Abstract
Application of plant growth regulators (PGRs) in apricot orchards is a common practice with a goal of improving yield and/or quality of fruits at harvest. However, the question of whether such treatment alters postharvest properties is seldom answered. The effects of an early application of PGRs on postharvest changes on apricots were investigated on cultivar NS-4, grown on Myrobalan rootstock with blackthorn interstock in a 5-year-old orchard. PGR treatments included 50 and 100 ppm of benzyladenine (BA) and 200 ppm of gibberellic acid (GA3), which were applied when the green ovary was surrounded by dying a sepal crown, at the stage where sepals beginning to fall. Apricots at the stage of commercial ripeness were used for the postharvest experiments. Analysis was performed at harvest, after 21 days of cold storage (at 1 ± 1 °C and 80 ± 10% RH), and after 3 days of shelf life (24 ± 2 °C). At harvest, significant differences were observed between treated and untreated fruits regarding flesh firmness, color, ethylene production and respiration rate, flavonoid, carotenoid and citric acid content, while application of BA100 changed TA and TSS. Prolonged cold storage reduced the initial differences in firmness, respiration rate, flavonoid and carotenoid contents, but new differences in fructose, malic and succinic acid contents began to appear. Shelf life reduced the difference in citric acid, but differences in TA, TSS, phenol and flavonoid content appeared. There is no difference in the sensory properties of treated and non-treated fruit after cold storage and shelf life.
1. Introduction
In order to improve apricot production, many contemporary systems and techniques were applied in orchards that have resulted in the intensification of fruit production, such as the use of different rootstocks, interstocks, the increase of tree density, irrigation, pruning, thinning, fertilization, grassing, mulching, and weed control [1,2,3,4]. Despite numerous advances in apricot production, the main challenges remained unsolved: (1) frost in the early fruit development phases (flowering) leading to 50% variation in yield coefficient and, in some years, in total loss of apricot fruit [5]; and (2) the relatively short postharvest life of apricot fruit, limited by fruit softening, tissue breakdown and browning [6]. In attempts to improve the postharvest life of apricots, ethylene blockers [7], and different packing solutions (which include a modified and controlled atmosphere), application of calcium, salicylic acid [8], melatonin [9], putrescine [10], as well as use of edible coatings [11], and, recently, irradiation [12] have been frequently used.
Among frequently used methods aiming to improve apricot production is the application of plant growth regulators (PGRs). The process of fruit ripening is guided by the succession of different plant hormones, in terms of their concentration and time of appearance. Fruit development begins with high concentrations of auxins, brassinosteroids, jasmonates and cytokinins, followed by an increase in the concentration of gibberellic acid [13]. The ripening phase is characterized by a high concentration of abscisic acid, followed by small peaks of auxins and jasmonates, while the maximum production of ethylene is present during the transition of fruit from the ripening to senescence stage [13]. The idea behind the external application of PGRs is to alter the natural development of fruit with a goal to improve its production. That improvement caused by application of PGRs does not always imply only a higher yield, but in some cases also improves the quality of the fruit [14].
The application of PGRs regulates plant metabolism, which consequently affects flowering, fruit set and development, as well as fruit abscission, which ultimately reflects on fruit size, composition, and color [15,16,17,18,19]. Application of PGRs in early phases of fruit development is performed with different goals. Thus, gibberellic acid (GA) applied to fully bloomed plants affected fruit set and yield in apricot [20], while its applications 10 or 21 days before harvest affected thinning, fruit size, and firmness [21]. Cytokinin applied at the pit hardening stage had an effect on fruit physiological, organoleptic, and phytochemical properties [22]. In respect to benzyladenine (BA), this plant hormone belongs to the group of cytokinins, and is well-known for its role in plant growth and development, while the studies on its effect on fruit development were mostly focused to climacteric fruits, where it would slow down ripening, possibly through suppressing biosynthesis of ethylene [13]. It was previously proven that BA has an effect on fruit set, development, and shape. Namely, the combination of gibberellins A4 and A7 with the cytokinin BA improved fruit appearance in Red Delicious apples. This success resulted in its different uses on a wide variety of crops [15]. Application of GA3 at 80% of full bloom affected fruit set, while its application 15 days prior to harvest had an effect on apricot shelf life at ambient temperature [23]. Noteworthy, some compounds with a similar structure to PGRs could have similar impact as PGRs on fruit storage [24].
However, studies examining an impact of PGR application on fruit quality after cold storage and shelf life are very rare, and mostly based on basic analysis of fruit, such as weight loss and spoilage [25]. Even rarer are the studies examining the relationship of an early PGR treatment with storage and shelf life on fruit quality and chemical composition [26]. Having in mind the frequent use of PGRs in practice today, and a lack of knowledge in terms of their effects on treated fruits, the results of the present study provide a glimpse and some interesting answers to the problems associated with PGR application and postharvest behavior of treated fruits. Therefore, this paper is expected to have a significant impact on today’s practice in apricot production.
