Identification of Nutritional Ingredients and Medicinal Components of Hawk Tea and Insect Tea Using Widely Targeted Secondary Metabolomics
Abstract
1. Introduction
2. Materials and Methods
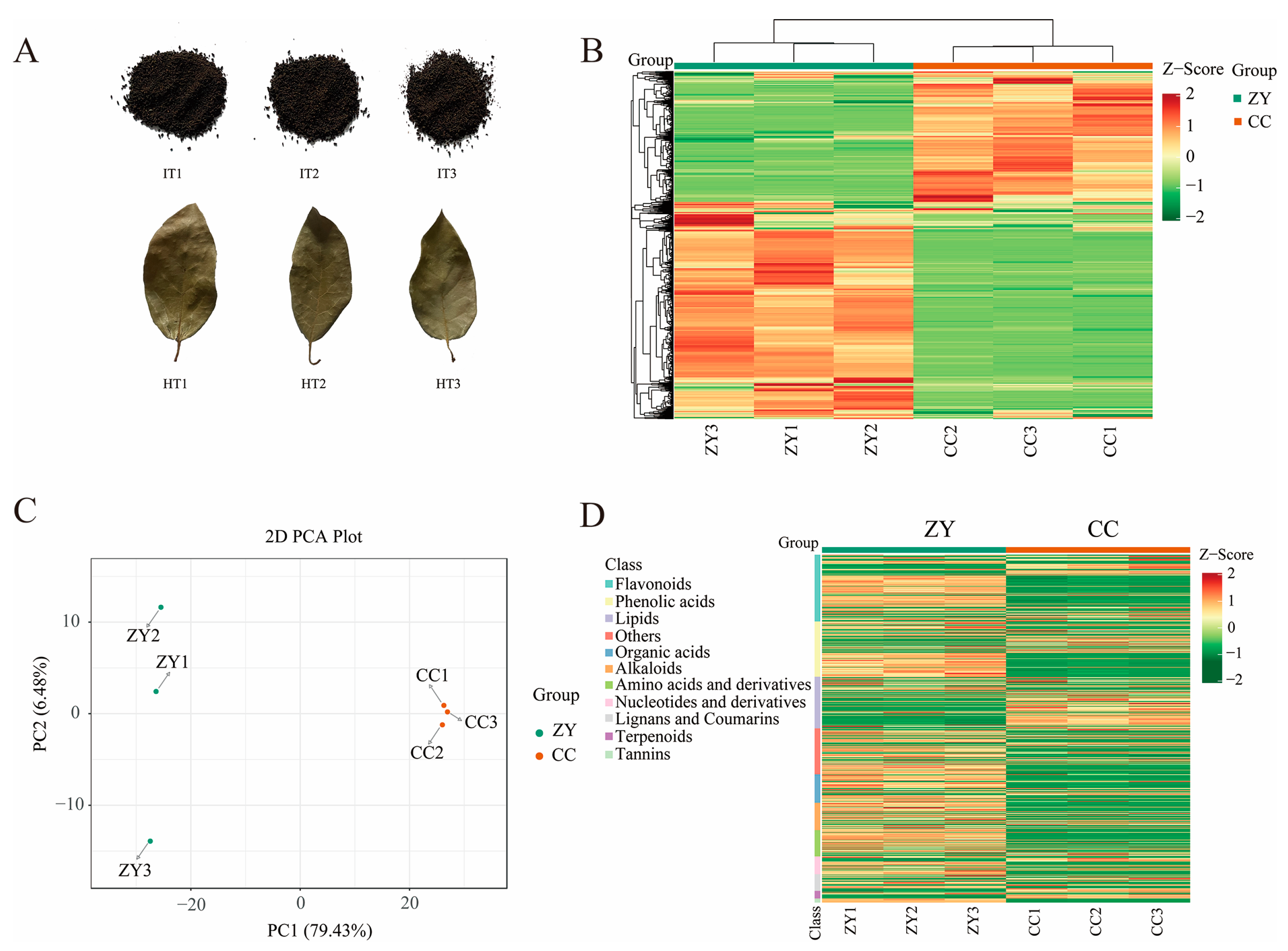
2.1. Plant Materials
2.2. Sample Preparation and Extraction
2.3. Extraction of Dried Hawk Tea Leaves Metabolites
- (1)
- Samples frozen at −80 °C were thawed.
- (2)
- The sample was mixed and 50 mg was placed in a 2 mL centrifuge tube.
- (3)
- A total of 1.2 mL of an internal standard extract in 70% methanol was added. Scroll for 15 min.
- (4)
- Centrifuged for 3 min at 12,000 rpm at 4 °C. A microporous filter membrane (0.22 μm) was used to filter the supernatant, which was placed in a flask designed for use with tandem mass spectrometry combined with liquid chromatography (LC-MS/MS), and stored at −80 °C until use.
2.4. Extraction of Insect Tea Metabolites
- (1)
- The sample was removed from storage at −80 °C and thawed on ice.
- (2)
- After mixing, 20 (±1 mg) of the sample was placed in a 2 mL centrifuge tube.
- (3)
- An internal standard was added in a volume of 400 μL of 70% methanol and vortexed for 3 min.
- (4)
- The sample was then sonicated for 10 min in an ice water bath and incubated stationary for 30 min at −20 °C.
- (5)
- The sample was centrifuged for 10 min at 12,000 rpm at 4 °C and the supernatant was collected. A volume of 300 μL was added to a new centrifuge tube.
- (6)
- The sample was finally centrifuged for 3 min at 12,000 rpm at 4 °C and the supernatant was collected for analysis.
