Fatty Acids and Minerals as Markers Useful to Classify Hass Avocado Quality: Ripening Patterns, Internal Disorders, and Sensory Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Conditions
2.2. Postharvest Quality
2.2.1. Sampling
2.2.2. Storage and Ripening
2.2.3. Some Fruit Postharvest Quality Analysis
- Internal defects or disorders
- Sensory analysis
- Flesh mineral content
- Fatty acid methyl esters (FAMEs)
2.3. Statistical Analysis
3. Results
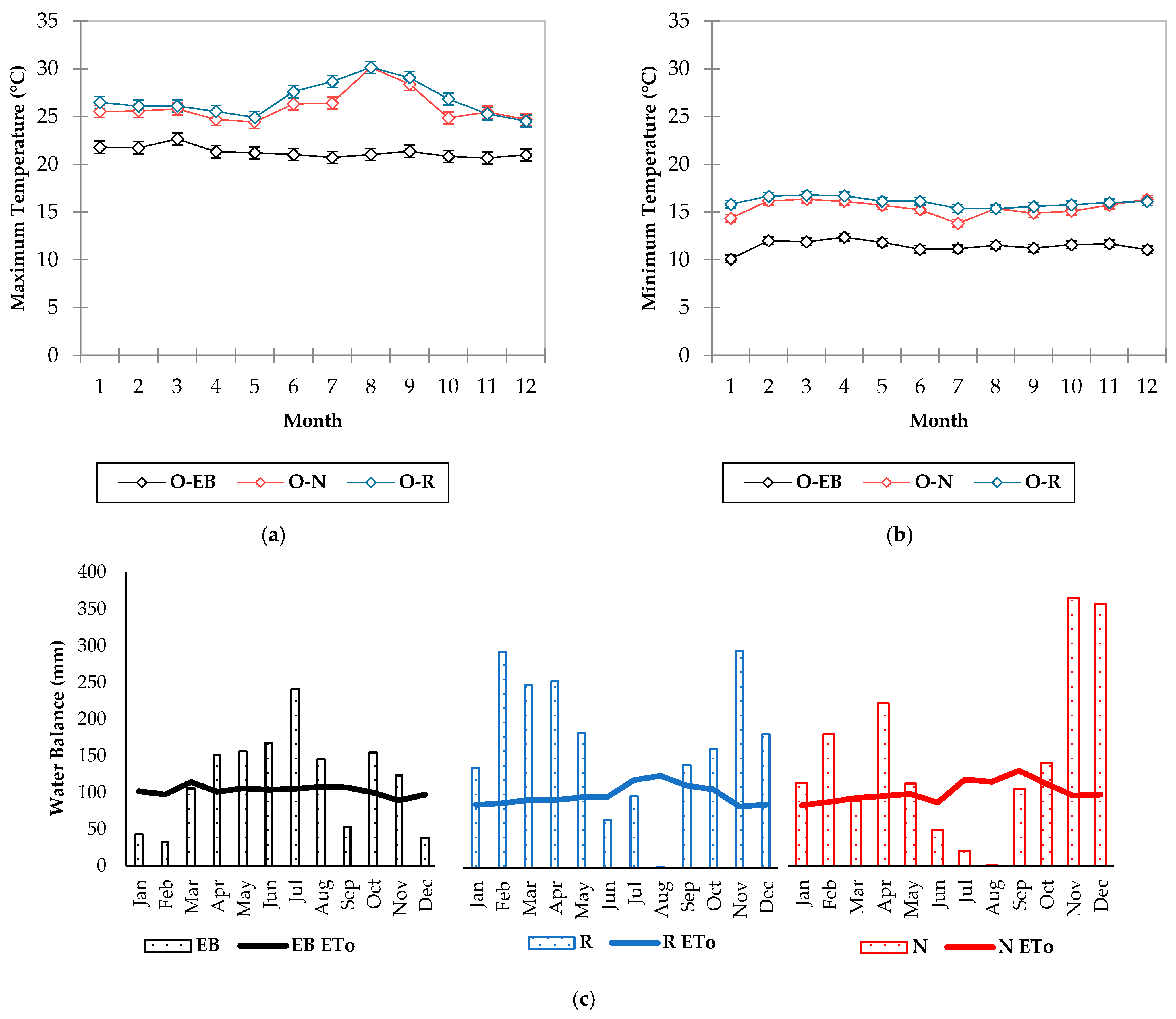
3.1. Field Conditions
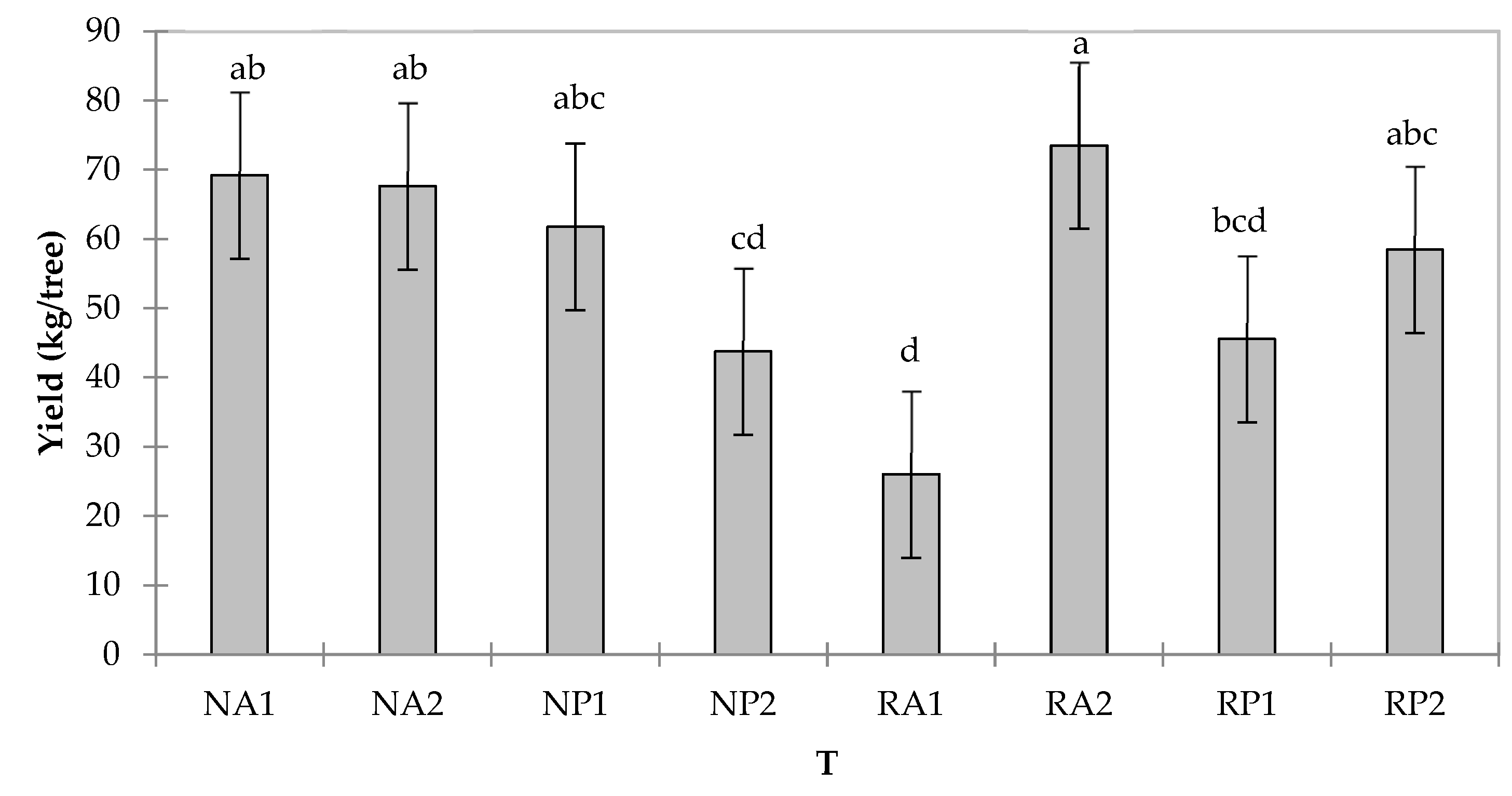
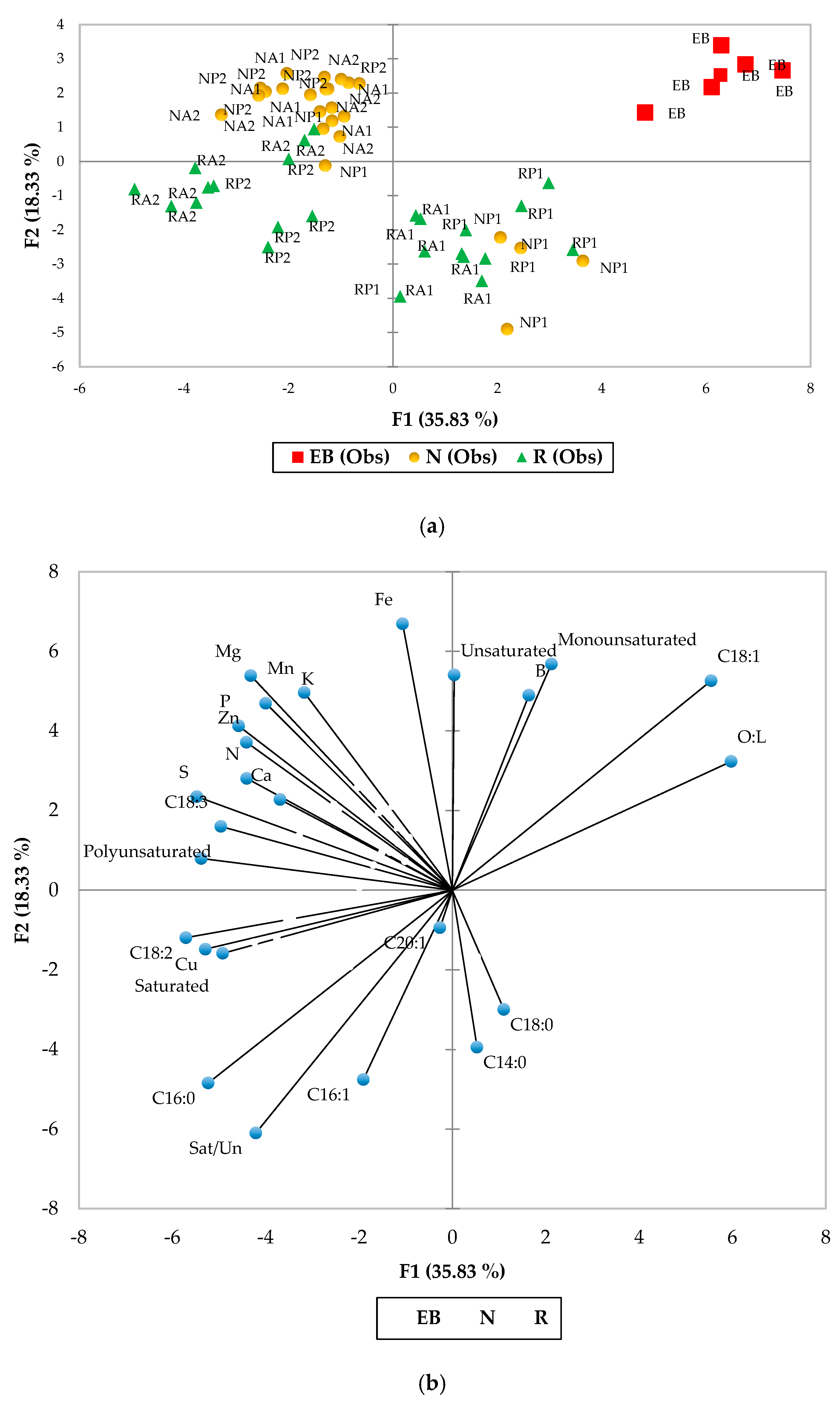
3.2. Postharvest Quality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anonymous. Avocado—World Statistics—Summer 2021 and Winter 2021–2022. Frutitrop Online 2022, 283, 98–110. [Google Scholar]
- Selladurai, R.; Awachare, C.M. Nutrient Management for Avocado (Persea Americana Miller). J. Plant Nutr. 2020, 43, 138–147. [Google Scholar] [CrossRef]
- Salazar, S.; Lazcano, I. Identifiying Fruit Mineral Removal Differences in Four Avocado Cultivars. Better Crop. Int. 2001, 15, 28–31. [Google Scholar]
- Salazar, S.; Lazcano, I. La Fertilizacion En “Sitio Especifico” Incrementa Los Rendimientos Y El Tamaño De La Fruta Del Aguacate En Mexico. Actas V Congr. Mund. Aguacate 2003, 35, 373–379. [Google Scholar]
- Hofman, P.J.; Vuthapanich, S.; Whiley, A.W.; Klieber, A.; Simons, D.H. Tree Yield and Fruit Minerals Concentrations Influence “Hass” Avocado Fruit Quality. Sci. Hortic. 2002, 92, 113–123. [Google Scholar] [CrossRef]
- Hofman, P. (Ed.) Optimising the Posharvest Qualities of Hass Avocado through Improved Calcium Nutrition, 1st ed.; Horticultural Australia Ltd: Sydney, Australia, 2007; ISBN 0734114389. [Google Scholar]
- Joyce, D.C. Improved Fruit Quality and Robustness in Avocado Supply Chains (Mineral Nutrition), 7th ed.; Horticulture Innovation: Sydney, Australia, 2021; ISBN 9780734146854. [Google Scholar]