In our previous study, the effects of PGRs applied in the early stages of fruit development were investigated in apricot fruit after 15 days of cold storage followed by several days of shelf life [27]. The treatment had an impact on flesh firmness at harvest, but recorded differences were diminished after shelf life. No difference in titratable acidity (TA) and pH were observed. This study aimed to examine the impact of gibberellic acid (GA3) and 6-benzyladenine (BA) on the physical characteristics and composition of apricot fruit after prolonged cold storage (i.e., 21 days) and shelf life.
2. Materials and Methods
2.1. Apricot Production and Preharvest Reatments
Apricot fruits (Prunus armeniaca L.) cv. NS-4 were obtained from trees grown at the Experimental field for fruit growing, Faculty of Agriculture, Novi Sad, located at Rimski Šančevi (45°3382′ N and 19°8445′ E, 86 m a.s.l.), Republic of Serbia. Cultivar NS-4 used Myrobalan seedlings (P. cerasifera Ehrh.) as a rootstock with blackthorn (Prunus spinosa L.) as an interstock. The orchard was established under black anti-hail nets and with drip irrigation. The lanes were covered with grass, while the space under the trees within each row was treated with herbicides. The standard agro-technical procedures were performed annually. The experiment was set up by using 5-year-old apricot trees grown at 4 × 2 m planting distance (1250 trees ha−1). The one year trial was set up in a completely randomized design with six single trees used per treatment. Plant hormone treatments included 6-benzyladenine in two concentrations (50 mg L−1—BA50 and 100 mg L−1—BA100, Gerba 4LG—4% active ingredient 6-benzyladenine; L-Gobbi, Campo ligure, Italy), 200 mg L−1 gibberellin (GA3, Gibberellin −1.8% active ingredient gibberellin; L-Gobbi, Campo ligure, Italy) and corresponding, non-treated control. PGRs were applied once by spraying to whole trees when the green ovaries were surrounded by dying sepal crown, just before the sepals begin to fall (stone fruit, principal grown stage 7: development of fruit, code 72, according to Meier [28]). Treatments were applied with a backpack sprayer (Stihl SR-420) and 4 L was used per treatment on six whole trees per treatment. In order to determine the effects on apricot fruits at harvest and after the prolonged storage period, fruit in commercial ripeness (IAD 0.4–0.8), determined by DA-meter (TR Turoni, Bologna, Italy) were harvested [29].
For postharvest analysis, fruits were distributed in wooden crates 50 × 30 × 8 cm holding approximately 5 kg of apricots and placed in the cooling chamber at 1 ± 1 °C and 80 ± 10% RH for 21 days. Subsequently, the fruits were removed from cold storage and exposed for 3 days to shelf life at room temperature (24 ± 2 °C). In order to perform chemical analysis at each sampling period, quarters of 20 fruits was homogenized and immediately frozen in dry ice.
2.2. Fruit Color, Texture and Weight Loss
Fruit color was determined by performing two measurements on the opposite sides of the equatorial region, using CR-400 Chroma Meter (Konica-Minolta, Osaka, Japan). Flesh firmness was measured at the equatorial region of each fruit after peeling off a small circle of skin. A penetration test was performed with an 8 mm diameter stainless steel rounded cylinder probe with TA.XT Plus Texture Analyser (Stable Micro Systems, England, UK). Both analyses were performed on 20 whole apricots.
Weight loss was determined by daily measurement of fruit weight after harvest and after 21 days of cold storage at room temperature (24 ± 2 °C).
2.3. Ethylene Production and Respiration Rate
For determination of ethylene and respiration rate, approximately 250–300 g of fruits were placed in a 770 mL container, hermetically sealed with multilayer foil at 24 ± 2 °C. CO2 measurement was performed by direct puncture of the sealed foil with a sampling needle of OXYBABY® 6.0 (WIT-Gasetechnik GmbH & Co KG T, Witten, Germany). Ethylene was analyzed from 2 mL of gas sampled by a plastic syringe and injected into a 10 mL headspace vial sealed with silicone septa. Ethylene was determined by gas chromatography (GC 7890, Agilent, Santa Clara, USA), equipped with a FID detector (Agilent, Santa Clara, USA) and auto sampler (COMBIPAL, CTC Analytics AG, Zwingen, Switzerland).
2.4. Chemical Analysis
Total soluble solids (TSS; %) were determined by a digital refractometer ATR-ST plus (Schmidt and Haensch, Berlin, Germany) on previously homogenized apricot samples at 20 °C. Titratable acidity (TA; g malic acid/100 g) was measured from 3 g of sample dissolved in 30 mL of deionized water. After homogenization, the sample was centrifuged (Centrifuge 5804R, Ependorf, Hamburg, Germany) at 13,776 g for 5 min, and 10 mL of supernatant was used for titration with 0.1 M NaOH.
Carotenoid content was analyzed according to Costache et al. [30], while phenol and flavonoid contents were extracted according to [31]. Phenol content was determined according to the Folin–Ciocalteu method [32], while the Pękal and Pyrzynska procedure [33] was used for flavonoids. A brief explanation of the methods used is described earlier in [6,27,29].
