Abstract
Sages are medicinal and aromatic plants that constitute a large pool from which active compounds of great pharmaceutical potential can be derived, while at the same time, they also have ornamental value. The purpose of this study was to develop the micropropagation protocols of Salvia fruticosa, S. officinalis, S. ringens, S. tomentosa, and S. pomifera ssp. pomifera to facilitate their exploitation in the pharmaceutical and floriculture industries. In vitro cultures of S. ringens and S. pomifera ssp. pomifera was studied for the first time. Shoot tips and single node explants from in vitro seedlings were initially cultured on hormone free (Hf)-MS medium, followed by subcultures on MS medium supplemented with 6-benzyladenine (BA) for all species, as well as with zeatin (ZEA), kinetin (KIN), 6-(γ,γ-dimethylallyamino) purine (2iP), or meta-topolin (mT) for S. fruticosa and S. officinalis, at concentrations 0.0 to 3.2 mg L−1, in combination with 0.01 mg L−1 1-naphthaleneacetic acid (NAA). S. officinalis was the most efficient in shoot multiplication of all the studied species. The highest multiplication indices were found using 0.8 mg L−1 BA for S. fruticosa, 0.4 mg L−1 BA, or mT for S. officinalis, and lower than 0.8 mg L−1 BA for the other three species. Hyperhydricity was a problem at the multiplication stage, and was most pronounced in single node explants, increasing in proportion to cytokinin concentration. Microshoots rooted at high percentages (75–85%) on half-strength MS medium with 0.0 or 0.5 mg L−1 Indole-3-butyric acid (IBA), except for those of S. ringens, which rooted best at 1.0–2.0 mg L−1 IBA. Ex vitro acclimatization was highly successful (80–95%) on peat–perlite substrate (1:1 v/v). Thus, the present study resulted in efficient micropropagation protocols for five Mediterranean sage species native to Greece, which will facilitate breeding programs and the promotion of these species in the floriculture and pharmaceutical industries.
1. Introduction
The genus Salvia (tribe Mentheae) is the largest in the Lamiaceae family, with 986 accepted species names [] displaying a remarkable range of variation; it is not a monophyletic genus []. It has undergone marked species radiations in three regions of the world, Mediterranean and Central Asia, Eastern Asia, and Central and South America []. Salvia is distinguished from other Mentheae by its staminal structure, having two stamens instead of the four found in most members of the tribe Menthae, and a distinct stamen morphology []. The name Salvia comes from the Latin salvus (healthy), and a plant called “Salvia” by the Romans, likely the type species for the genus Salvia, S. officinalis, was described for the first time by Pliny the Elder. The common name in English, sage, is attributed to different species of the genus Salvia, which are widely used as ornamental or medicinal plants [].
Sages are medicinal and aromatic plants from which active compounds of great pharmaceutical potential can be derived. The leaves of sages are rich in essential oils and secondary metabolites such as phenolics and terpenoids, and there is widespread knowledge and use of many native Salvia spp. in various countries for medicinal and perfumery purposes, as well as culinary plants, supported by a huge number of scientific publications [,,,,,,,]. In addition, numerous cultivars, varieties, and hybrids of Salvia are widely used worldwide as ornamental plants for their flower interest and fragrant foliage. Further, Salvia spp. are valuable along with other aromatic herbs for healing gardens and therapeutic landscapes, which are gaining ground internationally [].
The climate crisis has reinforced the need for the ecological and sustainable design of green projects. Therefore, Salvia spp. native to eastern Mediterranean regions are worth promoting for use in these landscape regeneration contexts, due to their resistance to xerothermic conditions, low maintenance needs, and their contribution to the rescue of insect pollinators [,,,,]. In addition, the floriculture industry is constantly looking for new plant species to compete in international markets, and the Mediterranean flora is an inexhaustible reservoir.
In Greece are found 30 taxa (species and subspecies) of the genus Salvia []. Two of these are widely known commercially as medicinal and ornamental plants, S. officinalis and S. fruticosa (S. triloba), and there are hundreds of research papers on their properties [,,,,,,,,]. However, a number of the other native sages of Greece have also been studied for their medicinal, cosmetic, and floricultural value [,,,,,,,,,,,].
The research project SALVIA-BREED-GR (https://www.salvia-breed-gr.com/el/) was carried out for four years (2019–2022) in order to explore the native sages of Greece, with the aim of promoting to the ornamental plant industry new sage products of high ornamental value suitable for xeriscaping. Four species, S. fruticosa Mill., S. officinalis L., S. ringens Sibth., and Sm., S. tomentosa Mill.; and one subspecies, S. pomifera L. ssp. pomifera, members of macchia vegetation, were selected for this project, and among other parameters, their seed and clonal propagation were studied.
S. fruticosa (Greek sage Figure 1a), is a strongly aromatic perennial evergreen shrub (120 cm tall), bearing lilac or pink flowers (Figure 1b) in early spring. It is endemic to the Mediterranean coastal zone, with a wider distribution from Sicily to Israel. In Greece, it is found in Central Greece, the Peloponnese, and the Aegean islands []. It is widely used for the preparation of an herbal tea (faskomilo).

Figure 1.
Native to Greece Salvia spp.; S. fruticosa plant (a) and inflorescence (b), S. officinalis plant (c) and inflorescence (d), S. ringens plant (e) and inflorescence (f), S. tomentosa plant (g) and inflorescence (h), S. pomifera ssp. pomifera plant (i) and inflorescence (j).
S. officinalis (Dalmatian sage, common sage, sage, (Figure 1c) is a strongly aromatic perennial evergreen shrub (60 cm tall), bearing violet-blue flowers (Figure 1d) in May–July. It is naturally widespread on the Apennines and the eastern Adriatic coast, but naturalized in many places throughout the world []. In Greece, it is found in the north and the east, and in the Ionian islands. Since antiquity, it has been used as a medicinal plant, while nowadays is cultivated in many varieties as a medicinal and ornamental plant, being one of the most important Salvia species worldwide.
S. ringens (Figure 1e) is a perennial evergreen plant with a slightly woody base and low vegetation (30 cm). Its leaves have a light aroma. It bears a few violet-blue flowers on branching long (60 cm) stocks (Figure 1f) during late spring through summer. It is found in the southern and eastern parts of the Balkan Peninsula. In Greece, it spreads north, and in the highlands of Macedonia, Epirus, and Central Greece [].
S. tomentosa (Figure 1g) is a strongly aromatic perennial evergreen plant (80 cm tall), bearing violet flowers with reddish-brown calyces (Figure 1h) in late spring or early summer. Its geographical distribution extends from South-Eastern Europe to Transcaucasia []. In Greece, it is found in the central and north-eastern areas, and in Eastern Aegean Islands [].
S. pomifera ssp. pomifera (Figure 1i) is an endemic shrub of Greece found in Crete and the Peloponnese []. It is a strongly aromatic perennial evergreen plant (80 cm tall), bearing pink or violet flowers (Figure 1j) in spring to early summer on slightly curving flower stocks.
All the above sages have been found to be suitable for use as ornamental plants under limited water supply [,,,], which makes them valuable plants for xeriscaping and landscaping in arid and semi-arid regions. Research on their propagation using stem cuttings has shown that this is a suitable method for their commercial production [], although its effectiveness may depend on the season or climatic conditions. In contrast, the seed propagation of these sage species was found to be unstable in terms of germination rates, and in most species, especially in S. ringens and S. tomentosa, the germination rate was extremely low [,,], verifying that seed germination is a global problem of the Salvia spp. [,]. Concerning the micropropagation of these Mediterranean Salvia spp., there are many works published on the in vitro culture of S. officinalis [,,,,,,,,,], two on S. fruticosa [,], and one on S. tomentosa [] while there were no reports found on the micropropagation of S. ringens and S. pomifera ssp. pomifera.
The commercial production of many ornamental, medicinal, and aromatic plants is based on the use of tissue culture and micropropagation at some stage of their development; micropropagation technique is important for developing, selecting, multiplying and conserving the critical genotypes of these plants [,,]. In medicinal plants, tissue culture is used to produce active compounds for pharmaceutical and herbal industries. In addition, in vitro culture techniques are applied for the conservation of the genetic material of many threatened medicinal plants [].
Shoot proliferation in most micropropagation protocols proposed for S. officinalis was low, as in most cases, up to three shoots per explant were produced using shoot tip or nodal explants on MS medium [] supplemented with rather low concentrations (0.5 to 1.5 mg L−1) of 6-benzyladenine (BA), alone or in combination with a low concentration (0.1 mg L−1) of 1-naphthaleneacetic acid (NAA) [,,,,]. Even lower BA concentrations (0.2–0.5 mg L−1) were found to be appropriate for the shoot proliferation of S. fruticosa [], inducing lower proliferation indices compared to S. officinalis. In S. officinalis, a liquid culture in MS with BA and a low concentration of auxin resulted in similarly low proliferation, as in solid medium [], while the proliferation was doubled when triacontanol at 20 mg L-I was added into the liquid medium []. Similarly increased proliferation was achieved when adventitious shoots were induced from nodal or leaf explants excised from in vitro seedlings on MS medium with 1.5 mg L−1 thidiazuron (TDZ), combined with 0.1 mg L−1 indole-3-acetic acid (IAA) [].