2.5. Ultra Performance Liquid Chromatography (UPLC) CONDITIONS
2.6. ESI-Q TRAP-MS/MS
2.7. Quantitative and Qualitative Determination of Metabolites
2.8. Identification of the Key Active Ingredients in Hawk Tea and Insect Tea That Were Used in Traditional Chinese Medicines
2.9. Identification of the Active Pharmaceutical Ingredients for Seven Major Types of Disease Resistance in Hawk Tea and Insect Tea
2.10. Principal Component Analysis
2.11. Hierarchical Cluster Analysis and Pearson Correlation Coefficients
2.12. Differential Metabolite Analysis
2.13. Determination of In Vitro Antioxidant Activity
3. Results and Discussion
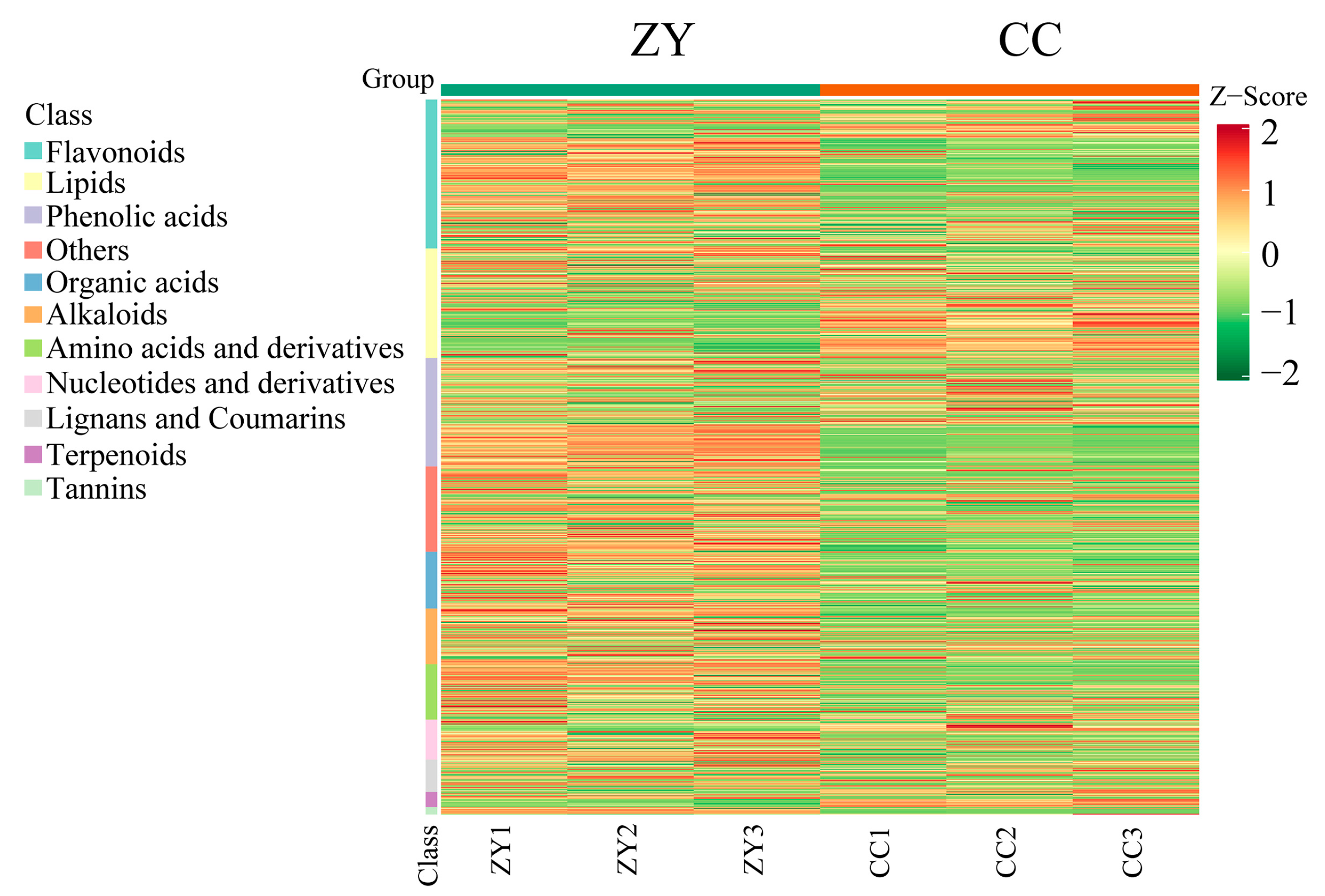
3.1. Detection of Metabolites in HT and IT
3.2. Identification of the Key Active Ingredients That Belong to TCMs in HT and IT
3.3. Identification of the Active Pharmaceutical Ingredients for Resistance to Major Diseases in HT and IT
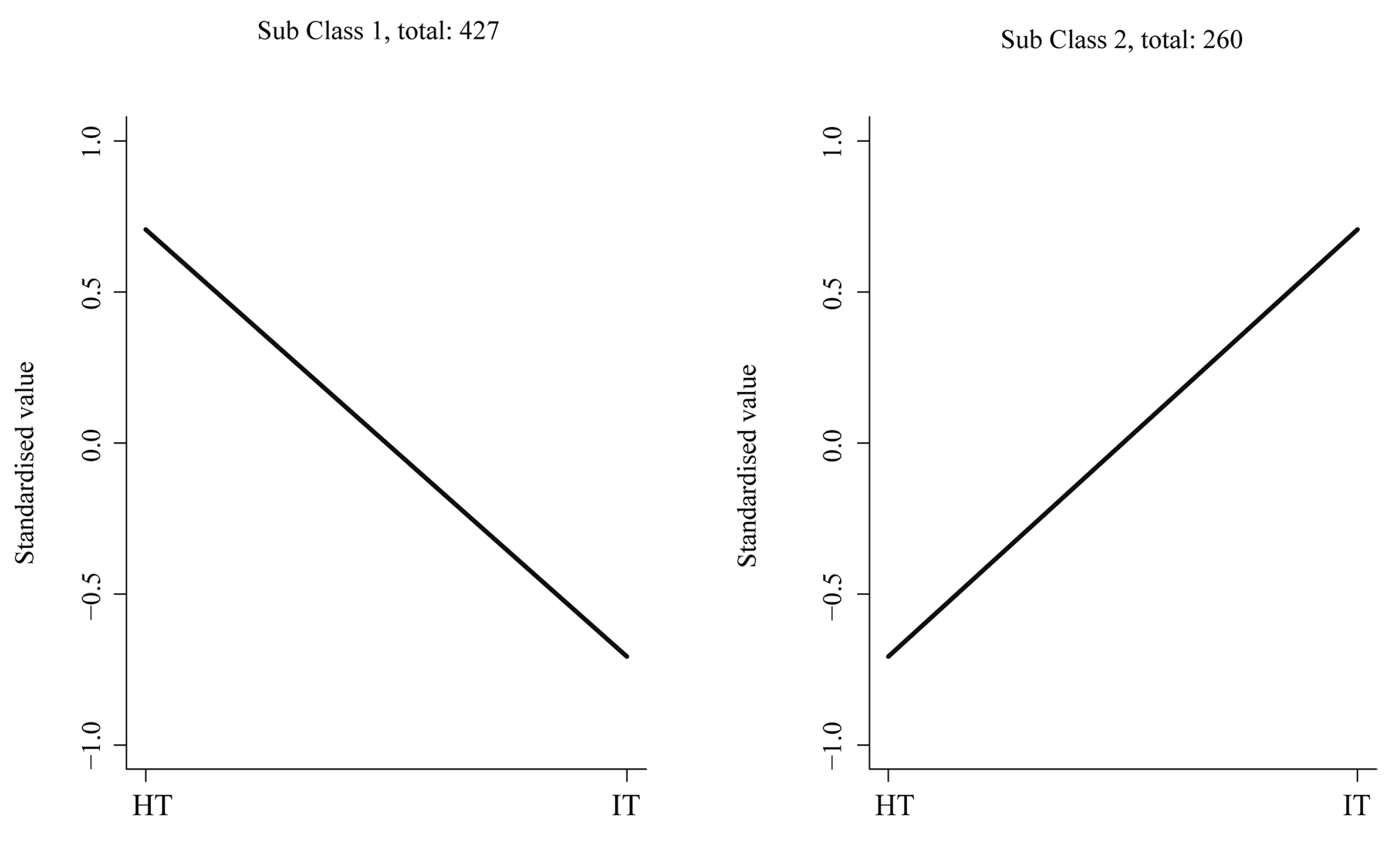
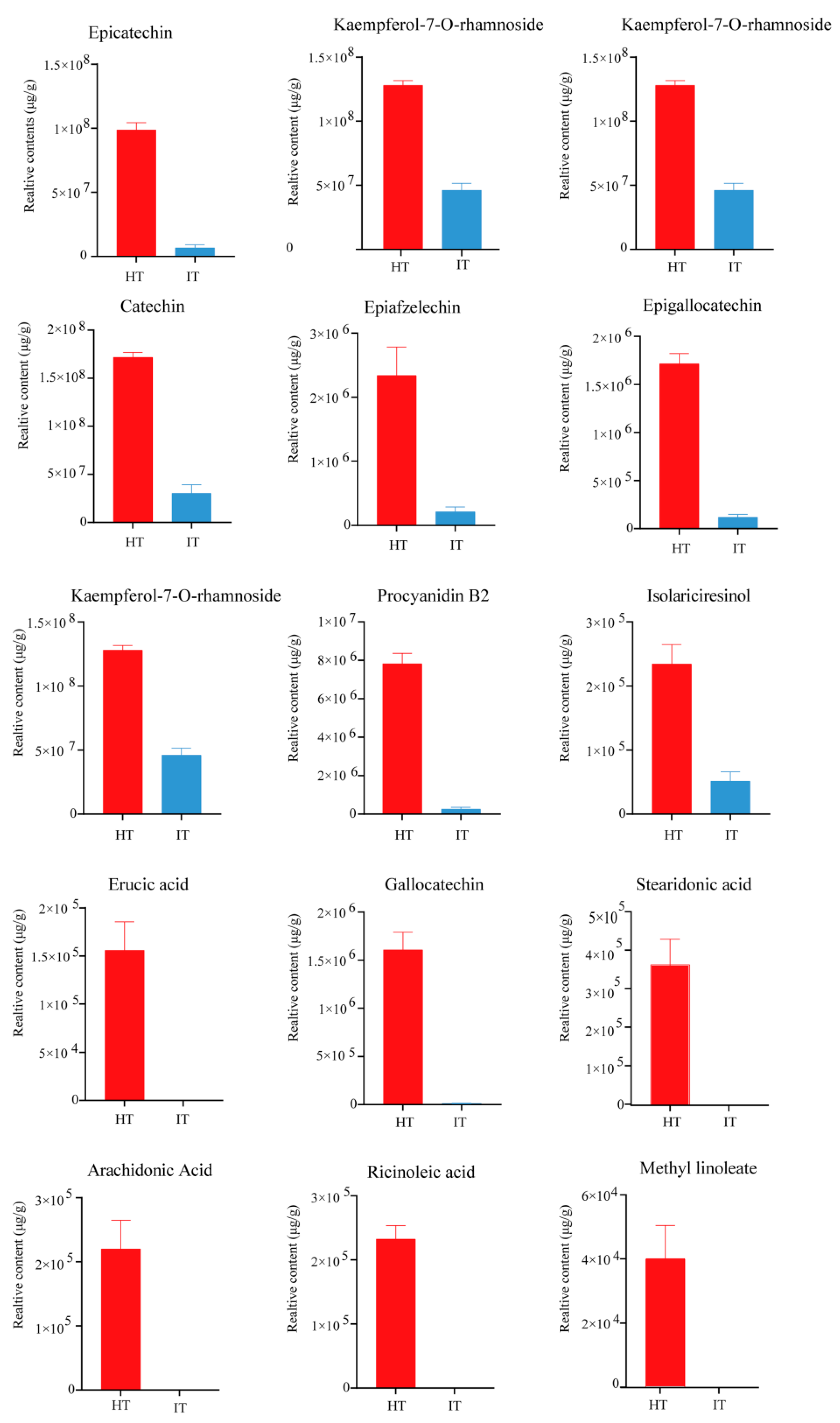
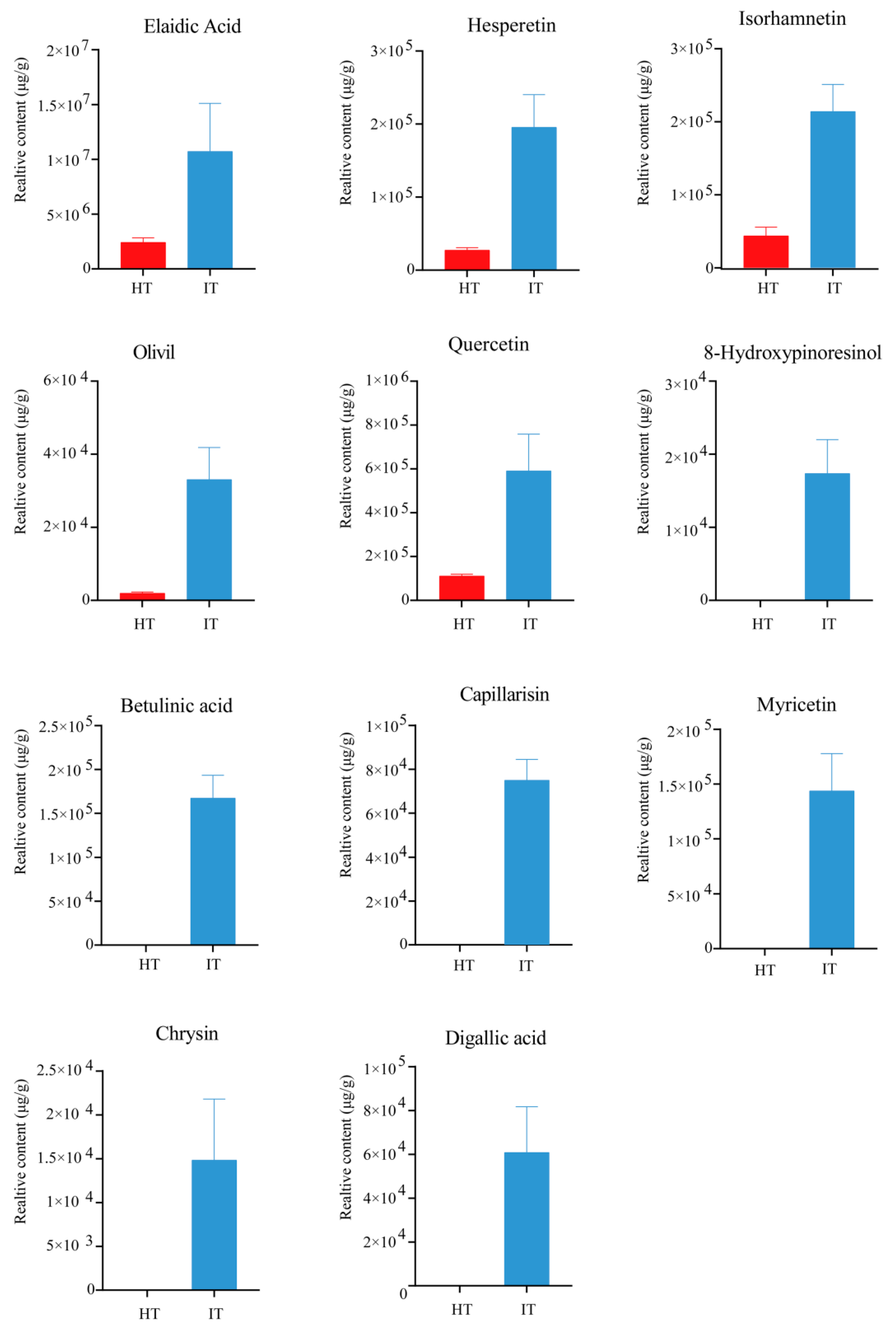
3.4. Profiles of Differential Metabolites in HT and IT
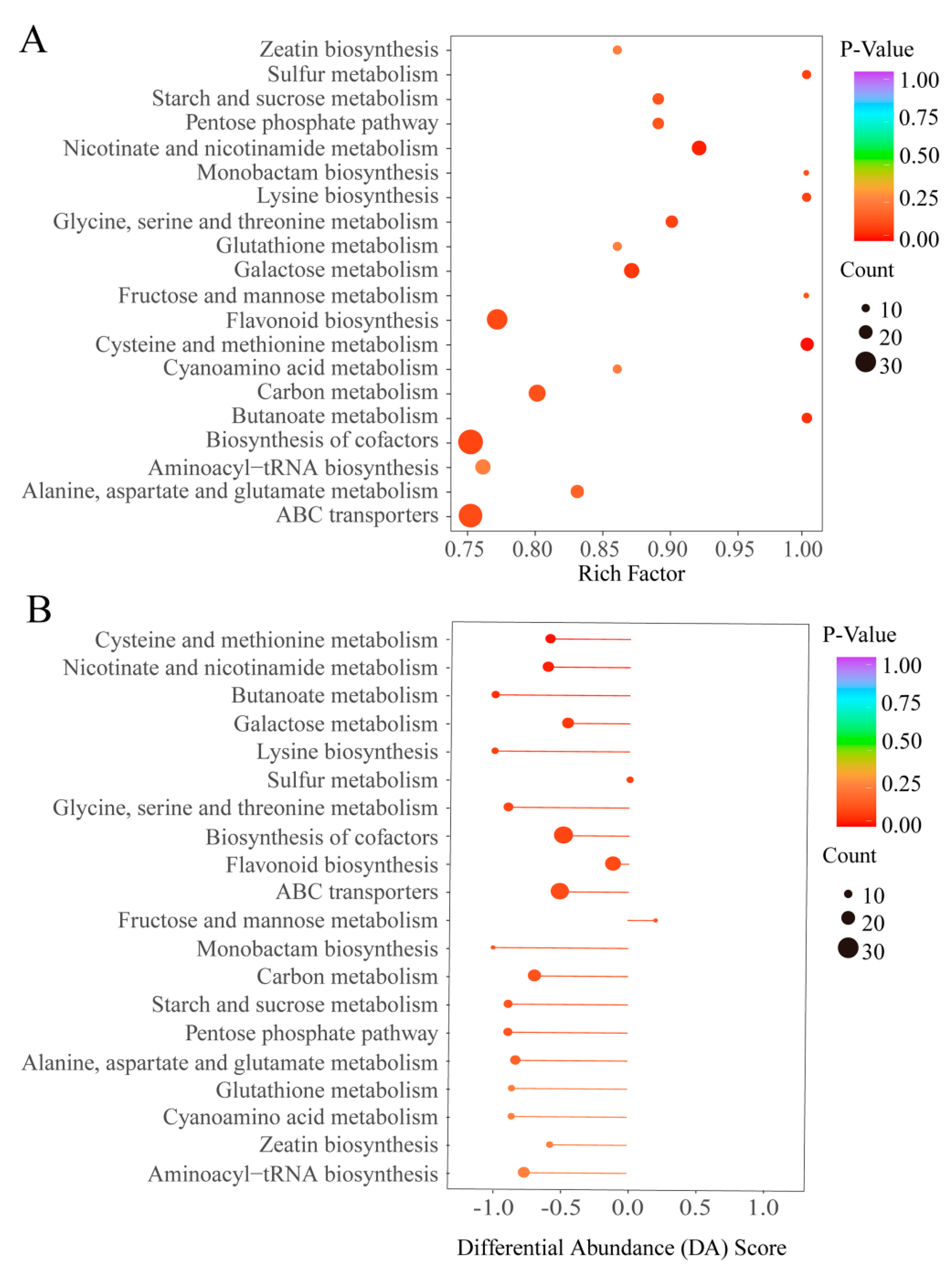
3.5. Characterization of the Differential Metabolites in HT and IT
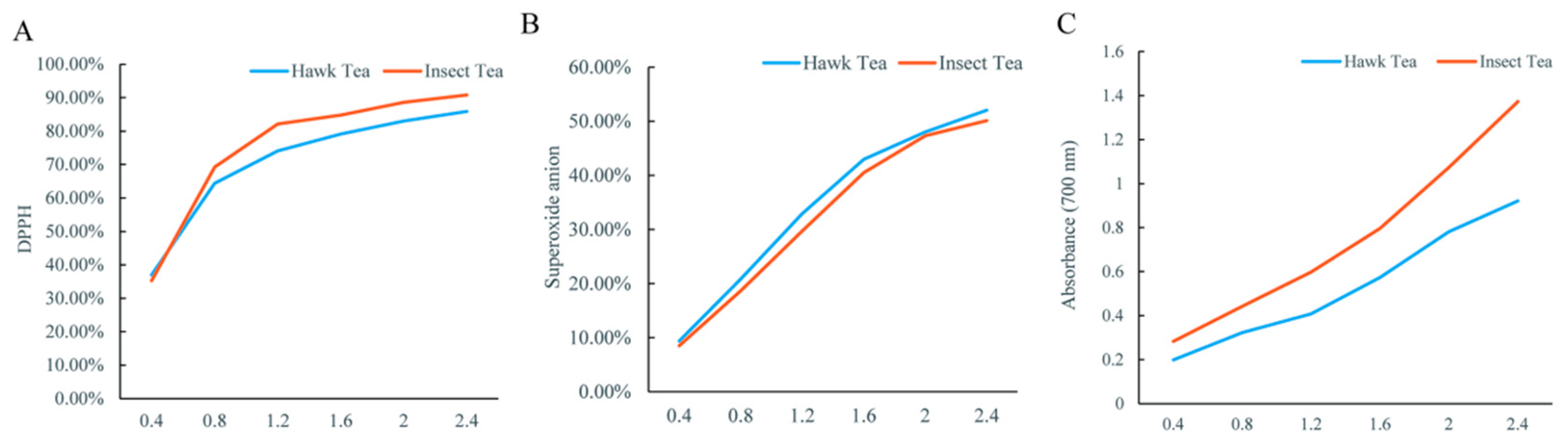
3.6. In Vitro Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guan, X.; Zhao, S.W.; Yang, J.F.; Xie, X.Y.; Lin, C.; Liu, Y.F. A New Framework for the Research of Tea Consumption: Based on the Two-way change of Tea Drinking Habit. J. Tea Commun. 2019, 46, 48–54. [Google Scholar]
- Yu, B.; Zhang, D.; Yan, X.W.; Wang, J.W.; Yao, L.; Tan, L.H.; Zhao, S.P.; Li, N.; Cao, W.G. Comparative evaluation of the chemical composition, antioxidant and antimicrobial activities of the volatile oils of Hawk tea from six botanical origins. Chem. Biodivers. 2016, 13, 1573–1583. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Z. Compendium of Materia Medica (Volume 2); People’s Health Publishing House: Beijing, China, 1982. [Google Scholar]
- Liu, J.; Yang, M.; Hu, J.; Shang, X.; Song, Q.; Li, B.; Sang, W. Investigation on the resource and utilization situation of insect tea in Guizhou. Guizhou Agric. Sci. 2015, 43, 62–65. [Google Scholar]
- Fedenko, V.S.; Shemet, S.A.; Landi, M. UV–vis spectroscopy and colorimetric models for detecting anthocyanin-metal complexes in plants: An overview of in vitro and in vivo techniques. J. Plant Physiol. 2017, 212, 13–28. [Google Scholar] [CrossRef]
- Ma, Y.; Shang, Y.; Zhong, Z.; Zhang, Y.; Yang, Y.; Feng, J.; Wei, Z. A new isoflavone glycoside from flowers of Pueraria Montana var. lobata (Willd.) Sanjappa & Pradeep. Nat. Prod. Res. 2021, 35, 1459–1464. [Google Scholar]