- Hernández, I.; Fuentealba, C.; Olaeta, J.A.; Lurie, S.; Defilippi, B.G.; Campos-Vargas, R.; Pedreschi, R. Factors Associated with Postharvest Ripening Heterogeneity of “Hass” Avocados (Persea Americana Mill). Fruits 2016, 71, 259–268. [Google Scholar] [CrossRef]
- Rodríguez-Fonseca, P.E.; Martínez-álvarez, O.L.; Ocampo-Arango, C.M.; Ardila-Castañeda, M.P.; Escobar-Calderón, J.V. Effect of Harvest Index and Storage Duration on Sensory Quality of Hass Avocado Grown in Colombia. DYNA 2021, 88, 131–139. [Google Scholar] [CrossRef]
- Hausch, B.J.; Arpaia, M.L.; Campisi-Pinto, S.; Obenland, D.M. Sensory Characterization of Two California-Grown Avocado Varieties (Persea Americana Mill.) over the Harvest Season by Descriptive Analysis and Consumer Tests. J. Food Sci. Wiley 2021, 86, 4134–4147. [Google Scholar] [CrossRef]
- Donetti, M.; Terry, L.A. Biochemical Markers Defining Growing Area and Ripening Stage of Imported Avocado Fruit Cv. Hass. J. Food Compos. Anal. 2014, 34, 90–98. [Google Scholar] [CrossRef]
- Allen, R.G.; Pruitt, W.O.; Wright, J.L.; Howell, T.A.; Ventura, F.; Snyder, R.; Itenfisu, D.; Steduto, P.; Berengena, J.; Yrisarry, J.B.; et al. A Recommendation on Standardized Surface Resistance for Hourly Calculation of Reference ETo by the FAO56 Penman-Monteith Method. Agric. Water Manag. 2006, 81, 1–22. [Google Scholar] [CrossRef]
- Kenworthy, A. Leaf Analysis as an Aid in Fertilizing Orchards. In Soil Testing and Plant Analysis; Walsh, L.M., Beaton, J.D., Eds.; Soil Science Society of America: Madison, WI, USA, 1973; pp. 381–392. [Google Scholar]
- FAO Codex Alimentarius CXS-197-1995; Normas Internacionales de Los Alimentos. IFFO: London, UK, 1995; pp. 3–4.
- Waissbluth, R.J.; Valenzuela, J. Determinación del porcentaje mínimo de materia seca para autorizar la cosecha de paltas cv. Hass para ser exportadas. In Proceedings of the VI World Avocado Congress, Viña del Mar, Chile, 12–16 November 2007; pp. 957–978. [Google Scholar]
- AOAC. Official Method of Analysis, 18th ed.; AOAC: Washington, DC, USA, 2005. [Google Scholar]
