp. 274–305. [Google Scholar]
- Shahidi, F.; Zhong, Y. Novel antioxidants in food quality preservation and health promotion. Eur. J. Lipid Sci. Technol. 2010, 112, 930–940. [Google Scholar] [CrossRef]
- Rubel, F.; Kottek, M. Observed and projected climate shifts 1901-2100 depicted by world maps of the Köppen-Geiger climate classification. Meteorol. Z. 2010, 19, 135–141. [Google Scholar] [CrossRef]
- Botu, M.; Alabedallat, Y.F.J.; Bucura, F.; Geană, E.I.; Vladu, M. The productive capacity and quality of several walnut cultivars (Juglans regia L.) grown in North Oltenia, Romania. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 574–579. [Google Scholar] [CrossRef]
- Bernard, A.; Lheureux, F.; Dirlewanger, E. Walnut: Past and future of genetic improvement. Tree Genet. Genomes 2018, 14, 1. [Google Scholar] [CrossRef]
- Giosanu, D.; Bărbuceanu, M.; Anghel, M.; Vîjan, L. The determination of the content of phenolic compounds from different Romanian wines using Folin-Ciocîlteu method. Curr. Trends Nat. Sci. 2018, 7, 155–159. [Google Scholar]
- Mazilu, I.E.; Vîjan, L.E.; Cosmulescu, S. The influence of harvest moment and cultivar on variability of some chemical constituents and antiradical activity of dehydrated chokeberry pomace. Horticulturae 2022, 8, 544. [Google Scholar] [CrossRef]
- Jan, R.; Ara, T.; Mir, J.I. Quality profiling and estimation of total phenols, flavonoids, flavonols and antioxidative potential of walnut kernel (Juglans regia) from Kashmir Valley. J. Nuts 2022, 13, 211–225. [Google Scholar] [CrossRef]
- Beyhan, O.; Gozlekci, S.; Gundogdu, M.; Ercisli, S. Physico-chemical and antioxidant characteristics in fruits of walnut (Juglans regia L.) genotypes from Inner Anatolia. Not. Bot. Horti Agrobot. Cluj-Napoca 2016, 44, 586–592. [Google Scholar] [CrossRef]
- Trandafir, I.; Cosmulescu, S.; Botu, M.; Nour, V. Antioxidant activity, and phenolic and mineral contents of the walnut kernel (Juglans regia L.) as a function of the pellicle color. Fruits 2016, 71, 177–184. [Google Scholar] [CrossRef]
- Yang, J.; Liu, R.H.; Halim, L. Antioxidant and antiproliferative activities of common edible nut seeds. LWT—Food Sci. Technol. 2009, 42, 1–8. [Google Scholar] [CrossRef]