The nature and origin of explants has been shown to significantly influence the in vitro response [,,,], as the ability for totipotency differs in plant cells. In the micropropagation of S. officinalis, both shoot tip explants [,,] and nodal explants [,,,] have been used, and more often, shoot tips showed higher response rates [], as has also been shown in other medicinal Lamiaceae herbs [,]. In S. fruticosa [] and other Mediterranean sages [,], as well as in other medicinal Lamiaceae herbs, nodal explants have been shown to produce more shoots than the shoot tip explants [,,,,]. Further, the juvenile or adult origins of the explants may affect their response to micropropagation differently. Explants originating from seedlings often present high shoot proliferation, as has been shown for some native Mediterranean species [,]. The use of seedlings as mother plants is recommended when native species are reintroduced to the landscape, because a higher genetic diversity is promoted. In addition, seedling mother material contributes to the selection of specific genotypes that present special characteristics as high medicinal value.
The in vitro rooting of medicinal Lamiaceae herbs, including Salvia spp., presented no difficulties [,,,,,,,,,,,,,], and in a number of these species, auxin supplementation was not necessary for rooting [,,,,]. In contrast, S. fruticosa microshoots rooted only in the presence of auxin []. Half-strength MS medium supplemented with IBA at 0.5–1.5 mg L−1 and occasionally up to 4.0 mg L−1 has been the most appropriate protocol, with a few exceptions in which full-strength MS medium was used [,].
The aim of this work was to develop protocols for the micropropagation of S. fruticosa, S. officinalis, S. ringens, S. tomentosa, and S. pomifera ssp. Pomifera, in order to facilitate their introduction into the pharmaceutical and floriculture industries, by providing high-value plant material for cultivation without seasonal constraints and the selection and conservation of critical genotypes. The micropropagation of S. ringens and S. pomifera ssp. pomifera were studied for the first time to our knowledge. The in vitro micropropagation studies of S. fruticosa and S. officinalis aimed to further improve the protocols due to the commercial importance of these species. Thus, five types of cytokinins at various concentrations were tested as for their efficacy in shoot proliferation from shoot tip and singe node explants of seedling origin. In vitro rooting and ex vitro acclimatization were also studied, and the responses of the five species to all stages of micropropagation were compared and discussed.
2. Materials and Methods
2.1. In Vitro Culture Establishment Stage
The establishment of in vitro cultures was performed on hormone free (Hf)-MS medium [] with 30 g L−1 sucrose, under the culture conditions described below. Shoot tip and single node explants of the 1st, 2nd, or 3rd visible node below the shoot tip, about 0.6 cm long, excised from 3-month-old in vitro grown seedlings of S. fruticosa, S. officinalis, S. ringens, S. tomentosa, and S. pomifera ssp. pomifera were used as explants. The seedlings were germinated in vitro, following the method described by Vlachou et al. [], and grown on Hf-MS medium with 30 g L−1 sucrose.
2.2. Shoot Multiplication Stage
Aiming to increase the plant material to be used in experiments on shoot proliferation, up to six subcultures (the number depending on Salvia spp.) on MS medium supplemented with 0.4 mg L−1 6-benzyladenine (BA) and 0.01 mg L−1 1-naphthaleneacetic acid (NAA) were performed for each Salvia species.
The media used in the shoot multiplication stage were based on preliminary work, and on previous reports on Mediterranean sages and other Lamiaceae [,,,,].
2.2.1. Effect of Explant Type and BA Concentration on the Proliferation of the Five Salvia spp.
The aim of this experiment was to investigate the effect of BA concentration in relation to explant type (shoot tip and single node) and Salvia species, on shoot proliferation. Shoot tip and single node explants of the five Salvia spp. were cultured, either on Hf-MS medium or on MS medium supplemented with BA at four concentrations, i.e., 0.4, 0.8, 1.6, and 3.2 mg L−1, in combination with 0.01 mg L−1 NAA.
The explants were 0.6 cm long and they were excised from microshoots grown on MS medium supplemented with 0.4 mg L−1 BA and 0.01 mg L−1 NAA.
2.2.2. Effect of Explant Type, and Cytokinin Type and Concentration on the Proliferation of S. fruticosa and S. officinalis
Shoot tip and single node explants, 0.6 cm long, were excised from the microshoots of in vitro cultures (on MS medium with 0.4 mg L−1 BA and 0.01 mg L−1 NAA) of S. fruticosa and S. officinalis, and cultured on MS medium with 30 g L−1 sucrose. The medium was either Hf (control) or supplemented with 0.01 mg L−1 1-naphthaleneacetic acid (NAA) in combination with a cytokinin of five different types, i.e., BA, zeatin (ZEA), kinetin (KIN), 6-(γ,γ-dimethylallylamino) purine (2iP), or meta-topolin (mT), at four concentrations, i.e., 0.4, 0.8, 1.6, and 3.2 mg·L−1.
2.2.3. Explant Number and Data Collection at the Shoot Multiplication Stage
The number of explants used in each experiment at the multiplication stage is presented at the base of each relevant data table.
Data were collected after 30 d of culture. The percentage of explants that responded to form shoots, the percentage of those that formed normal shoots or normal, together with hyperhydrated shoots and the percentage of explants that formed only hyperhydrated shoots (Figure 2), were recorded. The number of normal and hyperhydrated shoots produced, shoot length, and the number of nodes per shoot of the normal shoots were also recorded. The “multiplication index” of each culture was calculated by multiplying the percentage of explants that produced normal shoots (i.e., explants with all shoots hyperhydrated were not included) by the mean number of normal shoots per responding explant, and by the mean node number per normal shoot.

Figure 2.
Responses of single node explants of S. officinalis during the multiplication stage to produce normal and hyperhydrated shoots, (a) or hyperhydrated shoots only (b). NSh = normal shoot, HSh = hyperhydrated shoot. Size bars= 1.0 cm.
2.3. In Vitro Rooting
For the experiment on in vitro root induction and development, microshoots produced on MS medium supplemented with 0.4 or 0.8 mg L−1 BA and 0.01 mg L−1 NAA, approximately 2.0 cm long, were used. Microshoots were cultured on half-strength MS medium with 20 g L−1 sucrose supplemented with various concentrations of IBA, i.e., 0.0, 0.5, 1.0, 2.0, or 4.0 mg L−1.
Three replicates of 10 microshoots were used for each treatment. Data were collected after 30 d of culture. The percentage of explants that formed roots, and the number and length of roots were recorded.
2.4. Ex Vitro Acclimatization
For ex vitro acclimatization, the rooted microshoots of all rooting media after being rinsed thoroughly with running tap water to remove growth medium were transferred to 500 mL containers (eight plantlets per container), on peat (high-moor with adjusted pH of up to 5.5–6.5, Klasmann-Delimann Gmbh, Geeste, Germany) and perlite (particles diameter 1–5 mm, Perloflor, ISOCON S.A., Athens, Greece) substrate 1:1 (v/v), were transferred ex vitro into trays (eight plantlets per 500 mL volume tray) with a mixture of peat: perlite (1:1, v/v). The trays were covered with plastic wrap (SANITAS; Sarantis S.A., Greece) and placed in a growth chamber (20 °C and 16 h cool white fluorescent light, 37.5 mmol·m−2·s−1) for 1 week. Then, the plastic wrap was removed and transferred to a heated glasshouse (37°58′58.0″ N, 23°42′19.2″ E) for an additional 4 weeks.
Six to 10 replicates of eight rooted microshoots were used, and their survival was estimated at 30 d after transfer to the greenhouse.
2.5. In Vitro Culture Conditions
All media were solidified with 8 g L−1 agar, and their pH was adjusted to 5.7 before agar addition and autoclaving (121 °C for 20 min). Initial cultures from in vitro seedlings, subcultures, and rooting experiments took place in 145 mL glass vessels with 25 mL medium (four explants or microshoots per vessel), covered with a magenta plastic cap. The cultures were maintained at 25 ± 2 °C with a 16 h photoperiod at 37.5 μmol m−2 s−1 fluorescent light, provided by cool-white fluorescent lamps.
2.6. Statistical Analysis
A completely randomized design was used. The significance of the results was tested through either one-, two-, or three-way analysis of variance (ANOVA), and the means of the treatments were compared via Student’s t test at p ≤ 0.05 (JMP 13.0 software, SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Establishment Stage
For the establishment of in vitro cultures, seedlings that germinated and grew in vitro were used as mother plants. The germination rates of S. ringens, S. pomifera ssp. pomifera, and S. tomentosa were very low, and especially in S. tomentosa, it was lower than 10% []. Thus, for these sage species, the mother material was limited. At the culture establishment stage, which took place on Hf-MS medium, shoot tip explants of all five Salvia spp. responded at higher percentages to produce normal shoots compared to single node explants (26–93% vs. 10–75%, depending on the species), as the latter showed higher hyperhydricity, which in the case of S. pomifera ssp. pomifera and S. tomentosa reached 40–50%. Apart from hyperhydricity problems, S. tomentosa also showed much a lower explant response to producing shoots, compared to all other species (Table 1). In all of the sage species, single node explants, which bear two buds due to the phyllotaxis of Salvia spp., produced more shoots compared to shoot tip explants, but shoots on single node explants were shorter and with fewer nodes (Table 1, Figure 3) and thus, the shoot tip explants presented higher multiplication indices compared to single node explants (Table 1), with the exception of S. officinalis.

Table 1.
Effect of Salvia species and explant type on axillary shoot production of explants excised from in vitro seedlings of Greek sage species, at the establishment stage of in vitro cultures on a hormone-free MS medium.