For determination of fructose, glucose, sucrose, citric, malic, and succinic acid content, the sample was extracted according to [34]. Separation was performed using HPLC Agilent 1200 series, (Agilent, Santa Clara, CA, USA). For sugar analysis, Zorbax Carbohydrate was used, (4.6 × 250 mm, 5 μm column; Agilent Technologies, Vienna, Austria), along with an evaporative light scattering detector (ELSD), while organic acids were analyzed on a NUCLEOGEL SUGAR 810 H (Macherey-Nagel, Dueren, Germany) column with diode array detector (DAD).
2.5. Sensory Evaluation
Sensory evaluation of apricot fruits after cold storage and shelf life was conducted by 12 trained panelists (6 women and 6 men), aged 20–65 years, according to the methodology adopted by Melgarejo et al. [35]. The panelists were asked to score visual appearance (tissue breakdown and browning) on halved fruits, and intensity of 5 fruit attributes (sweetness, acidity, apricot flavor, crispiness, and off flavor) on fruit slices. Evaluation was performed on a continuous scale ranging from 0 (lowest score) to 100 (highest score). The process was carried out at room temperature (20 °C) in individual cabins under white lighting. The evaluation was performed in two separated sessions within the same day. All participants received written information about the study, and they signed informed consent to participate.
2.6. Statistical Analysis
Obtained results were analyzed using two-way ANOVA. Duncan’s multiple range test was used for testing the significance of differences between average values, while for weight loss, respiration, and ethylene, Tukey’s HSD test was used. Principal component analysis (PCA) was performed on data collected at the same time and frequency. Statistical calculations were performed using TIBCO Data Science—Workbench (Statistica® 14.0.0).
3. Results
3.1. Weight Loss, Respiration Rate and Ethylene Production
Physiological parameters (weight loss, respiration rate, and ethylene production) were differently affected by the treatments to which were apricots subjected (Figure 1). Significant impact on weight loss at the end of shelf life was observed only between the control and BA100-treated fruits (Figure 1A,B).
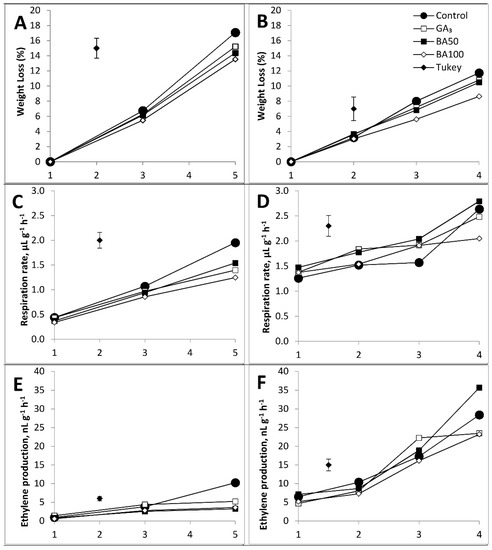
Figure 1.
Weight loss, respiration rate, and ethylene production in PGR-treated apricots during shelf life. Fruit weight loss (A,B), respiration rate (C,D), and ethylene production (E,F) after harvest and cold storage, respectively. PGR treatment: control—untreated apricots; GA3—200 mg L−1 of gibberellin A3; BA50—50 mg L−1 of 6-benzyladenine; BA100—100 mg L−1 of 6-benzyladenine. Differences higher than Tukey mark are significantly different (p < 0.05).
Respiration rate was significantly decreased at the end of shelf life after the harvest in all treated apricots versus the control (Figure 1C), while after the cold storage, this effect was present only in BA100-treated fruits (Figure 1D).
After the harvest, ethylene production increased on the 5th day of shelf life, but only in the control when compared to the rest of the PGR-treated groups (Figure 1E). After cold storage, its production increased after the 2nd day of shelf life in all groups, but it became especially prominent on the 4th day in the control and BA50-treated fruits (Figure 1F).
3.2. Physicochemical Properties
The PGR treatments showed different effects on physical properties and chemical composition on apricots at harvest, and after cold storage and shelf life (Table 1). In general, PGR treatments alone affected hue°, TA, and the contents of phenols, flavonoids, fructose, sucrose, and all organic acids in treated apricots. As expected, cold storage changed all analyzed parameters in all fruits, except the glucose content. Interaction between the PGRs and cold storage significantly affected TA, phenols, flavonoids, carotenoids, fructose, sucrose, malic, citric, and succinic acids, thereby suggesting that detected changes during cold storage are PGR-dependent.

Table 1.
Effects of application of GA and BA on color, texture, quality and chemical composition of apricot fruit.
3.2.1. Physical Properties
At harvest all PGR-treated fruits changed color towards red (hue°). The application of PGR did not affect the lightness of skin color, but since the hue° values were lower, the treated fruits appeared as more red, compared to the control (Table 1). Nevertheless, initial color differences between control and treated fruits faded away after cold storage and shelf life.
Flesh firmness of apricots after harvest was higher in GA3- and BA100-treated fruits versus the control. After cold storage, fruit firmness was the highest in BA100-treated apricots and was only significant if compared to the second BA treatment. After shelf life, all differences between the treatments were diminished (Table 1).