- White, A.; Woolf, A.; Hofman, P.; Arpaia, M.L. The International Avocado Quality; Postharves, White, A., Woolf, A., Research, P.F., Hofman, P., Fisheries, Q.P.I., Arpaia, M.L., Eds.; Plant & Food Research: Auckland, New Zealand, 2009. [Google Scholar]
- ISO (4121); Sensory Analysis-Guidelines for the Use of Quantitative Response Scales. Sensory Analysis. Technical Committee, I. 34/SC 12. ISO: Geneva, Switzerland, 2003; Volume 34.
- Obenland, D.; Collin, S.; Sievert, J.; Negm, F.; Arpaia, M.L. Influence of Maturity and Ripening on Aroma Volatiles and Flavor in ‘Hass’ Avocado. Postharvest Biol. Technol. 2012, 71, 41–50. [Google Scholar] [CrossRef]
- Escobar, J.V.; Cortés, M.; Correa, G.; Rondón, T.; Rodríguez, P. ‘Hass’ Avocado Internal Disorders under Simulated Export Conditions and Its Relationship with Flesh Mineral Content and Preharvest Variables. Horticulturae 2021, 7, 77. [Google Scholar] [CrossRef]
- Mckean, S. Thinking with the Saint: The Miracle of Saint Januarius of Naples and Science in Early Modern Europe. Early Sci. Med. 1993, 19, 133–173. [Google Scholar] [CrossRef]
- Gamble, J.; Harker, F.R.; Jaeger, S.R.; White, A.; Bava, C.; Beresford, M.; Stubbings, B.; Wohlers, M.; Hofman, P.J.; Marques, R.; et al. The Impact of Dry Matter, Ripeness and Internal Defects on Consumer Perceptions of Avocado Quality and Intentions to Purchase. Postharvest Biol. Technol. 2010, 57, 35–43. [Google Scholar] [CrossRef]
- Matsui, T.; Kamata, T.; Koseki, S.; Koyama, K. Development of Automatic Detection Model for Stem-End Rots of ‘Hass’ Avocado Fruit Using X-Ray Imaging and Image Processing. Postharvest Biol. Technol. 2022, 192, 111996. [Google Scholar] [CrossRef]
- Eslava, J. Some Particular Climatic Features of the Colombian Pacific Region. Atmósfera 1994, 17, 45–63. [Google Scholar]
- Mejia, F.; Mesa, O.; Poveda, G.; Velez, J.; Hoyos, C.; Mantilla, R.; Barco, J.; Cuartas, A.; Montoya, M.; Botero, B. Distribución Espacial y Ciclos Anual y Semianual de La Precipitación En Colombia. DYNA 1999, 127, 7–26. [Google Scholar]
- León, G.E.; Zea, J.A.; Eslava, J.A. Circulación General Del Tropico y La Zona de Confluencia Intertropical En Colombia. Meteorol. Colomb. 2000, 1, 31–38. [Google Scholar]