Figure 3.
Typical responses of shoot tip and single node explants excised from in vitro seedlings of Salvia spp., during the in vitro establishment stage on hormone-free MS medium. Size bars = 1.0 cm.
3.2. Shoot Multiplication on BA Media
3.2.1. S. fruticosa
In S. fruticosa, both type of explants, shoot tip, and single node, responded at high percentages (70–100%) to produce shoots in all media tested, with single node explants showing a decrease in response with increasing BA concentration, which was significantly lower compared to the shoot tip explants in all BA media (Figure 4a). Some of the explants produced only hyperhydrated shoots, while others produced both normal and hyperhydrated shoots. An increase in BA concentration resulted in an increase in hyperhydrated explants that was more pronounced in single node ones, which at the two highest BA concentrations, only 20–30% produced normal shoots and 40–50% produced only hyperhydrated shoots (Figure 4b). In contrast, at the highest BA concentration, shoot tip explants produced normal shoots in 68%, and only hyperhydrated shoots in 32% (Figure 4b).
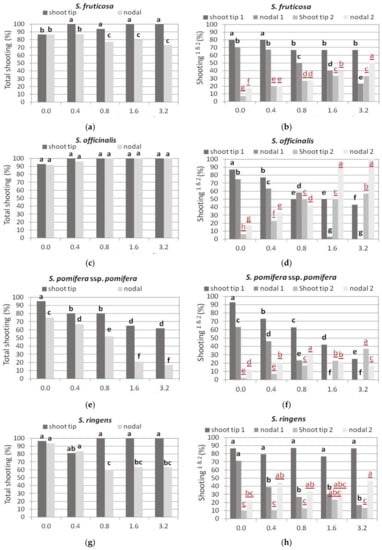
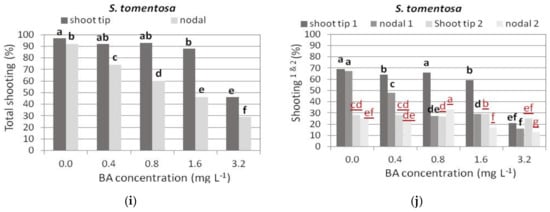
Figure 4.
Effect of 6-benzyladenine (BA) concentration on explant response percentage for shoot production when shoot tip or single node (nodal) explants of each Salvia species shown were cultured on MS medium, either hormone free or supplemented with 0.01 mg L−1 1-naphthaleneacetic acid (NAA) and BA concentration marked. Total shooting (%): percentage of explants that responded for shoot production. Explants formed both normal and hyperhydrated shoots. Explants formed only hyperhydrated shoots. Response of explants (black bold letters) was statistically analyzed separately from the respective explants (red underlined letters). Mean separation using Student’s t, p ≤ 0.05; means followed by the same letter are not significantly different at p ≤ 0.05, n = 30.
The number of shoots per responding explant was higher in single node explants when normal and hyperhydrated shoots were counted together (Figure 5a and Figure 6a), but as single node explants formed more hyperhydrated shoots compared to the tip explants, the production of normal shoots was not statistically significantly different between the two types of explants (Figure 5b). At 0.8 mg L−1 BA; however, single node explants produced significantly more normal shoots compared to the tip ones (Figure 5b).
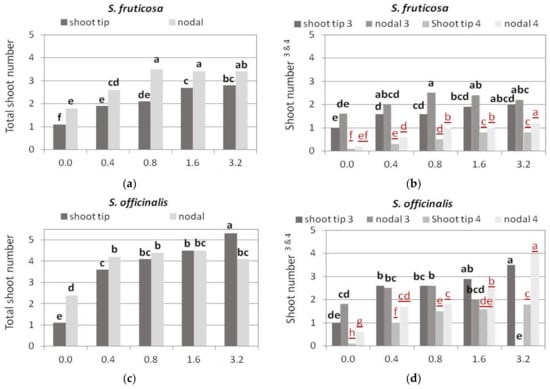
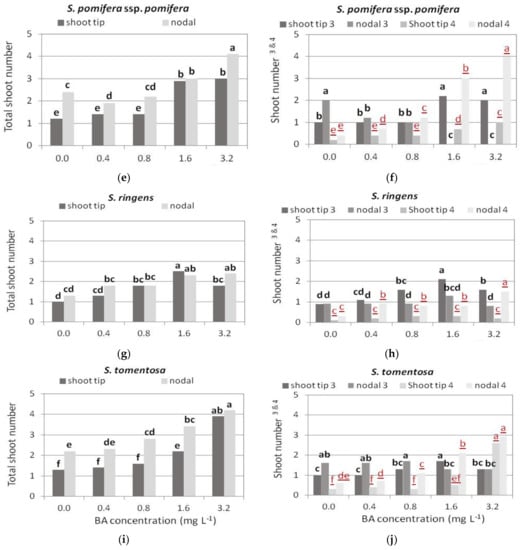
Figure 5.
Effect of BA concentration on normal, hyperhydrated, and total number of shoots produced per explant of Salvia spp. shown when shoot tip or single node (nodal) explants were cultured on MS medium, either hormone free or supplemented with 0.01 mg L−1 NAA and BA concentration marked. Response for normal and hyperhydrated shoot production was statistically analyzed separately (black bold letters and red underlined letters, respectively). Mean separation via Student’s t, p ≤ 0.05; means followed by the same letter are not significantly different at p ≤ 0.05, n = 30. Normal shoots per explant. Hyperhydrated shoots per explant.

Figure 6.
Characteristic response of shoot tip and single node explants of Salvia fruticosa (a), S. officinalis (b), S. pomifera spp. pomifera (c), S. ringens (d) and S. tomentosa (e) during the multiplication stage, when cultured on MS medium either hormone free (Hf) or supplemented with 0.01 mg L−1 NAA and BA concentration (mg L−1) marked. Size bar s = 1.0 cm.
Shoot length and number of nodes per shoot were the highest in Hf medium, while all media with BA reduced to a similar extent the shoot length and number of nodes in the generated shoots, to half of that of Hf medium (Figure 7a,b).
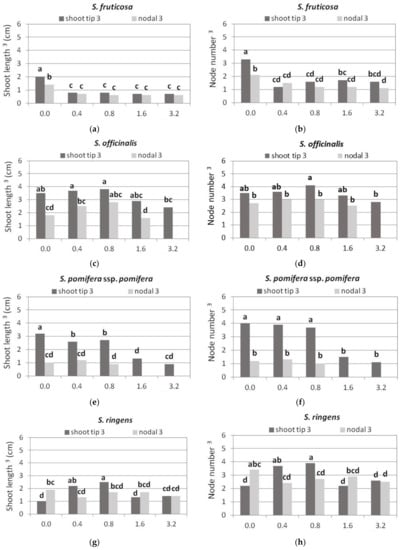
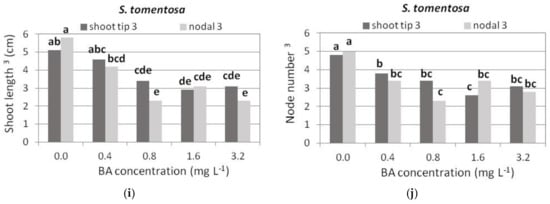
Figure 7.
Effect of BA concentration on shoot length (cm) and node number per shoot, produced using shoot tip or single node (nodal) explants of each Salvia species shown cultured on MS medium, either hormone free or supplemented with 0.01 mg L−1 NAA and BA concentration marked. Only normal shoots 3 were recorded. Mean separation using Student’s t, p ≤ 0.05; means followed by the same letter are not significantly different at p ≤ 0.05, n = 30. 3 Normal shoots per explant.
Both types of explants gave a similar multiplication index in Hf and low BA media, while at 1.6 and 3.2 mg L−1 BA, the multiplication index was significantly higher when shoot tip explants were used (Figure 8a).
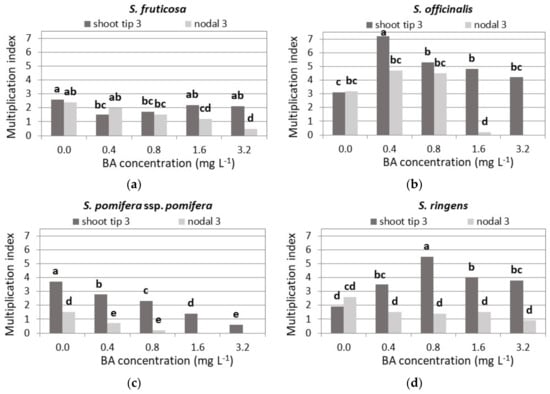
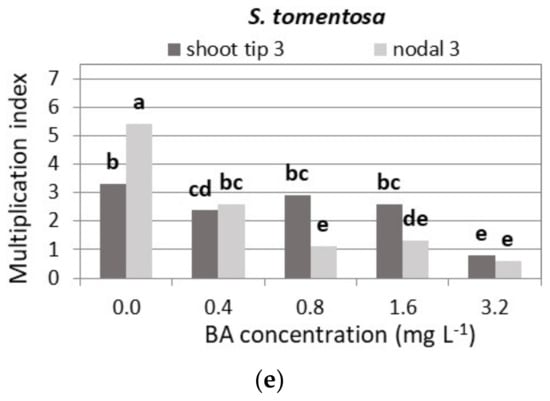
Figure 8.
