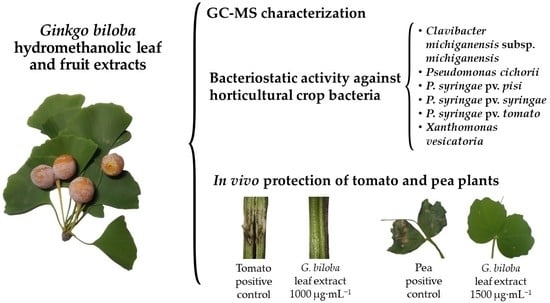
Antibacterial Activity of Ginkgo biloba Extracts against Clavibacter michiganensis subsp. michiganensis, Pseudomonas spp., and Xanthomonas vesicatoria
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Reagents
2.2. Bacterial Strains
2.3. Extract Preparation
2.4. Extract Characterization
2.5. In Vitro Antibacterial Activity Assessment
2.6. In Vivo Experiments on Tomato and Pea Plants under Greenhouse Conditions
3. Results
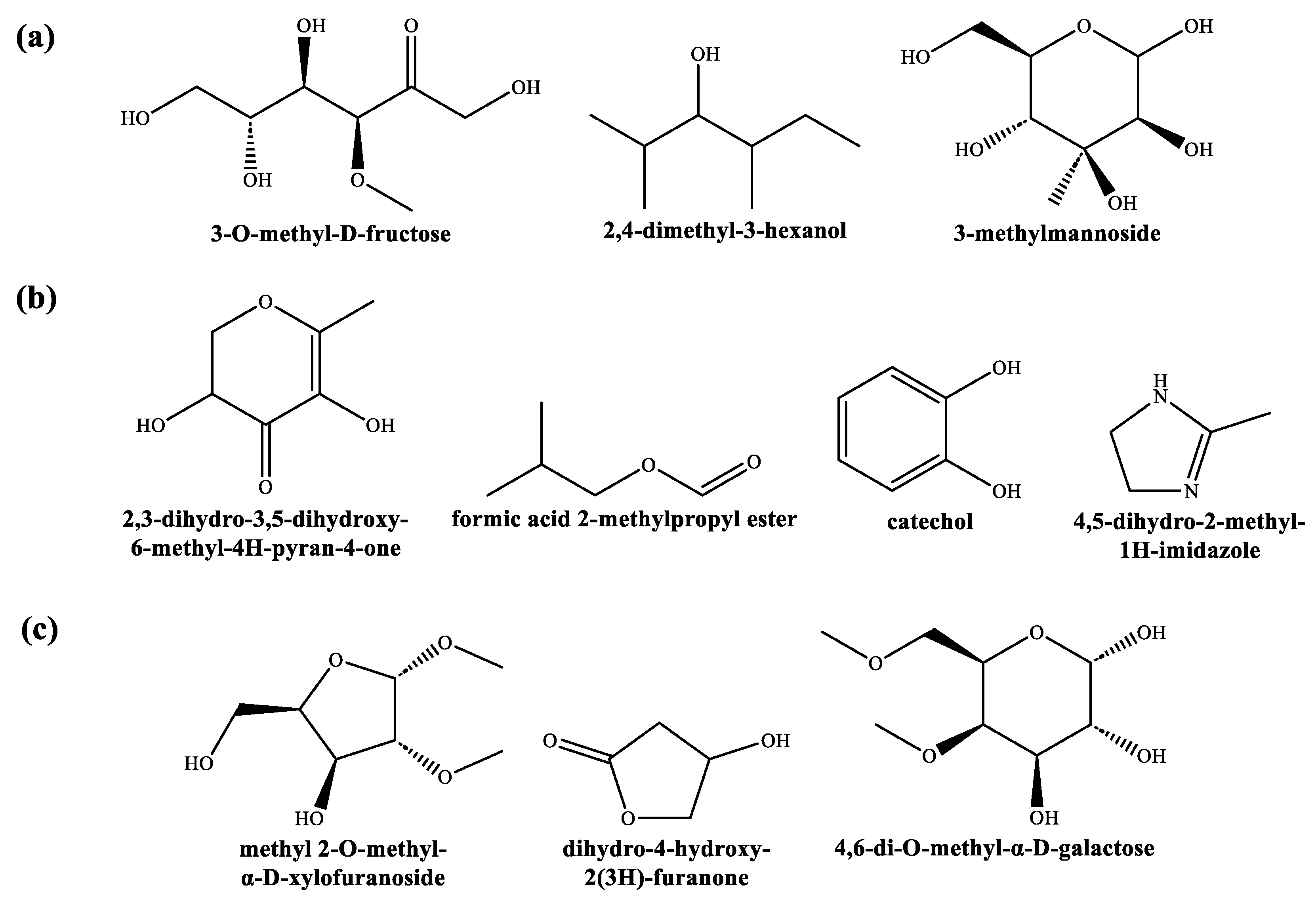
3.1. Extract Phytoconstituents Elucidation by GC−MS
3.2. Leaves Vibrational Spectra
3.3. Antibacterial Activity of the Extracts
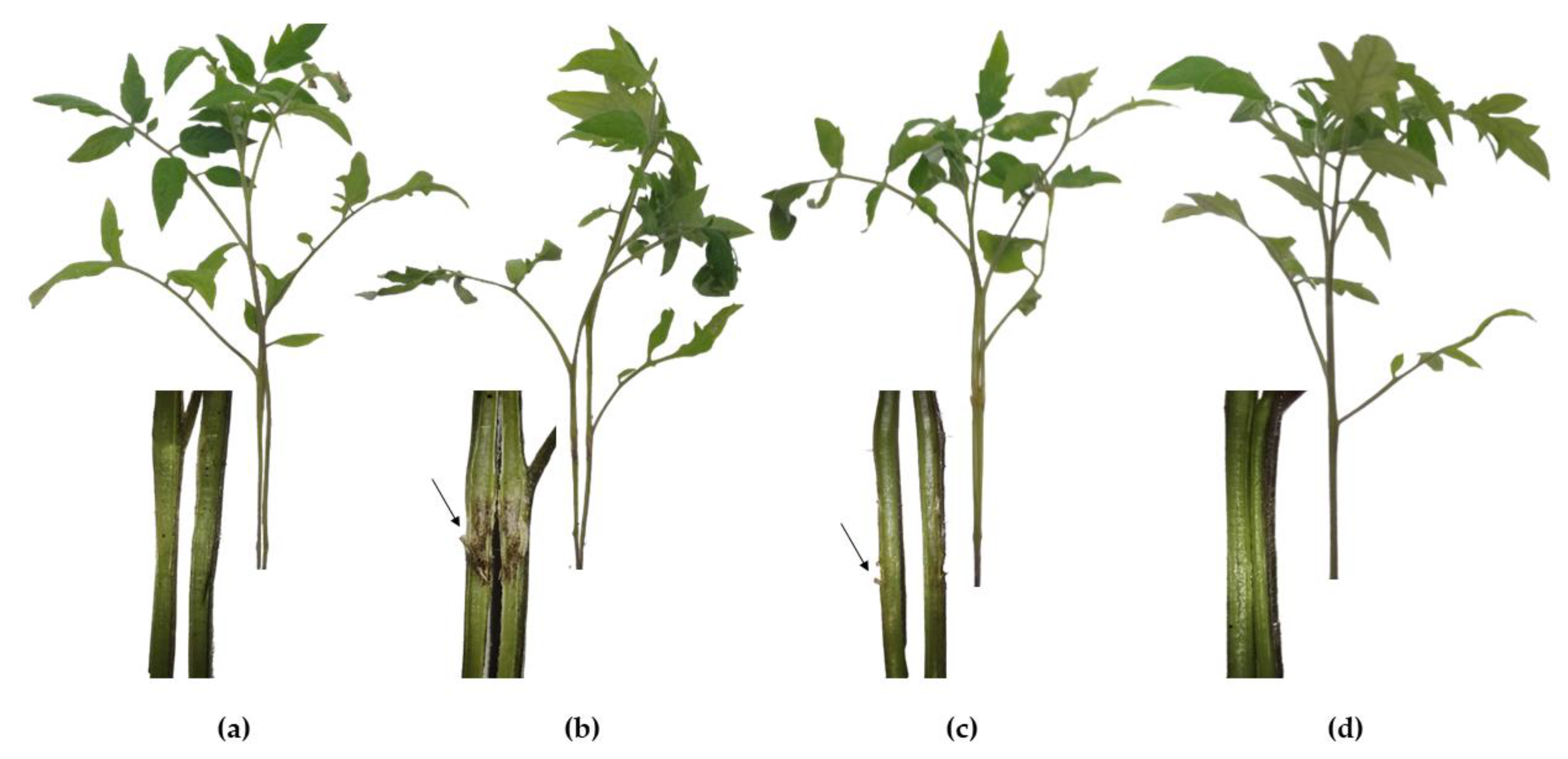
3.4. Tomato Plant Protection against C. michiganensis subsp. michiganensis
3.5. Pea Plant Protection against P. syringae pv. pisi
4. Discussion
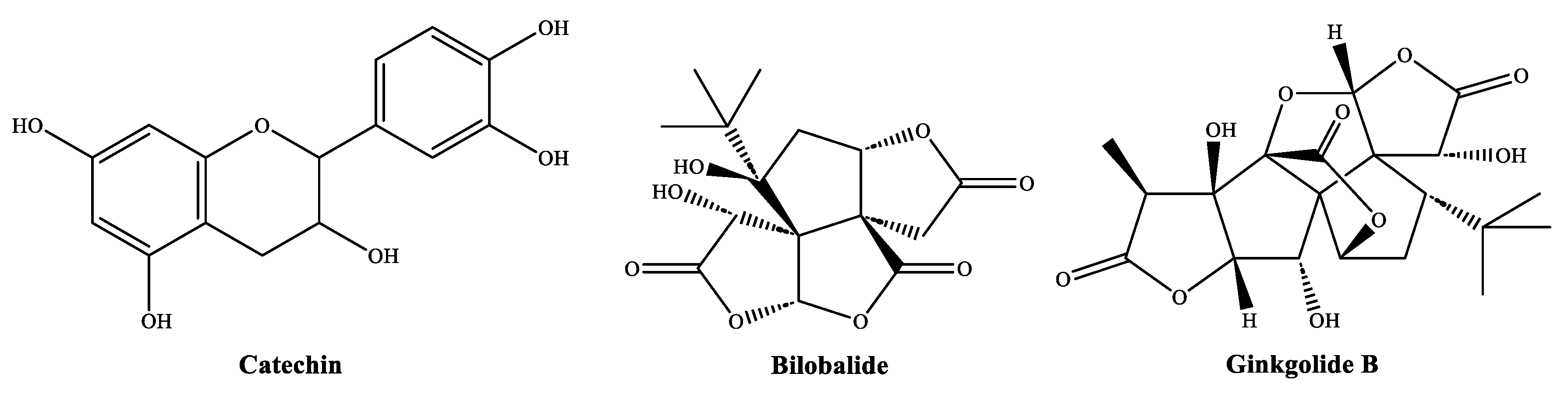
4.1. On the Identified Phytochemicals and Their Antimicrobial Activity
4.2. On the Antimicrobial Activity of G. biloba Extracts
4.3. Comparison with Conventional Antibiotics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, B.; Kaur, P.; Gopichand; Singh, R.D.; Ahuja, P.S. Biology and chemistry of Ginkgo biloba. Fitoterapia 2008, 79, 401–418. [Google Scholar] [CrossRef]
