Effective Priming Techniques to Enhance Ghaf (Prosopis cineraria L. Druce) Seed Germination for Mass Planting
Abstract
1. Introduction
2. Materials and Methods
2.1. Place and Experiment Conditions
2.2. Biological Material and Substances Used for Seed Priming
2.3. Seeds Priming Experimental Set-Up
2.3.1. Hydro-Priming (With Hot Water)
2.3.2. Osmo-Priming
2.3.3. Acid-Priming with Sulfuric Acid
2.3.4. Hormone Priming
2.3.5. Acid-Hormone Priming
2.4. Seed Response to Priming Treatments
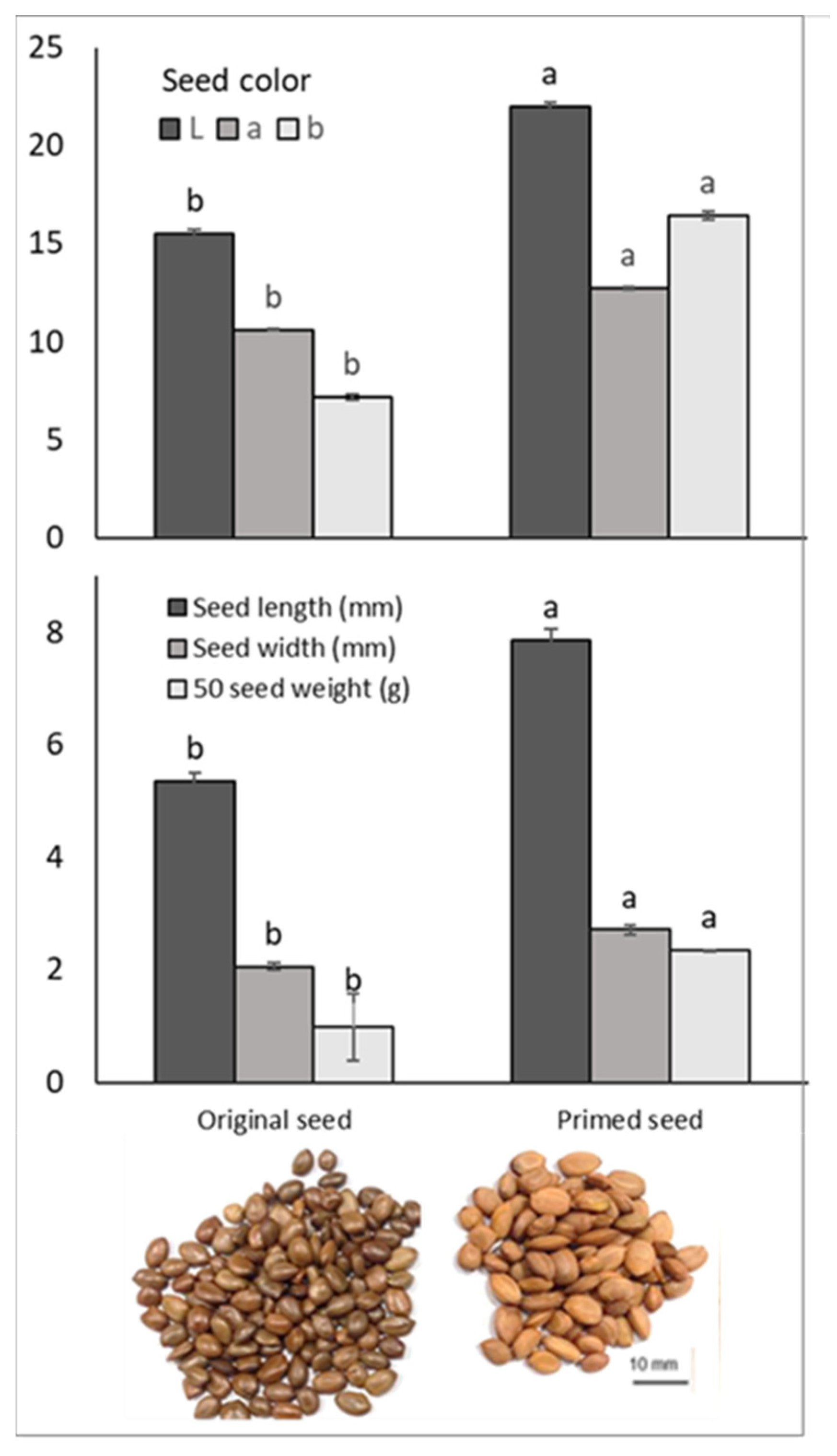
2.4.1. Color, Size, and Physical Changes
total number of seeds × 100.
2.4.2. Seeds Germination Indicators and Seedling Growth
Germination in Petri Dishes and the Primary Radicle Length
interval of trial/number of initial sowed seeds × 100.
Seedlings Emergence in Sandy Soil
interval of trial/number of initial seeds planted × 100.