Effect of BA concentration on the shoot multiplication index of Salvia fruticosa (a), S. officinalis (b), S. pomifera spp. pomifera (c), S. ringens (d) and S. tomentosa (e), when shoot tip or single node (nodal) explants were cultured on MS medium, either hormone free or supplemented with 0.01 mg L−1 NAA and BA concentration marked. Mean separation using Student’s t, p ≤ 0.05; means followed by the same letter are not significantly different at p ≤ 0.05, n = 30. Multiplication index = shoot-producing explants 1 (%) × mean shoot number 3 × mean node number 1. Explants that formed both normal and hyperhydrated shoots. 3 Normal shoots per explant.
3.2.2. S. officinalis
In S. officinalis, almost all explants of both types produced shoots (Figure 4c), but some of the explants produced only hyperhydrated shoots (Figure 4d). The explants showed the highest percentage of normal shoot production on Hf medium, which gradually decreased with the gradual increase in BA concentration, while the production of hyperhydrated explants gradually increased (Figure 4d). Shoot tip explants showed higher response percentages for normal shoot production compared to single node explants, while the latter showed higher percentages of hyperhydration, except in the medium with 0.8 mg L−1 BA (Figure 4d). At the highest BA concentrations, hyperhydration was 50–57 and 90% for shoot tip and single node explants, respectively (Figure 4d).
Increasing the BA concentration in the medium resulted in an increase in shoot production per explant (Figure 5c and Figure 6b), and at the same time, an increase in the hyperhydrated shoots (Figure 5d). So, at the two highest BA concentrations, the approximately five shoots formed per explant 2–4 were hyperhydrated, and in single node explants at 3.2 mg L−1 BA, all shoots were hyperhydrated (Figure 5d).
There was an indication that shoot length and node number per shoot were higher in shoot tip explants compared to single node explants, and this decreased at the highest BA concentration (Figure 7c,d).
The highest multiplication index was shown with 0.4 mg L−1 BA using shoot tip explants (Figure 8b).
3.2.3. S. pomifera ssp. pomifera
In S. pomifera ssp. pomifera, shoot tip explants showed the highest response to produce shoots (94%) on Hf medium (Figure 4e), and only 2% produced only hyperhydrated shoots (Figure 4f), while single node explants responded in a lower percentage (74%), and 12% of the responded explants produced only hyperhydrated shoots (Figure 4e,f). The gradual increase in BA concentration induced a proportional decrease in explant response, and an increase in hyperhydration (Figure 4e,f). The negative effect of BA on shoot production was stronger in single node explants, which at the two highest BA concentrations, responded at very low percentages (20%) and produced only hyperhydrated shoots (Figure 4e,f).
Single node explants produced higher shoot numbers at almost all BA concentrations compared to shoot tip explants (Figure 5e), but most of them were hyperhydrated, especially at the two highest BA concentrations (Figure 5f). Single node explants on Hf medium and shoot tip explants on the two highest BA concentrations produced the highest number of normal shoots (two shoots per explant, Figure 5f), while single node explants at the two highest BA concentrations produced double the number (3–4) shoots per explant, but they were all hyperhydrated (Figure 5f and Figure 6c).
Shoot tip explants gave longer shoots with a higher number of nodes compared to single node explants (Figure 7e,f). Shoot length gradually decreased with increasing BA concentration (Figure 7e). The number of nodes per shoot was similar in the HF medium and in the media with the two lowest BA concentrations (Figure 7f). Both shoot length and number of nodes were significantly reduced in the media with the two highest BA concentrations (Figure 7e,f).
Shoot tip explants showed much higher multiplication indices compared to single node explants. The multiplication index was highest in the HF medium and gradually decreased with increasing BA concentration (Figure 8c).
3.2.4. S. ringens
In S. ringens, the shoot tip explants had a consistent response for shoot production in all media tested compared to single node explants, whose response decreased at BA concentrations of 0.8 mg L−1 and higher (Figure 4g). In addition, shoot tip explants produced normal shoots at much higher percentages (78–88%), compared to single node explants (Figure 4h). Further, hyperhydration was more pronounced in single node explants, especially at the highest BA concentration (Figure 4h).
Increasing BA up to 1.6 mg L−1 resulted in a slight increase in both normal and hyperhydrated shoots (Figure 5g,h). Most normal shoots were produced at 1.6 mg L−1 BA (Figure 5h). In the two lowest BA concentrations, shoot tip explants gave slightly longer shoots with more nodes than single node explants, and the highest elongation and number of nodes (Figure 6d and Figure 7g,h).
Shoot tip explants resulted in higher multiplication indices than single node explants, except in the HF medium, which reached its highest value in the 0.8 mg L−1 BA medium (Figure 8d).
3.2.5. S. tomentosa
In S. tomentosa, shoot tip explants responded at higher percentages to produce shoots, both normal and hyperhydrated, compared to single node explants, and Hf medium induced the highest response, similar to 0.4 and 0.8 mg L−1 BA media, while the response percentage was almost halved in the medium with 3.2 mg L−1 BA (Figure 4i,j). The percentage of hyperhydrated explants was not affected by the medium plant growth regulators in both explant types (Figure 4j).
Single node explants produced a greater number of shoots in all media compared to shoot tip explants, and both explant types produced the highest number of shoots at the highest BA concentration (Figure 5i). Single node explants also produced more normal shoots compared to shoot tip explants in HF medium and in the two lowest BA concentrations, while in the two highest BA concentrations, most of the shoots produced were hyperhydrated; the number of hyperhydrated shoots in both explant types gradually increased with increasing BA concentration (Figure 5j).
The shoot length was greater in HF medium and at the lowest BA concentration (Figure 6e and Figure 7i), and the node number was greater in HF medium and similar in all BA media (Figure 7j).
The multiplication index was highest when single node explants were cultured on HF medium, halved when 0.4 mg L−1 BA was added to the medium, and lowest at the highest BA concentration in both types of explant (Figure 8e).
3.3. Effects of Cytokinin Type and Concentration on the Multiplication of S. fruticosa and S. officinalis
Aiming to increase shoot proliferation in the commercial species S. fruticosa and S. officinalis, the effect of BA on the response of shoot tip and single node explants for shoot production was compared with that of four other cytokinins, i.e., ZEA, Kin, 2iP, and mT. Three-way ANOVA showed in most cases a significant interaction of the experimental factors (cytokinin type, cytokinin concentration, and explant type). In both species, factor means indicated that a gradual increase in cytokinin concentration induced a gradual decrease in the percentage of explants responding to produce normal shoots, and an increase in hyperhydration expressed as the percentage of explants producing only hyperhydrated shoots or the number of hyperhydrated shoots produced per explant (Table 2 and Table 3). Furthermore, a gradual increase in cytokinin concentration, although causing a gradual increase in the number of normal and hyperhydrated shoots, simultaneously reduced the length and number of nodes per shoot, leading to a decrease in the multiplication index (Table 2 and Table 3). Concerning the cytokinin type, in both sage species, KIN induced the highest percentage of response to produce normal shoots, and the lowest percentage of explants that produced only hyperhydrated shoots (Table 2 and Table 3). In S. fruticosa, BA, ZEA, and mT produced the highest number of normal and hyperhydrated shoots per explant, and KIN and mT, the longest shoots; while in S. officinalis, BA and mT produced the highest numbers of normal and hyperhydrated shoots per explant, and ZEA produced the longest ones. mT in S. fruticosa and BA in S. officinalis achieved the highest multiplication index (Table 2 and Table 3). In both sage species, shoot tip explants responded at a higher percentage than single node explants to produce normal shoots, while single node explants showed higher hyperhydration levels. Further, shoot tip explants produced slightly fewer shoots, both normal and hyperhydrated (statistically not significant), which were longer and had more nodes compared to single node explants and resulted in a slightly higher multiplication index than single node explants (Table 2 and Table 3).

Table 2.
The effect of the experimental factors, i.e., cytokinin type, BA, zeatin (ZEA), kinetin (KIN), 6-(γ,γ-dimethylallyamino) purine (2iP), and meta-topolin (mT), cytokinin concentration (0.4, 0.8, 1.6, and 3.2 mg L−1) and explant type (shoot tip and single node) on shoot multiplication of S. fruticosa. All media except BA also contained 0.01 mg L−1 NAA.

Table 3.
The effect of the experimental factors, i.e., cytokinin type (BA, ZEA, KIN, 2iP, and mT), cytokinin concentration (0.4, 0.8, 1.6, and 3.2 mg L−1) and explant type (shoot tip and single node) on shoot multiplication of S. officinalis. All media except BA also contained 0.01 mg L−1 NAA.
Due to interactions between experimental factors, the results were further analyzed using one-way ANOVA, and the treatment means were compared.
In S. fruticosa, all cytokinins, with the exception of mT, affected the percentage of explant response to produce normal and hyperhydrated shoots in a similar manner; i.e., at the lowest concentration (0.8 mg L−1) they induced high response percentages for normal shoot production, such as Hf medium, while as the concentration increased, there was a proportional decrease in the response percentage for the production of normal shoots and an increase in explant hyperhydration (Table 4). mT at the lowest concentration caused a lower response percentage compared to other cytokinins and the control, which increased when 0.8 mg L−1 was used. As in previous experiments with BA, shoot tip explants responded at higher percentages compared to single node explants, while single node explants showed higher hyperhydration. An increase in cytokinin concentration caused a gradual increase in shoot number in both normal and hyperhydrated shoots, and a decrease in shoot length. Although ZEA at the highest concentration resulted in the highest number of shoots per explant, half of them were hyperhydrated, so that normal shoot production on ZEA media was similar to that of BA, while the other cytokinins produced slightly lower shoot numbers (Table 4 and Figure 9). Multiplication indices were higher in Hf medium, as well as those with low cytokinin concentrations, and reached the highest value in the medium, with 0.8 mg L−1 mT (Table 4).

