Effect of Nanopriming with Selenium Nanocomposites on Potato Productivity in a Field Experiment, Soybean Germination and Viability of Pectobacterium carotovorum
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanocomposites
2.2. Potato Nanopriming and Field Experiment Conditions
2.3. Analysis of Microorganisms Collected from Potato Tubers
2.4. Experiments with Bacteria Pectobacterium carotovorum
2.5. Soybean Nanopriming
3. Results
3.1. Effect of NCs on Potato Productivity
3.2. Effect of NC on the Microbial Profile of Potato Tubers
3.3. Influence of Se/AG NC on Phytopathogen Pectobacterium carotovorum
3.4. Influence of Se/AG NC on Soybean Germination
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Füleky, G. Cultivated plants, primarily as food sources. In Cultivated Plants, Primarily as Food Sources; Fuleky, G., Ed.; EOLSS Publishers Co Ltd.: Oxford, UK, 2009; Volume 1, pp. 1–41. [Google Scholar]
- Goffart, J.-P.; Haverkort, A.; Storey, M.; Haase, N.; Martin, M.; Lebrun, P.; Ryckmans, D.; Florins, D.; Demeulemeester, K. Potato production in Northwestern Europe (Germany, France, the Netherlands, United Kingdom, Belgium): Characteristics, issues, challenges and opportunities. Potato Res. 2022, 65, 503–547. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.J.; Suarez, V. The rise of Asia as the centre of global potato production and some implications for industry. Potato J. 2012, 39, 1–22. [Google Scholar]
- Wang, Z.-J.; Liu, H.; Zeng, F.-K.; Yang, Y.-C.; Xu, D.; Zhao, Y.-C.; Liu, X.-F.; Kaur, L.; Liu, G.; Singh, J. Potato processing industry in China: Current scenario, future trends and global impact. Potato Res. 2022, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Devaux, A.; Goffart, J.-P.; Kromann, P.; Andrade-Piedra, J.; Polar, V.; Hareau, G. The potato of the future: Opportunities and challenges in sustainable agri-food systems. Potato Res. 2021, 64, 681–720. [Google Scholar] [CrossRef]
- Sun, J.; Mooney, H.; Wu, W.; Tang, H.; Tong, Y.; Xu, Z.; Huang, B.; Cheng, Y.; Yang, X.; Wei, D.; et al. Importing food damages domestic environment: Evidence from global soybean trade. Proc. Natl. Acad. Sci. USA 2018, 115, 5415–5419. [Google Scholar] [CrossRef]
- Campos, H.; Ortiz, O. (Eds.) The Potato Crop. Its Agricultural, Nutritional and Social Contribution to Humankind; Springer: Cham, Switzerland, 2020; 518p. [Google Scholar] [CrossRef]
- Dilawari, R.; Kaur, N.; Priyadarshi, N.; Prakash, I.; Patra, A.; Mehta, S.; Singh, B.; Jain, P.; Islam, M.A. Soybean: A key player for global food security. In Soybean Improvement; Wani, S.H., Sofi, N.u.R., Bhat, M.A., Lin, F., Eds.; Springer: Cham, Switzerland, 2022; pp. 1–46. [Google Scholar] [CrossRef]
- Paparella, S.; Araújo, S.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed priming: State of the art and new perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef]
