Variability in Productive and Biochemical Traits of Vicia faba L. Landraces from Apulia Region (South Italy)
Abstract
1. Introduction
2. Materials and Methods
2.1. Germplasm and Location of Experiment
2.2. Productive Characterization
2.3. Chemical Characterization
2.3.1. Sample Preparation
2.3.2. Determination of Dry Matter and Crude Protein Content
2.3.3. Extraction for Phenolic Analyses
2.3.4. Determination of Total Phenolic Content
2.3.5. Determination of Total Flavonoid Content
2.3.6. Determination of Condensed Tannin Content
2.3.7. Determination of L-DOPA Content and Yield
2.4. Statistical Analysis
3. Results
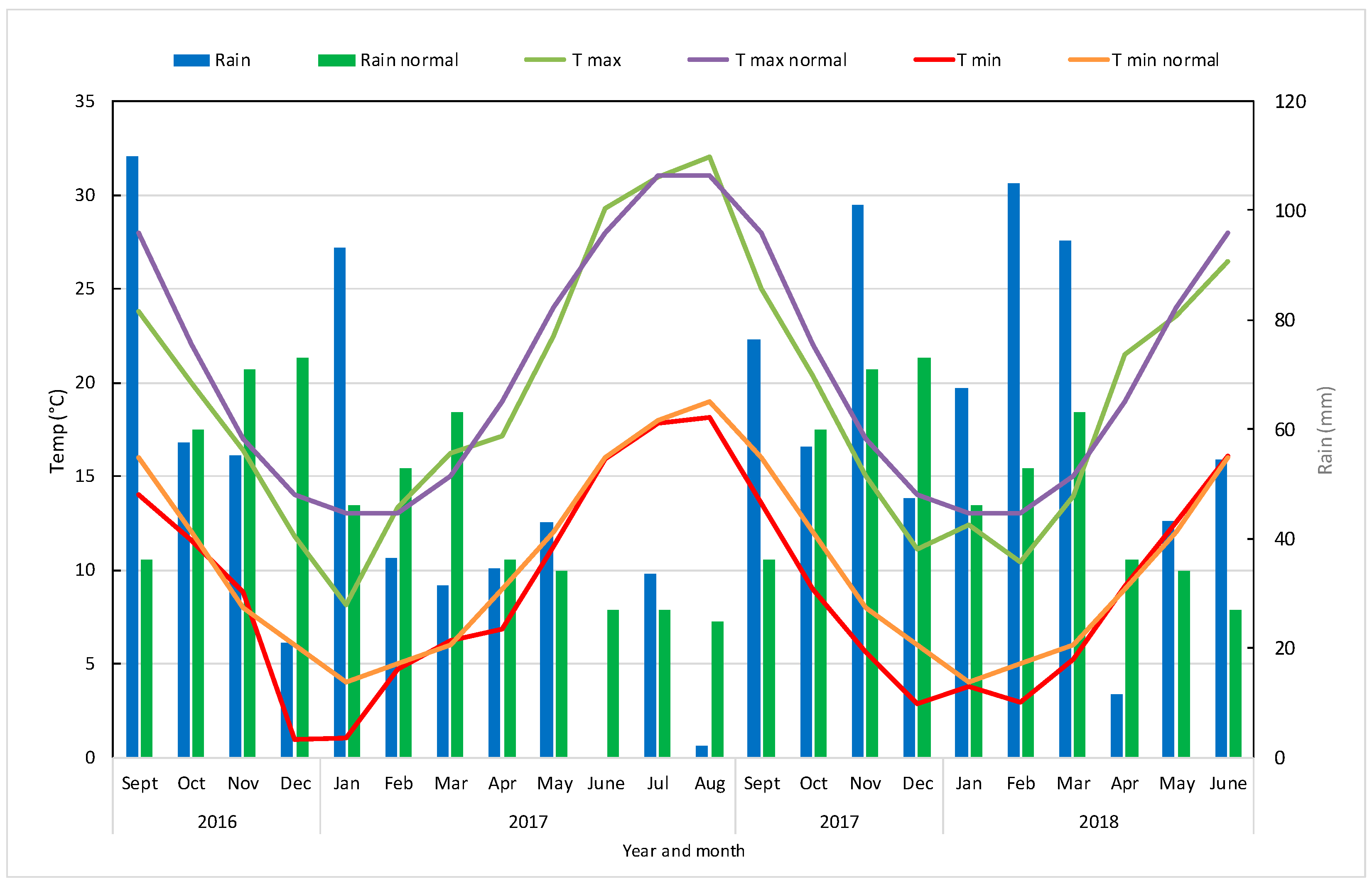
3.1. Meteorological Trend
3.2. Productive Component Measurements
3.3. Chemical Characterization
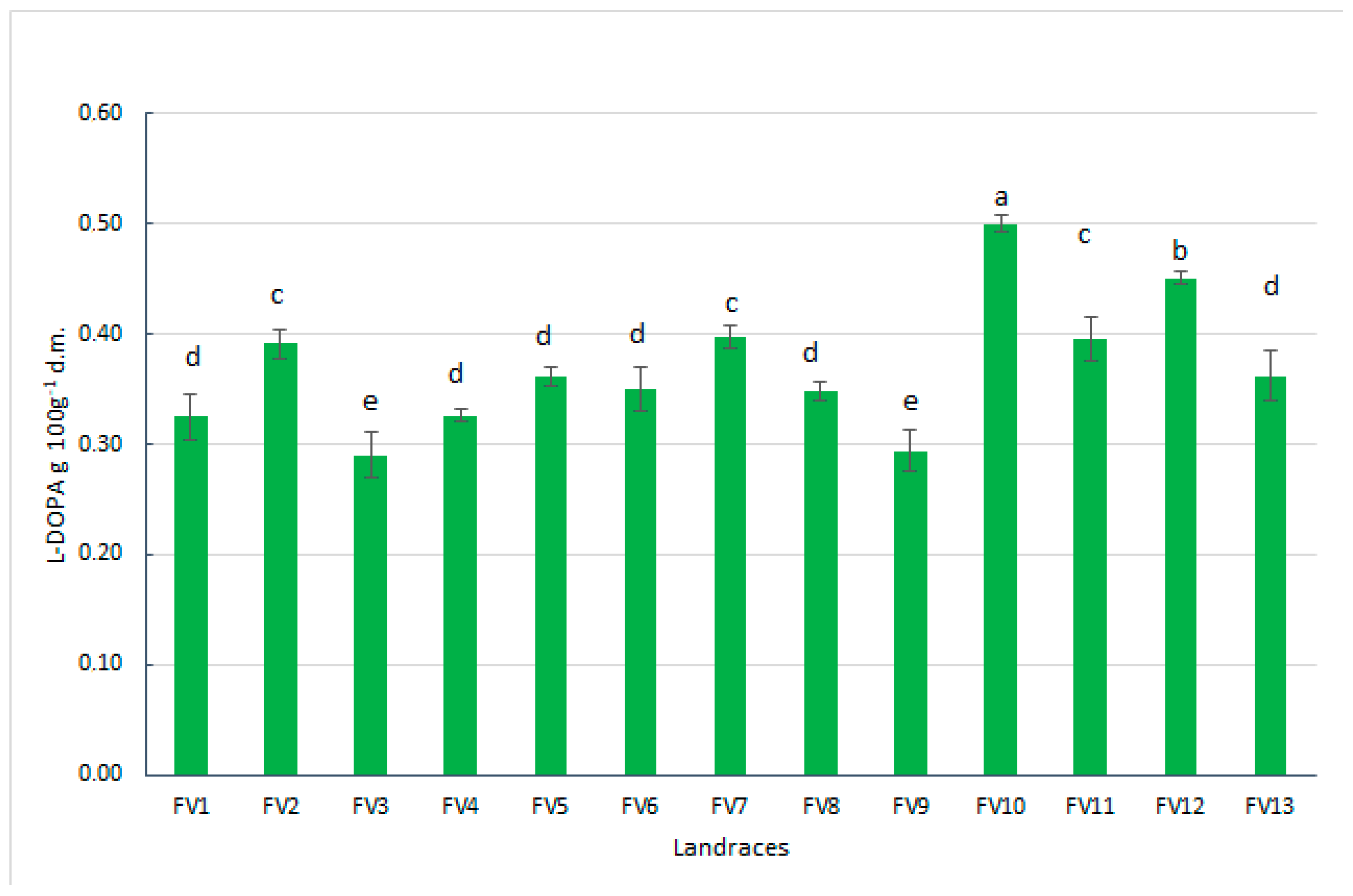
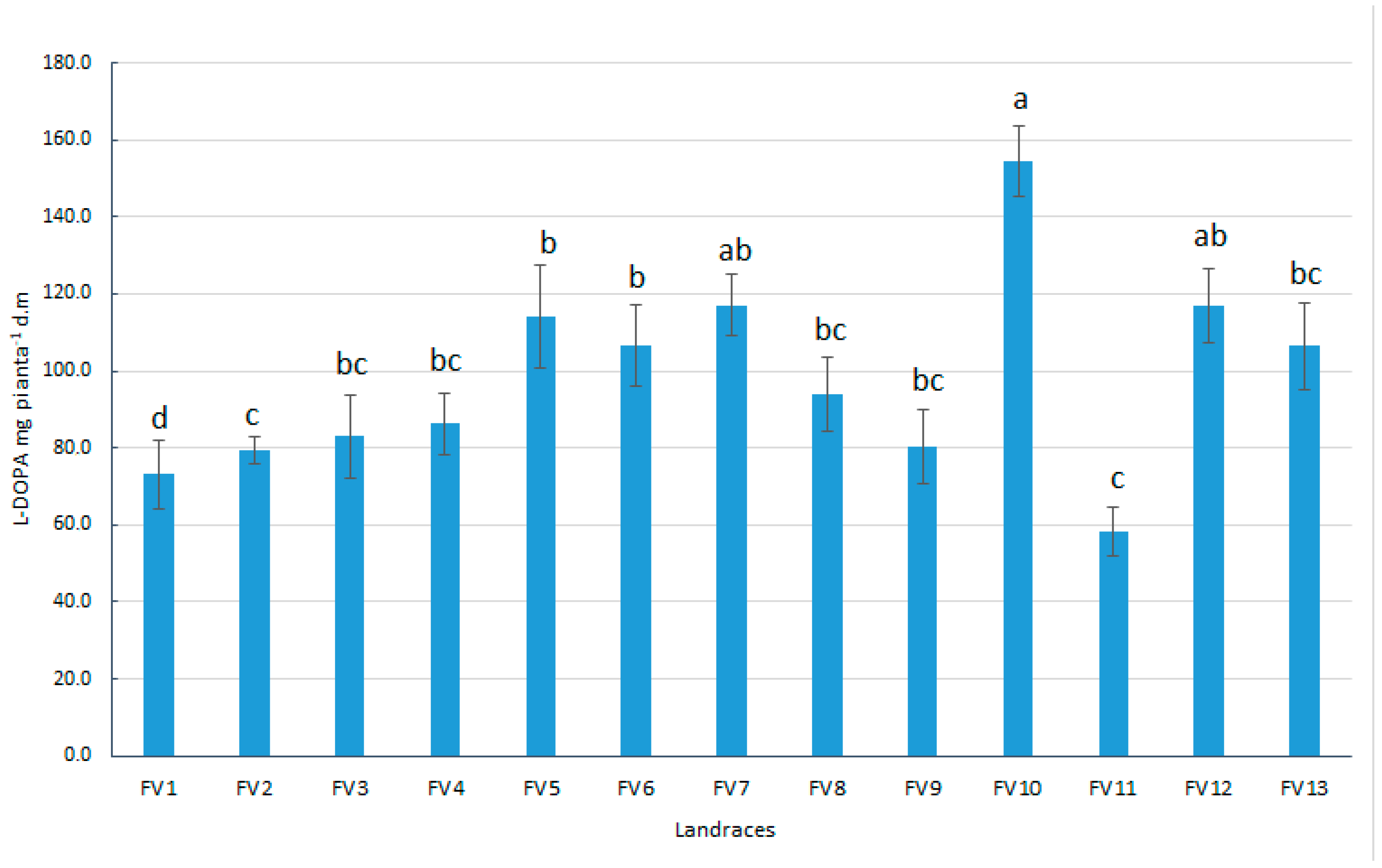
3.4. L-DOPA Content and L-DOPA Yield
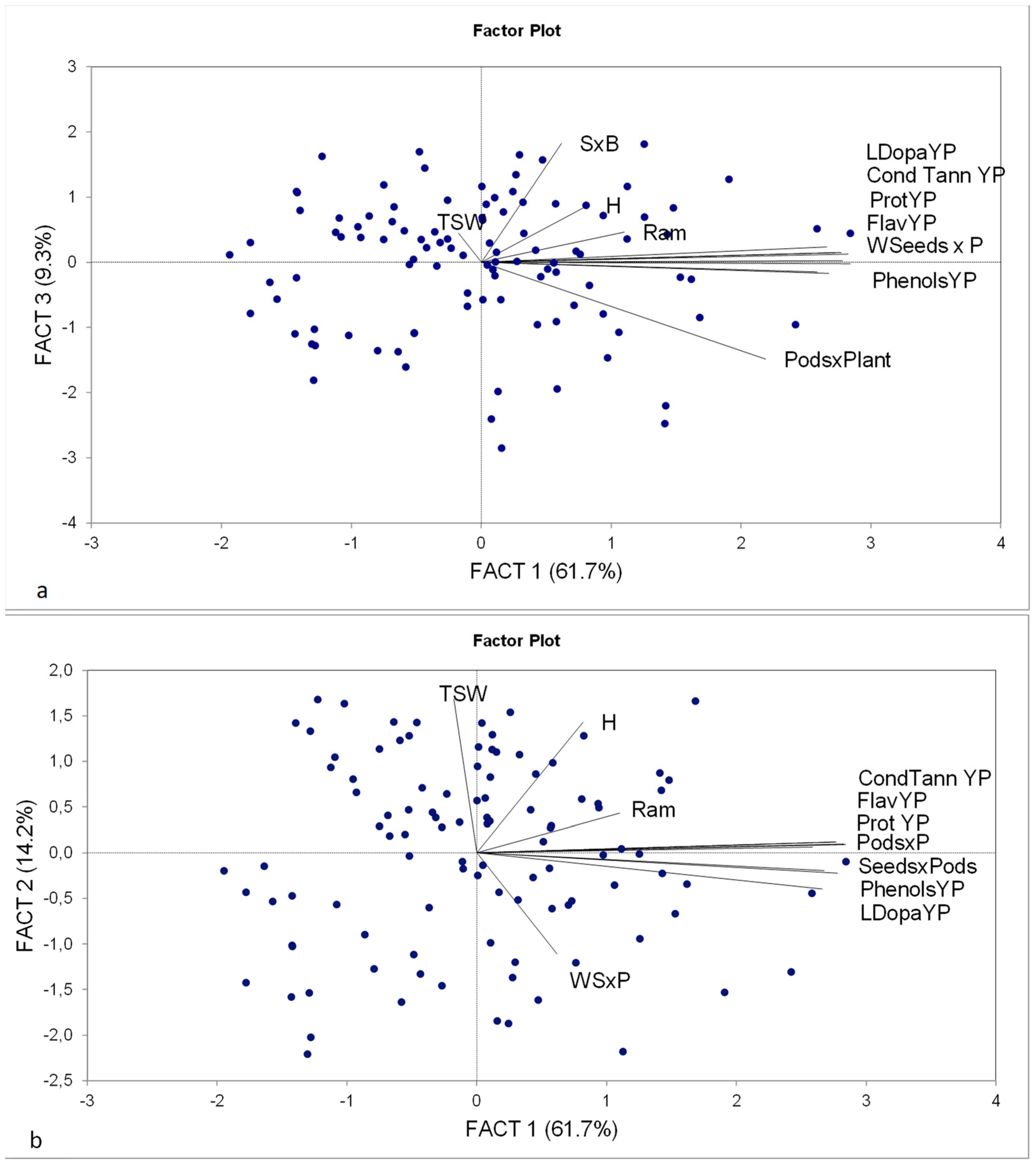
3.5. PCA Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT. Statistical Data. Food and Agriculture Organization of the United Nations. 2017. Available online: http://faostat.fao.org (accessed on 4 September 2019).
- Eurostat. Database. European Commission. Brussels, Belgium. 2019. Available online: https://ec.europa.eu/eurostat/data/database (accessed on 4 September 2019).