Table 4.
Effect of cytokinin type and concentration on shoot multiplication of S. fruticosa shoot tip or single node explants, excised from culture established from in vitro grown seedlings, in the presence of 0.01 mg L−1 NAA.

Figure 9.
Characteristic shoot multiplication of shoot tip and single node explants of S. fruticosa cultured on MS medium supplemented with 0.01 NAA (mg.L−1), and cytokinin type and concentration (mg.L−1) marked. Size bars= 1.0 cm.
In S. officinalis, at the two highest concentrations of cytokinins, especially in BA, the single node explants had a lower response percentage to produce normal shoots than the shoot tip explants, as they showed very high hyperhydricity percentages reaching 100%. Increasing the concentrations of all cytokinins gradually decreased the percentage of explants that gave normal shoots, and increased the percentage of hyperhydrated explants (Table 5). At the same time, there was a small increase in the number of normal and hyperhydrated shoots, and a generally small decrease in their length (Table 5 and Figure 10). S. officinalis showed higher proliferation indices than S. fruticosa, which were highest in 0.4 mg L−1 BA in both shoot tip and single node explants (Table 5).

Table 5.
Effect of cytokinin concentration and type on shoot multiplication of shoot tip or single node explants of S. officinalis excised from culture established from in vitro grown seedlings, in the presence of 0.01 mg L−1 NAA.

Figure 10.
Characteristic shoot multiplication of shoot tip and single node explants of S. officinalis cultured on MS medium supplemented with 0.01 NAA (mg.L−1) and cytokinin type and concentration (mg.L−1) marked. Size bars= 1.0 cm.
3.4. In Vitro Rooting
3.4.1. S. fruticosa
One-third of S. fruticosa microshoots rooted on Hf ½ MS medium, while the highest percentage of rooting (75%) was achieved on medium supplemented with the lowest IBA concentration tested, i.e., 0.5 mg L−1. An increase in IBA concentration resulted in a corresponding decrease in rooting, although the two highest IBA concentrations (2 and 4 mg L−1) resulted in the same percentage of rooting (Figure 11). The number of roots formed in a microshoot increased, while the root length decreased proportionally with increasing IBA concentration in the medium (Figure 11 and Figure 12a).
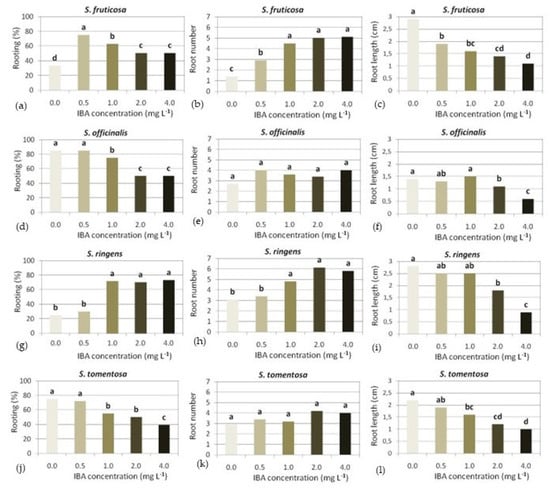
Figure 11.
Effect of IBA concentration on in vitro rooting of marked Salvia spp. microshoots, on ½MS medium supplemented with indole-3-butyric acid (IBA) concentration marked (n = 15–30).

Figure 12.
Typical in vitro rooting of Salvia fruticosa (a), S. officinalis (b), S. ringens (c) and S. tomentosa (d) microshoots, cultured on ½MS medium supplemented with IBA at concentration (mg.L−1) marked (left), and ex vitro acclimatized plantlets about 6 weeks old (right). Size bars = 1.0 cm.
3.4.2. S. officinalis
S. officinalis microshoots, in contrast to S. fruticosa, rooted at the highest percentage (85%) on Hf medium, as well as on a medium with 0.5 mg L−1 IBA, and a gradual increase in IBA resulted in a corresponding reduction in rooting, but as in S. fruticosa, the two highest IBA concentrations led to the same percentage of rooting (Figure 11). IBA resulted in an increase in root number regardless of concentration, while the root length was similar in all media except that with the highest IBA concentration, which resulted in a halving of root length (Figure 11 and Figure 12b).
3.4.3. S. ringens
In S. ringens, higher rooting percentages (70–73%) were induced using IBA concentrations of higher than 0.5 mg L−1, which also resulted in a greater number of roots, while the root length was similar in Hf medium and those with the two lowest IBA concentrations, and decreased in those with the two highest concentrations (Figure 11 and Figure 12c).
3.4.4. S. tomentosa
S. tomentosa microshoots rooted at the highest percentage (75%) on Hf medium, and a gradual increase in IBA concentration in the medium resulted in a corresponding decrease in rooting (Figure 11). Root number was not affected by IBA, while root length decreased gradually with increasing IBA concentration (Figure 11 and Figure 12d).
3.4.5. S. pomifera ssp. pomifera
Shoot tip explants of S. pomifera ssp. pomifera rooted at 80% when cultured on Hf MS medium, and formed numerous and long roots (Figure 6c). The small number of mother plants of this Salvia species available for the establishment of in vitro cultures due to the very low percentage of seed germination, as well as the low percentage of shoot multiplication, along with the high percentage of hyperhydrated shoots, did not allow for the production of sufficiently long normal shoots that could be used for rooting. As a result, in vitro rooting was not tested further for this species.
3.5. Ex Vitro Acclimatization
In all five Salvia spp., in vitro rooted microshoots coming from MS medium supplemented with 0.4 or 0.8 mg L−1 BA and 0.01 mg L−1 NAA, except for S. pomifera ssp. pomifera, whose microshoots were obtained from the Hf MS medium, acclimatized ex vitro at percentages of 80–95% (Figure 12 and Figure 13).
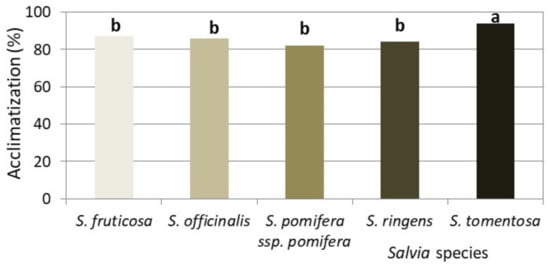
Figure 13.
Ex vitro acclimatization of rooted microshoots of Salvia spp., on a peat–perlite substrate 1:1 (v/v) (n = 6–10 repetitions of 8 rooted microshoots).
4. Discussion
In vitro cultures of all five Salvia spp. were successfully established on Hf-MS medium from either shoot tip or single node explants excised from in vitro-grown seedlings, but explant response varied with Salvia species and explant type. Most shoot tip explants elongated when placed in culture, while single node explants produced two shoots, as expected due to the phyllotaxis of Salvia sp., confirming a previous report for S. officinalis []. Previous reports also showed reduced establishment rates due to explant necrosis, browning, and hyperhydricity []. In our work, almost all (96–100%) S. officinalis and S. pomifera ssp. pomifera explants of both types produced shoots, while in S. fruticosa, the response was slightly reduced (78–87%), as was the case for the shoot tip explants of S. ringens (87% response) too, while single node explants of S. ringens and both explant types of S. tomentosa responded at much lower percentages (36–50%) (Table 1). A problem at the establishment stage was the hyperhydricity of the produced shoots that was more pronounced in single node explants (18–50%), with the exception of S. fruticosa, which developed hyperhydrated shoots at a quite high percentage (17%) in shoot tip explants as well. The growth conditions mostly associated with hyperhydricity are limited aeration; high levels of total nitrogen, ammonium nitrogen, and cytokinin in the medium; and ethylene accumulation in the aerial part of the culture vessel [,,,,]. As the medium of the establishment stage did not contain cytokinin, and vessels were covered with magenta caps that allowed for aeration, the most possible reason for the appearance of hyperhydricity seems to be the nitrogen richness of the MS medium. Nitrogen levels have been positively correlated with the increased occurrence of hyperhydricity in a number of species [,,,]. However, a low-nitrogen medium may not be suitable for shoot production, as in a previous work with S. fruticosa, MS medium was found superior to low-nitrogen media such as Nitsch and Nitsch and B5 [].
Pronounced hyperhydricity has been reported in the micropropagation of several species of macchia, such as Globularia alypum [], Lithodora zahnii [], Anthyllis barba-jovis [,], Calamintha cretica [], and Clinopodium nepeta [].
At the shoot multiplication stage, in the presence of BA, shoot tip explants responded at higher percentages for shoot production compared to single node explants, with the exception of S. officinalis, where explants of both types responded at 100% (Figure 4). Increasing the BA concentration resulted in a corresponding decrease in explant response that was more pronounced in single node explants and in S. pomifera ssp. pomifera and S. tomentosa, and at the same time, it proportionally increased the number of shoots produced per explant while reducing their length and number of nodes. As in the establishment stage, the hyperhydricity was particularly pronounced in single node explants and increased proportionally with increasing BA concentration. Thus, the multiplication index in S. pomifera ssp. pomifera and S. tomentosa decreased with increasing BA concentration, and in S. fruticosa it was rather indifferent; while in S. officinalis, for both explant types, and in S. ringens, only for shoot tip explants, low BA concentrations (0,4 or 0.8 mg L−1, respectively) resulted in increased multiplication indices compared to Hf-medium and a medium with higher BA content, indices that were the highest among all species.