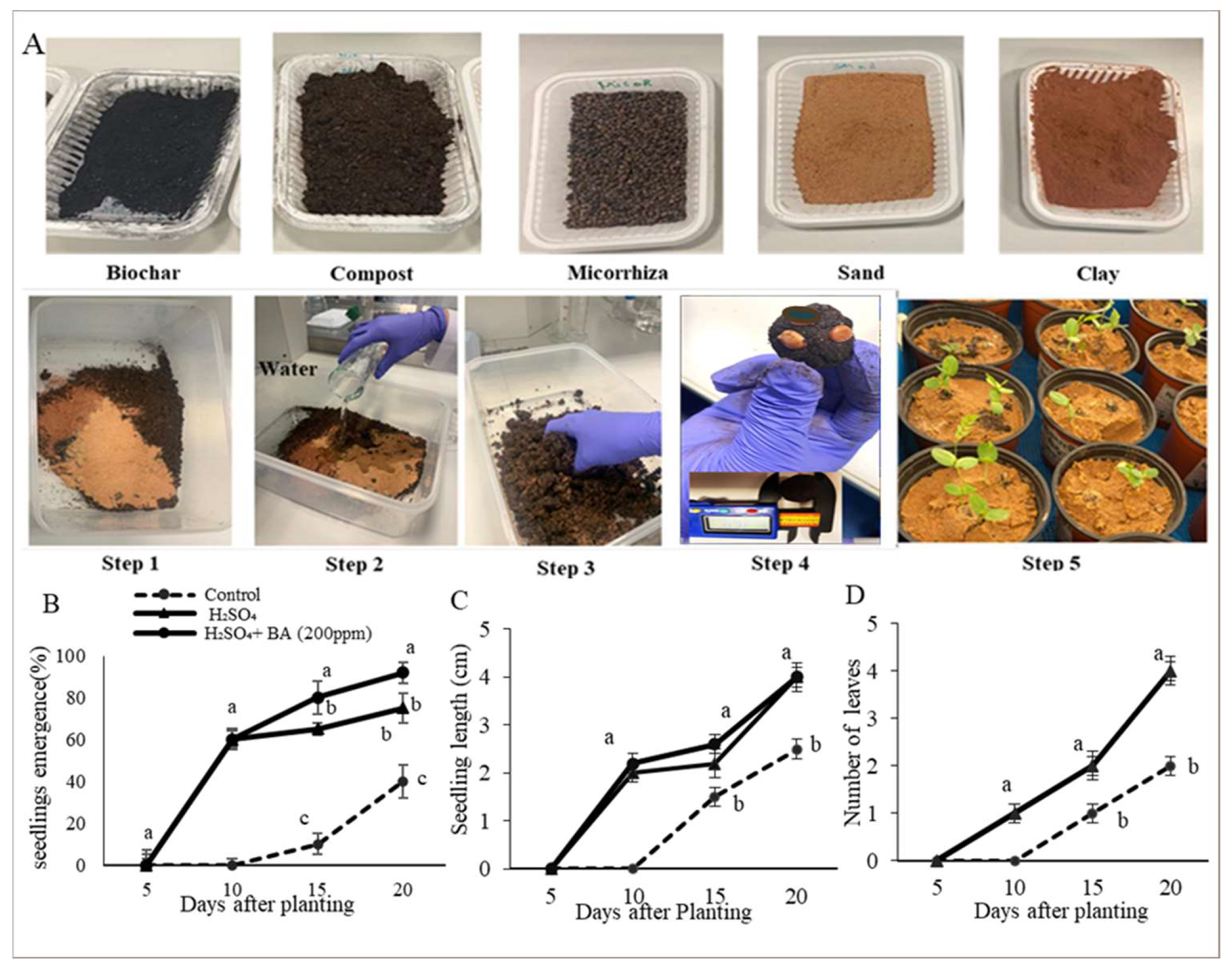
Seed Ball Preparation and Seedlings Emergence in Sandy Soil Substrate
2.5. Effect of Primed Seed Storage (Conditions and Duration) on Germination Parameters
2.6. Statistical Analysis
3. Results
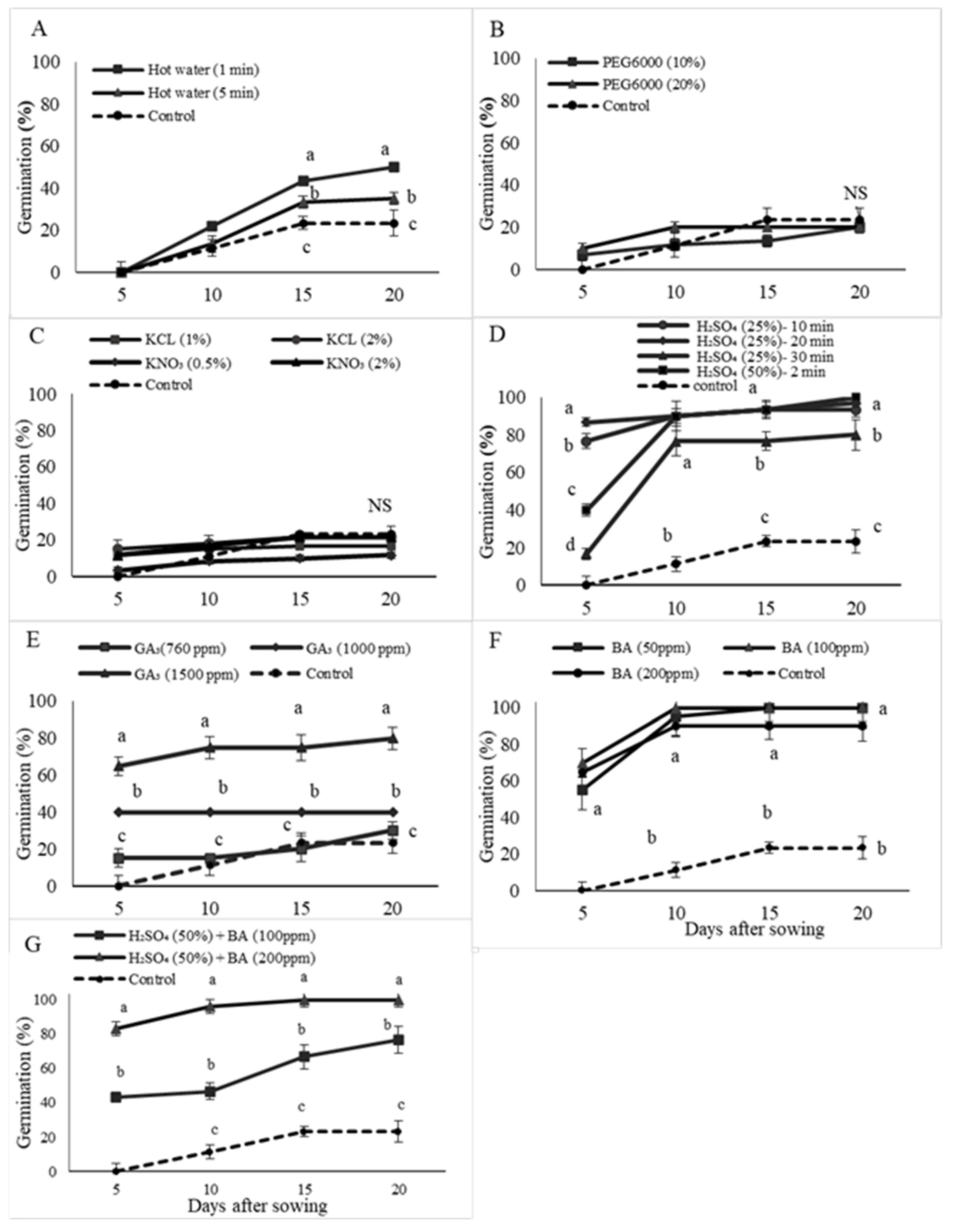
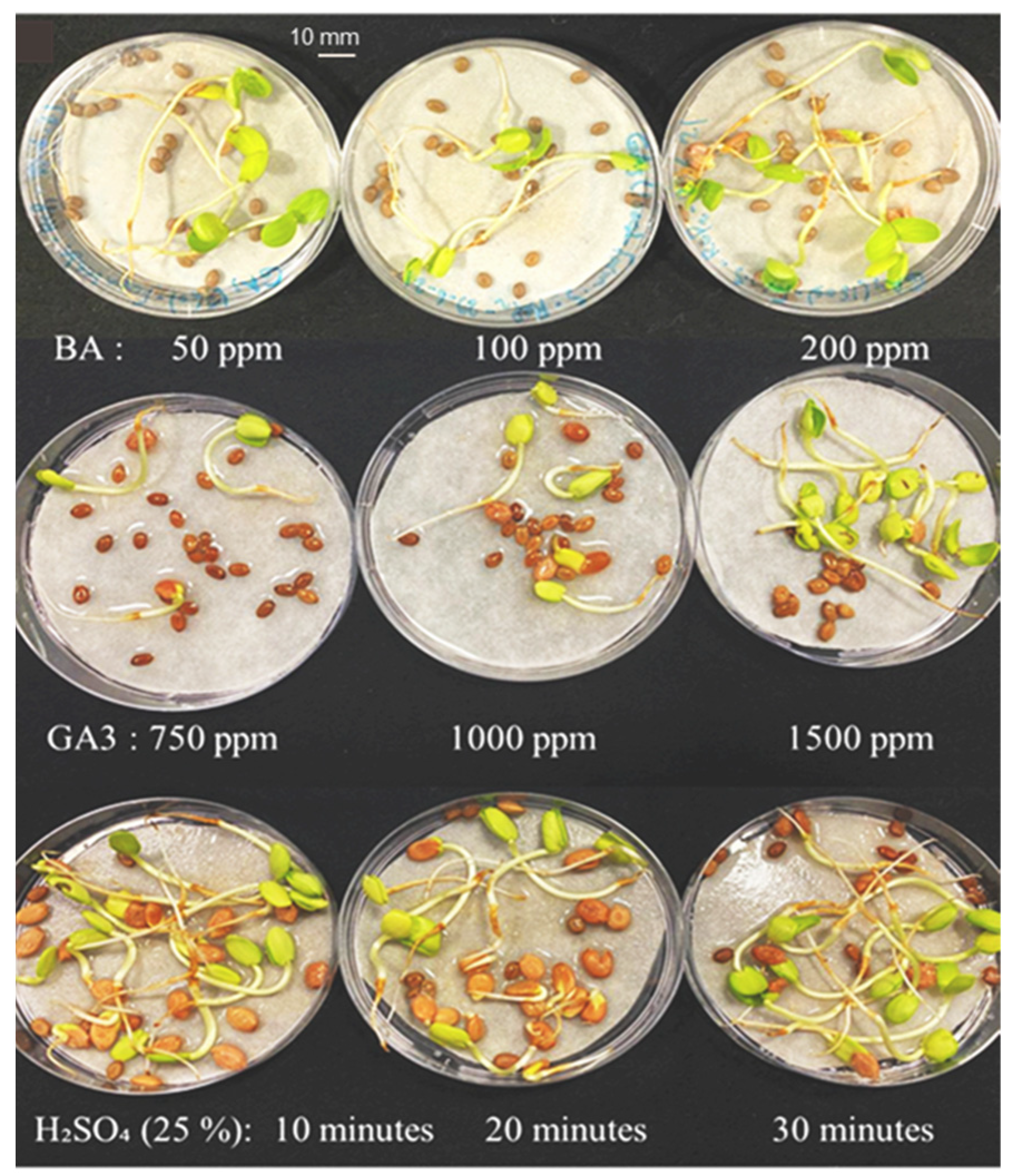
3.1. Seed Response to Priming Treatment
3.2. Seed Germination in Petri Dishes and Primary Root Length
3.3. Seedling Emergencein Sandy Soil
3.4. Seed Ball Experiment Results
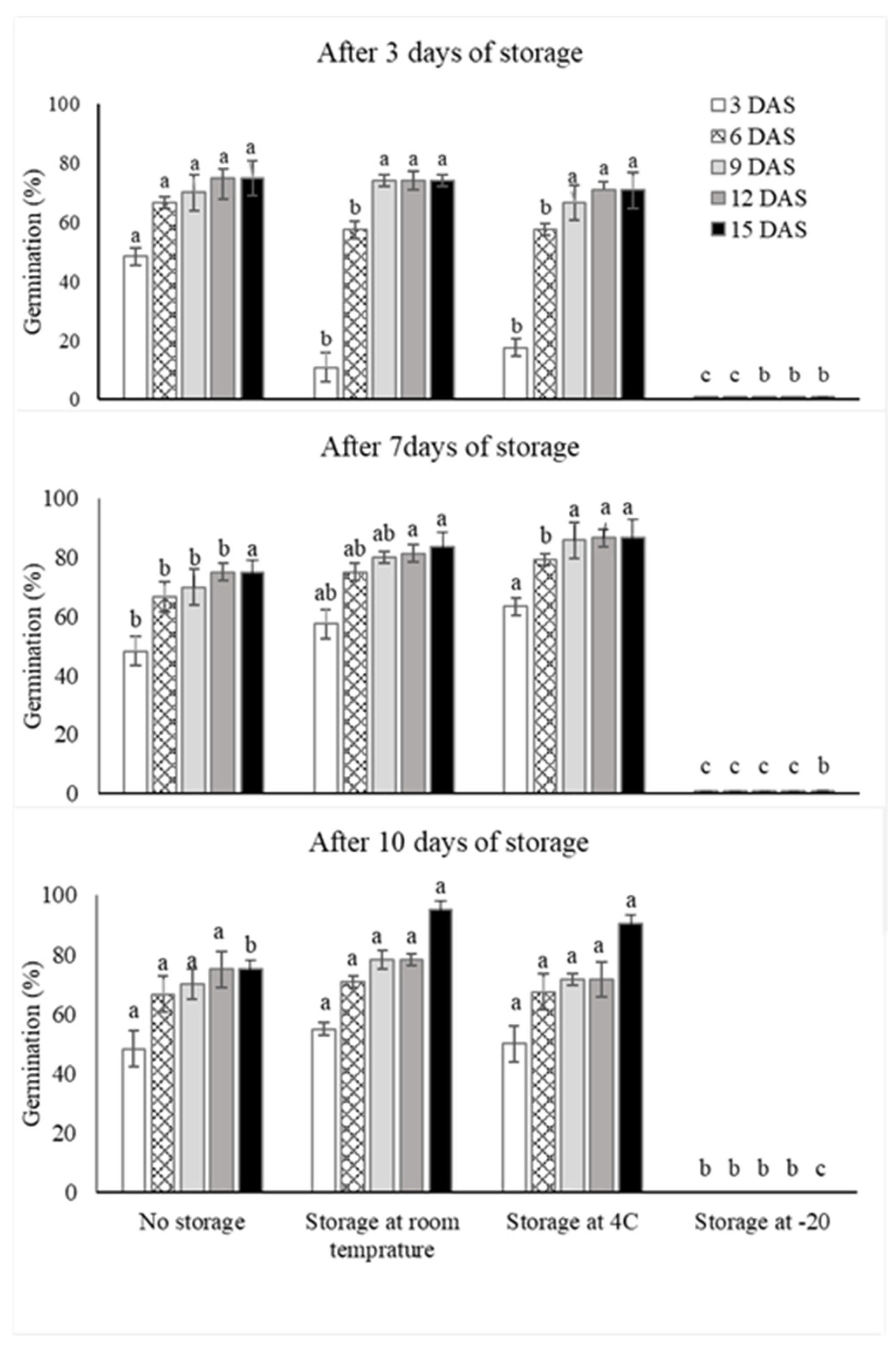
3.5. Storage of Primed Seeds
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gallacher, D.J.; El-Keblawy, A. Macromorphology and recruitment of Prosopis cineraria in the United Arab Emirates. Eco. Env. Cons. 2016, 22, 499–504. [Google Scholar]
- Al Yamani, W.; Green, S.; Pangilinan, R.; Dixon, S.; Shahid, S.A.; Kemp, P.; Clothier, B. Water use of Al Ghaf (Prosopis cineraria) and Al Sidr (Ziziphus spina-christi) forests irrigated with saline groundwater in the hyper-arid deserts of Abu Dhabi. Agri. Water Manag. 2018, 203, 105–114. [Google Scholar] [CrossRef]
- Afifi, H.S.A.; Al-rub, I.A. Prosopis Cineraria as an Unconventional Legumes, Nutrition and Health Benefits; IntechOpen: London, UK, 2018. [Google Scholar]
- Baibout, M.; Corcket, E.; Kothari, S.L.; Fievet, V. Ecosystem services provided by Prosopis cineraria (L.) Druce in the drylands of Southern and Western Asia. Bot. Lett. 2022, 169, 30–42. [Google Scholar] [CrossRef]
- Ahmed, Z.F.R.; Kaur, N.; Hassan, F.E. Ornamental date palm and sidr trees: Fruit elements composition and concerns regarding consumption. Int. J. Fruit Sci. 2022, 22, 17–34. [Google Scholar] [CrossRef]
- Puri, S.; Kumar, A. Establishment of Prosopis cineraria (L.) druce in the hot deserts of India. New For. 1995, 9, 21–33. [Google Scholar] [CrossRef]