- Özrenk, K.; Javidipour, I.; Yarilgac, T.; Balta, F.; Gündoğdu, M. Fatty acids, tocopherols, selenium and total carotene of pistachios (P. vera L.) from Diyarbakır (Southestern Turkey) and walnuts (J. regia L.) from Erzincan (Eastern Turkey). Food Sci. Technol. Int. 2012, 18, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, I.B.; Tlili, N.; Martinez-Force, E.; Rubio, A.G.P.; Perez-Camino, M.C.; Albouchi, A.; Boukhchina, S. Content of carotenoids, tocopherols, sterols, triterpenic and aliphatic alcohols, and volatile compounds in six walnuts (Juglans regia L.) varieties. Food Chem. 2015, 173, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Beaudry, R.M.; Payne, J.A.; Kays, S.J. Variation in the respiration of harvested pecans due to genotype and kernel moisture level. HortScience 1985, 20, 752–754. [Google Scholar] [CrossRef]
| Biochemical Parameters | Storage Duration | Storage Temperature | Cultivar | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ‘Vina’ | ‘Jupanesti’ | ‘Unival’ | ‘Sarmis’ | ‘Franquette’ | ‘Valcor’ | ‘Ferjean’ | ‘Fernor’ | |||
| TPC | Before the storage experiment (mid-December) | 4342.16 ± 0.52 | 2294.12 ± 0.54 | 2339.27 ± 0.90 | 2793.96 ± 0.57 | 3915.31 ± 0.73 | 4454.66 ± 0.78 | 3640.32 ± 0.87 | 2798.16 ± 0.19 | |
| After three months | 20–22 °C | 5725.86 ± 0.61 | 2698.91 ± 0.84 | 3665.91 ± 4.53 | 3392.25 ± 0.56 | 4621.87 ± 4.07 | 4815.86 ± 0.60 | 3771.59 ± 0.66 | 2992.58 ± 0.68 | |
| 3–4 °C | 4653.41 ± 0.83 | 2713.39 ± 0.93 | 4056.37 ± 0.54 | 3540.83 ± 0.23 | 4357.99 ± 0.61 | 5593.81 ± 0.80 | 4715.42 ± 0.76 | 3985.98 ± 0.69 | ||
| −20 to −18 °C | 4815.43 ± 0.49 | 2873.18 ± 0.53 | 4801.48 ± 0.64 | 3573.25 ± 0.64 | 5190.36 ± 0.59 | 5729.20 ± 0.19 | 5543.58 ± 0.50 | 4535.65 ± 6.33 | ||
| After five months | 20–22 °C | 2095.74 ± 1.52 | 1647.16 ± 0.79 | 1834.20 ± 0.98 | 1789.78 ± 1.50 | 1828.97 ± 1.90 | 2551.76 ± 1.59 | 1841.40 ± 1.63 | 1585.68 ± 1.21 | |
| 3–4 °C | 3110.05 ± 1.00 | 1787.42 ± 0.3 | 3496.21 ± 0.59 | 2484.07 ± 0.60 | 4759.50 ± 4.80 | 4383.84 ± 0.96 | 4033.09 ± 0.43 | 3118.39 ± 0.33 | ||
| −20 to −18 °C | 4399.04 ± 0.88 | 2884.38 ± 0.46 | 4259.77 ± 0.42 | 2865.06 ± 0.18 | 5335.45 ± 0.87 | 4692.62 ± 0.50 | 5277.94 ± 0.12 | 3623.63 ± 0.47 | ||
| After six months | 20–22 °C | 781.35 ± 0.05 | 484.93 ± 0.06 | 530.26 ± 0.08 | 489.54 ± 0.04 | 1142.38 ± 0.45 | 1621.81 ± 1.96 | 797.87 ± 0.04 | 771.22 ± 0.14 | |
| 3–4 °C | 970.86 ± 0.20 | 975.29 ± 0.16 | 1095.76 ± 0.19 | 685.64 ± 1.12 | 2078.17 ± 0.35 | 2009.56 ± 0.77 | 1282.29 ± 0.69 | 1333.54 ± 0.61 | ||