Multiplication indices, in terms of normal shoots (excluding hyperhydrated shoots) in the most productive treatment for each species, were rather low in S. fruticosa (2.6) and S. pomifera ssp. pomifera (3.8), average in S. ringens (5.5) and S. tomentosa (5.4), and high in S. officinalis (7.2). These indices are considered satisfactory, as in all previous works on the in vitro propagation of Mediterranean sages, the multiplication indices were in almost all cases much lower than the present ones [,,,,,].
Regarding the effect of different types of cytokinin, i.e., BA, ZEA, KIN, 2iP, and mT, on the shoot multiplication of S. fruticosa and S. officinalis, it was found that mT at 0.8 mg L−1 induced the highest multiplication indices in S. fruticosa, both in shoot tip and single node explants, while in S. officinalis, mT at the lowest concentration of 0.4 mg L−1 also induced high multiplication indices, but lower than those induced by 0.4 mg L−1 BA. BA was superior for shoot multiplication in S. officinalis compared to all other cytokinins tested, in agreement with previous works, which also showed that BA or BAP in combination, as in the present study, with an auxin in low concentration, was superior to KIN [,,], thidiazuron (TDZ) [,], 2iP, and ZEA [] for proliferation. To the contrary, for adventitious shoots regeneration through callus, TDZ was more suitable than BA and KIN []. In S. fruticosa, a previous work had shown that BA is superior to KIN for proliferation [], while in our work, BA, KIN, ZEA, and 2iP induced a similar response.
As discussed above for BA, increasing the concentration of cytokinin of all types in the medium resulted in an analogous increase in shoot number per explant, with a simultaneous decrease in shoot length and number of nodes, and a significant increase in hyperhydricity leading to a decrease in the multiplication indices. Single node explants showed a slightly lower response rate for shoot production, and more pronounced hyperhydricity compared to shoot tip explants in all cytokinin types, while at the highest cytokinin concentrations, the production of normal shoots was very low from single node explants due to hyperhydricity. In S. fruticosa, Arikat et al. [] reported that single node explants almost failed to produce shoots when BA, KIN, or TDZ were used at concentrations of higher than 1 mg L−1, but he did not report on hyperhydricity. Hyperhydricity has been reported previously in S. officinalis [,,], as well as in S. scarlea [].
Cytokinins has been shown to induce hyperhydricity in some plant species during in vitro propagation, depending on the cytokinin type and concentration [,,,,]. Decreasing the concentration of cytokinin in the medium or using a different type of cytokinin resulted in a reduction in hyperhydricity [,,], often with a simultaneous reduction in shoot production [,], as shown in the present work. Therefore, measures aimed at eliminating hyperhydricity should be complex and specific for each plant species.
In vitro rooting was successful (a rooting percentage of higher than 80%) in all five species. S. officinalis, S. tomentosa, and S. pomifera ssp. pomifera rooted at high percentages (75–83%) on Hf medium, or in the presence of the lowest IBA concentration tested (0.5 mg L−1), while S. ringens and S. fruticosa rooted at low percentages on Hf medium (35 and 35%, respectively) and at 1.0 and 0.5 mg L−1 IBA, respectively, were required to achieve high rooting percentages. Our results confirmed those of Avato et al. [], who also found that auxin supplementation was not necessary for rooting in S. officinalis. Arikat et al. [] also found that S. fruticosa rooted only in the presence of a low concentration of auxin (0.5 mg L−1 IBA). An equally low concentration of IBA (0.5–1.0 mg L−1) was shown to induce maximum rooting rates in the microshoots of S. officinalis (65–70%) [] and Salvia hispanica (65–70%) [], while similar concentrations of IBA induced the highest percentage of rooting in S. scarlea, which however, did not exceed 40% []. IBA at low concentrations (up to 1 mg L−1) was effective for the rooting of other Mediterranean medicinal Lamiaceae as well [,,,,,,]. Half-strength MS medium was used in most of the above works, as in the present study, as rather low concentrations of nutrients positively affect rooting [].
In S. officinalis and S. tomentosa, where auxin supplementation was not necessary for rooting, root number was indifferent to auxin concentration in the medium, while in S. fruticosa and S. ringens, which showed low rooting ability in auxin-free medium, increasing auxin content, in addition to higher rooting rates, also induced a higher number of roots per microshoot. In contrast, a gradual increase in IBA resulted in a gradual decrease in root length in all five species, as previously found for S. scarlea [], Teucrium capitatum [], and C. cretica [].
Rooted microshoots of all five sage species acclimatized ex vitro at percentages of higher than 80%, retaining the characteristics of the mother plants, as has been shown for other sages [,,,,,,] and a number of other medicinal Lamiaceae [,,,].
5. Conclusions
The five Mediterranean sages, S. fruticosa, S. officinalis, S. ringens, S. tomentosa, and S. pomifera spp. pomifera, native to Greece, showed many similarities, but also some quantitative differences in their in vitro propagation. All were successfully established on Hf-MS medium in the initial culture from either shoot tip or single node explants excised from seedlings grown in vitro, showing low proliferation indices, except for S. officinalis, which stood out with the highest indices. In the multiplication stage, the explant response for shoot production was high, but the number of shoots produced per explants was rather low. Supplementing the medium with various types and concentrations of cytokinin in combination with auxin did not significantly increase the proliferation indices, as the number of shoots produced per explant remained below five, and a significant proportion of them were hyperhydrated. Shoot tip explants produced normal shoots at much higher percentages compared to single node explants. All five sages rooted readily in vitro, either without or under low auxin, and they acclimatized ex vitro at high percentages. Thus, the present study led to efficient micropropagation protocols for five Mediterranean sage species native to Greece, which are expected to facilitate their sustainable exploitation in the pharmaceutical and floriculture industries.
Author Contributions
Conceptualization, M.P., G.V. and A.N.M.; methodology, M.P., G.V. and A.N.M.; validation, M.P., G.V. and A.N.M.; formal analysis, G.V. and A.N.M.; investigation, M.P., G.V. and A.N.M.; resources, M.P.; data curation, G.V. and A.N.M.; writing—original draft preparation, M.P., G.V. and A.N.M.; writing—review and editing, M.P., G.V. and A.N.M.; visualization, M.P., G.V. and A.N.M.; supervision, M.P.; project administration, M.P.; funding acquisition, M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was co-financed by the European Regional Development Fund of the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call RESEARCH—CREATE—INNOVATE (project code: T1EDK-04923, project: SALVIA-BREED-GR).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- The Plant List. Version 1.1. 2013. Available online: http://www.theplantlist.org/ (accessed on 29 April 2022).
- Walker, J.B.; Sytsma, K.J.; Treutlein, J.; Wink, M. Salvia (Lamiaceae) is not monophyletic: Implications for the systematics, radiation, and ecological specializations of Salvia and tribe Mentheae. Am. J. Bot. 2004, 91, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Bentham, G. Labiatae. In Genera Plantarum; Bentham, G., Hooker, J.D., Eds.; Reeve and Co.: London, UK, 1876; Volume 2, pp. 1160–1196. [Google Scholar]
- Online Etymology Dictionary. Available online: https://www.etymonline.com/word/salvia (accessed on 11 December 2021).