- Rafay, M.; Malik, Z.; Riaz, U.; Abid, M.; Farooqi, M.; Akhtar, S. Propagation techniques and their efficacy rate in Prosopis cineraria (L.) druce -a multipurpose tree of Cholistan desert, Pakistan. Appl. Ecol. Environ. Res. 2019, 18, 2463–2474. [Google Scholar] [CrossRef]
- Manga, V.; Sen, D. Improving germination by acid scarification in Prosopis cineraria. J. Tree Sci. 1998, 17, 52–55. [Google Scholar]
- Waqas, M.; Korres, N.E.; Khan, M.D.; Nizami, A.S.; Deeba, F.; Ali, I.; Hussain, H. Advances in the concept and methods of seed priming. In Priming and Pretreatment of Seeds and Seedlings: Implication in Plant Stress Tolerance and Enhancing Productivity in Crop Plants; Hasanuzzaman, M., Fotopoulos, V., Eds.; Springer: Singapore, 2019; pp. 11–41. [Google Scholar]
- Dawood, M.G. Stimulating plant tolerance against abiotic stress through seed priming. In Advances in Seed Priming; Springer: Berlin/Heidelberg, Germany, 2018; pp. 147–183. [Google Scholar]
- Tian, Y.; Guan, B.; Zhou, D.; Yu, J.; Li, G.; Lou, Y. Responses of seed germination, Sseedling growth, and seed yield traits to seed pretreatment in maize (Zea mays L.). Sci. World J. 2014, 2014, 834630. [Google Scholar] [CrossRef] [PubMed]
- Lutts, S.; Benincasa, P.; Wojtyla, L.; Kubala, S.; Pace, R.; Lechowska, K.; Quinet, M.; Garnczarska, M. Seed priming: New comprehensive approaches for an old empirical technique. New Chall. Seed Biol. 2016, 1, 46. [Google Scholar]
- Singh, H.; Jassal, R.K.; Kang, J.; Sandhu, S.; Kang, H.; Grewal, K. Seed priming techniques in field crops-A review. Agric. Rev. 2015, 36, 251–264. [Google Scholar] [CrossRef]
- Damalas, C.A.; Koutroubas, S.D.; Fotiadis, S. Hydro-priming effects on seed germination and field performance of baba bean in spring sowing. Agriculture 2019, 9, 201. [Google Scholar] [CrossRef]
- Singh, A.; Dahiru, R.; Musa, M.; Sani Haliru, B. Effect of osmo priming duration on germination, emergence, and early growth of cowpea (Vigna unguiculata (L.) Walp.) in the Sudan Savanna of Nigeria. Inter. J. Agronomy 2014, 2014, 841238. [Google Scholar] [CrossRef]