3.2.2. Chemical Properties
After harvest, only BA100-treated apricots had higher TA and lower TSS than control apricots. Cold storage decreased TA only in BA50-treated apricots, but after shelf life, it increased again in all fruits, except in BA100-treated fruits (Table 1). In respect to TSS, cold storage increased this parameter only in GA3- and BA50-treated fruits, but after shelf life, all treated apricots has significantly higher values of TSS compared to the control. Applied PGRs also changed the chemical composition by increasing the contents of phenol, flavonoid, carotenoid, fructose, and citric acid.
At harvest, the highest phenol content was present in apricots treated with GA3 and the lowest in the control (Table 1). After cold storage, no significant differences were observed among the experimental groups, but after shelf life, phenol content significantly increased in GA3- and BA100-treated fruits versus the control. Similarly to phenols at harvest, flavonoids were the highest in GA3-, followed by BA100-treated apricots, while the lowest content was recorded in the control (Table 1). Unlike the phenol content, which increased after cold storage and shelf life, flavonoid content had a decreasing trend. As a result, the lowest loss in these bioactive compounds was detected in GA3- and BA100-treated apricots comparing to the rest of the groups. In respect to carotenoid content, all treated fruits had higher values than control fruit (Table 1). However, all recorded differences were eliminated after cold storage, and the slight increase in its content after shelf life did not make any difference among the treatments (Table 1).
In respect to sugar contents, only glucose did not change throughout the experiment (Table 1). Slightly higher fructose content was present at harvest in all treated fruits versus the control. During the cold storage, fructose content increased in all fruits, except in GA3-treated fruits, but after shelf life, the highest increase in its content was detected in GA3- and BA100-treated apricots. Sucrose level at harvest was similar for all fruits; it decreased during cold storage and after shelf life, with the highest content present in BA100-treated apricots versus the rest of the fruits.
In general, each of the examined organic acids did not show any decline toward the end of the experiment (Table 1). At harvest, contents of malic acid was similar among all fruits. After cold storage, GA3- and BA100-treated apricots were characterized with a higher content of malic acid then the other two groups, while after shelf life, all treated apricots exhibited a significantly higher content of malic acid versus the control. Content of citric acid was higher in treated apricots at harvest as well as after cold storage versus the control, with BA100 group standing out due to its highest content of this acid. Shelf life increased the level of citric acid only in the control fruits. Succinic acid content was similar in all fruits at harvest. During cold storage, its content increased for all treated fruits. After shelf life, succinic acid increased in all apricots, with the highest content being recorded in BA100-, while the lowest in GA3-treated apricots
3.3. Sensory Properties
According to the results of sensory analysis, cold storage affected all examined traits, except inappropriate taste and browning, while PGR treatments only had an impact on tissue breakdown (Table 2).

Table 2.
Effects of application of GA and BA on sensory properties of apricot fruit.
After cold storage, the only difference among the examined parameters was detected regarding aroma between BA50- and BA100-treated apricots. After shelf life, significant changes in sweetness, sourness, and aroma were recorded in BA100-treated fruits as well as in aroma in GA3-treated apricots, when compared to initial estimation. After cold storage, both BA concentrations decreased tissue break down, but the only significant difference was noted when apricots from the GA3 and BA100 groups were compared.
Following shelf life, only fruits treated with BA100 had significantly lower tissue breakdown when compared to the rest of the apricots. Still, tissue break down after shelf life was quite high for all treatments.
3.4. PCA Analysis
Based on the PCA analysis (Figure 2), the first two factors explained more than 70% of variability in the case of color, texture, and all indicators of metabolic activities: TA, TSS, phenols, flavonoids, carotenoids, sugars, and organic acids sampled at three sampling points (Figure 2A). In the case of sensory descriptors assessed after cold storage and shelf life, the first two factors explain more than 90% (Figure 2B). It is noticeable that most of the variation is explained by Factor 1 (55.66% and 75.98%, respectively). Given the nature of the sample separation in both cases, it could be tentatively assumed that Factor 1 represents the sampling time, while Factor 2 may represent PGR treatments.
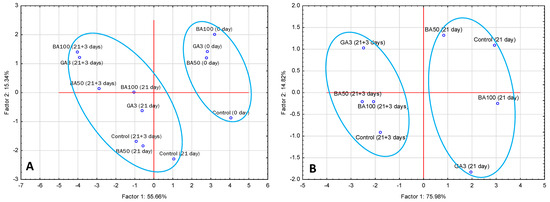
Figure 2.
Principal component analysis (PCA) of apricot fruit separation at three sampling points based on color, texture, quality, and chemical composition (A) and two sampling points based on sensory characteristics (B). Treatments: GA3—200 mg L−1 of gibberellin A3; BA50—50 mg L−1 of 6-benzyladenine; BA100—100 mg L−1 of 6-benzyladenine; storage: 0—at harvest; 21 day of cold storage; 21 + 3—21 day of cold storage followed by 3 days of shelf life. Circled treatments represent the samples belonging to the following groups: before and after cold storage (A) and after cold storage and after shelf life (B), respectively.