- Dhull, S.B.; Kidwai, M.K.; Noor, R.; Chawla, P.; Rose, P.K. A review of nutritional profile and processing of faba bean (Vicia faba L.). Legume Sci. 2021, 4, e129. [Google Scholar] [CrossRef]
- Giménez, M.A.; Drago, S.R.; De Greef, D.; Gonzalez, R.J.; Lobo, M.O.; Samman, N.C. Rheological, functional and nutritional properties of wheat/broad bean (Vicia faba) flour blends for pasta formulation. Food Chem. 2012, 134, 200–206. [Google Scholar] [CrossRef]
- Lizarazo Torres, C.; Lampi, A.-M.; Liu, J.; Sontag-Strohm, T.; Piironen, V.; Stoddard, F. Nutritive quality and protein production from grain legumes in a boreal climate. J. Sci. Food Agric. 2015, 95, 2053–2064. [Google Scholar] [CrossRef] [PubMed]
- Dillard, C.J.; German, J.B. Phytochemicals: Nutraceuticals and human health. J. Sci. Food Agric. 2000, 80, 1744–1756. [Google Scholar] [CrossRef]
- Madhujith, T.; Amarowicz, R.; Shahidi, F. Phenolic antioxidants in beans and their effects on inhibition of radical induced DNA damage. J. Am. Oil Chem. Soc. 2004, 81, 691–696. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Contreras, M.M.; ArráezRomán, D.; Fernández-Gutiérrez, A. UHPLCESI-QTOF-MS-based metabolic profiling of Vicia faba L. (Fabaceae) seeds as a key strategy for characterization in foodomics. Electrophoresis 2014, 35, 1571–1581. [Google Scholar] [CrossRef]