- Maji, A.K.; Pandit, S.; Banerji, P.; Banerjee, D. Pueraria tuberosa: A review on its phytochemical and therapeutic potential. Nat. Prod. Res. 2014, 28, 2111–2127. [Google Scholar] [CrossRef] [PubMed]
- Leisso, R.; Rudell, D.; Mazzola, M. Targeted metabolic profiling indicates apple rootstock genotype-specific differences in primary and secondary metabolite production and validate quantitative contribution from vegetative growth. J. Fron. Plant Sci. 2018, 9, 1336. [Google Scholar] [CrossRef]
- Poiroux-Gonord, F.; Bidel, L.P.; Fanciullino, A.L.; Gautier, H.; Lauri-Lopez, F.; Urban, L. Health benefits of vitamins and secondary metabolites of fruits and vegetables and prospects to increase their concentrations by agronomic approaches. J. Agric. Food Chem. 2010, 58, 12065–12082. [Google Scholar] [CrossRef]
- Robe, K.; Izquierdo, E.; Vignols, F.; Rouached, H.; Dubos, C. The coumarins: Secondary metabolites playing a primary role in plant nutrition and health. Trends Plant Sci. 2021, 26, 248–259. [Google Scholar] [CrossRef]
- Xin, Z.; Li, G.J. Comparison of Antioxidant Effects of insect tea and Its Raw Tea of Kuding Tea. Food Ind. 2015, 36, 235–238. [Google Scholar]
- Zhao, X.; Song, J.L.; Yi, R.; Li, G.; Sun, P.; Park, K.Y.; Suo, H. Comparison of antioxidative effects of insect tea and its raw tea (Kuding tea) polyphenols in Kunming mice. Molecules 2018, 23, 204. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Zhang, J.; Fu, X.; Yi, R.; Sun, P.; Zou, M.; Long, X.; Zhao, X. Preventive effect of raw Liubao tea polyphenols on mouse gastric injuries induced by HCl/ethanol via anti-oxidative stress. Molecules 2018, 23, 2848. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.Y.; Wen, L.Z.; Sheng, L.I.; Qin, L.H.; Deng, B.X.; Peng, Z.P.; Jiang, M.L.; Shi, B.J. Experimental Research of Sanye Worm-eaten Tea on Immuno-neurologic Regulation of Renal Hypertension in Rats. J. Hunan Univ. Chin. Med. 2006, 26, 4–6. [Google Scholar]
- Zhu, D.F.; Wen, L.Z.; Bi, B.F.; Xu, H.; Zhang, J. Hypoglycemic effects of Sanye insect tea and its tea-producing plants. Publ. Cent. South Univ. 2010, 6, 53–57. [Google Scholar]
- Xu, L.; Pan, H.; Lei, Q.; Xiao, W.; Peng, Y.; Xiao, P. Insect tea, a wonderful work in the Chinese tea culture. Food Res. Int. 2013, 53, 629–635. [Google Scholar] [CrossRef]
- Feng, X.; Luo, M.; Zhao, X. Inbibitional effect of sandy tea on the carcinoma cells growth and tumor metastasis. Mod. Food Sci. Technol. 2013, 29, 1898–1901+1905. [Google Scholar]