- Luo, Y.; Smith, J.V. Studies on molecular mechanisms of Ginkgo biloba extract. Appl. Microbiol. Biotechnol. 2004, 64, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.-C.; Xia, Q.; Fu, P.P. Ginkgo biloba leave extract: Biological, medicinal, and toxicological effects. J. Environ. Sci. Health C 2007, 25, 211–244. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, S.; Park, Y.; Park, Y. Modulation of cholesterol metabolism by Ginkgo biloba L. nuts and their extract. Food Res. Int. 2008, 41, 89–95. [Google Scholar] [CrossRef]
- Gülz, P.G.; Müller, E.; Schmitz, K.; Marner, F.J.; Güth, S. Chemical composition and surface structures of epicuticular leaf waxes of Ginkgo biloba, Magnolia grandiflora and Liriodendron tulipifera. Z. Nat. C 1992, 47, 516–526. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Chen, P.; Ozcan, M.; Harnly, J.M. Chromatographic profiles and identification of new phenolic components of Ginkgo biloba leaves and selected products. J. Agric. Food. Chem. 2008, 56, 6671–6679. [Google Scholar] [CrossRef]
- Drieu, K. Preparation and definition of Ginkgo biloba extract. In Rökan: Ginkgo biloba Recent Results in Pharmacology and Clinic; Fünfgeld, E.W., Ed.; Springer: Berlin/Heidelberg, Germany, 1988; pp. 32–36. [Google Scholar] [CrossRef]
- DeFeudis, F.V. Ginkgo biloba Extract (EGb 761): From Chemistry to the Clinic; Ullstein Medical: Wiesbaden, Germany, 1998; Volume 25, p. 401. [Google Scholar]