- Borowska, J.; Giczewska, A.; Zadernowski, R. Nutritional value of broad bean seeds. Part 2: Selected biologically active components. Nahr. Food 2003, 47, 98–101. [Google Scholar] [CrossRef]
- Merghem, R.; Jay, M.; Brun, N.; Voirin, B. Qualitative analysis and HPLC isolation and identification of procyanidins from Vicia faba. Phytochem. Anal. 2004, 15, 95–99. [Google Scholar] [CrossRef]
- Arts, I.C.W.; van de Putte, B.; Hollman, P.C.H. Catechin contents of foods commonly consumed in the Netherlands. 1. Fruits, vegetables, staple foods, and processed foods. J. Agric. Food Chem. 2000, 48, 1746–1751. [Google Scholar] [CrossRef]
- Karamac, M.; Kosinska, A.; Rybarczyk, A.; Ryszard, A. Extraction and chromatograhic separation of tannin fractions from tannin-rich plant material. Pol. J. Food Nutr. Sci. 2007, 57, 471–474. [Google Scholar]
- J Tebib, K.; Bitri, L.; Besançon, P. Rouanet. Polymeric grape seed tannins prevent plasma cholesterol changes in high-cholesterol-fed rats. Food Chem. 1994, 49, 403–406. [Google Scholar] [CrossRef]
- Papadopoulou, A.; Frazier, R.A. Characterization of protein–polyphenol interactions. Trends Food Sci. Technol. 2004, 15, 186–190. [Google Scholar] [CrossRef]
- Singh, A.K.; Bharati, R.C.; Manibhushan, N.C.; Pedpati, A. An assessment of faba bean (Vicia faba L.) current status and future prospect. Afr. J. Agric. Res. 2013, 8, 6634–6641. [Google Scholar]