- Deng, X.X.; Zhao, X. Gastric Injury Preventive Effect of Different Concentrations of Sandy Tea in SD-rats. J. Beijing Union Univ. 2013, 26, 4–6. [Google Scholar]
- Suo, H.; Sun, P.; Wang, C.; Peng, D.; Zhao, X. Apoptotic effects of insect tea in HepG2 human hepatoma cells. CyTA-J. Food. 2016, 14, 169–175. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, X. In vitro anticancer effect of different concentrations of sandy tea in MCF-7 human breast adenocarcinoma cells. Food Res. Dev. 2014, 35, 16–19. [Google Scholar]
- Deng, M.; Zhang, X.; Luo, J.; Liu, H.; Wen, W.; Luo, H.; Yan, J.; Xiao, Y. Metabolomics analysis reveals differences in evolution between maize and rice. Plant J. 2020, 103, 1710–1722. [Google Scholar] [CrossRef]
- Hu, H.; Wang, J.; Hu, Y.; Xie, J. Nutritional component changes in Xiangfen 1 banana at different developmental stages. Food Funct. 2020, 11, 8286–8296. [Google Scholar] [CrossRef]
- Razzaq, A.; Sadia, B.; Raza, A.; Khalid, H.M.; Saleem, F. Metabolomics: A way forward for crop improvement. Metabolism 2019, 9, 303. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Iqbal, M.; Rasheed, R.; Hussain, L.; Riaz, M.; Arif, M.S. Environmental stress and secondary metabolites in plants: An overview. In Plant Metabolites and Regulation under Environmental Stress; Academic Press: Cambridge, MA, USA, 2018; pp. 153–167. [Google Scholar]
- Li, C.F.; Zhu, Y.; Yu, Y.; Zhao, Q.Y.; Wang, S.J.; Wang, X.C.; Yao, M.S.; Luo, X.L.; Li, X.; Chen, L.; et al. Global transcriptome and gene regulation network for secondary metabolite biosynthesis of tea plant (Camellia sinensis). BMC Genom. 2015, 16, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Arkorful, E.; Yu, Y.; Chen, C.; Lu, L.; Hu, S.; Yu, H.; Ma, Q.; Thangaraj, K.; Periakaruppan, R.; Jeyaraj, A.; et al. Untargeted metabolomic analysis using UPLC-MS/MS identifies metabolites involved in shoot growth and development in pruned tea plants (Camellia sinensis (L.) O. Kuntz). Sci. Hortic. 2020, 264, 109164. [Google Scholar] [CrossRef]
- Liu, D.; Ma, L.; Zhou, Z.; Liang, Q.; Xie, Q.; Ou, K.; Liu, Y.; Su, Y. Starch and mineral element accumulation during root tuber expansion period of Pueraria thomsonii Benth. Food Chem. 2021, 343, 128445. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zeng, M.; Ye, Y.; Liu, J.; Xu, P. Antiviral activity of puerarin as potent inhibitor of influenza virus neuraminidase. Phytother. Res. 2020, 35, 324–336. [Google Scholar] [CrossRef]