- Nile, S.H.; Thiruvengadam, M.; Wang, Y.; Samynathan, R.; Shariati, M.A.; Rebezov, M.; Nile, A.; Sun, M.; Venkidasamy, B.; Xiao, J.; et al. Nano-priming as emerging seed priming technology for sustainable agriculture—Recent developments and future perspectives. J. Nanobiotechnol. 2022, 20, 254. [Google Scholar] [CrossRef]
- Pagano, A.; Macovei, A.; Xia, X.; Padula, G.; Hołubowicz, R.; Balestrazzi, A. Seed priming applied to onion-like crops: State of the art and open questions. Agronomy 2023, 13, 288. [Google Scholar] [CrossRef]
- Jisha, K.C.; Vijayakumari, K.; Puthur, J.T. Seed priming for abiotic stress tolerance: An overview. Acta Physiol. Plant 2013, 35, 1381–1396. [Google Scholar] [CrossRef]
- Zulfiqar, F. Effect of seed priming on horticultural crops. Sci. Hortic. 2021, 286, 110197. [Google Scholar] [CrossRef]
- Liu, X.; Quan, W.; Bartels, D. Stress memory responses and seed priming correlate with drought tolerance in plants: An overview. Planta 2022, 255, 45. [Google Scholar] [CrossRef] [PubMed]
- Singhal, R.K.; Kumar, M.; Bose, B.; Mondal, S.; Srivastava, S.; Dhankher, O.P.; Tripathi, R.D. Heavy metal (loid)s phytotoxicity in crops and its mitigation through seed priming technology. Int. J. Phytoremediation 2023, 25, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Adetunji, A.E.; Adetunji, T.L.; Varghese, B.; Sershen, N.; Pammenter, N.W. Oxidative Stress, Ageing and Methods of Seed Invigoration: An Overview and Perspectives. Agronomy 2021, 11, 2369. [Google Scholar] [CrossRef]
- Sen, A.; Puthur, J.T. Influence of different seed priming techniques on oxidative and antioxidative responses during the germination of Oryza sativa varieties. Physiol. Mol. Biol. Plants 2020, 26, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Mombeini, M.; Ansari, N.A.; Abdossi, V.; Naseri, A. Effects of chemical seed priming on germination and antioxidant enzymes activity of two cucumber (Cucumis sativus L.) cultivars. Int. J. Hortic. Sci. Technol. 2022, 9, 165–175. [Google Scholar] [CrossRef]
- Szőllősi, R.; Molnár, Á.; Kondak, S.; Kolbert, Z. Dual effect of nanomaterials on germination and seedling growth: Stimulation vs. phytotoxicity. Plants 2020, 9, 1745. [Google Scholar] [CrossRef]
- Khan, I.; Awan, S.A.; Rizwan, M.; Hassan, Z.U.; Akram, M.A.; Tariq, R.; Brestic, M.; Xie, W. Nanoparticle’s uptake and translocation mechanisms in plants via seed priming. foliar treatment. and root exposure: A review. Environ. Sci. Pollut. Res. Int. 2022, 29, 89823–89833. [Google Scholar] [CrossRef]
- Kandhol, N.; Singh, V.P.; Ramawat, N.; Prasad, R.; Chauhan, D.K.; Sharma, S.; Grillo, R.; Sahi, S.; Peralta-Videa, J.; Tripathi, D.K. Nano-priming: Impression on the beginner of plant life. Plant Stress 2022, 5, 100091. [Google Scholar] [CrossRef]