- Maitra, I.; Marcocci, L.; Droy-Lefaix, M.T.; Packer, L. Peroxyl radical scavenging activity of Ginkgo biloba extract EGb 761. Biochem. Pharmacol. 1995, 49, 1649–1655. [Google Scholar] [CrossRef]
- Krauze-Baranowska, M.; Wiwart, M. Antifungal activity of biflavones from Taxus baccata and Ginkgo biloba. Z. Nat. C 2003, 58, 65–69. [Google Scholar] [CrossRef]
- Oh, T.-S.; Koo, H.-M.; Yoon, H.-R.; Jeong, N.-S.; Kim, Y.-J.; Kim, C.-H. Antifungal action of Ginkgo biloba outer seedcoat on rice sheath blight. Plant Pathol. J. 2015, 31, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Ražná, K.; Sawinska, Z.; Ivanišová, E.; Vukovic, N.; Terentjeva, M.; Stričík, M.; Kowalczewski, P.Ł.; Hlavačková, L.; Rovná, K.; Žiarovská, J.; et al. Properties of Ginkgo biloba L.: Antioxidant characterization, antimicrobial activities, and genomic MicroRNA based marker fingerprints. Int. J. Mol. Sci. 2020, 21, 3087. [Google Scholar] [CrossRef]
- Martins, P.M.M.; Merfa, M.V.; Takita, M.A.; De Souza, A.A. Persistence in phytopathogenic bacteria: Do we know enough? Front. Microbiol. 2018, 9, 1099. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.-F.; Kvitko, B.; He, S.Y. Pseudomonas syringae: What it takes to be a pathogen. Nat. Rev. Microbiol. 2018, 16, 316–328. [Google Scholar] [CrossRef]