- Mohseni-Mehran, S.M.; Golshani, B. Simultaneous determination of Levodopa and Carbidopa from fava bean, green peas and green beans by high per formance liquid gas chromatography. J. Clin. Diagn. Res. 2013, 7, 1004–1007. [Google Scholar]
- Etemadi, F.; Masoud, H.; Randhir, R.; ZandVakili, O.; Ebadi, A. Accumulation of L-DOPA in various organs of faba bean and influence of drought, nitrogen stress, and processing methods on L-DOPA yield. Crop J. 2018, 6, 426–434. [Google Scholar] [CrossRef]
- Knowles, W.S. Asymmetric Hydrogenations—The Monsanto L-Dopa Process. In Asymmetric Catalysis on Industrial Scale; Blaser, H.-U., Schmidt, E., Eds.; Wiley-VCH Verlag GmbH Co. KGaA: Weinheim, Germany, 2004; pp. 21–38. [Google Scholar]
- Parkinson’s Disease Drugs Market. Available online: https://www.fortunebusinessinsights.com/enquiry/request-sample-pdf/parkinson-s-disease-387drugs-market-100655 (accessed on 20 October 2019).
- Patil, S.A.; Apine, O.A.; Surwase, S.N.; Jadhav, J.P. Biological sources of L-DOPA: An alternative approach. Adv. Park. Dis. 2013, 2, 81–87. [Google Scholar] [CrossRef]
- Apaydin, H.; Ertan, S.; Özekmekçi, S. Broad bean (Vicia faba)—A natural source of L-392 DOPA-prolongs ‘on’ periods in patients with Parkinson’s disease who have ‘on-off’ fluctuations. Mov. Disord. 