- Chen, K.; Fessehaie, A.; Arora, R. Dehydrin metabolism is altered during seed osmopriming and subsequent germination under chilling and desiccation in Spinacia oleracea L. cv. Bloomsdale: Possible role in stress tolerance. Plant Sci. 2012, 183, 27–36. [Google Scholar] [CrossRef]
- Mirmazloum, I.; Kiss, A.; Erdélyi, É.; Ladányi, M.; Németh, É.Z.; Radácsi, P. The Effect of Osmopriming on Seed Germination and Early Seedling Characteristics of Carum carvi L. Agriculture 2020, 10, 94. [Google Scholar] [CrossRef]
- Tanaka-Oda, A.; Kenzo, T.; Fukuda, K. Optimal germination condition by sulfuric acid pretreatment to improve seed germination of Sabina vulgaris. Ant. J. Forest Res. 2009, 14, 251–256. [Google Scholar] [CrossRef]
- Oboho, E.; Ahanon, E. Effect of different pre-treatments on seed germination and watering regime on growth of Adansonia digitata (Linn.) seedlings. Asian J. Sci. Technol. 2017, 8, 4569–4573. [Google Scholar]
- Ahmed, Z.F.R.; Palta, J.P. Hormone-like effect of a natural lipid, lysophosphatidylethanolamine, can mitigate calcium deficiency injury in potato shoot cultures. Acta Hortic. 2017, 1187, 107–114. [Google Scholar] [CrossRef]
- Ibrahim, M.S.; Moses, N.; Ikhajiagbe, B. Seed priming with phytohormones. In Plant Hormones-Recent Advances, New Perspectives and Applications; Christophe, H., Ed.; IntechOpen: London, UK, 2022; 99p. [Google Scholar]
- Khalil, H.A.; El-Ansary, D.O.; Ahmed, Z.F.R. Mitigation of salinity stress on pomegranate (Punica granatum L. cv. Wonderful) plant using salicylic acid foliar spray. Horticulturae 2022, 8, 375. [Google Scholar] [CrossRef]
- Javed, T.; Afzal, I.; Shabbir, R.; Ikram, K.; Saqlain Zaheer, M.; Faheem, M.; Ali, H.; Iqbal, J. Seed coating technology: An innovative and sustainable approach for improving seed quality and crop performance. J. Saudi Soci. Agri. Sci. 2022, 21, 536–545. [Google Scholar] [CrossRef]