- Poveda, G.; Vélez, J.I.; Mesa, O.J.; Cuartas, A.; Barco, J.; Mantilla, R.I.; Mejía, J.F.; Hoyos, C.D.; Ramírez, J.M.; Ceballos, L.I.; et al. Linking Long-Term Water Balances and Statistical Scaling to Estimate River Flows along the Drainage Network of Colombia. J. Hydrol. Eng. 2007, 12, 4–13. [Google Scholar] [CrossRef]
- Poveda, G.; Álvarez, D.M.; Rueda, Ó.A. Hydro-Climatic Variability over the Andes of Colombia Associated with ENSO: A Review of Climatic Processes and Their Impact on One of the Earth’s Most Important Biodiversity Hotspots. Clim. Dyn. 2011, 36, 2233–2249. [Google Scholar] [CrossRef]
- Whiley, A.W.; Chapman, K.R.; Saranah, J.B. Water Loss by Floral Structures of Avocado (Persea Americana Cv. Fuerte) during Flowering. Aust. J. Agric. Res. 1988, 39, 457–467. [Google Scholar] [CrossRef]
- Blanke, M.M.; Lovatt, C.J. Anatomy and Transpiration of the Avocado Inflorescence. Ann. Bot. 1993, 71, 543–547. [Google Scholar] [CrossRef]
- Lahav, E.; Whiley, A.W.; Turner, D.W. Irrigation and Mineral Nutrition. In Avocado: Botany, Production and Uses; Schaffer, B., Wolstenholme, B.N., Whiley, A.W., Eds.; CABI: London, UK, 2013; pp. 301–341. [Google Scholar]
- Schaffer, B.; Gil, P.; Mickelbart, M.V.; Whiley, A.W. Ecophysiology. In Avocado: Botany, Production and Uses; Schaffer, B., Wolstenholme, B.N., Whiley, A.W., Eds.; CABI: London, UK, 2013; Volume 1, pp. 168–199. ISBN 9788578110796. [Google Scholar]
- Silber, A.; Naor, A.; Cohen, H.; Bar-Noy, Y.; Yechieli, N.; Levi, M.; Noy, M.; Peres, M.; Duari, D.; Narkis, K.; et al. Avocado Fertilization: Matching the Periodic Demand for Nutrients. Sci. Hortic. 2018, 241, 231–240. [Google Scholar] [CrossRef]
- Cantuarias-Avilés, T.; da Silva, S.R.; Angolini, S.F.; do Amaral Brogio, B.; Baptista, E.G.; Micheletti, L.B. Water Status and Productivity of “Hass” Avocado Trees in Response to Supplemental Irrigation during Winter. Pesqui. Agropecu. Bras. 2019, 54, e00237. [Google Scholar] [CrossRef]