2000, 15, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Burbano, C.; Cuadrado, C.; Muzquiz, M.; Cubero, J.I. Variation of favism-inducing factors (vicine, convicine and L-DOPA) during pod development in Vicia faba L. Plant Foods Hum. Nutr. 1995, 47, 265–275. [Google Scholar] [CrossRef]
- Polanowska, K.; Łukasik, R.M.; Kuligowski, M.; Nowak, J. Development of a sustainable, simple, and robust method for efficient L-DOPA extraction. Molecules 2019, 24, 2325. [Google Scholar] [CrossRef]
- De Cillis, F.; Leoni, B.; Massaro, M.; Renna, M.; Santamaria, P. Yield and quality of faba bean (Vicia faba L. var. major) genotypes as a vegetable for fresh consumption: A comparison between Italian landraces and commercial varieties. Agriculture 2019, 9, 253. [Google Scholar] [CrossRef]
- Randhir, R.; Shetty, P.; Shetty, K. L-DOPA and total phenolic stimulation in dark germinated fava bean in response to peptide and phytochemical elicitors. Process Biochem. 2002, 37, 1247–1256. [Google Scholar] [CrossRef]
- Randhir, R.; Shetty, K. Microwave-induced stimulation of L-DOPA, phenolics and antioxidant activity in fava bean (Vicia faba) for Parkinson’s diet. Process Biochem. 2004, 39, 1775–1784. [Google Scholar] [CrossRef]
- Database “SaVeGraINPuglia”. Available online: http://bancadati.savegrainpuglia.it/it/signin (accessed on 10 February 2023).