- Greipsson, S. Effects of stratification and GA3 on seed germination of a sand stabilising grass Leymus arenarius used in reclamation. Seed Sci.Technol. 2001, 29, 1–10. [Google Scholar]
- Ahmed, Z.F.R.; Kaur, N.; Maqsood, S.; Schmeda-Hirschmann, G. Preharvest applications of chitosan, salicylic acid, and calcium chloride have a synergistic effect on quality and storability of date palm fruit (Phoenix dactylifera L.). HortScience 2022, 57, 22–30. [Google Scholar] [CrossRef]
- Nadeem, A.; Ahmed, Z.F.R.; Hussain, S.B.; Omar, A.K.; Amin, M.; Javed, S.; Ali, A.; Ullah, S.; Razzaq, K.; Rajwana, I.A.; et al. On-tree fruit bagging and cold storage maintain the postharvest quality of mango fruit. Horticulturae 2022, 8, 814. [Google Scholar] [CrossRef]
- Sacheti, U.; Al-Areimi, M.S. The influence of high storage and germination temperatures on the germination of Prosopis cineraria seeds from northern Oman. J. Tropical Forest Sci. 2000, 12, 191–193. [Google Scholar]
- Patil, K.L.; Anilkumar, R.; Trivedi, V.; Hirpara, A.; Sasidharan, N.J.I. Effect of seed priming treatment in chickpea (Cicer arietinum L.). Inter. J. Chemical Studies. 2018, 6, 1064–1069. [Google Scholar]
- Thornton, J.M.; Collins, A.R.S.; Powell, A.A. The effect of aerated hydration on DNA synthesis in embryos of Brassica oleracea L. Seed Sci. Res. 1993, 3, 195–199. [Google Scholar] [CrossRef]
- Tahaei, A.; Soleymani, A.; Shams, M. Seed germination of medicinal plant, fennel (Foeniculum vulgare Mill), as affected by different priming techniques. Appl. Biochem. Biotechnol. 2016, 180, 26–40. [Google Scholar] [CrossRef]
- Blandino, C.; Fernández-Pascual, E.; Marin; Vernet, M.A.; Pritchard, H.W. Seed ecology of the geophyte Conopodium majus (Apiaceae), indicator species of ancient woodland understories and oligotrophic meadows. Plant Biol. 2019, 21, 487–497. [Google Scholar] [CrossRef]