- Hikichi, Y.; Wali, U.M.; Ohnishi, K.; Kiba, A. Mechanism of disease development caused by a multihost plant bacterium, Pseudomonas cichorii, and its virulence diversity. J. Gen. Plant Pathol. 2013, 79, 379–389. [Google Scholar] [CrossRef]
- Nakayinga, R.; Makumi, A.; Tumuhaise, V.; Tinzaara, W. Xanthomonas bacteriophages: A review of their biology and biocontrol applications in agriculture. BMC Microbiol. 2021, 21, 291. [Google Scholar] [CrossRef] [PubMed]
- Peritore-Galve, F.C.; Tancos, M.A.; Smart, C.D. Bacterial canker of tomato: Revisiting a global and economically damaging seedborne pathogen. Plant Dis. 2021, 105, 1581–1595. [Google Scholar] [CrossRef]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.A.X.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol 2012, 13, 614–629. [Google Scholar] [CrossRef]
- Boateng, I.D.; Yang, X.M. Effect of different drying methods on product quality, bioactive and toxic components of Ginkgo biloba L. seed. J. Sci. Food Agric. 2021, 101, 3290–3297. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Hernández, E.; Martín-Ramos, P.; Martín-Gil, J.; Santiago-Aliste, A.; Hernández-Navarro, S.; Oliveira, R.; González-García, V. Bark extract of Uncaria tomentosa L. for the control of strawberry phytopathogens. Horticulturae 2022, 8, 672. [Google Scholar] [CrossRef]
- CLSI M07; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically (11th ed.). Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018.