- Signore, A.; Renna, M.; Santamaria, P. Agrobiodiversity of vegetable crops: Aspect, needs, and future perspectives. Annu. Plant Rev. Online 2019, 2, 1–24. [Google Scholar] [CrossRef]
- ISTA. International Rules for Seed Testing, 2016 ed.; International Seed Testing Association: Basserdorf, Switzerland, 2016. [Google Scholar]
- AOAC (Association of Official Agricultural Chemists). Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaitherburg, MD, USA, 2000; Volume I. [Google Scholar]
- Lavelli, V.; Hidalgo, A.; Pompei, C.; Brandolini, A. Radical scavenging activity of einkorn (Triticum monococcum L. subsp. monococcum) wholemeal flour and its relationship to soluble phenolic and lipophilic antioxidant content. J. Cereal Sci. 2009, 49, 319–321. [Google Scholar] [CrossRef]
- Hidalgo, A.; Brandolini, A.; Pompei, C.; Piscozzi, R. Carotenoids and tocols of einkorn wheat (Triticum monococcum ssp. monococcum L.). J. Cereal Sci. 2006, 44, 182–193. [Google Scholar] [CrossRef]
- Roberts, I.M. Iso-butanol saturated water: A simple procedure for increasing staining intensity of resin sections for light and electron microscopy. J. Microsc. 2002, 207, 97–107. [Google Scholar] [CrossRef]
- Xu, B.J.; Chang, S.K.C. A Comparative Study on Phenolic Profiles and Antioxidant Activities of Legumes as Affected by Extraction Solvents. J. Food Sci. 2007, 72, 159–166. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Heimler, D.; Vignolini, P.; Dini, M.G.; Romani, A. Rapid tests to assess the antioxidant activity of Phaseolus vulgaris L. dry beans. J. Agric. Food Chemestry 2005, 53, 3053–3056. [Google Scholar] [CrossRef]
- Broadhurst, R.B.; Jones, W.T. Analysis of condensed tannins using acidified vanillin. J. Sci. Food Agric. 1978, 29, 788–794. [Google Scholar] [CrossRef]
- Marquardt, R.R.; Frohlich, A.A. Rapid reversed-phase high-performance liquid chromatography method for the quantitation of vicine, convicine and related compounds. J. Chromatogr. A 1981, 208, 373–379. [Google Scholar] [CrossRef]
- Karkanis, A.; Ntatsi, G.; Lepse, L.; Fernández, J.A.; Vågen, I.M.; Rewald, B.; Savvas, D. Faba bean cultivation–revealing novel managing practices for more sustainable and competitive European cropping systems. Front. Plant Sci. 2018, 9, 1115. [Google Scholar] [CrossRef] [PubMed]
- Mansour, E.; Desoky, E.S.M.; Ali, M.M.; Abdul-Hamid, M.I.; Ullah, H.; Attia, A.; Datta, A. Identifying drought-tolerant genotypes of faba bean and their agro-physiological responses to different water regimes in an arid Mediterranean environment. Agric. Water Manag. 2021, 247, 106754. [Google Scholar] [CrossRef]
- Almeida, D.; Greiner, R.; Furtunado, D.; Trigueiro, I.; Araújo, M. Content of some antinutritional factors in bean cultivars frequently consumed in Brazil. Int. J. Food Sci. Technol. 2008, 43, 243–249. [Google Scholar] [CrossRef]
- Avola, G.; Gresta, F.; Abbate, V. Diversity examination based on physical, technological and chemical traits in a locally grown landrace of faba bean (Vicia faba L. var. major). Int. J. Food Sci. Technol. 2009, 44, 2568–2576. [Google Scholar] [CrossRef]