- Viémont, J.D.; Crabbé, J. Dormancy in Plants: From Whole Plant Behaviour to Cellular Control; CABI Pub: Egham, UK, 2000. [Google Scholar]
- Demir, I.; Ellialtioglu, S.; Tipirdamaz, R. The effect of different priming treatments on reparability of aged eggplant seeds. In Proceedings of theInternational Symposium on Agrotechnics and Storage of Vegetable and Ornamental Seeds, Bari, Italy, 1 June 1994; Volume 362, pp. 205–212. [Google Scholar]
- Rhaman, M.S.; Imran, S.; Rauf, F.; Khatun, M.; Baskin, C.C.; Murata, Y.; Hasanuzzaman, M. Seed priming with Phytohormones: An Effective Approach for the Mitigation of Abiotic Stress. Plants. 2021, 10, 37. [Google Scholar] [CrossRef]
- Toklu, F. Effects of Different priming Treatments on Seed Germination Properties, Yield Components and Grain Yield of Lentil (Lens culinaris Medik.). Not. Bot. Horti Agrobot. Cluj Napoca 2015, 43, 153–158. [Google Scholar] [CrossRef]
- Mangena, P. Effect of hormonal seed priming on germination, growth, yield and biomass allocation in soybean grown under induced drought stress. Indian J. Agril. Res. 2020, 54, 592–598. [Google Scholar] [CrossRef]
- Sepehri, A.; Rouhi, H. Effect of cytokinin on morphological and physiological characteristics and antioxidant enzymes activity of aged groundnut (Arachis hypogaea L.) seeds under drought stress. Iranian J. Seed Sci. 2016, 5, 181–198. [Google Scholar]
- Gorim, L.; Asch, F. Seed Coating Increases Seed Moisture Uptake and Restricts Embryonic Oxygen Availability in Germinating Cereal Seeds. Biology 2017, 6, 31. [Google Scholar] [CrossRef]
- Cony, M.A.; Trione, S.O. Germination with respect to temperature of two Argentinian Prosopis species. J. Arid. Environ. 1996, 33, 225. [Google Scholar] [CrossRef]