| −20 to −18 °C | 1092.77 ± 0.24 | 1569.20 ± 6.21 | 1202.90 ± 0.41 | 988.95 ± 0.64 | 2388.58 ± 0.57 | 2515.19 ± 1.82 | 1728.35 ± 0.69 | 1655.69 ± 0.78 | ||
| TFC | Mid-December | 1131.93 ± 6.45 | 740.36 ± 3.83 | 828.50 ± 4.64 | 821.04 ± 4.17 | 973.46 ± 4.18 | 981.76 ± 6.20 | 924.91 ± 5.46 | 700.67 ± 3.83 | |
| After three months | 20–22 °C | 1153.92 ± 8.54 | 859.61 ± 1.88 | 1046.19 ± 4.48 | 1014.61 ± 2.62 | 1352.87 ± 28.76 | 1487.33 ± 22.58 | 1172.88 ± 1.89 | 875.00 ± 2.62 | |
| 3–4 °C | 1309.00 ± 14.64 | 998.89 ± 6.13 | 1263.45 ± 2.82 | 1082.47 ± 6.64 | 1243.74 ± 12.33 | 1418.62 ± 65.78 | 1270.25 ± 8.28 | 1143.27 ± 5.64 | ||
| −20 to −18 °C | 1221.21 ± 6.83 | 1061.69 ± 5.6 | 1287.95 ± 14.15 | 999.69 ± 4.50 | 1326.47 ± 11.45 | 1372.22 ± 11.37 | 1237.91 ± 9.02 | 1037.91 ± 9.02 | ||
| After five months | 20–22 °C | 986.70 ± 11.81 | 342.16 ± 9.72 | 381.88 ± 3.51 | 364.66 ± 1.37 | 582.91 ± 4.67 | 636.58 ± 3.31 | 367.43 ± 3.77 | 267.19 ± 3.72 | |
| 3–4 °C | 1278.06 ± 12.89 | 760.42 ± 5.01 | 1180.06 ± 5.52 | 906.42 ± 5.15 | 1093.79 ± 7.30 | 1205.93 ± 48.08 | 1070.32 ± 5.28 | 937.30 ± 4.62 | ||
| −20 to −18 °C | 1206.03 ± 6.82 | 858.29 ± 8.93 | 1245.61 ± 31.19 | 962.79 ± 3.66 | 1278.62 ± 6.29 | 1231.56 ± 7.70 | 1205.02 ± 10.92 | 1142.99 ± 2.21 | ||
| After six months | 20–22 °C | 467.14 ± 3.53 | 188.54 ± 3.49 | 267.87 ± 1.46 | 183.89 ± 3.00 | 285.55 ± 1.61 | 283.40 ± 2.13 | 268.43 ± 1.54 | 139.64 ± 1.15 | |
| 3–4 °C | 633.52 ± 0.32 | 640.41 ± 5.19 | 803.02 ± 1.50 | 733.99 ± 0.63 | 816.29 ± 1.09 | 754.23 ± 1.88 | 749.10 ± 0.73 | 608.27 ± 0.44 | ||
| −20 to −18 °C | 618.70 ± 1.39 | 756.85 ± 3.08 | 856.85 ± 3.08 | 857.71 ± 11.92 | 925.23 ± 7.50 | 768.49 ± 9.89 | 830.40 ± 12.97 | 704.02 ± 15.08 | ||
| TTC | Mid-December | 1420.42 ± 1.46 | 1815.07 ± 1.61 | 996.89 ± 0.42 | 1723.66 ± 2.16 | 1685.12 ± 1.59 | 2100.53 ± 1.12 | 2278.12 ± 0.43 | 1395.67 ± 0.34 | |
| After three months | 20–22 °C | 3367.82 ± 2.83 | 1904.91 ± 1.28 | 1307.13 ± 0.25 | 1943.79 ± 0.67 | 1943.79 ± 0.67 | 2251.15 ± 3.63 | 2511.56 ± 2.00 | 1894.37 ± 1.49 | |
| 3–4 °C | 2257.23 ± 3.65 | 1912.58 ± 4.23 | 2207.02 ± 0.04 | 2017.97 ± 0.87 | 2649.48 ± 0.15 | 3076.83 ± 0.54 | 3180.70 ± 1.34 | 2510.22 ± 1.73 | ||
| −20 to −18 °C | 3586.81 ± 4.79 | 2154.39 ± 2.78 | 2813.77 ± 2.17 | 2374.94 ± 2.19 | 2596.77 ± 2.28 | 3299.57 ± 3.44 | 3486.87 ± 12.32 | 3011.90 ± 3.04 | ||