- do Espirito Santo Pereira, A.; Oliveira, H.C.; Fernandes Fraceto, L.; Santaella, C. Nanotechnology potential in seed priming for sustainable agriculture. Nanomaterials 2021, 11, 267. [Google Scholar] [CrossRef]
- Acharya, P.; Jayaprakasha, G.K.; Crosby, K.M.; Jifon, J.L.; Patil, B.S. Nanoparticle-mediated seed priming improves germination. growth. yield. and quality of watermelons (Citrullus lanatus) at multi-locations in Texas. Sci. Rep. 2020, 10, 5037. [Google Scholar] [CrossRef]
- Farhana; Munis, M.F.H.; Alamer, K.H.; Althobaiti, A.T.; Kamal, A.; Liaquat, F.; Haroon, U.; Ahmed, J.; Chaudhary, H.J.; Attia, H. ZnO nanoparticle-mediated seed priming induces biochemical and antioxidant changes in chickpea to alleviate Fusarium Wilt. J. Fungi 2022, 8, 753. [Google Scholar] [CrossRef] [PubMed]
- Salam, A.; Khan, A.R.; Liu, L.; Yang, S.; Azhar, W.; Ulhassan, Z.; Zeeshan, M.; Wu, J.; Fan, X.; Gan, Y. Seed priming with zinc oxide nanoparticles downplayed ultrastructural damage and improved photosynthetic apparatus in maize under cobalt stress. J. Hazard Mater. 2022, 423 Pt A, 127021. [Google Scholar] [CrossRef]
- Zhou, X.; Jia, X.; Zhang, Z.; Chen, K.; Wang, L.; Chen, H.; Yang, Z.; Li, C.; Zhao, L. AgNPs seed priming accelerated germination speed and altered nutritional profile of Chinese cabbage. Sci. Total Environ. 2022, 808, 151896. [Google Scholar] [CrossRef] [PubMed]
- Venzhik, Y.; Deryabin, A.; Popov, V.; Dykman, L.; Moshkov, I. Priming with gold nanoparticles leads to changes in the photosynthetic apparatus and improves the cold tolerance of wheat. Plant Physiol. Biochem. 2022, 190, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Perfileva, A.I.; Tsivileva, O.M.; Nozhkina, O.A.; Karepova, M.S.; Graskova, I.A.; Ganenko, T.V.; Sukhov, B.G.; Krutovsky, K.V. Effect of natural polysaccharide matrix-based selenium nanocomposites on Phytophthora cactorum and rhizospheric microorganisms. Nanomaterials 2021, 11, 2274. [Google Scholar] [CrossRef] [PubMed]
- Perfileva, A.I.; Nozhkina, O.A.; Graskova, I.A.; Sidorov, A.V.; Lesnichaya, M.V.; Aleksandrova, G.P.; Dolmaa, G.; Klimenkov, I.V.; Sukhov, B.G. Synthesis of selenium and silver nanobiocomposites and their influence on phytopathogenic bacterium Clavibacter michiganensis subsp. sepedonicus. Russ. Chem. Bull. 2018, 67, 157–163. [Google Scholar] [CrossRef]
- Perfileva, A.I.; Nozhkina, O.A.; Tretyakova, M.S.; Graskova, I.A.; Klimenkov, I.V.; Sudakov, N.P.; Alexandrova, G.P.; Sukhov, B.G. Biological activity and environmental safety of selenium nanoparticles encapsulated in starch macromolecules. Nanotechnol. Russ. 2020, 15, 96–104. [Google Scholar] [CrossRef]