- Cromarty, A.S.; Ellis, R.H.; Roberts, E.H. The Design of Seed Storage Facilities for Genetic Conservation; Bioversity International: Rome, Italy, 1982. [Google Scholar]

| Treatments | Responsive Seed (%) |
|---|---|
| Control | 20.11 ± 1.11 g |
| Hot water (1 min) | 48.22 ± 2.13 d |
| Hot water (5 min) | 33.72 ± 2.71 f |
| KCl (1%) 24 h | 14.88 ± 1.07 h |
| KCl (2%) 24 h | 20.99 ± 1.33 g |
| KNO3 (0.5%) 24 h | 10.60 ± 0.84 h |
| KNO3 (2%) 24 h | 19.45 ± 1.73 g |
| PEG6000 (10%) 24 h | 20 ± 1.28 g |
| PEG6000 (20%) 24 h | 20 ± 0.98 g |
| H2SO4 (25%), 10 min | 33 ± 0.91 f |
| H2SO4 (25%), 20 min | 40 ± 1.01 e |
| H2SO4 (25%), 30 min | 64 ± 1.32 c |
| H2SO4 (50%), 2 min | 73 ± 0.68 b |
| GA3 (750 ppm) | 30 ± 1.10 f |
| GA3 (1000 ppm) | 40 ± 1.15 e |
| GA3 (1500 ppm) | 80 ± 1.28 a |
| Cytokinin BA (50 ppm) | 27 ± 1.04 f |
| Cytokinin BA (100 ppm) | 35 ± 0.88 f |
| Cytokinin BA (200 ppm) | 32 ± 1.21 f |
| H2SO4 (50%) for 2 min + BA (100 ppm) | 70.2 ± 1.77 b |
| H2SO4 (50%) for 2 min + BA (200 ppm) | 82 ± 5.13 a |
| Treatments | Day 0 | Germination (%), 5 DAS | Germination (%), 20 DAS | Primary Root Length (cm)_10 DAS |
|---|---|---|---|---|
| Control | 10 | 11 ± 0.73 g | 23.33 ± 1.11 gh | 0.55 ± 0.01 e |
| Hot water (1 min) | 10 | 20 ± 1.2 f | 50 ± 4.46 e | 0.65 ± 0.09d e |
| Hot water (5 min) | 10 | 21 ± 0.8 f | 35 ± 1.23 fg | 0.50 ± 0.06 e |
| KCl (1%) 24 h | 5 | 12 ± 0.73 g | 16.67 ± 0.98 h | 1.15 ± 0.11 bc |
| KCl (2%) 24 h | 5 | 15 ± 0.52 g | 21.67 ± 1.21 h | 1.15 ± 0.13 bc |
| KNO3 (0.5%) 24 h | 5 | 3 ± 0.25 h | 11.67 ± 0.79 h | 0.7 ± 0.03 d |
| KNO3 (2%) 24 h | 5 | 7 ± 0.53 gh | 21.67 ± 1.03 h | 0.9 ± 0.07 cd |
| PEG6000 (10%) 24 h | 5 | 7 ± 0.34 gh | 20 ± 1.07 h | 1.1 ± 0.20 c |
| PEG6000 (20%) 24 h | 5 | 10 ± 0.33 g | 20 ± 1.07 h | 1.15 ± 0.13 bc |
| H2SO4 (25%), 10 min | 5 | 47 ± 2.7 d | 93.33 ± 1.97 b | 0.85 ± 0.07 d |
| H2SO4 (25%), 20 min | 5 | 47 ± 2.5 d | 96.67 ± 2.25 b | 1.05 ± 0.08 c |