- Siah, S.; Wood, A.J.; Agboola, S.; Konczak, I.; Blanchard, C.L. Effects of soaking, boiling and autoclaving on the phenolic contents and antioxidant activities of faba beans (Vicia faba L.) differing in seed coat colours. Food Chem. 2014, 142, 461–468. [Google Scholar] [CrossRef]
- Baginsky, C.; Pena-Neira, A.; Cáceres, A.; Hernández, T.; Estrella, I.; Moralesb, H.; Pertuzé, R. Phenolic compound composition in immature seeds of fava bean (Vicia faba L.) varieties cultivated in Chile. J. Food Compos. Anal. 2013, 31, 1–6. [Google Scholar] [CrossRef]
- Chaieb, N.; González, J.L.; López-Mesas, M.; Bouslama, M.; Valiente, M. Polyphenols content and antioxidant capacity of thirteen faba bean (Vicia faba L.) genotypes cultivated in Tunisia. Food Res. Int. 2011, 44, 970–977. [Google Scholar] [CrossRef]
- Bond, D.A.; Duc, G. Plant breeding as a means of reducing antinutritional factors in grain legumes. In Recent Advances of Research in Antinutritional Factors in Legume Seeds; Van der Poel, A.F.B., Huisman, J., Raini, H.S., Eds.; Wageningen Pers (Pbs.): Wageningen, The Netherlands, 1993; pp. 379–396. [Google Scholar]
- Duc, G.; Marget, P.; Esnault, R.; Le Guen, J.; Bastianelli, D. Genetic variability for feeding value of faba bean seeds (Vicia faba L.): Comparative chemical composition of isogenics involving zero-tannin and zero-vicine genes. J. Agric. Sci. 1999, 133, 185–196. [Google Scholar] [CrossRef]
- Capo-chichi, E.M.; Carsky, R.J.; Gilbert, R.A.; Maasdorp, B. Effect of genotype and environment on L-DOPA Concentration in Mucunas’s (Mucuna sp.) seeds. Trop. Subtrop. Agroecosyst. 2003, 1, 319–328. [Google Scholar]
- Chinapolaiah, A.; Hima Bindu, K.; Manjesh, G.N.; Thondaiman, V.; Keshava, R.V.; Hariprasad, R.N.; Sudheer, K.S. Variability in L-Dopa and other biochemical composition of Mucuna pruriens (L.) an underutilized tropical legume. Ind. Crops Prod. 2019, 138, 111447. [Google Scholar] [CrossRef]
- Goyoaga, C.; Burbano, C.; Cuadrado, C.; Alejandro, V.; Guillamón, E.; Pedrosa, M.M.; Muzquiz, M. Content and distribution of vicine, convicine and L-DOPA during germination and seedling growth of two Vicia faba L. Varieties. Eur. Food Res. Technol. 2008, 227, 1537–1542. [Google Scholar] [CrossRef]
| Accession Code | Collection Sites | Latitude | Longitude | * Altitude m.a.s.l. |
|---|---|---|---|---|
| FV1 | Carpino (FG) | 41°50′393″ N | 15°50′284″ E | 56 |
| FV2 | Locorotondo (BA)–Battaglini district | 40°44′368″ N | 17°19′001″ E | 365 |
| FV3 | Locorotondo (BA)–Ritunno district | 40°44′435″ N | 17°19′089″ E | 367 |
| FV4 | Locorotondo (BA)–San Marco district | 40°47′235″ N | 17°19′304″ E | 370 |
| FV5 | Locorotondo (BA)–Sant’Elia district | 40°45′704″ N | 17°18′164″ E | 395 |
| FV6 | Locorotondo (BA)–Spiano district | 40°45′079″ N | 17°17′858″ E | 401 |
| FV7 | Locorotondo (BA)–Spiano viola district | 40°45′079″ N | 17°17′858″ E | 401 |
| FV8 | Locorotondo (BA)–Tommasone district | 40°44′701″ N | 17°18′715″ E | 388 |
| FV9 | Putignano (BA) | 40°50′167″ N | 17°00′313″ E | 310 |
| FV10 | San Donato (LE) | 40°08′796″ N | 18°15′238″ E | 82 |
| FV11 | Troia (FG) | 41°24′552″ N | 15°23′719″ E | 211 |
| FV12 | Zollino (LE) | 40°08′868″ N | 18°15′067″ E | 78 |
| FV13 | Altamura (BA) | 40°53′805″ N | 16°23′678″ E | 422 |
| Accessions | Plant Density | Pods | Seeds | Grain Production | TSW | |