- Hamidpour, M.; Hamidpour, R.; Hamidpour, S.; Shahlari, M. Chemistry, Pharmacology, and Medicinal Property of Sage (Salvia) to Prevent and Cure Illnesses such as Obesity, Diabetes, Depression, Dementia, Lupus, Autism, Heart Disease, and Cancer. J. Tradit. Complement. Med. 2014, 4, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Bonesi, M.; Loizzo, M.R.; Acquaviva, R.; Malfa, G.A.; Aiello, F.; Tundis, R. Anti-inflammatory and antioxidant agents from Salvia genus (Lamiaceae): An assessment of the current state of knowledge. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2017, 16, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L. Salvia (Sage): A review of its potential cognitive-enhancing and protective effects. Drugs R&D 2017, 17, 53–64. [Google Scholar] [CrossRef]
- Hao, D.C.; Ge, G.B.; Xiao, P.G. Anticancer drug targets of Salvia phytometabolites: Chemistry, biology and omics. Curr. Drug Targets 2018, 19, 1–20. [Google Scholar] [CrossRef]
- Ververis, A.; Savvidou, G.; Ioannou, K.; Nicolaou, P.; Christodoulou, K.; Plioukas, M. Greek sage exhibits neuroprotective activity against amyloid beta-induced toxicity. Evid. Based Complement. Altern. Med. 2020, 2020, 2975284. [Google Scholar] [CrossRef]
- Afonso, A.F.; Pereira, O.R.; Cardoso, S.M. Salvia species as nutraceuticals: Focus on antioxidant, antidiabetic and anti-obesity properties. Appl. Sci. 2021, 11, 9365. [Google Scholar] [CrossRef]
- Mervić, M.; Štefan, M.B.; Kindl, M.; Blažeković, B.; Marijan, M.; Vladimir-Knežević, S. Comparative Antioxidant, Anti-Acetylcholinesterase and Anti-α-Glucosidase Activities of Mediterranean Salvia Species. Plants 2022, 11, 625. [Google Scholar] [CrossRef]
- Karalija, E.; Dahija, S.; Tarkowski, P.; Ćavar Zeljkovic, S. Influence of Climate-Related Environmental Stresses on Economically Important Essential Oils of Mediterranean Salvia sp. Front. Plant Sci. 2022, 13, 864807. [Google Scholar] [CrossRef]
- Jiang, S. Therapeutic landscapes and healing gardens: A review of Chinese literature in relation to the studies in western countries. Front. Archit. Res. 2014, 3, 141–153. [Google Scholar] [CrossRef]
- Savi, T.; Andri, S.; Nardini, A. Impact of different green roof layering on plant water status and drought survival. Ecol. Eng. 2013, 57, 188–196. [Google Scholar] [CrossRef]
- Raimondo, F.; Trifilò, P.; Lo Gullo, M.A.; Andri, S.; Savi, T.; Nardini, A. Plant performance on Mediterranean green roofs: Interaction of species-specific hydraulic strategies and substrate water relations. AoB Plants 2015, 7, plv007. [Google Scholar] [CrossRef]
- Martini, A.N.; Tassoula, L.; Papafotiou, M. Adaptation of Salvia fruticosa, S. officinalis, S. ringens and interspecific hybrids in an extensive green roof under two irrigation frequencies. Notulae 2022, 50, 12767. [Google Scholar] [CrossRef]
- Papafotiou, M.; Martini, A.N.; Tassoula, L.; Stylias, E.G.; Kalantzis, A.; Dariotis, E. Acclimatization of Mediterranean native sages (Salvia spp.) and interspecific hybrids in an urban green roof under regular and reduced irrigation. Sustainability 2022, 14, 4978. [Google Scholar] [CrossRef]
- Rahimi, E.; Barghjelveh, S.; Dong, P. A review of diversity of bees, the attractiveness of host plants and the effects of landscape variables on bees in urban gardens. Agric. Food Secur. 2022, 11, 6. [Google Scholar] [CrossRef]
- Dimopoulos, P.; Raus, T.; Bergmeier, E.; Constantinidis, T.; Iatrou, G.; Kokkini, S.; Strid, S.; Tzanoudakis, D. Vascular Plants of Greece: An Annotated Checklist; Botanischer Garten und Botanisches Museum Berlin-Dahlem; Hellenic Botanical Society: Berlin, Germany, 2013. [Google Scholar]
- Chatzopoulou, F.M.; Makris, A.M.; Argiriou, A.; Degenhardt, J.; Kanellis, A.K. EST analysis and annotation of transcripts derived from a trichome-specific cDNA library from Salvia fruticosa. Plant Cell Rep. 2010, 29, 523–534. [Google Scholar] [CrossRef]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement Med. 2017, 7, 433–440. [Google Scholar] [CrossRef]
- Kemp, S.; Hadley, P.; Blanuša, T. The influence of plant type on green roof rainfall retention. Urban Ecosyst. 2019, 22, 355–366. [Google Scholar] [CrossRef]
- Göçer, H.; Yetişir, H.; Ulaş, A.; Arslan, M.; Aydin, A. Plant growth, ion accumulation and essential oil content of Salvia officinalis Mill. and S. tomentosa L. grown under different salt stress. KSU J. Agric. Nat. 2021, 24, 505–514. [Google Scholar]
- Papafotiou, M.; Martini, A.N.; Papanikolaou, E.; Stylias, E.G.; Kalantzis, A. Hybrids Development between Greek Salvia Species and Their Drought Resistance Evaluation along with Salvia fruticosa, under Attapulgite-Amended Substrate. Agronomy 2021, 11, 2401. [Google Scholar] [CrossRef]
- Putievsky, E.; Ravid, U.; Diwan-Rinzler, N.; Zohary, D. Genetic affinities and essential oil composition of Salvia officinalis L., S. fruticosa Mill., S. tomentosa Mill. and their hybrids. Flavour Fragr. J. 1990, 5, 121–123. [Google Scholar] [CrossRef]
- Karousou, R.; Vokou, D.; Kokkini, S. Distribution and essential oils of Salvia pomifera subsp. pomifera (Labiatae) on the island of Crete (S Greece). Biochem. Syst. Ecol. 1998, 26, 889–897. [Google Scholar] [CrossRef]
- Haznedaroglu, M.; Karabay, N.; Zeybek, U. Antibacterial activity of Salvia tomentosa essential oil. Fitoterapia 2001, 72, 829–831. [Google Scholar] [CrossRef] [PubMed]
- Tepe, B.; Daferera, D.; Sokmen, A.; Sokmen, M.; Polissiou, M. Antimicrobial and antioxidant activities of the essential oil and various extracts of Salvia tomentosa Miller (Lamiaceae). Food Chem. 2005, 90, 333–340. [Google Scholar] [CrossRef]
- Arslan, M. Cultivation potential of Salvia tomentosa and S. aramiensis under the Eastern Mediterranean conditions. Sci. Pap. Ser. A Agron. 2016, 59, 174–177. [Google Scholar]
- Hanlidou, E.; Karousou, R.; Lazari, D. Essential-oil diversity of Salvia tomentosa Mill. in Greece. Chem. Biodivers. 2014, 11, 1205–1215. [Google Scholar] [CrossRef]
- Bardakci, H.; Servi, H.; Polatoglu, K. Essential oil composition of Salvia candidissima Vahl. occidentalis Hedge, S. tomentosa Miller and S. heldreichiana Boiss. Ex Bentham from Turkey. J. Essent. Oil-Bear. Plants 2019, 22, 1467–1480. [Google Scholar] [CrossRef]
- Özcan, M.M.; Figueredo, G.; Chalchat, J.C.; Chalard, P.; Tugay, O.; Ceylan, D.A. Chemical constituents of essential oils of Salvia heldreichiana Boiss. Ex Bentham and Salvia tomentosa Mill. J. Agroaliment. Process. Technol. 2019, 25, 106–110. [Google Scholar]
- Grigoriadou, K.; Trikka, F.A.; Tsoktouridis, G.; Krigas, N.; Sarropoulou, V.; Papanastasi, K.; Maloupa, E.; Makris, A.M. Micropropagation and cultivation of Salvia sclarea for essential oil and sclareol production in northern Greece. In Vitro Cell. Dev. Biol. Plant 2020, 56, 51–59. [Google Scholar] [CrossRef]
- Thanos, C.A.; Doussi, M.A. Ecophysiology of seed germination in endemic labiates of Crete. Isr. J. Plant Sci. 1995, 43, 227–237. [Google Scholar] [CrossRef]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea, Volume 3 Diapenstaceae to Myoporaceae; Cambridge University Press: Cambridge, UK, 1972; pp. 188–190. [Google Scholar]
- Guner, A.; Ozhatay, N.; Ekim, T.; Baser, K.H.C. Flora of Turkey and the East Aegean Islands, Supplement II; Edinburg University Press: Edinburg, UK, 2000; Volume 11. [Google Scholar]
- Martini, A.N.; Bertsouklis, K.F.; Vlachou, G.; Dariotis, E.; Papafotiou, M. Comparative evaluation of rooting cuttings of five Mediterranean sage species (Salvia sp.) native to Greece. Acta Hortic. 2020, 1298, 587–592. [Google Scholar] [CrossRef]
- Vlachou, G.; Papafotiou, M.; Bertsouklis, K.F. Seed germination, micropropagation from adult and juvenile origin explants and address of hyperhydricity of the Cretan endemic herb Calamintha cretica. Not. Bot. Horti. Agrobot. 2020, 48, 1504–1518. [Google Scholar] [CrossRef]
- Kanellou, E.; Vlachou, G.; Martini, A.N.; Bertsouklis, K.F.; Papafotiou, M. Seed germination of five sage species (Salvia sp.) of populations native to Greece. Acta Hortic. 2022, 1345, 439–444. [Google Scholar] [CrossRef]
- Jaafar, A.; Mohsen, E.; Hossein, A.R.; Ali, A.J.; Mostafa, E.; Yaser, S.M.; Mohammad, A.S.B.G. Seed germination as the major conservation issue of endemic Iranian salvia species. J. Med. Plants Res. 2012, 6, 37–46. [Google Scholar] [CrossRef]