| After five months | 20–22 °C | 1824.54 ± 2.81 | 1354.25 ± 2.53 | 1261.57 ± 2.73 | 1684.97 ± 3.13 | 1158.22 ± 2.53 | 2188.79 ± 4.65 | 1138.75 ± 0.33 | 1380.89 ± 0.32 | |
| 3–4 °C | 1752.91 ± 0.30 | 1786.81 ± 0.19 | 1629.85 ± 0.57 | 1642.33 ± 2.59 | 3656.50 ± 3.93 | 2420.72 ± 1.99 | 2659.09 ± 5.01 | 2196.55 ± 0.15 | ||
| −20 to −18 °C | 3546.46 ± 2.87 | 2439.05 ± 2.59 | 1968.66 ± 1.19 | 2026.81 ± 2.23 | 3413.98 ± 3.01 | 2971.60 ± 1.23 | 3051.34 ± 5.40 | 2339.48 ± 1.49 | ||
| After six months | 20–22 °C | 556.65 ± 2.54 | 330.85 ± 0.33 | 426.75 ± 0.73 | 365.61 ± 0.30 | 908.17 ± 2.54 | 968.27 ± 0.70 | 638.46 ± 2.88 | 616.95 ± 2.27 | |
| 3–4 °C | 823.82 ± 3.02 | 592.19 ± 0.29 | 933.98 ± 0.59 | 548.47 ± 1.68 | 1716.86 ± 2.30 | 1212.31 ± 1.85 | 762.34 ± 0.25 | 964.49 ± 0.32 | ||
| −20 to −18 °C | 990.55 ± 2.55 | 1266.3 ± 3.94 | 954.81 ± 0.97 | 845.84 ± 0.51 | 2178.27 ± 2.76 | 1791.83 ± 0.27 | 904.06 ± 1.31 | 1118.81 ± 0.35 | ||
| Lycopene | Mid-December | 2.56 ± 0.00 | 1.81 ± 0.00 | 1.33 ± 0.00 | 2.56 ± 0.00 | 2.49 ± 0.00 | 2.84 ± 0.00 | 3.41 ± 0.01 | 3.10 ± 0.01 | |
| After three months | 20–22 °C | 2.39 ± 0.00 | 0.64 ± 0.00 | 1.22 ± 0.00 | 2.17 ± 0.00 | 2.45 ± 0.00 | 2.74 ± 0.00 | 1.08 ± 0.00 | 1.64 ± 0.00 | |
| 3–4 °C | 2.01 ± 0.00 | 0.58 ± 0.00 | 0.83 ± 0.00 | 1.98 ± 0.00 | 1.41 ± 0.00 | 1.46 ± 0.00 | 1.05 ± 0.00 | 0.86 ± 0.00 | ||
| −20 to −18 °C | 1.96 ± 0.00 | 0.61 ± 0.00 | 0.74 ± 0.01 | 1.34 ± 0.00 | 1.47 ± 0.00 | 1.43 ± 0.00 | 0.97 ± 0.00 | 1.10 ± 0.00 | ||
| After five months | 20–22 °C | 0.69 ± 0.00 | 0.33 ± 0.00 | 0.88 ± 0.01 | 2.13 ± 0.00 | 1.46 ± 0.00 | 0.56 ± 0.00 | 0.92 ± 0.00 | 1.28 ± 0.00 | |
| 3–4 °C | 0.39 ± 0.00 | 0.23 ± 0.00 | 0.29 ± 0.00 | 0.92 ± 0.00 | 0.78 ± 0.00 | 0.28 ± 0.00 | 1.02 ± 0.00 | 0.67 ± 0.00 | ||
| −20 to −18 °C | 0.19 ± 0.00 | 0.23 ± 0.01 | 0.27 ± 0.00 | 0.73 ± 0.00 | 0.90 ± 0.00 | 0.24 ± 0.00 | 0.80 ± 0.00 | 0.60 ± 0.00 | ||
| After six months | 20–22 °C | 0.41 ± 0.00 | 0.13 ± 0.00 | 0.55 ± 0.00 | 0.59 ± 0.00 | 0.72 ± 0.00 | 0.30 ± 0.00 | 0.49 ± 0.00 | 0.63 ± 0.00 | |
| 3–4 °C | 0.04 ± 0.00 | 0.02 ± 0.00 | 0.09 ± 0.00 | 0.05 ± 0.00 | 0.44 ± 0.00 | 0.21 ± 0.00 | 0.35 ± 0.00 | 0.29 ± 0.00 | ||
| −20 to −18 °C | 0.03 ± 0.00 | 0.02 ± 0.01 | 0.05 ± 0.00 | 0.03 ± 0.00 | 0.47 ± 0.00 | 0.18 ± 0.00 | 0.35 ± 0.00 | 0.24 ± 0.00 | ||
| β-carotene | Mid-December | 0.43 ± 0.01 | 0.07 ± 0.00 | 0.27 ± 0.00 | 0.35 ± 0.01 | 0.60 ± 0.01 | 0.44 ± 0.02 | 0.58 ± 0.01 | 0.38 ± 0.01 | |