|---|---|---|---|---|---|---|
| (n m−2) | (n. plant−1) | (n. pod−1) | (n. plant−1) | (g. m−2) | (g) | |
| FV1 | 20.0 a | 6.1 b–d | 3.0 b | 18.2 e,f | 398.1 b,c | 1238.3 h |
| FV2 | 19.6 a | 4.0 e,f | 2.9 b | 11.6 h | 426.3 b,c | 2089.4 a |
| FV3 | 19.2 a | 8.6 a | 2.0 c | 17.4 f | 593.0 a–c | 2002.7 b |
| FV4 | 20.0 a | 6.0 c,d | 2.9 b | 17.2 f | 582.2 a–c | 1884.3 c |
| FV5 | 19.2 a | 6.6 b–d | 3.5 b | 22.9 b | 686.9 a | 1705.4 e |
| FV6 | 19.4 a | 7.5 a–c | 3.1 b | 22.9 b | 670.8 a | 1668.2 e |
| FV7 | 19.6 a | 6.5 b–d | 3.0 b | 19.7 d | 655.6 a,b | 1909.1 c |
| FV8 | 19.4 a | 4.9 d–f | 3.2 b | 15.5 g | 560.5 a–c | 2063.8 a |
| FV9 | 19.2 a | 5.5 c–e | 3.4 b | 18.7 d | 572.5 a–c | 1778.5 d |
| FV10 | 19.6 a | 6.25 b–d | 4.1 a | 25.6 a | 666.6 a | 1498.0 g |
| FV11 | 20.0 a | 3.5 f | 3.2 b | 11.3 h | 322.2 c | 1602.0 f |
| FV12 | 19.6 a | 5.0 d–f | 4.2 a | 21.0 c | 554.1 b,c | 1487.4 g |
| FV13 | 19.2 a | 8.1 a,b | 3.0 b | 24.5 a,b | 639.7 a,b | 1513.0 g |
| Year effect (Y) | ns | * | ns | ns | * | ** |
| Accession effect (A) | ns | *** | ** | ** | *** | *** |
| A X Y | ns | ns | ns | ns | ns | * |
| Accessions | Crude Proteins | TPC | TFC | CTC |
|---|---|---|---|---|
| g 100 g−1 d.m. | mg GAE g−1 d.m. | mg CE g−1 d.m. | mg CE g−1 d.m. | |
| FV1 | 27.94 f–h | 12.35 a | 1.681 a,b | 0.779 a |
| FV2 | 30.10 a | 10.61 a–c | 1.381 b,c | 0.921 a |
| FV3 | 26.68 g,h | 12.20 a,b | 1.586 a,b | 0.822 a |
| FV4 | 27.65 e | 10.40 b | 1.503 a,b | 0.965 a |
| FV5 | 26.43 h | 11.46 a,b | 1.300 b,c | 0.830 a |
| FV6 | 27.40 e,f | 9.41 c | 1.661 a,b | 0.887 a |
| FV7 | 27.03 f,g | 10.60 a–c | 1.553 a,b | 0.742 a |
| FV8 | 28.3 c,d | 10.35 b,c | 1.656 a,b | 0.868 a |
| FV9 | 26.57 g,h | 11.57 a,b | 1.572 a,b | 0.952 a |
| FV10 | 28.3 c,d | 9.59 b,c | 1.725 a | 0.954 a |
| FV11 | 27.82 d,e | 10.88 a–c | 1.483 a,b,c | 0.882 a |
| FV12 | 28.96 b | 12.67 a | 1.121 c | 0.820 a |
| FV13 | 28.48 c | 10.52 a,b,c | 1.472 b,c | 0.899 a |
| Year effect (Y) | ** | * | ns | ns |
| Accession effect (A) | ** | *** | *** | * |
| A X Y | ns | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Cillis, F.; Ruta, C.; Pulvento, C.; Tedone, L.; De Mastro, G. Variability in Productive and Biochemical Traits of Vicia faba L. Landraces from Apulia Region (South Italy). Horticulturae 2023, 9, 601. https://doi.org/10.3390/horticulturae9050601
De Cillis F, Ruta C, Pulvento C, Tedone L, De Mastro G. Variability in Productive and Biochemical Traits of Vicia faba L. Landraces from Apulia Region (South Italy). Horticulturae. 2023; 9(5):601. https://doi.org/10.3390/horticulturae9050601
Chicago/Turabian StyleDe Cillis, Francesca, Claudia Ruta, Cataldo Pulvento, Luigi Tedone, and Giuseppe De Mastro. 2023. "Variability in Productive and Biochemical Traits of Vicia faba L. Landraces from Apulia Region (South Italy)" Horticulturae 9, no. 5: 601. https://doi.org/10.3390/horticulturae9050601
APA StyleDe Cillis, F., Ruta, C., Pulvento, C., Tedone, L., & De Mastro, G. (2023). Variability in Productive and Biochemical Traits of Vicia faba L. Landraces from Apulia Region (South Italy). Horticulturae, 9(5), 601. https://doi.org/10.3390/horticulturae9050601