- de León, L.; Siverio, F.; López, M.M.; Rodríguez, A. Comparative efficiency of chemical compounds for in vitro and in vivo activity against Clavibacter michiganensis subsp. michiganensis, the causal agent of tomato bacterial canker. Crop Prot. 2008, 27, 1277–1283. [Google Scholar] [CrossRef]
- Martin Sanz, A. Bacteriosis en Guisante (Pisum sativum L.): Situación en Castilla y León, Caracterización de los Patógenos Implicados y Búsqueda de Fuentes de Resistencia. Ph.D. Thesis, Universidad de León, León, Spain, 2008. [Google Scholar]
- Maréchal, Y.; Chanzy, H. The hydrogen bond network in I β cellulose as observed by infrared spectrometry. J. Mol. Struct. 2000, 523, 183–196. [Google Scholar] [CrossRef]
- Langa-Lomba, N.; Buzón-Durán, L.; Martín-Ramos, P.; Casanova-Gascón, J.; Martín-Gil, J.; Sánchez-Hernández, E.; González-García, V. Assessment of conjugate complexes of chitosan and Urtica dioica or Equisetum arvense extracts for the control of grapevine trunk pathogens. Agronomy 2021, 11, 976. [Google Scholar] [CrossRef]
- Lan, K.; Li, X.-J.; Du, G.; Xu, L. Characterizations of the hydrolyzed products of ginkgolide A and ginkgolide B by liquid chromatography coupled with mass spectrometry. J. Pharm. Biomed. Anal. 2016, 118, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.-J.; Li, L.; Ma, W.-H.; Han, T.; Qin, L.-P. Chemical constituents and bioactivities of the liposoluble fraction from different medicinal parts of Crocus sativus. Pharm. Biol. 2011, 49, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Kalaivanan, C.; Sundhararajan, A.; Kokila, S.; Ahmed, J.S. GC-MS analysis of Pentatropis Microphylla. Int. J. Eng. Res. Technol. 2017, 5, 1–2. [Google Scholar]
- Mannaa, M.; Oh, J.Y.; Kim, K.D. Microbe-mediated control of Aspergillus flavusin stored rice grains with a focus on aflatoxin inhibition and biodegradation. Ann. Appl. Biol. 2017, 171, 376–392. [Google Scholar] [CrossRef]
- Mannaa, M.; Oh, J.Y.; Kim, K.D. Biocontrol activity of volatile-producing Bacillus megaterium and Pseudomonas protegens against Aspergillus flavus and aflatoxin production on stored rice grains. Mycobiology 2018, 45, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Hernández, E.; González-García, V.; Martín-Gil, J.; Lorenzo-Vidal, B.; Palacio-Bielsa, A.; Martín-Ramos, P. Phytochemical screening and antibacterial activity of Taxus baccata L. against Pectobacterium spp. and Dickeya chrysanthemi. Horticulturae 2023, 9, 201. [Google Scholar] [CrossRef]
- Teixeira, A.; Sánchez-Hernández, E.; Noversa, J.; Cunha, A.; Cortez, I.; Marques, G.; Martín-Ramos, P.; Oliveira, R. Antifungal activity of plant waste extracts against phytopathogenic fungi: Allium sativum peels extract as a promising product targeting the fungal plasma membrane and cell wall. Horticulturae 2023, 9, 136. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Balduque-Gil, J.; González-García, V.; Barriuso-Vargas, J.J.; Casanova-Gascón, J.; Martín-Gil, J.; Martín-Ramos, P. Phytochemical profiling of Sambucus nigra L. flower and leaf extracts and their antimicrobial potential against almond tree pathogens. Int. J. Mol. Sci. 2023, 24, 1154. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Balduque-Gil, J.; Barriuso-Vargas, J.J.; Casanova-Gascón, J.; González-García, V.; Cuchí-Oterino, J.A.; Lorenzo-Vidal, B.; Martín-Gil, J.; Martín-Ramos, P. Holm oak (Quercus ilex subsp. ballota (Desf.) Samp.) bark aqueous ammonia extract for the control of invasive forest pathogens. Int. J. Mol. Sci. 2022, 23, 11882. [Google Scholar] [CrossRef]
- Kocaçalışkan, I.; Talan, I.; Terzi, I. Antimicrobial activity of catechol and pyrogallol as allelochemicals. Z. Nat. C 2006, 61, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Rashid, T.S. Bioactive metabolites from tomato endophytic fungi with antibacterial activity against tomato bacterial spot disease. Rhizosphere 2021, 17, 100292. [Google Scholar] [CrossRef]
- Oda, T.; Oda, K.; Yamamoto, H.; Matsuyama, A.; Ishii, M.; Igarashi, Y.; Nishihara, H. Hydrogen-driven asymmetric reduction of hydroxyacetone to (R)-1,2-propanediol by Ralstonia eutropha transformant expressing alcohol dehydrogenase from Kluyveromyces lactis. Microb. Cell Fact. 2013, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.H.; Cokcetin, N.N.; Pappalardo, M.; Campbell, L.T.; Brooks, P.; Carter, D.A.; Blair, S.E.; Harry, E.J. The antibacterial activity of Australian Leptospermum honey correlates with methylglyoxal levels. PLoS ONE 2016, 11, e0167780. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Buzón-Durán, L.; Lorenzo-Vidal, B.; Martín-Gil, J.; Martín-Ramos, P. Physicochemical characterization and antimicrobial activity against Erwinia amylovora, Erwinia vitivora, and Diplodia seriata of a light purple Hibiscus syriacus L. cultivar. Plants 2021, 10, 1876. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Buzón-Durán, L.; Cuchí-Oterino, J.A.; Martín-Gil, J.; Lorenzo-Vidal, B.; Martín-Ramos, P. Dwarf pomegranate (Punica granatum L. var. nana): Source of 5-HMF and bioactive compounds with applications in the protection of woody crops. Plants 2022, 11, 550. [Google Scholar] [CrossRef] [PubMed]
- Mainasara, M.M.; Abu Bakar, M.F.; Barau, A.I. GC-MS analysis of phytochemical constituents from ethyl acetate and methanol extract of Artocarpus altilis (Parkinson) Fosberg from Endau Rompin, Johor, Malaysia. Path Sci. 2019, 5, 3001–3010. [Google Scholar] [CrossRef]
- Hosoda, S.; Kawazoe, Y.; Shiba, T.; Numazawa, S.; Manabe, A. Anti-obesity effect of ginkgo vinegar, a fermented product of ginkgo seed coat, in mice fed a high-fat diet and 3T3-L1 preadipocyte cells. Nutrients 2020, 12, 230. [Google Scholar] [CrossRef]
- Fraise, A.P.; Wilkinson, M.A.C.; Bradley, C.R.; Oppenheim, B.; Moiemen, N. The antibacterial activity and stability of acetic acid. J. Hosp. Infect. 2013, 84, 329–331. [Google Scholar] [CrossRef]