- Bao, W.; Wan, S.X. The cultural connotation and integration of insect tea and civet coffee. Agric. Arch. 2016, 5, 216–217. [Google Scholar]
- Wang, D.D.; Zhang, L.X.; Huang, X.R.; Wang, X.; Yang, R.N.; Mao, J.; Wang, X.F.; Zhang, Q.; Li, P.W. Identification of Nutritional Components in Black Sesame Determined by Widely Targeted Metabolomics and Traditional Chinese Medicines. Molecules 2018, 23, 1180. [Google Scholar] [CrossRef]
- Zou, S.; Wu, J.; Shahid, M.Q.; He, Y.; Yang, X. Identification of key taste components in loquat using widely targeted metabolomics. Food Chem. 2020, 323, 126822. [Google Scholar] [CrossRef]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Yu, S.; Xiong, L.Z.; Luo, J. A novel integrated method for large-scale detection, identification, and quantification of widely targeted metabolites: Application in the study of rice metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef]
- Yan, N.; Du, Y.; Liu, X.; Chu, M.; Shi, J.; Zhang, H.; Liu, Y.; Zhang, Z. A comparative UHPLC-QqQ-MS-based metabolomics approach for evaluating Chinese and North American wild rice. Food Chem. 2019, 275, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Dadwal, V.; Joshi, R.; Gupta, M. A comparative metabolomic investigation in fruit sections of Citrus medica L. and Citrus maxima L. detecting potential bioactive metabolites using UHPLC-QTOF-IMS. Food Res. Int. 2022, 157, 111486. [Google Scholar] [CrossRef]
- Jie, Z.; Liu, J.; Shu, M.; Ying, Y.; Yang, H. Detection strategies for superoxide anion: A review. Talanta 2022, 236, 122892. [Google Scholar] [CrossRef]
- Gulcin, I.; Kirecci, E.; Akkemik, E.; Topal, F.; Hisar, O. Antioxidant, antibacterial, and anticandidal activities of an aquatic plant: Duckweed (Lemna minor L. Lemnaceae). Turk. J. Biol. 2010, 34, 175–188. [Google Scholar]
- Valenzuela, A.; Sanhueza, J.; Niet, S. Natural antioxidants in functional foods: From food safety to health benefits. Grasas Aceites 2003, 54, 295–303. [Google Scholar] [CrossRef]
- Olaiya, C.O.; Soetan, K.O.; Esan, A.M. The role of nutraceuticals, functional foods and value added food products in the prevention and treatment of chronic diseases. Afr. J. Food Sci. 2016, 10, 185–193. [Google Scholar] [CrossRef]
- Li, H.Y.; Lv, Q.L.; Liu, A.; Wang, J.R.; Sun, X.Q.; Deng, J.; Chen, Q.F.; Wu, Q. Comparative metabolomics study of Tartary (Fagopyrum tataricum (L.) Gaertn) and common (Fagopyrum esculentum Moench) buckwheat seed. Food Chem. 2022, 371, 131125. [Google Scholar] [CrossRef]