4. Discussion
Apricot perishability is one of the main causes limiting its storability and, consequently, market potential. This factor becomes even more important when apricots are exposed to ambient temperatures [36], and it is one of the key reasons why some studies have no records of apricot shelf life [37]. Despite this, there are reports on the decrease in physiological loss in apricot cv. Harcot, treated with GA3 following eight days of exposure to ambient temperature [23]. Our results also indicate a change in weight loss, but a decrease in weight loss in cv. NS-4 was caused by BA100, rather than GA3, compared to control (Figure 1A,B).
Application of PGRs often changes fruit texture and color at harvest. In the present study, PGRs changed fruit color to redder tones, and increased fruit firmness (Table 1). Similar changes in apricot color were reported after the treatment with 25, 50, 75, and 100 ppm of GA3 [38]. Obtained results of the increase in flesh firmness are similar to the previous reports, in which apricot fruit firmness increased at harvest as a result of application of 10 and 15 ppm GA3 [20], and also after application of 50 and 100 ppm BA and 50 and 100 ppm GA3 [39]. In apricots, beside different concentrations of PGRs, the time of the application also affects flesh firmness, which is shown in the case of GA [40].
The changes in color and texture at harvest are accompanied with the changes in chemical composition of apricot fruits; i.e., TA, TSS, phenols, flavonoids, carotenoids fructose, sucrose, malic, citric, and succinic acid contents (Table 1). Although recorded changes are not uncommon for apricot fruit, their trends are not always similar. Contrary to our results, treatment with BA did not change TA [41], nor did the treatment with GA3 [23]. On the other hand, the treatments with GA3 and BA reduced TA [39]. Furthermore, treatment with different concentrations of BA (50, 100 and 150 ppm) did not change TSS [41], nor did the treatment with 50 and 100 ppm of GA in two seasons of Patterson apricot [40]. Still, the treatment with 75 and 100 ppm of GA increased TSS in both applied timings [38]. Regarding the increase in total phenol content, similar findings were reported after application of 10 and 15 ppm GA3 [20], while other cytokinins (forchlorfenuron) had no effect in two apricot cultivars [22]. Glucose level was not changed by PGRs, which is consistent with reports where lower concentration of synthetic cytokinin did not change glucose, fructose, sucrose, or sorbitol contents [22]. Moreover, similar changes in quality, as a consequence of PGR treatment at harvest, were reported for litchi [18], mango [42], cherry [43], plum [26], and apricot [41]. Such different trends, despite of similar treatments, could be explained by several different aspects. Some of them may include the usage of different cultivars, different PGR concentrations and application time, but also the variations in climatic conditions should not be overlooked. Another important factor is the insufficiently defined ripening stage of apricot, which inevitably leads to different stages of fruit maturity at the moment of harvest, and contributes to inconsistent or contradictory results. Regarding this, the fruit selection may be resolved using a noninvasive method for determination of the ripening stage for each apricot individually [22].
Externally added PGRs interact with the naturally defined sequence of the occurrence of plant hormones and could lead to subtle changes in plant and fruit metabolism, as detected in this study and in previous reports. Fruit metabolism does not stop after its picking, thus it continues during the postharvest period, causing changes in almost all measured parameters (Figure 1, Table 1 and Table 2). Alongside 21 days of cold storage, pre-harvest treatment of apricots with PGRs reflected on a number of observed parameters during cold storage: TA, fructose and sucrose contents, citric, malic, and succinic acid contents, bioactive compounds (phenols, flavonoids), and carotenoids.
The mentioned changes caused by PGR application, according to PCA analysis, are tentatively assigned as Factor 2, which could explain only 15.34% and 14.82% of variability, respectively (Figure 2A,B). Such small percentages coupled with relatively close proximity of samples after cold storage and shelf life pointed out a small impact, and thereby the overall small difference caused by the application of PGRs.
Considering our previous study in which the apricots were stored for 15 days [27], 21 day of cold storage did not eliminate the differences in weight loss. Furthermore, some parameters appeared to be affected by PGRs as the storage period extended; i.e., TA, fructose and sucrose, organic acids, bioactive compounds (phenols, flavonoids), and carotenoids.
According to some reports, application of PGRs could have a negative effect on apricot storability, reflected by a reduction of firmness in NAA-treated apricots after 20 days of cold storage [44]. This effect was not recorded in this study, since the PGRs alone or in combination with 21 day of cold storage did not affect flesh firmness (Table 1).
Subsequent shelf life, as expected, increased the respiration rate and ethylene production when compared to the values before cold storage; also it reduced lightness, increased malic acid content, and, in the case of GA3 and BA100, increased TSS, phenol, flavonoid, and fructose contents. None of these differences could be observed by sensory analysis.
5. Conclusions
As expected, the application of PGR improved most of the quality traits and fruit composition of apricots at harvest. The significant differences between the treated and untreated fruits were observed at harvest in terms of their flesh firmness, color, ethylene production and respiration rate, flavonoid, carotenoid, and citric acid contents. Prolonged cold storage diminished the initial differences in firmness, respiration rate, flavonoid and carotenoid contents, but new differences in fructose, and malic and succinic acid contents began to appear. Noteworthy, shelf life reduced the difference in citric acid, but the differences in TA, TSS, phenol, and flavonoid contents were recorded. In terms of sensory properties, the treated and non-treated apricots did not show differences following the cold storage and shelf life. Despite the observed differences in chemical composition at the end of cold storage, fruit firmness, color, and other sensory traits did not differ between the treated and non-treated fruits. Differences between the treated and non-treated apricots were recorded after shelf life in respect to fruit color, more precisely, treated fruits had lower L*, which made them more dark than control fruits. Still, the recorded fluctuations in chemical composition needs another extensive and separate study, which will be focused on the underlying processes that defines the ripening and senescence, as well as the PGR treatment.