- Nozhkina, O.A.; Perfileva, A.I.; Graskova, I.A.; Nurminsky, V.N.; Klimenkov, I.V.; Dyakova, A.V.; Ganenko, T.V.; Borodina, T.N.; Aleksandrova, G.P.; Sukhov, B.G.; et al. The biological activity of a selenium nanocomposite encapsulated in carrageenan macromolecules with respect to ring rot pathogenesis of potato plants. Nanotechnol. Russ. 2019, 14, 255–262. [Google Scholar] [CrossRef]
- Graskova, I.A.; Perfilyeva, A.I.; Nozhkina, O.A.; Dyakova, A.V.; Nurminsky, V.N.; Klimenkov, I.V.; Sudakov, N.P.; Borodina, T.N.; Aleksandrova, G.P.; Lesnichaya, M.V.; et al. Impact of nano-sized selenium the ring rott pathogus and in vitro potatoes. Chem. Plant Raw Mater. 2019, 3, 345–354, (In Russian with English Abstract). [Google Scholar] [CrossRef]
- Perfileva, A.I.; Nozhkina, O.A.; Ganenko, T.V.; Sukhov, B.G.; Artem’ev, A.V.; Trofimov, B.A.; Krutovsky, K.V. Selenium nanocomposites in natural matrices as potato recovery agent. Int. J. Mol. Sci. 2021, 22, 4576. [Google Scholar] [CrossRef]
- Perfileva, A.I.; Graskova, I.A.; Sukhov, B.G.; Krutovsky, K.V. Effect of selenium nanocomposites based on natural polymer matrices on the biomass and storage of potato tubers in a field experiment. Agronomy 2022, 12, 1281. [Google Scholar] [CrossRef]
- Faithfull, T. Methods in Agricultural Chemical Analysis: A Practical Handbook; CABI Publishing: Oxford, UK, 2002; 304p. [Google Scholar] [CrossRef]
- Sanders, E.R. Aseptic Laboratory Techniques: Plating Methods. J. Vis. Exp. 2012, 63, e3064. [Google Scholar] [CrossRef]
- Sagdic, O.; Aksoy, A.; Ozkan, G. Evaluation of the antibacterial and antioxidant potentials of gilaburu (Viburnum opulus L.) fruit extract. Acta Aliment. 2006, 35, 487–492. [Google Scholar] [CrossRef]
- Shaginyan, I.A.; Danilina, G.A.; Chernukha, M.Y.; Alekseeva, G.V.; Batov, A.B. Biofilm formation by strains of Burkholderia cepacia complex in dependence of their phenotypic and genotypic characteristics. J. Microbiol. Epidemiol. Immunobiol. 2007, 1, 3–9, (In Russian with English Abstract). [Google Scholar]
- Defez, R.; Andreozzi, A.; Bianco, C. Quantification of triphenyl-2h-tetrazoliumchloride reduction activity in bacterial cells. Bio-protocol 2017, 7, e2115. [Google Scholar] [CrossRef] [PubMed]
- Zelentsov, S.V.; Moshnenko, E.V.; Trunova, M.V.; Bubnova, L.A.; Budnikov, E.N.; Lukomets, A.V.; Savichenko, V.G.; Dorofeev, N.V.; Katysheva, N.B.; Pomortsev, A.V. A cold-resistant soybean cultivar of the northern ecotype Sayana. Oil Crops 2021, 1, 95–102, (In Russian with English Abstract). [Google Scholar] [CrossRef]
- Boyarkin, A.N. A quick method for determining the activity of peroxidase. Biochemistry 1951, 16, 352. [Google Scholar]
- Hadwan, M.H.; Abed, H.N. Data supporting the spectrophotometric method for the estimation of catalase activity. Data Brief 2016, 6, 194–199. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.N.S.; Nam, K.; Ahn, D.U. Analytical methods for lipid oxidation and antioxidant capacity in food systems. Antioxidants 2021, 10, 1587. [Google Scholar] [CrossRef]