- Balamurugan, V.; Balakrishnan, V.; Sundaresan, A. GC-MS analysis of leaf and bark extract of Moringa concanensis Nimmo, a Siddha medicinal plant of South India. Eur. J. Biotechnol. Biosci. 2015, 3, 57–61. [Google Scholar]
- Johnston, J.C.; Welch, R.C.; Hunter, G.L.K. Volatile constituents of litchi (Litchi chinesis Sonn.). J. Agric. Food. Chem. 2002, 28, 859–861. [Google Scholar] [CrossRef]
- Habu, T.; Flath, R.A.; Mon, T.R.; Morton, J.F. Volatile components of Rooibos tea (Aspalathus linearis). J. Agric. Food. Chem. 1985, 33, 249–254. [Google Scholar] [CrossRef]
- Kaddes, A.; Fauconnier, M.-L.; Sassi, K.; Nasraoui, B.; Jijakli, M.H. Antifungal properties of two volatile organic compounds on barley pathogens and introduction to their mechanism of action. Int. J. Environ. Res. Public Health 2019, 16, 2866. [Google Scholar] [CrossRef] [PubMed]
- Meher, A.; Behera, B.; Nanda, B.K. GC–MS investigation of phytocomponents present in ethanolic extract of plant Ichnocarpus frutescens (L.) W. T. Aiton aerial part. Int. J. Pharm. Sci. Res. 2020, 11, 104–113. [Google Scholar]
- Ghosh, G.; Panda, P.; Rath, M.; Pal, A.; Sharma, T.; Das, D. GC-MS analysis of bioactive compounds in the methanol extract of Clerodendrum viscosum leaves. Pharmacogn. Res. 2015, 7, 110. [Google Scholar] [CrossRef]
- Bai, Y.; Tohda, C.; Zhu, S.; Hattori, M.; Komatsu, K. Active components from Siberian ginseng (Eleutherococcus senticosus) for protection of amyloid β(25–35)-induced neuritic atrophy in cultured rat cortical neurons. J. Nat. Med. 2011, 65, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Yusufzai, S.; Kaun, L.; Shah, M.; Idris, R. Chemical composition and antioxidant activity of essential oil of leaves and flowers of Alternanthera sessilis red from Sabah. J. Appl. Pharm. Sci. 2016, 6, 157–161. [Google Scholar] [CrossRef]
- Erlina, L.; Paramita, R.I.; Kusuma, W.A.; Fadilah, F.; Tedjo, A.; Pratomo, I.P.; Ramadhanti, N.S.; Nasution, A.K.; Surado, F.K.; Fitriawan, A.; et al. Virtual screening of Indonesian herbal compounds as COVID-19 supportive therapy: Machine learning and pharmacophore modeling approaches. BMC Complement. Med. Ther. 2022, 22, 207. [Google Scholar] [CrossRef]
- Brasch, J.; Christophers, E. Azelaic acid has antimycotic properties in vitro. Dermatology 1993, 186, 55–58. [Google Scholar] [CrossRef]
- Miller, S.A.; Ferreira, J.P.; LeJeune, J.T. Antimicrobial use and resistance in plant agriculture: A One Health perspective. Agriculture 2022, 12, 289. [Google Scholar] [CrossRef]
- Coleman, R.H.; Shaffer, J.; True, H. Properties of β-lactamase from Pseudomonas syringae. Curr. Microbiol. 1996, 32, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.S.H.; Morgan, R.L.; Sarkar, S.F.; Wang, P.W.; Guttman, D.S. Phylogenetic characterization of virulence and resistance phenotypes of Pseudomonas syringae. Appl. Environ. Microbiol. 2005, 71, 5182–5191. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.-F.; Chen, C.-Y.; Lee, Y.-S.; Lin, J.-W.; Tseng, Y.-H. Identification of a novel β-lactamase produced by Xanthomonas campestris, a phytopathogenic bacterium. Antimicrob. Agents Chemother. 1999, 43, 1792–1797. [Google Scholar] [CrossRef] [PubMed]