Author Contributions
Conceptualization and Experimental Design: J.M., Ž.K., B.M., N.M. and Z.K.; Experiment in the field: M.M., J.K. and G.B.; Postharvest experiment and laboratory analyses: Ž.K., A.B., G.B., M.M. and J.K.; Validation of laboratory methods R.K.; Results analysis and Data curation: J.M. and Ž.K.; Writing—Original Draft Preparation: Ž.K., J.M. and R.K.; Writing—Review & Editing: Ž.K., J.M. and R.K.; Supervision: J.M. and Ž.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financed by the Ministry of Education, Science and Technological Development of Republic of Serbia [Contract numbers: 451-03-68/2022-14/200222] and project “The use of plant growth regulators and biostimulants for the improvement of fruit quality and storage ability” funded from 2016–2019, by Provincial Secretariat for Higher Education and Scientific Research, Autonomous Province of Vojvodina, Republic of Serbia.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sansavini, S.; Giannerini, G.F. Advances in apricot growing and management. Acta Hortic. 1991, 293, 409–430. [Google Scholar] [CrossRef]
- Bassi, D.; Bartolini, S.; Viti, R. Recent advances on environmental and physiological challenges in apricot growing. Acta Hortic. 2006, 717, 23–32. [Google Scholar] [CrossRef]
- Moale, C.; Ghiurea, M.; Sîrbu, C.E.; Somoghi, R.; Cioroianu, T.M.; Faraon, V.A.; Lupu, C.; Trică, B.; Constantinescu-Aruxandei, D.; Oancea, F. Effects of Siliceous Natural Nanomaterials Applied in Combination with Foliar Fertilizers on Physiology, Yield and Fruit Quality of the Apricot and Peach Trees. Plants 2021, 10, 2395. [Google Scholar] [CrossRef]
- Mendelné Pászti, E.; Bujdosó, G.; Mendel, Á. Vegetative Characteristics of Three Apricot Cultivars Grafted on Six Different Rootstocks. Horticulturae 2022, 8, 1004. [Google Scholar] [CrossRef]
- Milatović, D.; Đurović, D.; Zec, G. Osetljivost sorti kajsije na zimski i pozni prolećni mraz. In Zbornik Radova IV Savetovanja „Inovacije u Voćarstvu”; Poljoprivredni fakultet Univerziteta u Beogradu: Belgrade, Serbia, 2013; pp. 239–247. [Google Scholar]
- Mastilović, J.; Kevrešan, Ž.; Milović, M.; Kovač, R.; Milić, B.; Magazin, N.; Plavšić, D.; Kalajdžić, J. Effects of ripening stage and postharvest treatment on apricot (Prunus armeniaca L.) cv. NS4 delivered to the consumers. J. Food Process. Preserv. 2022, 46, e16399. [Google Scholar] [CrossRef]
- Rebeaud, S.G.; Cioli, L.; Cotter, P.Y.; Christen, D. Cultivar, maturity at harvest and postharvest treatments influence softening of apricots. Postharvest Biol. Technol. 2023, 195, 112134. [Google Scholar] [CrossRef]
- Batool, M.; Bashir, O.; Amin, T.; Wani, S.M.; Masoodi, F.A.; Jan, N.; Bhat, S.A.; Gul, A. Effect of oxalic acid and salicylic acid treatments on the post-harvest life of temperate grown apricot varieties (Prunus armeniaca) during controlled atmosphere storage. Food Sci. Technol. Int. 2022, 28, 557–569. [Google Scholar] [CrossRef]
- Medina-Santamarina, J.; Zapata, P.J.; Valverde, J.M.; Valero, D.; Serrano, M.; Guillén, F. Melatonin treatment of apricot trees leads to maintenance of fruit quality attributes during storage at chilling and non-chilling temperatures. Agronomy 2021, 11, 917. [Google Scholar] [CrossRef]
- Hosseinifarahi, M.; Mousavi, S.M.; Mohsen, R.A.D.I.; Jowkar, M.M.; Romanazzi, G. Postharvest application of hot water and putrescine treatments reduce brown rot and improve shelf life and quality of apricots. Phytopathol. Mediterr. 2020, 59, 319–329. [Google Scholar]
- Algarni, E.H.; Elnaggar, I.A.; Abd El-wahed, A.E.W.N.; Taha, I.M.; Al-Jumayi, H.A.; Elhamamsy, S.M.; Mahmoud, S.F.; Fahmy, A. Effect of Chitosan Nanoparticles as Edible Coating on the Storability and Quality of Apricot Fruits. Polymers 2022, 14, 2227. [Google Scholar] [CrossRef]
- Muzzaffar, S.; Bhat, M.M.; Wani, T.A.; Wani, I.A.; Masoodi, F.A. Postharvest biology and technology of apricot. In Postharvest Biology and Technology of Temperate Fruits; Mir, S.A., Shah, M.A., Mir, M.M., Eds.; Springer: Cham, Switzerland, 2018; pp. 201–222. [Google Scholar]