- García-martínez, R. Yield, Quality and Post-Harvest Behavior of ‘Hass’ Avocado Fruits of Orchards with Different Fertilization. Rev. Mex. Cienc. Agrícolas 2021, 12, 205–218. [Google Scholar] [CrossRef]
- Maldonado-Torres, R.; Álvarez-Sánchez, M.E.; Almaguer-Vargas, G.; Barrientos-Priego, A.F.; García-Mateos, R. Estándares Nutricionales Para Aguacatero ‘HASS’. Rev. Chapingo Ser. Hortic. 2007, 13, 103–108. [Google Scholar] [CrossRef]
- Mariscal, J.L.C.; Sánchez, M.E.Á.; Torres, R.M.; Gustavo, A.V. Aplicación de Abonos Orgánicos En El Rendimiento y Desarrollo Radicular En El Cultivo de Aguacate. Rev. Mex. De Cienc. Agrícolas 2020, 11, 263–274. [Google Scholar]
- Medina-Carrillo, R.E.; Salazar-García, S.; Bonilla-Cárdenas, J.A.; Herrera-González, J.A.; Ibarra-Estrada, M.E.; Álvarez-Bravo, A. Secondary Metabolites and Lignin in ‘Hass’ Avocado Fruit Skin during Fruit Development in Three Producing Regions. HortScience 2017, 52, 852–858. [Google Scholar] [CrossRef]
- Rooyen, V.Z.; Bower, J.P. The Role of Fruit Mineral Composition on Fruit Softness and Mesocarp Discolouration in “Pinkerton” Avocado (Persea Americana Mill.). J. Hortic. Sci. Biotechnol. 2005, 80, 793–799. [Google Scholar] [CrossRef]
- Fuentealba, C.; Hernández, I.; Olaeta, J.A.; Defilippi, B.; Meneses, C.; Campos, R.; Lurie, S.; Carpentier, S.; Pedreschi, R. New Insights into the Heterogeneous Ripening in Hass Avocado via LC–MS/MS Proteomics. Postharvest Biol. Technol. 2017, 132, 51–61. [Google Scholar] [CrossRef]
- Ferreyra, R.; Sellés, G.; Saavedra, J.; Ortiz, J.; Zúñiga, C.; Troncoso, C.; Rivera, S.A.; González-Agüero, M.; Defilippi, B.G. Identification of Pre-Harvest Factors That Affect Fatty Acid Profiles of Avocado Fruit (Persea Americana Mill.) Cv. ‘Hass’ at Harvest. S. Afr. J. Bot. 2016, 104, 15–20. [Google Scholar] [CrossRef]
- Ferguson, I.; Volz, R.; Woolf, A. Preharvest Factors Affecting Physiological Disorders of Fruit. Postharvest Biol. Technol. 1999, 15, 255–262. [Google Scholar] [CrossRef]