- Kang, M.; Kim, S.; Lee, J.Y.; Yoon, S.; Kim, S.H.; Ha, J. Inactivation of Pectobacterium carotovorum subsp. carotovorum on Chinese cabbage (Brassica rapa L. subsp. pekinensis) by wash treatments with phenolic compounds. LWT 2018, 93, 229–236. [Google Scholar] [CrossRef]
- Markovic, S.; Milić Komić, S.; Jelušić, A.; Iličić, R.; Bagi, F.; Stanković, S.; Popović, T. First report of Pectobacterium versatile causing blackleg of potato in Serbia. Plant Dis. 2022, 106, 312. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Sun, D.; Zhang, X.; Hu, H.; Kong, L.; Rookes, J.E.; Xie, J.; Cahill, D.M. Stimulation of photosynthesis and enhancement of growth and yield in Arabidopsis thaliana treated with amine-functionalized mesoporous silica nanoparticles. Plant Physiol. Biochem. 2020, 156, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Shebl, A.; Hassan, A.; Salama, D.M.; Abd El-Aziz, M.E.; Abd Elwahed, M.S. Template-free microwave-assisted hydrothermal synthesis of manganese zinc ferrite as a nanofertilizer for squash plant (Cucurbita pepo L). Heliyon 2020, 6, e03596. [Google Scholar] [CrossRef]
- Feng, Y.; Kreslavski, V.D.; Shmarev, A.N.; Ivanov, A.A.; Zharmukhamedov, S.K.; Kosobryukhov, A.; Allakhverdiev, S.I.; Shabala, S. Effects of iron oxide nanoparticles (Fe3O4) on growth. photosynthesis. antioxidant activity and distribution of mineral elements in wheat (Triticum aestivum). Plants 2022, 11, 1894. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, Y.; Li, H.; Lu, J.; Zhao, H.; Liu, M.; Nechitaylo, G.S.; Glushchenko, N.N. New insights into the cellular responses to iron nanoparticles in Capsicum annuum. Sci. Rep. 2018, 8, 3228. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Singh, P.M.; Sagar, V.; Pandya, A.; Chinnappa, M.; Kumar, R.; Bahadur, A. Seed priming with ZnO and Fe3O4 nanoparticles alleviate the lead toxicity in Basella alba L. through reduced lead uptake and regulation of ROS. Plants 2022, 11, 2227. [Google Scholar] [CrossRef] [PubMed]
- Ghazal, B.; Saif, S.; Farid, K.; Khan, A.; Rehman, S.; Reshma, A.; Fazal, H.; Ali, M.; Ahmad, A.; Rahman, L.; et al. Stimulation of secondary metabolites by copper and gold nanoparticles in submerge adventitious root cultures of Stevia rebaudiana (Bert.). IET Nanobiotechnol. 2018, 12, 569–573. [Google Scholar] [CrossRef]
- Faizan, M.; Yu, F.; Chen, C.; Faraz, A.; Hayat, S. Zinc oxide nanoparticles help to enhance plant growth and alleviate abiotic stress: A review. Curr. Protein Pept. Sci. 2021, 22, 362–375. [Google Scholar] [CrossRef]
- Mehmood, A. Brief overview of the application of silver nanoparticles to improve growth of crop plants. IET Nanobiotechnol. 2018, 12, 701–705. [Google Scholar] [CrossRef]