- Ji, Y.; Xu, M.; Wang, A. Recent advances in the regulation of climacteric fruit ripening: Hormone, transcription factor and epigenetic modifications. Front. Agric. Sci. Eng. 2021, 8, 314–334. [Google Scholar]
- Milić, B.; Tarlanović, J.; Keserović, Z.; Magazin, N.; Miodragović, M.; Popara, G. Bioregulators can improve fruit size, yield and plant growth of northern highbush blueberry (Vaccinium corymbosum L.). Sci. Hortic. 2018, 235, 214–220. [Google Scholar] [CrossRef]
- Nickel, L. Plant Growth Regulators in Agriculture and Horticulture. In Bioregulators for Crop Protection and Pest Control, Proceedings of the Developed from a Symposium Sponsored by the Division of Agrochemicals at the 205th National Meeting of the American Chemical Society, Denver, CO, USA, 28 march–2 April 1993; Hedin, P.A., Ed.; American Chemical Society: Washington, DC, USA, 1993; pp. 1–14. [Google Scholar]
- Abd El-Naby, S.K.M.; Mohamed, A.A.A.; El-Naggar, Y.I.M. Effect of melatonin, GA3 and NAA on vegetative growth, yield and quality of ‘canino’ apricot fruits. Acta Sci. Pol. Hortorum Cultus 2019, 18, 167–174. [Google Scholar] [CrossRef]
- Mhetre, V.B.; Patel, V.B.; Singh, S.K.; Verma, M.K.; Mishra, G.P.; Dahuja, A.; Kumar, C. Effect of new generation bio-regulators on anthocyanins and berry quality of grape cv. Beauty Seedless. Indian J. Agric. Sci. 2021, 91, 920–923. [Google Scholar] [CrossRef]
- Gupta, A.; Tripathi, V.K.; Shukla, J.K. Influence of GA3, Zinc and Boron on Fruit drop, Yield and Quality of Litchi (Litchi chinensis Sonn.). Biol. Forum-Int. J. 2022, 14, 1079–1083. [Google Scholar]
- Laňar, L.; Scháňková, K.; Náměstek, J. Searching for new possibilities of bloom delay in apricots. Acta Hortic. 2022, 1344, 183–188. [Google Scholar] [CrossRef]
- Devrari, N.; Negi, M.; Thakur, N. Studies on effect of gibberellic acid and naphthalene acetic acid spray on fruit set and yield of apricot (Prunus armeniaca L.). Int. J. Agric. Sci. 2017, 7, 59–64. [Google Scholar]
- Southwick, S.M.; Yeager, J.T. Use of gibberellin formulations for improved fruit firmness and chemical thinning in ‘Patterson’ apricot. ISHS Acta Hortic. X Int. Symp. Apric. Cult. 1993, 384, 425–430. [Google Scholar] [CrossRef]
- Roussos, P.A.; Ntanos, E.; Denaxa, N.K.; Tsafouros, A.; Bouali, I.; Nikolakakos, V.; Assimakopoulou, A. Auxin (triclopyr) and cytokinin (forchlorfenuron) differentially affect fruit physiological, organoleptic and phytochemical properties of two apricot cultivars. Acta Physiol. Plant. 2021, 43, 25. [Google Scholar] [CrossRef]
- Lal, S.; Kumar, D.; Singh, D.B.; Ahmed, N.; Kumar, R.; Dar, G.A. Effect of pre-harvest application of calcium chloride and gibberellic acid on shelf-life and post-harvest quality of apricot (Prunus armeniaca L.) cv. Harcot. J. Hortic. Sci. 2011, 6, 46–51. [Google Scholar]
- Kevrešan, Ž.; Milić, B.; Bajić, A.; Kovač, R.; Milović, M.; Kalajdžić, J.; Barać, G. Does application of naphthenic acids in early fruit development stage result in prolonged effect on cold storage and shelf life of apricot fruit? Food Feed. Res. 2022, 49, 139–153. [Google Scholar]
- Garhwal, O.P.; Choudhary, M.R.; Bairwa, L.N.; Kumawat, K.L.; Kumar, P.; Basile, B.; Corrado, G.; Rouphael, Y.; Gora, J.S. Effects of Time of Pruning and Plant Bio-Regulators on the Growth, Yield, Fruit Quality, and Post-Harvest Losses of Ber (Ziziphus mauritiana). Horticulturae 2022, 8, 809. [Google Scholar]
- Barać, G.; Mastilović, J.; Kevrešan, Ž.; Milić, B.; Kovač, R.; Milović, M.; Kalajdžić, J.; Bajić, A.; Magazon, N.; Keserović, Z. Effects of Plant Growth Regulators on Plum (Prunus domestica L.) Grown on Two Rootstocks at Harvest and at the Postharvest Period. Horticulturae 2022, 8, 621. [Google Scholar] [CrossRef]