- Drori, N.; Kramer-Haimovich, H.; Rollins, J.; Dinoor, A.; Okon, Y.; Pines, O.; Prusky, D. External PH and Nitrogen Source Affect Secretion of Pectate Lyase by Colletotrichum Gloeosporioides. Appl. Environ. Microbiol. 2003, 69, 3258–3262. [Google Scholar] [CrossRef]
- Van Rooyen, Z.; Bower, J.P. The Role of Fruit Mineral Composition, Phenolic Concentration and Polyphenol Oxidase Activity on Mesocarp Discolouration in ‘Pinkerton’. S. Afr. Avocado Grow. Assoc. Yearb. 2003, 26, 72–75. [Google Scholar]
- Arpaia, M.L.; Collin, S.; Sievert, J.; Obenland, D. Influence of Cold Storage Prior to and after Ripening on Quality Factors and Sensory Attributes of ‘Hass’ Avocados. Postharvest Biol. Technol. 2015, 110, 149–157. [Google Scholar] [CrossRef]
- Rodriguez, P.; Henao, J.C.; Correa, G.; Aristizabal, A. Identification of Harvest Maturity Indicators for ‘Hass’ Avocado Adaptable to Field Conditions. Horttechnology 2018, 28, 815–821. [Google Scholar] [CrossRef]
- Metlenkin, D.A.; Platov, Y.T.; Platova, R.A.; Zhirkova, E.V.; Teneva, O.T. Non-Destructive Identification of Defects and Classification of Hass Avocado Fruits with the Use of a Hyperspectral Image. Agron. Res. 2022, 20, 326–340. [Google Scholar] [CrossRef]
- Rozenstvet, O.A.; Saksonov, S.V.; Filin, V.R.; Dembitsky, V.M. Seasonal Changes of Lipid Content in the Leaves of Some Ferns. Physiol. Plant. 2000, 113, 59–63. [Google Scholar] [CrossRef]
- Kaiser, C.; Wolstenholme, B.N. Aspects of Delayed Harvest of “Haas” Avocado (Persea Americana Mill.) Fruit in a Cool Subtropical Climate. I.Fruit Lipid and Fatty Acid Accumulation. J. Hortic. Sci. 1994, 69, 437–445. [Google Scholar] [CrossRef]
- Pedreschi, R.; Hollak, S.; Harkema, H.; Otma, E.; Robledo, P.; Westra, E.; Somhorst, D.; Ferreyra, R.; Defilippi, B.G. Impact of Postharvest Ripening Strategies on ‘Hass’ Avocado Fatty Acid Profiles. South Afr. J. Bot. 2016, 103, 32–35. [Google Scholar] [CrossRef]