- Yu, P.; Yeo, A.S.; Low, M.Y.; Zhou, W.B. Identifying key non-volatile compounds in ready-to-drink green tea and their impact on taste profile. Food Chem. 2014, 155, 9–16. [Google Scholar] [CrossRef]
- Batra, P.; Sharma, A.K. Anti-cancer potential of flavonoids: Recent trends and future perspectives. 3 Biotech. 2013, 3, 439–459. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Shishodia, S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006, 71, 1397–1421. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.Y.; Yu, Z.; Liu, X.Y.; Zeng, L.; Cheng, S.; Li, J.L.; Yang, Z.Y. Effect of major tea insect attack on formation of quality-related nonvolatile specialized metabolites in tea (Camellia sinensis) leaves. J. Agric. Food Chem. 2019, 67, 6716–6724. [Google Scholar] [CrossRef] [PubMed]
| Class | Anticancer Ingredients | Antidiabetic Ingredients | Anticardiovascular Ingredients | Antihypertensive Ingredients | Anti-Atheroscleroticingredients | Antithrombotic Ingredients | Antipain Ingredients |
|---|---|---|---|---|---|---|---|
| Phenolic acids(20) | Salicylic acid | Salicylic acid | Salicylic acid | ||||
| Protocatechuald- ehyde | |||||||
| Isovanillin | Isovanillin | Isovanillin | |||||
| 4-Methoxycinn- amaldehyde | 4-Methoxycinn- namaldehyde | 4-Methoxycinn- amaldehyde | |||||
| p-Coumaric acid | p-Coumaric acid | p-Coumaric acid | |||||
| Terephthalic acid | Terephthalic acid | Terephthalic acid | |||||
| 2-Hydroxy-3- phenylpropanoic acid | 2-Hydroxy-3- phenylpropanoic acid | 2-Hydroxy-3- phenylpropanoic acid | |||||
| Vanillic acid | Vanillic acid | ||||||
| 3-Hydroxy-4- methoxybenzoic acid | 3-Hydroxy-4- methoxybenzoic acid | 3-Hydroxy-4- methoxybenzoic acid | |||||
| 4-Methoxycinna-mic acid | 2-Methoxycinna- mic acid | 4-Methoxycinn- amic acid | |||||
| 2-Methoxycinna-mic acid | 2-Methoxycinna- mic acid | 2-Methoxycinna- mic acid | |||||
| Caffeic acid | Caffeic acid | Caffeic acid | Caffeic acid | ||||
| 4-O-Methylgallic acid | 4-O-Methylgallic acid | 4-O-Methylgallic acid | |||||
| Dimethyl phthalate | Dimethyl phthalate | ||||||
| Methyl caffeate | Methyl caffeate | Methyl caffeate | |||||
| Syringic acid | Syringic acid | ||||||
| Ferulic acid methyl ester | Ferulic acid methyl ester | ||||||
| Sinapinaldehyde | Sinapinaldehyde | Sinapinaldehyde | |||||
| Digallic acid | Digallic acid | ||||||
| Rosmarinic acid | Rosmarinic acid | Rosmarinic acid | |||||
| Flavnoids (16) | Chalcone | ||||||
| Chrysin | Chrysin | ||||||
| Epiafzelechin | Epiafzelechin | Epiafzelechin | |||||
| Epicatechin | Epicatechin | ||||||
| Catechin | Catechin | Catechin | |||||
| Chrysoeriol | Chrysoeriol | Chrysoeriol | |||||