- Milić, B.; Mastilović, J.; Kevrešan, Ž.; Kovač, R.; Bajić, A.; Keserović, Z.; Magazin, N.; Milović, M.; Kalajdžić, J.; Barać, G. Consequences of NAA, BA and GA3 treatment in early fruit development phase on postharvest properties of apricot cv. NS4. Acta Sci. Pol. Hortorum Cultus 2022, 21, 49–59. [Google Scholar] [CrossRef]
- Meier, U. Growth Stages of Mono- and Dico-Tyledonous Plants; Blackwell Wissenschafts-Verlag: Berlin, Germany, 1997. [Google Scholar]
- Kovač, R.; Kevrešan, Ž.; Mastilović, J.; Magazin, N.; Milić, M.; Milović, M.; Bajić, A.; Kalajdžić, J.; Barać, G.; Keserović, Z. IAD values of apricot (Prunus armeniaca L.) at harvest in relation to fruit quality and sensory properties during cold storage and shelf life. N. Z. J. Crop Hortic. Sci. 2022, 50, 205–222. [Google Scholar] [CrossRef]
- Costache, M.A.; Campeanu, G.; Neata, G. Studies concerning the extraction of chlorophyll and total carotenoids from vegetables. Rom. Biotechnol. Lett. 2012, 17, 7702–7708. [Google Scholar]
- Larrauri, J.A.; Rupérez, P.; Saura-Calixto, F. Mango peel fibers with antioxidant activity. Z. Lebensm. Forsch. A 1997, 205, 39–42. [Google Scholar] [CrossRef]
- Singleton, V.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Pękal, A.; Pyrzynska, K. Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Milenković, L.; Mastilović, J.; Kevrešan, Ž.; Bajić, A.; Gledić, A.; Stanojević, L.; Cvetković, D.; Šunić, L.; Ilić, S.Z. Effect of shading and grafting on yield and quality of tomato. J. Sci. Food Agric. 2020, 100, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Melgarejo, P.; Calín-Sánchez, Á.; Carbonell-Barrachina, Á.A.; Martínez-Nicolás, J.J.; Legua, P.; Martínez, R.; Hernández, F. Antioxidant activity, volatile composition and sensory profile of four new very-early apricots (Prunus armeniaca L.). J. Sci. Food Agric. 2014, 94, 85–94. [Google Scholar] [CrossRef]
- Ishaq, S.; Rathore, H.A.; Masud, T.; Ali, S. Influence of postharvest calcium chloride application, ethylene absorbent and modified atmosphere on quality characteristics and shelf life of apricot (Prunus armeniaca L.) fruit during storage. Pak. J. Nutr. 2009, 8, 861–865. [Google Scholar] [CrossRef]
- Nagy, N. Effect of preharvest applications of calcium, anti-ethylene compounds and their combinations on “Canino” apricot fruit quality and storability. Zagazig J. Agric. Res. 2018, 45, 1609–1631. [Google Scholar] [CrossRef]
- Khajehyar, R.; Rahemi, M.; Fallahi, E. The impact of various rates and dates of gibberellic acid applications on fruit set in apricot. Int. J. Fruit Sci. 2015, 15, 324–338. [Google Scholar] [CrossRef]
- Abdel-Mohsen, M.A.; Kamel, H.M. Fruit set and quality of ‘canino’ apricot fruits as affected by spraying with yeast, growth regulators and micronutrients. J. Plant Prod. 2015, 6, 1431–1441. [Google Scholar] [CrossRef]
- Southwick, S.M.; Yeager, J.T.; Weis, K.G. Use of gibberellins on ‘Patterson’ apricot (Prunus armeniaca) to reduce hand thinning and improve fruit size and firmness: Effects over three seasons. J. Hortic. Sci. 1997, 72, 645–652. [Google Scholar] [CrossRef]
- Canli, F.A.; Sahin, M.; Temurtas, N.; Pektas, M. Improving fruit quality of apricot by means of preharvest benzyladenine and benzyladenine plus gibberellin applications. Horttechnology 2014, 24, 424–427. [Google Scholar] [CrossRef]
- Bezerra, J.B.N.; Jesus, P.R.R.D.; Souza, I.D.; Bezerra, W.C.; Martins, G.C.S.B.; Ribeiro, V.G. Plant regulators on the growth, quality and production of ‘Tommy Atkins’ mango fruits. Rev. Bras. Frutic. 2021, 43, e546. [Google Scholar] [CrossRef]
- Webster, A.D.; Spencer, J.E.; Dover, C.; Atkinson, C.J. The influence of sprays of gibberellic acid (GA3) and aminoethoxyvinylglycine (AVG) on fruit abscission, fruit ripening and quality of two sweet cherry cultivars. Acta Hortic. 2006, 727, 467–472. [Google Scholar] [CrossRef]
- Mesa, K.; Reginato, G.; Contador, L.; Infante, R. Prohexadione Calcium and Naphthalene Acetic Acid Sprays Improve Fruit Size and Maintain Fruit Quality of ‘Castlebrite’ Apricot. Eur. J. Hortic. Sci. 2012, 77, 115. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).