| H2SO4 (25%), 30 min | 5 | 64 ± 1.9 c | 80 ± 2.34 c | 1.15 ± 0.13 bc |
| H2SO4 (50%), 2 min | 5 | 73 ± 4.1 b | 100 ± 3.06 a | 1.2 ± 0.11 b |
| GA3 (750 ppm) | 5 | 3 ± 0.07 h | 30 ± 1.10 g | 0.55 ± 0.05 e |
| GA3 (1000 ppm) | 5 | 8 ± 1.0 gh | 40 ± 1.15 f | 0.70 ± 0.05 d |
| GA3 (1500 ppm) | 5 | 13 ± 1.11 g | 80 ± 1.28 c | 0.75 ± 0.03 d |
| Cytokinin BA (50 ppm) | 5 | 27 ± 2.1 e | 60 ± 5.81 e | 0.50 ± 0.02 e |
| Cytokinin BA (100 ppm) | 5 | 35 ± 1.8 e | 70 ± 3.11 d | 0.50 ± 0.04 e |
| Cytokinin BA (200 ppm) | 5 | 32 ± 2.4 e | 100 ± 4.37 a | 1.15 ± 0.12 bc |
| H2SO4 (50%) for 2 min + BA (100 ppm) | 5 | 43 ± 4.6 d | 76.7 ± 2.21 cd | 1.2 ± 0.13 b |
| H2SO4 (50%) for 2 min + BA (200 ppm) | 5 | 83 ± 5.4 a | 100 ± 4.65 a | 1.45 ± 0.10 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, F.E.; Alyafei, M.A.S.; Kurup, S.; Jaleel, A.; Al Busaidi, N.; Ahmed, Z.F.R. Effective Priming Techniques to Enhance Ghaf (Prosopis cineraria L. Druce) Seed Germination for Mass Planting. Horticulturae 2023, 9, 542. https://doi.org/10.3390/horticulturae9050542
Hassan FE, Alyafei MAS, Kurup S, Jaleel A, Al Busaidi N, Ahmed ZFR. Effective Priming Techniques to Enhance Ghaf (Prosopis cineraria L. Druce) Seed Germination for Mass Planting. Horticulturae. 2023; 9(5):542. https://doi.org/10.3390/horticulturae9050542
Chicago/Turabian StyleHassan, Fatima E., Mohammed A. S. Alyafei, Shyam Kurup, Abdul Jaleel, Nabra Al Busaidi, and Zienab F. R. Ahmed. 2023. "Effective Priming Techniques to Enhance Ghaf (Prosopis cineraria L. Druce) Seed Germination for Mass Planting" Horticulturae 9, no. 5: 542. https://doi.org/10.3390/horticulturae9050542
APA StyleHassan, F. E., Alyafei, M. A. S., Kurup, S., Jaleel, A., Al Busaidi, N., & Ahmed, Z. F. R. (2023). Effective Priming Techniques to Enhance Ghaf (Prosopis cineraria L. Druce) Seed Germination for Mass Planting. Horticulturae, 9(5), 542. https://doi.org/10.3390/horticulturae9050542