| Quercetin | Quercetin | Quercetin | Quercetin | Quercetin | |||
| Hesperetin | Hesperetin | ||||||
| Gallocatechin | Gallocatechin | ||||||
| Epigallocatechin | Epigallocatechin | Epigallocatechin | |||||
| Isorhamnetin | Isorhamnetin | Isorhamnetin | |||||
| Myricetin | Myricetin | ||||||
| Kaempferol-7-O-rhamnoside | |||||||
| Catechin gallate | |||||||
| Quercetin-3-O- glucuronide | Quercetin- 3-O- glucuronide | Quercetin-3-O- glucuronide | |||||
| Isorhamnetin-3-O-neohesperidoside | |||||||
| Terpenoids (3) | Ursolic acid | Ursolic acid | |||||
| Betulinic acid | |||||||
| Cycloartenol | Cycloartenol | ||||||
| Lipids (15) | Tridecanoic acid | ||||||
| Myristic acid | Myristic acid | Myristic acid | |||||
| Pentadecanoic acid | Pentadecanoic acid | Pentadecanoic acid | |||||
| Palmitoleic acid | Palmitoleic acid | Palmitoleic acid | |||||
| Palmitic acid | Palmitic acid | Palmitic acid | |||||
| Methyl palmitate | Methyl palmitate | ||||||
| Stearidonic acid | Stearidonic acid | Stearidonic acid | |||||
| Elaidic acid | Elaidic acid | Elaidic acid | |||||
| Stearic acid | Stearic acid | Stearic acid | |||||
| Methyl linolenate | Methyl linolenate | Methyl linolenate | |||||
| Methyl linoleate | Methyl linoleate | Methyl linoleate | |||||
| Phytol | |||||||
| Ricinoleic acid | Ricinoleic acid | Ricinoleic acid | |||||
| Arachidonic acid | Arachidonic acid | Arachidonic acid | |||||
| Erucic acid | Erucic acid | Erucic acid | |||||
| Lignans and (9) coumarins | Coumarin | Coumarin | Coumarin | Coumarin | |||
| 4-Hydroxycou- marin | 4-Hydroxycou- marin | 4-Hydroxycou- marin | 4-Hydroxycoumarin | ||||
| Umbelliferone | Umbelliferone | Umbelliferone | Umbelliferone | ||||
| Esculetin | Esculetin | Esculetin | |||||
| Isofraxidin | Isofraxidin | Isofraxidin | Isofraxidin | ||||
| Isolariciresinol | Isolariciresinol | Isolariciresinol | |||||
| 8-Hydroxypinor- esinol | 8-Hydroxypinor- esinol | 8-Hydroxypinor-esinol | 8-Hydroxypinor- esinol | ||||
| Olivil | |||||||
| Syringaresinol | Syringaresinol | Syringaresinol | |||||
| Organic acids (11) | 3-Hydroxy-butyric acid | 3-Hydroxybutyric acid | 3-Hydroxybutyric acid | ||||
| Malonic acid | |||||||
| 2-Furoic acid | |||||||
| Succinic acid | |||||||
| 2-Hydroxyphen- ylacetic acid | 2-Hydroxyphen- ylacetic acid | 2-Hydroxyphen- ylacetic acid | |||||
| Phenylpyruvic acid | Phenylpyruvic acid | Phenylpyruvic acid | Phenylpyruvic acid | ||||
| Shikimic acid | Shikimic acid | ||||||
| Isocitric acid | Isocitric acid | Isocitric acid | |||||