- Hausch, B.J.; Arpaia, M.L.; Kawagoe, Z.; Walse, S.; Obenland, D. Chemical Characterization of Two California-Grown Avocado Varieties (Persea Americana Mill.) over the Harvest Season with an Emphasis on Sensory-Directed Flavor Analysis. J. Agric. Food Chem. 2020, 68, 15301–15310. [Google Scholar] [CrossRef] [PubMed]
- Pisani, C.; Ritenour, M.A.; Stover, E.; Plotto, A.; Alessandro, R.; Kuhn, D.N.; Schnell, R.J. Postharvest and Sensory Evaluation of Selected ‘Hass’ × ‘Bacon’ and ‘Bacon’ × ‘Hass’ Avocado Hybrids Grown in East-Central Florida. HortScience 2017, 52, 880–886. [Google Scholar] [CrossRef]
- Yang, S.; Hallett, I.; Rebstock, R.; Oh, H.E.; Kam, R.; Woolf, A.B.; Wong, M. Cellular Changes in “Hass” Avocado Mesocarp During Cold-Pressed Oil Extraction. J. Am. Oil Chem. Soc. 2018, 95, 229–238. [Google Scholar] [CrossRef]
- Cervantes-Paz, B.; Yahia, E.M. Avocado Oil: Production and Market Demand, Bioactive Components, Implications in Health, and Tendencies and Potential Uses. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4120–4158. [Google Scholar] [CrossRef] [PubMed]

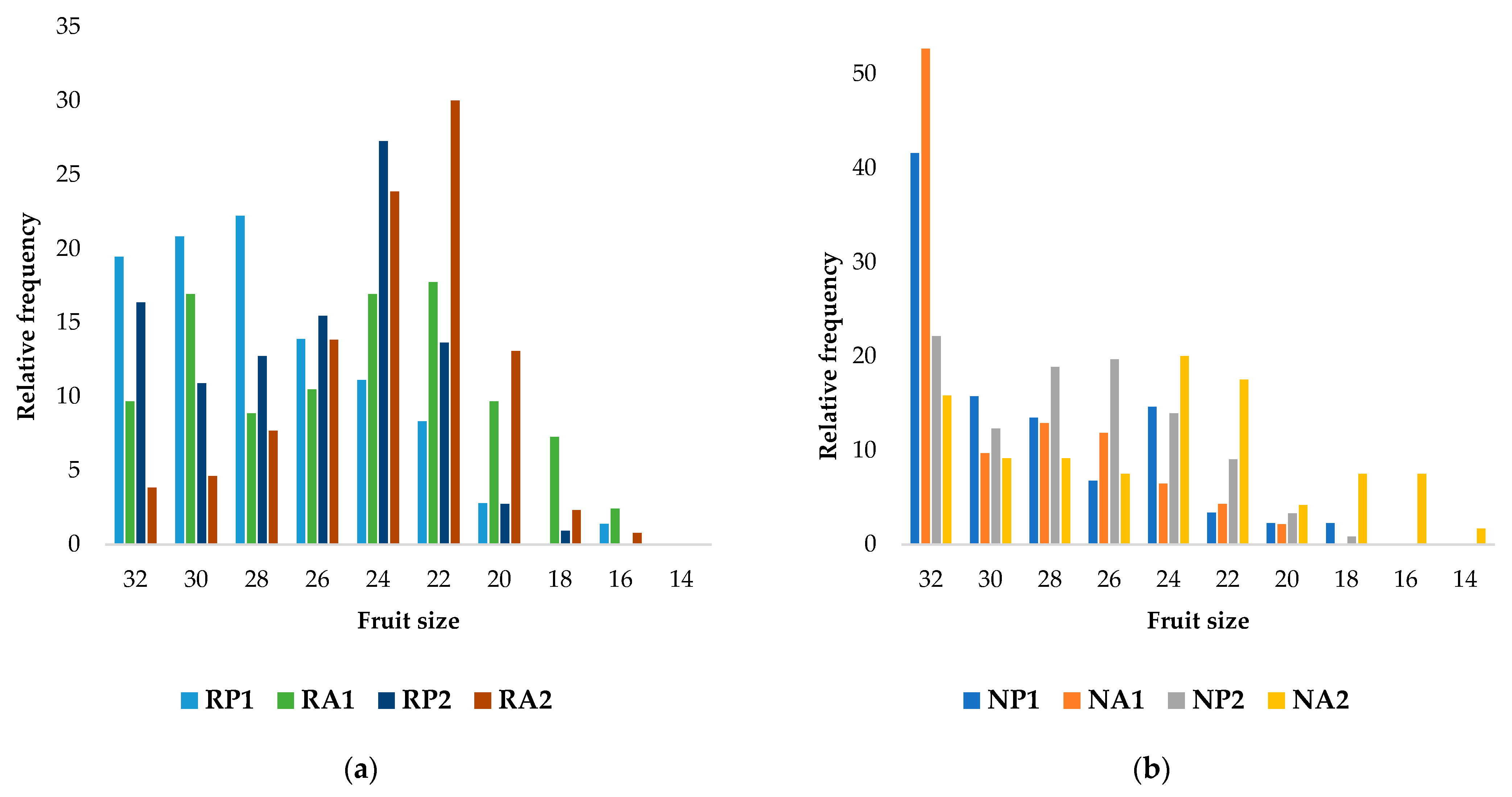


| Mineral | R Min–Max | N Min–Max | EB Min–Max |
|---|---|---|---|
| Depth (cm) | 0.45 | 0.45 | 0.45 |
| pH | 5.42 ± 0.10 | 5.50 ± 0.06 | 5.12 ± 0.05 |
| EC (ds/m) | 0.33 ± 0.10 | 0.35 ± 0.06 | 1.05 ± 0.11 |
| OM (%) | 12.36 ± 0.47 | 14.85 ± 0.88 | 12.53 ± 0.69 |
| P * | 4.69 ± 0.72 | 5.45 ± 0.70 | 37.79 ± 5.08 |
| S * | 67.03 ± 7.4 | 75.13 ± 7.9 | 30.52 ± 1.83 |
| Mg ** | 0.97 ± 0.15 | 0.99 ± 0.04 | 1.64 ± 0.27 |
| Ca ** | 2.87± 0.40 | 3.90 ± 0.5 | 4.04 ± 0.61 |
| K ** | 0.62 ± 0.09 | 0.44 ± 0.12 | 0.62 ± 0.05 |
| Na ** | 0.09 ± 0.003 | 0.08 ± 0.008 | 0.10 ± 0.001 |
| CEC ** | 4.64 ± 1.00 | 5.29 ± 0.6 | 6.98 ± 0.82 |
| B * | 0.22 ± 0.04 | 0.26 ± 0.03 | 0.33 ± 0.06 |
| Mn * | 2.39 ± 0.30 | 2.19 ± 0.20 | 13.05 ± 0.88 |
| Cu * | 2.49 ± 0.90 | 0.69 ± 0.20 | 5.65 ± 0.41 |
| Fe * | 101.95 ± 2.72 | 80.20 ± 5.60 | 309.32 ± 22.23 |
| Zn * | 1.54 ± 0.20 | 3.76 ± 1.20 | 8.56 ± 1.35 |
| Soil texture | SL | SL | SL |
| Fatty Acids | Orchards | ||
|---|---|---|---|
| R | N | EB | |
| Saturated | 27.531 ± 0.547 a | 24.465 ± 0.489 b | 19.017 ± 1.262 c |