| Bacteria | Leaf Extract | Sarcotesta Extract | Sclerotesta Extract |
|---|---|---|---|
| Clavibacter michiganensis subsp. michiganensis | 500 | n.a. | n.a. |
| Pseudomonas cichorii | 750 | n.a. | n.a. |
| Pseudomonas syringae pv. pisi | 750 | n.a. | n.a. |
| Pseudomonas syringae pv. syringae | 750 | n.a. | n.a. |
| Pseudomonas syringae pv. tomato | 750 | n.a. | n.a. |
| Xanthomonas vesicatoria | 1000 | n.a. | n.a. |
| Bacteria | 2,4-Dimethyl-3-Hexanol | Catechol |
|---|---|---|
| Clavibacter michiganensis subsp. michiganensis | 375 | 187.5 |
| Pseudomonas cichorii | 750 | 500 |
| Pseudomonas syringae pv. pisi | 750 | 375 |
| Pseudomonas syringae pv. syringae | 750 | 500 |
| Pseudomonas syringae pv. tomato | 500 | 500 |
| Xanthomonas vesicatoria | 250 | 187.5 |
| Bacteria | PG | AM | GM | CI | TC |
|---|---|---|---|---|---|
| Clavibacter michiganensis subsp. michiganensis | 0.125 | 0.094 | 0.38 | 0.38 | 0.19 |
| Pseudomonas cichorii | ≥32 | ≥256 | 3 | 6 | 24 |
| Pseudomonas syringae pv. pisi | ≥32 | 48 | 1 | 0.25 | 1 |
| Pseudomonas syringae pv. syringae | ≥32 | 48 | 0.25 | 0.047 | 1 |
| Pseudomonas syringae pv. tomato | ≥32 | 16 | 0.125 | 0.047 | 0.75 |
| Xanthomonas vesicatoria | ≥32 | ≥256 | 0.75 | 0.125 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Hernández, E.; González-García, V.; Palacio-Bielsa, A.; Lorenzo-Vidal, B.; Buzón-Durán, L.; Martín-Gil, J.; Martín-Ramos, P. Antibacterial Activity of Ginkgo biloba Extracts against Clavibacter michiganensis subsp. michiganensis, Pseudomonas spp., and Xanthomonas vesicatoria. Horticulturae 2023, 9, 461. https://doi.org/10.3390/horticulturae9040461
Sánchez-Hernández E, González-García V, Palacio-Bielsa A, Lorenzo-Vidal B, Buzón-Durán L, Martín-Gil J, Martín-Ramos P. Antibacterial Activity of Ginkgo biloba Extracts against Clavibacter michiganensis subsp. michiganensis, Pseudomonas spp., and Xanthomonas vesicatoria. Horticulturae. 2023; 9(4):461. https://doi.org/10.3390/horticulturae9040461
Chicago/Turabian StyleSánchez-Hernández, Eva, Vicente González-García, Ana Palacio-Bielsa, Belén Lorenzo-Vidal, Laura Buzón-Durán, Jesús Martín-Gil, and Pablo Martín-Ramos. 2023. "Antibacterial Activity of Ginkgo biloba Extracts against Clavibacter michiganensis subsp. michiganensis, Pseudomonas spp., and Xanthomonas vesicatoria" Horticulturae 9, no. 4: 461. https://doi.org/10.3390/horticulturae9040461
APA StyleSánchez-Hernández, E., González-García, V., Palacio-Bielsa, A., Lorenzo-Vidal, B., Buzón-Durán, L., Martín-Gil, J., & Martín-Ramos, P. (2023). Antibacterial Activity of Ginkgo biloba Extracts against Clavibacter michiganensis subsp. michiganensis, Pseudomonas spp., and Xanthomonas vesicatoria. Horticulturae, 9(4), 461. https://doi.org/10.3390/horticulturae9040461