| Citric acid | Citric acid | Citric acid | |||||
| Quinic acid | Quinic acid | ||||||
| Amino acids (9) | L-Serine | L-Serine | |||||
| L-Proline | |||||||
| L-Valine | L-Valine | L-Valine | |||||
| L-Asparagine | L-Aspara- gine | L-Asparagine | |||||
| L-Lysine | |||||||
| L-Glutamic acid | L-Glutamic acid | L-Glutamic acid | |||||
| L-Histidine | L-Histidine | ||||||
| L-Arginine | L-Arginine | ||||||
| L-Tryptophan | L-Tryptophan | L-Tryptophan | |||||
| Nucleotides (7) | Uracil | ||||||
| Adenine | Adenine | ||||||
| Hypoxanthine | Hypoxanthine | ||||||
| Guanine | Guanine | ||||||
| Thymidine | Thymidine | ||||||
| Uridine | |||||||
| Uridine 5’- monophosphate | |||||||
| Tannins (2) | Procyanidin B2 | Procyanidin B2 | |||||
| Procyanidin B1 | Procyanidin B1 | Procyanidin B1 | |||||
| Alkaloids (1) | Indole-3- carboxylic acid | ||||||
| Stilbene (2) | Resveratrol | Resveratrol | |||||
| Pterostilbene | Pterostilbene | Pterostilbene | |||||
| Saccharide and alcohol (1) | D-sorbitol | D-sorbitol | |||||
| Vitamin (2) | Nicotinamide | Nicotinamide | Nicotinamide | ||||
| Pyridoxine | Pyridoxine | Pyridoxine | Pyridoxine | ||||
| Others (4) | 5-Hydroxyme- thylfurfural | 5-Hydroxyme- thylfurfural | 5-Hydroxyme- thylfurfural | ||||
| Butylideneph- thalide | |||||||
| Sarisan | |||||||
| Capillarisin | Capillarisin | Capillarisin | |||||
| Total numbers | 83 | 5 | 69 | 24 | 17 | 39 | 41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, Q.; Yao, X.; Chen, H.; Tang, H.; Lu, L. Identification of Nutritional Ingredients and Medicinal Components of Hawk Tea and Insect Tea Using Widely Targeted Secondary Metabolomics. Horticulturae 2023, 9, 457. https://doi.org/10.3390/horticulturae9040457
Sheng Q, Yao X, Chen H, Tang H, Lu L. Identification of Nutritional Ingredients and Medicinal Components of Hawk Tea and Insect Tea Using Widely Targeted Secondary Metabolomics. Horticulturae. 2023; 9(4):457. https://doi.org/10.3390/horticulturae9040457
Chicago/Turabian StyleSheng, Qi, Xinzhuan Yao, Hufang Chen, Hu Tang, and Litang Lu. 2023. "Identification of Nutritional Ingredients and Medicinal Components of Hawk Tea and Insect Tea Using Widely Targeted Secondary Metabolomics" Horticulturae 9, no. 4: 457. https://doi.org/10.3390/horticulturae9040457
APA StyleSheng, Q., Yao, X., Chen, H., Tang, H., & Lu, L. (2023). Identification of Nutritional Ingredients and Medicinal Components of Hawk Tea and Insect Tea Using Widely Targeted Secondary Metabolomics. Horticulturae, 9(4), 457. https://doi.org/10.3390/horticulturae9040457