| After three months | 20–22 °C | 0.37 ± 0.01 | 0.06 ± 0.00 | 0.24 ± 0.01 | 0.26 ± 0.00 | 0.53 ± 0.03 | 0.34 ± 0.01 | 0.42 ± 0.01 | 0.30 ± 0.02 | |
| 3–4 °C | 0.29 ± 0.00 | 0.05 ± 0.00 | 0.11 ± 0.00 | 0.19 ± 0.00 | 0.19 ± 0.00 | 0.14 ± 0.00 | 0.09 ± 0.00 | 0.13 ± 0.00 | ||
| −20 to −18 °C | 0.25 ± 0.00 | 0.02 ± 0.00 | 0.08 ± 0.00 | 0.18 ± 0.00 | 0.19 ± 0.00 | 0.12 ± 0.01 | 0.37 ± 0.00 | 0.22 ± 0.00 | ||
| After five months | 20–22 °C | 0.12 ± 0.00 | 0.02 ± 0.00 | 0.13 ± 0.00 | 0.17 ± 0.00 | 0.22 ± 0.00 | 0.09 ± 0.00 | 0.08 ± 0.00 | 0.15 ± 0.01 | |
| 3–4 °C | 0.07 ± 0.00 | 0.02 ± 0.00 | 0.06 ± 0.00 | 0.06 ± 0.00 | 0.11 ± 0.01 | 0.07 ± 0.00 | 0.07 ± 0.00 | 0.05 ± 0.00 | ||
| −20 to −18 °C | 0.07 ± 0.00 | 0.01 ± 0.00 | 0.06 ± 0.00 | 0.08 ± 0.00 | 0.10 ± 0.00 | 0.06 ± 0.00 | 0.14 ± 0.00 | 0.12 ± 0.00 | ||
| After six months | 20–22 °C | 0.08 ± 0.00 | 0.01 ± 0.00 | 0.05 ± 0.00 | 0.11 ± 0.00 | 0.10 ± 0.00 | 0.07 ± 0.00 | 0.05 ± 0.00 | 0.02 ± 0.00 | |
| 3–4 °C | 0.04 ± 0.01 | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.01 | 0.03 ± 0.00 | 0.04 ± 0.00 | 0.00 ± 0.00 | ||
| −20 to −18 °C | 0.04 ± 0.00 | 0.00 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.01 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.01 ± 0.01 | 0.07 ± 0.01 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vijan, L.E.; Giura, S.; Mazilu, I.C.; Botu, M. Effect of Temperature and Storage Time on Some Biochemical Compounds from the Kernel of Some Walnut Cultivars Grown in Romania. Horticulturae 2023, 9, 544. https://doi.org/10.3390/horticulturae9050544
Vijan LE, Giura S, Mazilu IC, Botu M. Effect of Temperature and Storage Time on Some Biochemical Compounds from the Kernel of Some Walnut Cultivars Grown in Romania. Horticulturae. 2023; 9(5):544. https://doi.org/10.3390/horticulturae9050544
Chicago/Turabian StyleVijan, Loredana Elena, Simona Giura, Ivona Cristina Mazilu, and Mihai Botu. 2023. "Effect of Temperature and Storage Time on Some Biochemical Compounds from the Kernel of Some Walnut Cultivars Grown in Romania" Horticulturae 9, no. 5: 544. https://doi.org/10.3390/horticulturae9050544
APA StyleVijan, L. E., Giura, S., Mazilu, I. C., & Botu, M. (2023). Effect of Temperature and Storage Time on Some Biochemical Compounds from the Kernel of Some Walnut Cultivars Grown in Romania. Horticulturae, 9(5), 544. https://doi.org/10.3390/horticulturae9050544