- Salem, N.M.; Albanna, L.S.; Awwad, A.M.; Ibrahim, Q.M.; Abdeen, A.O. Green synthesis of nano-sized sulfur and its effect on plant growth. J. Agric. Sci. 2016, 8, 188–194. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Shafeev, G.A.; Glinushkin, A.P.; Shkirin, A.V.; Barmina, E.V.; Rakov, I.I.; Simakin, A.V.; Kislov, A.V.; Astashev, M.E.; Vodeneev, V.A.; et al. Production and use of selenium nanoparticles as fertilizers. ACS Omega 2020, 5, 17767–17774. [Google Scholar] [CrossRef] [PubMed]
- Ghasemian, S.; Masoudian, N.; Nematpour, F.S.; Afshar, A.S. Selenium nanoparticles stimulate growth, physiology, and gene expression to alleviate salt stress in Melissa officinalis. Biologia 2021, 76, 2879–2888. [Google Scholar] [CrossRef]
- Feng, T.; Chen, S.; Gao, D.; Liu, G.; Bai, H.; Li, A.; Peng, L.; Ren, Z. Selenium improves photosynthesis and protects photosystem II in pear (Pyrus bretschneideri), grape (Vitis vinifera), and peach (Prunus persica). Photosynthetica 2015, 53, 609–612. [Google Scholar] [CrossRef]
- Hussein, H.A.; Darwesh, O.M.; Mekki, B.B.; El-Hallouty, S.M. Evaluation of cytotoxicity, biochemical profile and yield components of groundnut plants treated with nano-selenium. Biotechnol. Rep. 2019, 12, e00377. [Google Scholar] [CrossRef]
- Chouhan, D.; Mandal, P. Applications of chitosan and chitosan based metallic nanoparticles in agrosciences—A review. Int. J. Biol. Macromol. 2021, 166, 1554–1569. [Google Scholar] [CrossRef] [PubMed]
- Landa, P. Positive effects of metallic nanoparticles on plants: Overview of involved mechanisms. Plant Physiol. Biochem. 2021, 161, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Hashmi, S.S.; Palma, J.M.; Corpas, F.J. Influence of metallic, metallic oxide, and organic nanoparticles on plant physiology. Chemosphere 2022, 290, 133329. [Google Scholar] [CrossRef] [PubMed]
- Verma, K.K.; Song, X.P.; Joshi, A.; Tian, D.D.; Rajput, V.D.; Singh, M.; Arora, J.; Minkina, T.; Li, Y.R. Recent trends in nano-fertilizers for sustainable agriculture under climate change for global food security. Nanomaterials 2022, 12, 173. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef]
- Antisari, L.V.; Carbone, S.; Gatti, A.; Vianello, G.; Nannipieri, P. Uptake and translocation of metals and nutrients in tomato grown in soil polluted with metal oxide (CeO2, Fe3O4, SnO2, TiO2) or metallic (Ag, Co, Ni) engineered nanoparticles. Environ. Sci. Pollut. Res. 2015, 22, 1841–1853. [Google Scholar] [CrossRef]
- Buchholz, F.; Junker, R.; Samad, A.; Antonielli, L.; Sarić, N.; Kostić, T.; Sessitsch, A.; Mitter, B. 16S rRNA gene-based microbiome analysis identifies candidate bacterial strains that increase the storage time of potato tubers. Sci. Rep. 2021, 11, 3146. [Google Scholar] [CrossRef]