| Monounsaturated | 57.093 ± 1.207 b | 57.062 ± 1.079 b | 71.453 ± 2.787 a |
| Polyunsaturated | 15.377 ± 0.482 a | 13.901 ± 0.431 a | 9.693 ± 1.114 b |
| Unsaturated | 72.469 ± 1.443 b | 70.963 ± 1.291 b | 81.148 ± 3.333 a |
| Sat/Un | 0.381 ± 0.008 a | 0.349 ± 0.007 a | 0.235 ± 0.018 b |
| C14:0 | 0.199 ± 0.046 a | 0.204 ± 0.041 a | 0.131 ± 0.107 a |
| C16:0 | 26.127 ± 0.327 a | 24.174 ± 0.293 b | 17.340 ± 0.756 c |
| C16:1 | 12.012 ± 0.243 a | 10.103 ± 0.217 b | 7.043 ± 0.561 c |
| C18:0 | 0.599 ± 0.118 a | 0.866 ± 0.106 a | 0.789 ± 0.274 a |
| C18:1 | 44.883 ± 0.548 c | 49.498 ± 0.490 b | 64.164 ± 1.265 a |
| C18:2 | 14.371 ± 0.337 a | 13.436 ± 0.302 a | 9.177 ± 0.779 b |
| C18:3 | 1.043 ± 0.035 a | 1.008 ± 0.031 a | 0.708 ± 0.080 b |
| C20:1 | 0.139 ± 0.015 a | 0.168 ± 0.013 a | 0.158 ± 0.034 a |
| O:L | 3.225 ± 0.117 c | 3.748 ± 0.105 b | 7.096 ± 0.271 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez, P.; Soto, I.; Villamizar, J.; Rebolledo, A. Fatty Acids and Minerals as Markers Useful to Classify Hass Avocado Quality: Ripening Patterns, Internal Disorders, and Sensory Quality. Horticulturae 2023, 9, 460. https://doi.org/10.3390/horticulturae9040460
Rodríguez P, Soto I, Villamizar J, Rebolledo A. Fatty Acids and Minerals as Markers Useful to Classify Hass Avocado Quality: Ripening Patterns, Internal Disorders, and Sensory Quality. Horticulturae. 2023; 9(4):460. https://doi.org/10.3390/horticulturae9040460
Chicago/Turabian StyleRodríguez, Pablo, Iris Soto, Jairo Villamizar, and Alexander Rebolledo. 2023. "Fatty Acids and Minerals as Markers Useful to Classify Hass Avocado Quality: Ripening Patterns, Internal Disorders, and Sensory Quality" Horticulturae 9, no. 4: 460. https://doi.org/10.3390/horticulturae9040460
APA StyleRodríguez, P., Soto, I., Villamizar, J., & Rebolledo, A. (2023). Fatty Acids and Minerals as Markers Useful to Classify Hass Avocado Quality: Ripening Patterns, Internal Disorders, and Sensory Quality. Horticulturae, 9(4), 460. https://doi.org/10.3390/horticulturae9040460