- Kõiv, V.; Roosaare, M.; Vedler, E.; Ann Kivistik, P.; Toppi, K.; Schryer, D.; Remm, M.; Tenson, T.; Mäe, A. Microbial population dynamics in response to Pectobacterium atrosepticum infection in potato tubers. Sci. Rep. 2015, 5, 11606. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Kobayashi, Y.O.; Someya, N.; Ikeda, S. Community analysis of root- and tuber-associated bacteria in field-grown potato plants harboring different resistance levels against common scab. Microbes Environ. 2015, 30, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fu, L.; Chen, C.; Sun, W.; Tian, Y.; Xie, H. Characteristics and rapid diagnosis of Pectobacterium carotovorum ssp. associated with bacterial soft rot of vegetables in China. Plant Dis. 2020, 104, 1158–1166. [Google Scholar] [CrossRef]
- El-Naggar, N.E.-A.; Bashir, S.I.; Rabei, N.H.; Saber, W.E.I.A. Innovative biosynthesis, artificial intelligence-based optimization, and characterization of chitosan nanoparticles by Streptomyces microflavus and their inhibitory potential against Pectobacterium carotovorum. Sci. Rep. 2022, 12, 21851. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Fu, X.; Gao, Y.; Shi, L.; Liu, Q.; Yang, W.; Feng, J. Synthesis, antibacterial evaluation, and safety assessment of CuS NPs against Pectobacterium carotovorum subsp. carotovorum. Pest Manag. Sci. 2022, 78, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Jia, H.; Zhang, H. Green synthesis of silver nanoparticles from Mahonia fortunei extracts and characterization of its inhibitory effect on chinese cabbage soft rot pathogen. Front. Microbiol. 2022, 13, 1030261. [Google Scholar] [CrossRef]
- Coman, V.; Oprea, I.; Leopold, L.F.; Vodnar, D.C.; Coman, C. Soybean interaction with engineered nanomaterials: A literature review of recent data. Nanomaterials 2019, 9, 1248. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, L.; Baschieri, A.; Amorati, R. Antioxidat activity of nanomaterials. J. Mater. Chem. B 2018, 6, 2036–2051. [Google Scholar] [CrossRef]
- Konopko, A.; Kusio, J.; Litwinienko, G. Antioxidant activity of metal nanoparticles coated with tocopherol-like residues—The importance of studies in homo- and heterogeneous systems. Antioxidants 2020, 9, 5. [Google Scholar] [CrossRef]
- Mahlangeni, N.T.; Moodley, R. Biosynthesis of manganese oxide nanoparticles using Urginea sanguinea and their effects on cytotoxicity and antioxidant activity. Adv. Nat. Sci. Nanosci. Nanotechnol. 2021, 12, 015015. [Google Scholar] [CrossRef]
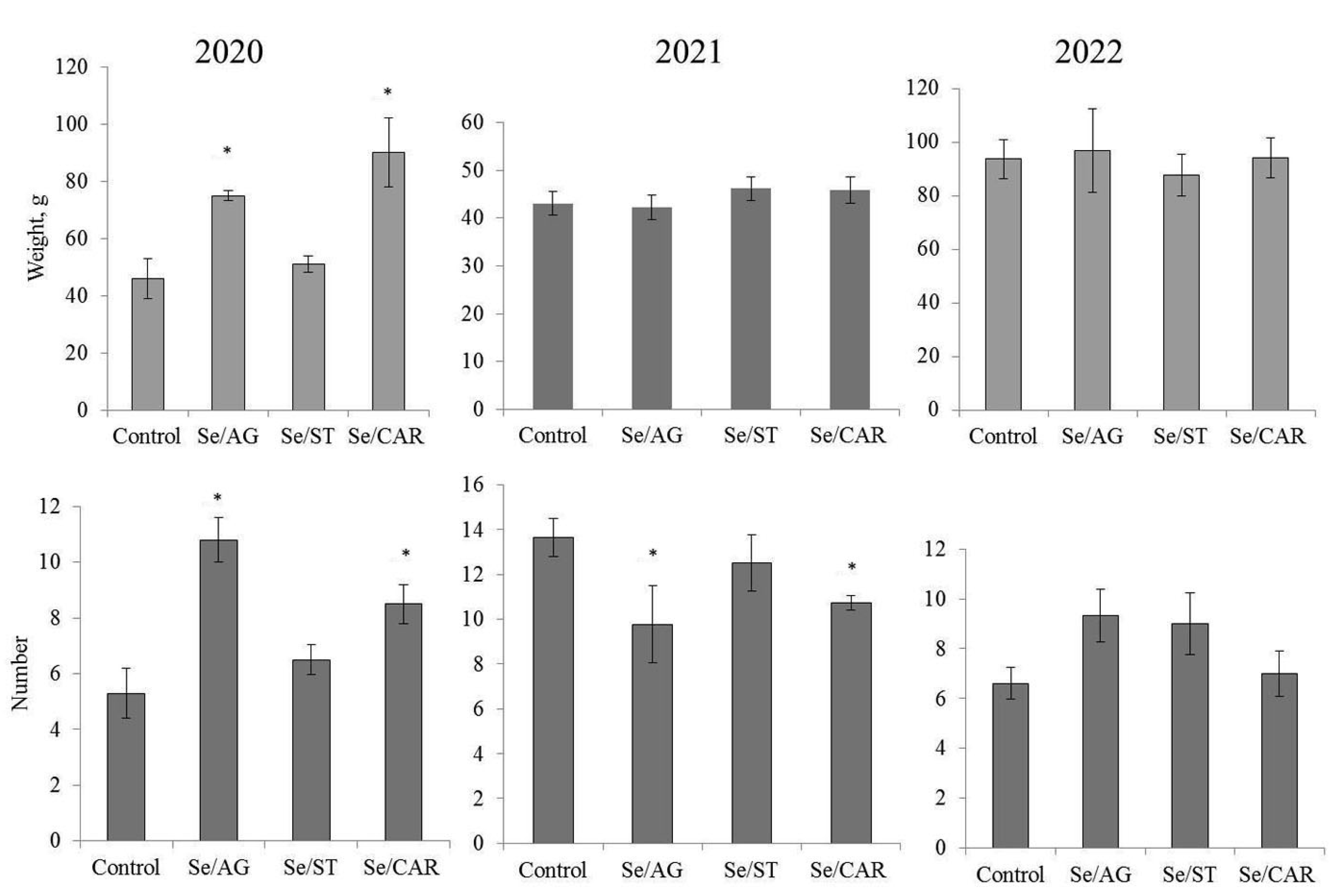
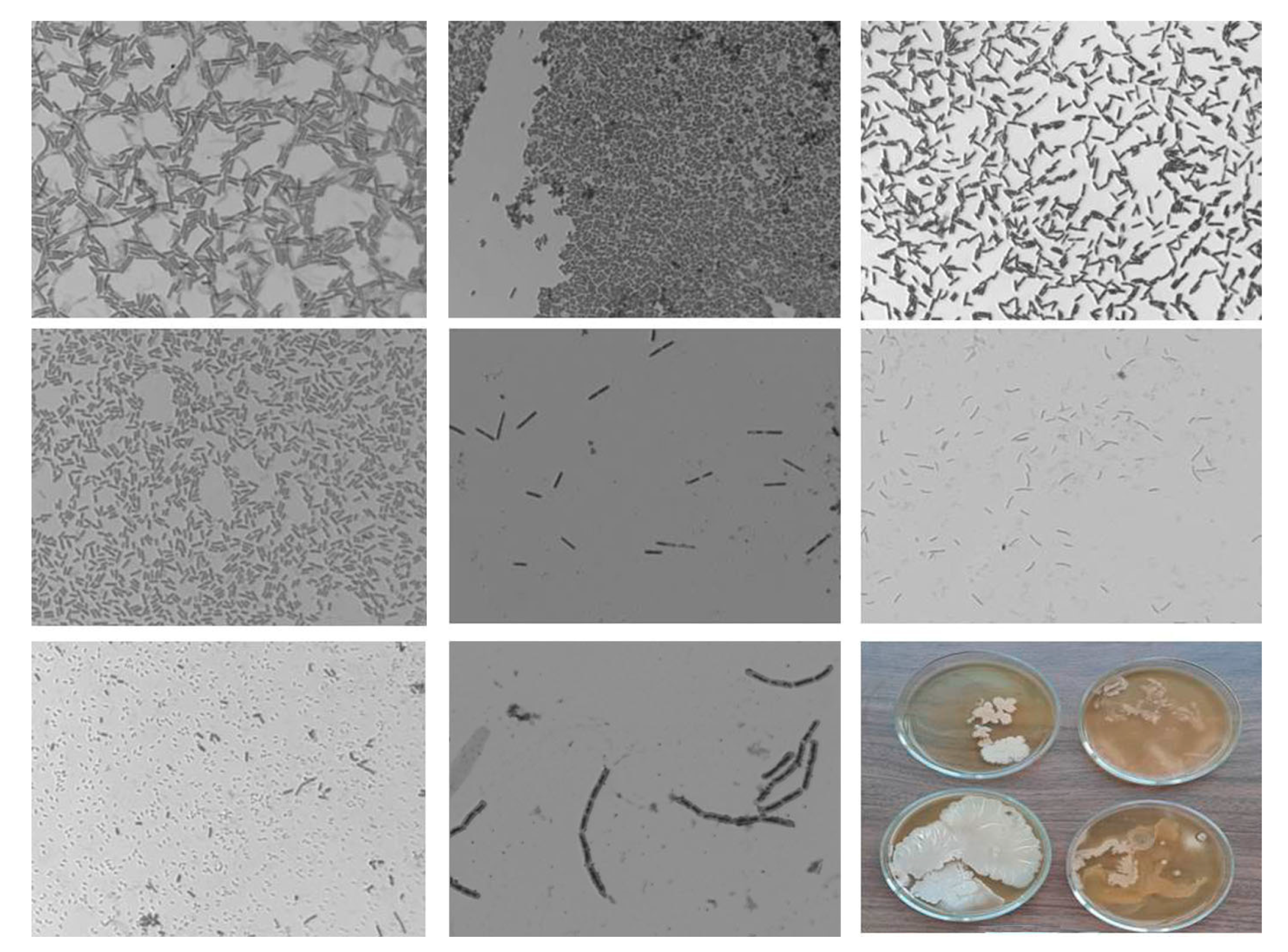



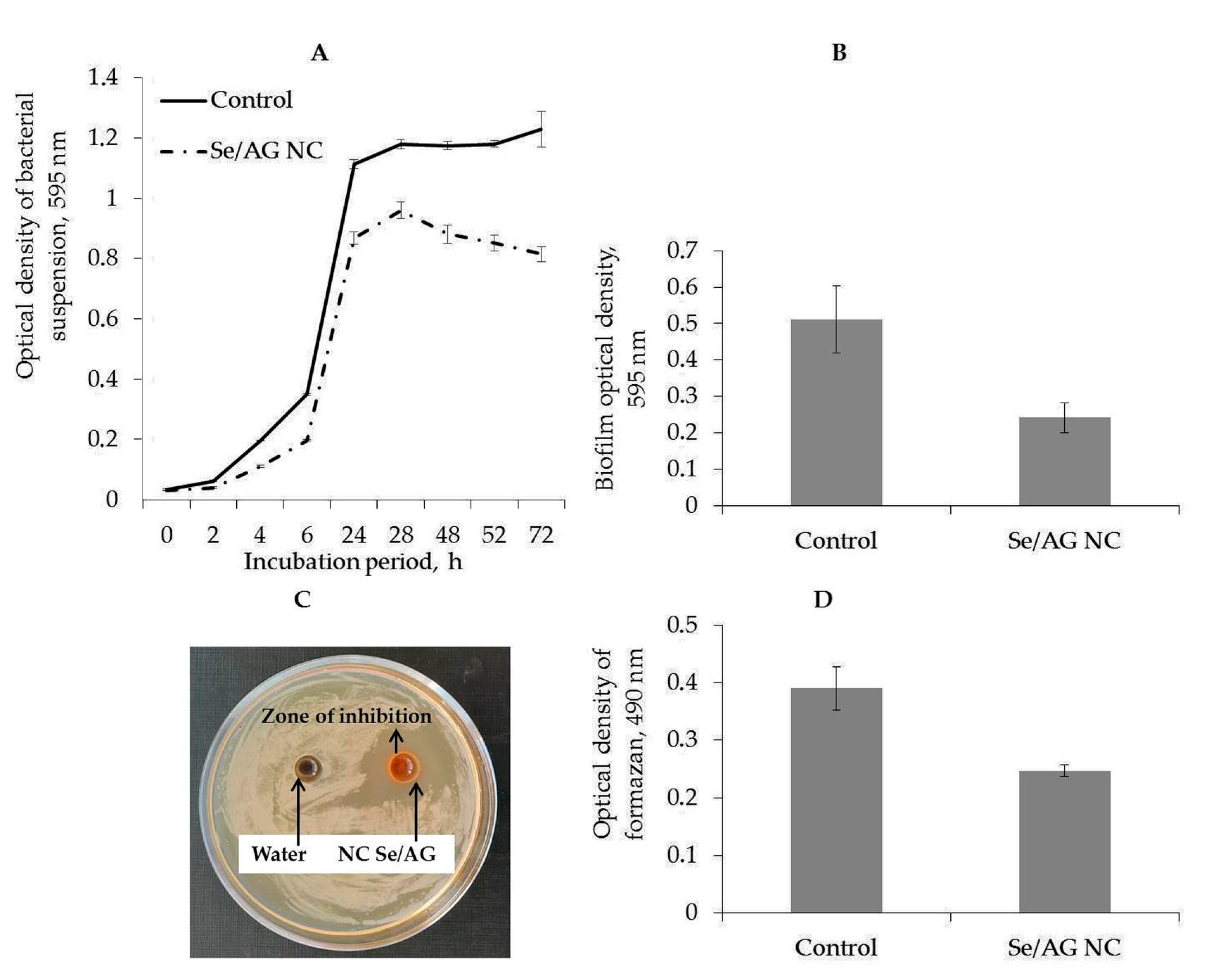
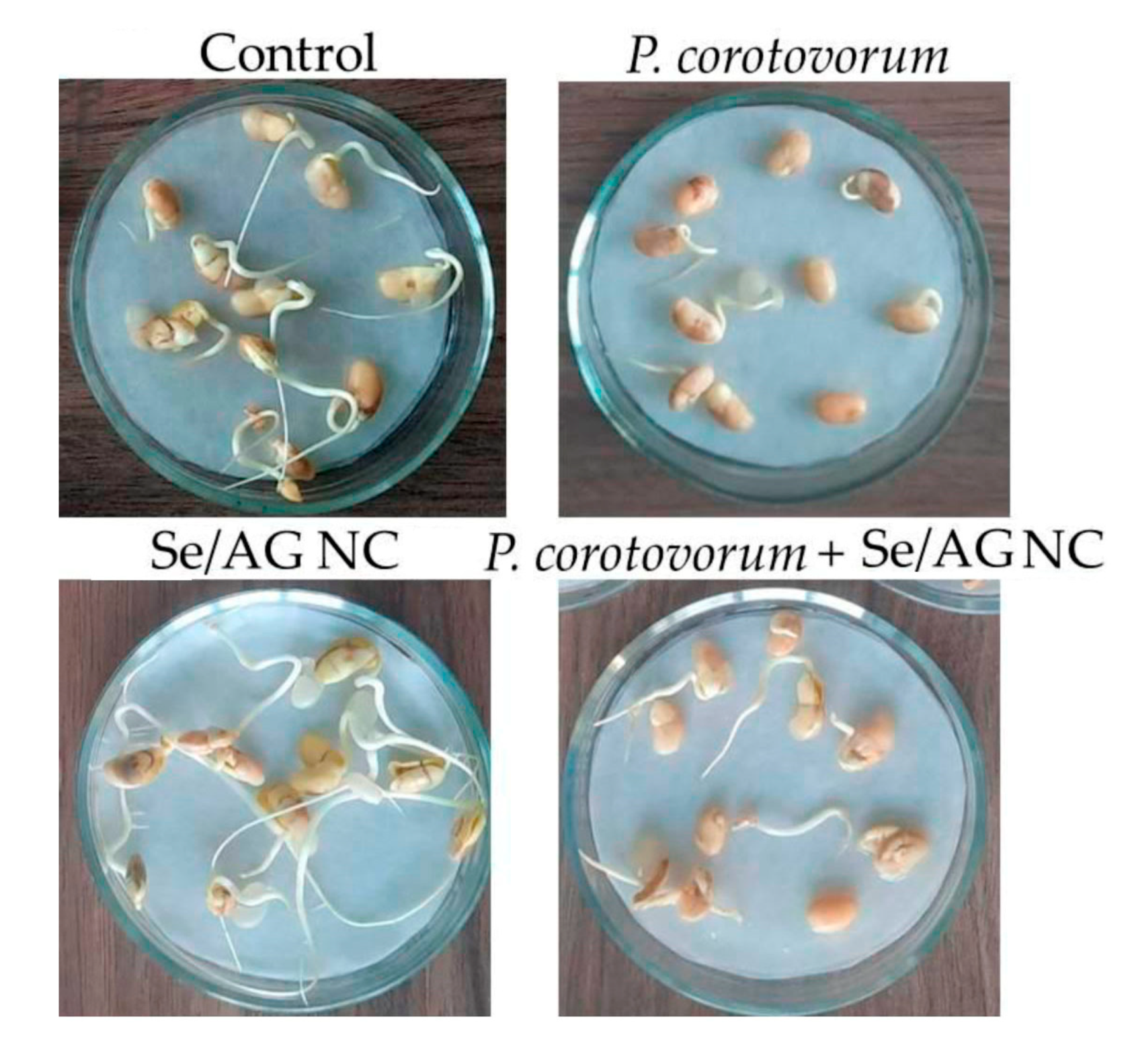
| Tubers\Year | Control | Se/AG NC | Se/ST NC | Se/CAR NC | Mean ± S.D. for All NCs | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2020 | 2021 | 2022 | Mean ± S.D. | 2020 | 2021 | 2022 | Mean ± S.D. | 2020 | 2021 | 2022 | Mean ± S.D. | 2020 | 2021 | 2022 | Mean ± S.D. | ||
| marketable | 35 | 11 | 30 | 25.3 ± 7.3 | 40 | 9 | 32 | 27.0 ± 9.3 | 33.5 | 11 | 23 | 17.0 ± 4.9 | 36 | 14 | 35 | 28.3 ± 7.2 | 25.1 ± 3.5 |
| seed | 20 | 21.5 | 20 | 20.3 ± 0.3 | 23 | 23 | 19 | 21.7 ± 1.3 | 24 | 28 | 19 | 23.7 ± 2.6 | 16.5 | 19 | 16 | 17.2 ± 0.9 | 20.7 ± 1.0 |
| Treatment | Unsprouted Seeds, % | Length, cm | Weight, g | ||
|---|---|---|---|---|---|
| Hypocotyl | Roots | Hypocotyl | Roots | ||
| Control | 32 ± 9 | 2.80 ± 0.32 | 3.26 ± 0.69 | 0.05 ± 0.22 | 0.03 ± 0.00 |
| Se/AG NC (a) | 22 ± 5 | 2.66 ± 0.37 | 3.73 ± 0.48 | 0.05 ± 0.01 | 0.04 ± 0.01 * |
| P. carotovorum (b) | 56 ± 9 | 1.03 ± 0.17 ** | 1.07 ± 0.22 * | 0.01 ± 0.00 ** | 0.01 ± 0.00 ** |
| P. carotovorum + Se/AG NC (c) | 48 ± 7 | 1.66 ± 0.16 * | 1.69 ± 0.18 * | 0.03 ± 0.00 ** | 0.02 ± 0.00 * |
| Treatment | Peroxidase Activity, c.u. | Catalase Activity, mCat | DC Content, nmol/g Fresh Weight × 106 | |||
|---|---|---|---|---|---|---|
| Hypocotyl | Roots | Hypocotyl | Roots | Hypocotyl | Roots | |
| Control | 0.85 ± 0.03 | 0.76 ± 0.03 | 1.19 ± 0.22 | 1.53 ± 0.02 | 0.39 ± 0.02 | 1.08 ± 0.05 |
| Se/AG NC (a) | 0.80 ± 0.04 | 1.13 ± 0.05 ** | 3.98 ± 0.35 ** | 0.76 ± 0.02 ** | 0.30 ± 0.01 ** | 1.10 ± 0.08 |
| P. carotovorum (b) | 0.93 ± 0.03 | 0.97 ± 0.09 * | 5.61 ± 0.39 ** | 1.55 ± 0.30 | 0.38 ± 0.10 | 6.65 ± 0.22 ** |
| P. carotovorum + Se/AG NC (c) | 0.92 ± 0.04 | 0.92 ± 0.06 | 5.07 ± 0.48 | 1.26 ± 0.19 * | 0.36 ± 0.00 ** | 4.90 ± 0.20 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perfileva, A.I.; Kharasova, A.R.; Nozhkina, O.A.; Sidorov, A.V.; Graskova, I.A.; Krutovsky, K.V. Effect of Nanopriming with Selenium Nanocomposites on Potato Productivity in a Field Experiment, Soybean Germination and Viability of Pectobacterium carotovorum. Horticulturae 2023, 9, 458. https://doi.org/10.3390/horticulturae9040458
Perfileva AI, Kharasova AR, Nozhkina OA, Sidorov AV, Graskova IA, Krutovsky KV. Effect of Nanopriming with Selenium Nanocomposites on Potato Productivity in a Field Experiment, Soybean Germination and Viability of Pectobacterium carotovorum. Horticulturae. 2023; 9(4):458. https://doi.org/10.3390/horticulturae9040458
Chicago/Turabian StylePerfileva, Alla I., Anastasia R. Kharasova, Olga A. Nozhkina, Alexander V. Sidorov, Irina A. Graskova, and Konstantin V. Krutovsky. 2023. "Effect of Nanopriming with Selenium Nanocomposites on Potato Productivity in a Field Experiment, Soybean Germination and Viability of Pectobacterium carotovorum" Horticulturae 9, no. 4: 458. https://doi.org/10.3390/horticulturae9040458
APA StylePerfileva, A. I., Kharasova, A. R., Nozhkina, O. A., Sidorov, A. V., Graskova, I. A., & Krutovsky, K. V. (2023). Effect of Nanopriming with Selenium Nanocomposites on Potato Productivity in a Field Experiment, Soybean Germination and Viability of Pectobacterium carotovorum. Horticulturae, 9(4), 458. https://doi.org/10.3390/horticulturae9040458