- Jaafar, A.; Seyed, H.T.; Mohsen, E.; Hossein, A.R.; Ahamad, R.D.T.; Mehdi, M.; Mostafa, E.; Amir, H.D.; Asma, B. Effect of drought stress on germination and seedling growth of Salvia species. Afr. J. Agric. Res. 2012, 7, 5719–5725. [Google Scholar] [CrossRef]
- Kintzios, S.; Nikolau, A.; Skoula, M. Somatic embryogenesis and in vitro rosmarinic acid accumulation in Salvia officinalis and S. fruticosa leaf callus cultures. Plant Cell Rep. 1999, 18, 462–466. [Google Scholar] [CrossRef]
- Santos-Gomes, P.C.; Seabra, R.M.; Andrade, P.B.; Ferreira, M.F. Phenolic antioxidant compounds produced by in vitro shoots of sage (Salvia officinalis L.). Plant Sci. 2002, 162, 981–987. [Google Scholar] [CrossRef]
- Grzegorczyk, I.; Bilichowski, I.; Mikiciuk-Olasik, E.; Wysokinska, H. The effect of triacontanol on shoot multiplication and production of antioxidant compounds in shoot cultures of Salvia officinalis L. Acta Soc. Bot. Pol. 2006, 75, 11–15. [Google Scholar] [CrossRef]
- Avato, P.; Fortunato, I.M.; Ruta, C.; D’ Elia, R. Glandular hairs and essential oils in micropropagated plants of Salvia officinalis L. Plant Sci. 2005, 169, 29–36. [Google Scholar] [CrossRef]
- Tawfik, A.A.; Mohamed, F.M. Regeneration of salvia (Salvia officinalis L.) via induction of meristematic callus. In Vitro Cell. Dev. Biol. Plant 2007, 43, 21–27. [Google Scholar] [CrossRef]
- Gostin, I. Effects of different plant hormones on Salvia officinalis cultivated in vitro. Int. J. Bot. 2008, 4, 430–436. [Google Scholar]
- Grzegorczyk, I.; Wysokinska, H. Liquid shoot culture of Salvia officinalis L. for micropropagation and production of antioxidant compounds; effect of triacontanol. Acta Soc. Bot. Pol. 2008, 77, 99–104. [Google Scholar] [CrossRef]
- Cristea, O.T.; Prisecaru, M.; Ambarus, S.; Calin, M.; Brezeanu, C.; Brezeanu, M.; Florin, G. Vegetative multiplication of Salvia officinalis L. for the obtaining of true-to-type plants. Biologie 2014, 23, 104–107. [Google Scholar]
- Petrova, M.; Nikolova, M.; Dimitrova, L.; Zayova, E. Micropropagation and evaluation of flavonoid content and antioxidant activity of Salvia officinalis L. Genet. Plant Physiol. 2015, 5, 48–60. [Google Scholar]
- Mohamed, M.A.-H.; Aly, M.K.; Ahmed, E.T.; Abd El-latif, S.A.H. Effect of plant growth regulators on organogenesis of Salvia officinalis L. plants. Minia J. Agric. Res. Develop. 2019, 3, 401–414. [Google Scholar] [CrossRef]
- Arikat, N.A.; Jawad, F.M.; Karam, N.S.; Shibli, R.A. Micropropagation and accumulation of essential oils in wild sage (Salvia fruticosa Mill.). Sci. Hortic. 2004, 100, 193–202. [Google Scholar] [CrossRef]
- Martini, A.N.; Vlachou, G.; Papafotiou, M. Effect of Explant Origin and Medium Plant Growth Regulators on In Vitro Shoot Proliferation and Rooting of Salvia tomentosa, a Native Sage of the Northeastern Mediterranean Basin. Agronomy 2022, 12, 1889. [Google Scholar] [CrossRef]
- Kitto, S.L. Commercial micropropagation. HortScience 1997, 32, 1012–1014. [Google Scholar] [CrossRef]
- Sidhu, Y. In vitro micropropagation of medicinal plants by tissue culture. Plymouth Stud. Sci. 2011, 4, 432–449. [Google Scholar]
- Grigoriadou, K.; Krigas, N.; Sarropoulou, V.; Papanastasi, K.; Tsoktouridis, G.; Maloupa, E. In vitro propagation of medicinal and aromatic plants: The case of selected Greek species with conservation priority. In Vitro Cell. Dev. Biol. Plant 2019, 55, 635–646. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Grzegorczyk, I.; Wysokinska, H. Micropropagation of Salvia officinalis L. by shoot tips. Biotechnologia 2004, 2, 212–218. [Google Scholar]
- CachiŢĂ-Cosma, D. The effect of the nature and origin of explants on micropropagation. In High-Tech and Micropropagation I. Biotechnology in Agriculture and Forestry; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1991; Volume 17. [Google Scholar]
- Papafotiou, M.; Martini, A. Effect of position and orientation of leaflet explants with respect to plant growth regulators on micropropagation of Zamioculcas zamiifolia Engl. (ZZ). Sci. Hortic. 2009, 120, 115–120. [Google Scholar] [CrossRef]
- Kartsonas, E.; Papafotiou, M. Mother plant age and seasonal influence on in vitro propagation of Quercus euboica Pap., an endemic, rare and endangered oak species of Greece. Plant Cell Tissue Organ Cult. (PCTOC) 2007, 90, 111–116. [Google Scholar] [CrossRef]
- Martini, A.N.; Papafotiou, M.; Vemmos, S.N. Season and explant origin affect phenolic content, browning of explants, and micropropagation of ×Malosorbus florentina (Zucc.) Browicz. HortScience 2013, 48, 102–107. [Google Scholar] [CrossRef]
- Vlachou, G.; Papafotiou, M.; Bertsouklis, K.F. In Vitro Propagation of Calamintha nepeta. Acta Hort. 2016, 1113, 189–194. [Google Scholar] [CrossRef]
- Vlachou, G.; Papafotiou, M.; Bertsouklis, K.F. In Vitro Propagation of Ballota acetabulosa. Acta Hort. 2016, 1113, 171–174. [Google Scholar] [CrossRef]
- Cuenca, S.; Amo-Marco, J.B. In vitro propagation of two Spanish endemic species of Salvia through bud proliferation. In Vitro Cell. Dev. Biol. Plant 2000, 36, 225–229. [Google Scholar] [CrossRef]
- Ghanbar, T.; Hosseini, B.; Jabbarzadeh, Z.; Farokhzad, A.; Sharafi, A. High-frequency in vitro direct shoots regeneration from axillary nodal and shoot tip explants of clary sage (Salvia sclarea L.). Bulg. J. Agric. Sci. 2016, 22, 73–78. [Google Scholar]
- Raja, H.D.; Arockiasamy, D.I. In vitro propagation of Mentha viridis L. from nodal and shoot tip explants. Plant Tissue Cult. Biotechnol. 2008, 18, 1–6. [Google Scholar] [CrossRef]
- Sujana, P.; Naidu, C.V. High frequency rapid plant regeneration from shoot tip and nodal explants of Mentha piperita (L.)—An important multipurpose medicinal plant. J. Phytol. 2011, 3, 9–13. [Google Scholar]
- Moharami, L.; Hosseini, B.; Ghotbi Ravandi, E.; Jafari, M. Effects of plant growth regulators and explant types on in vitro direct plant regeneration of Agastache foeniculum, an important medicinal plant. In Vitro Cell. Dev. Biol. Plant 2014, 50, 707–711. [Google Scholar] [CrossRef]
- Sharma, U.; Mohan, J.S.S. Reduction of vitrification in in vitro raised shoots of Chlorophytum borilivinum (Sant. and Fernand.), a rare potent medicinal herb. Indian J. Exp. Biol. 2006, 44, 499–505. [Google Scholar] [PubMed]
- Islam, A.T.M.R.; Islam, M.M.; Alam, M.F. Rapid in vitro clonal propagation of herbal spice, Mentha piperita L. using shoot tip and nodal explants. Res. Plant Biol. 2017, 5, 43–50. [Google Scholar] [CrossRef]
- Papafotiou, M.; Martini, A.N. In vitro seed and clonal propagation of the Mediterranean aromatic and medicinal plant Teucrium capitatum. HortScience 2016, 51, 403–411. [Google Scholar] [CrossRef]
- Papafotiou, M.; Bertsouklis, K.F.; Trigka, M. Micropropagation of Arbutus unedo, A. andrachne, and their natural hybrid, A. x andrachnoides from seedling explants. J. Hort. Sci. Biot. 2013, 88, 768–775. [Google Scholar] [CrossRef]
- Sharma, S.; Shahzad, A.; Kumar, J.; Anis, M. In vitro propagation and synseed production of scarlet salvia (Salvia splendens). Rend. Fis. Acc. Lincei 2014, 25, 359–368. [Google Scholar] [CrossRef]
- Vlachou, G.; Papafotiou, M.; Bertsouklis, K. Studies on seed germination and micropropagation of Clinopodium nepeta: A medicinal and aromatic plant. HortScience 2019, 54, 1558–1564. [Google Scholar] [CrossRef]
- Zayova, E.; Nikolova, M.; Dimitrova, L.; Petrova, M. Comparative study of in vitro, ex vitro and in vivo propagated Salvia hispanica (Chia) plants: Morphometric analysis and antioxidant activity. AgroLife Sci. J. 2016, 5, 166–174. [Google Scholar]
- Vlachou, G.; Martini, A.N.; Dariotis, E.; Papafotiou, M. Comparative evaluation of seed germination of five Mediterranean sage species (Salvia sp.) native to Greece. Acta Hortic. 2020, 1298, 593–598. [Google Scholar] [CrossRef]
- Lai, C.C.; Lin, H.M.; Nalawade, S.M.; Fang, W.; Tsay, H.S. Hyperhydricity in shoot cultures of Scrophularia yoshimurae can be effectively reduced by ventilation of culture vessels. J. Plant Physiol. 2005, 162, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, M.; van Staden, J. Effect of ammonium ions and cytokinins on hyperhydricity and multiplication rate of in vitro regenerated shoots of Aloe polyphylla. Plant Cell Tissue Organ Cult. (PCTOC) 2008, 92, 227–231. [Google Scholar] [CrossRef]
- Ivanova, M.; van Staden, J. Influence of gelling agent and cytokinins on the control of hyperhydricity in Aloe polyphylla. Plant Cell Tissue Organ Cult. (PCTOC) 2011, 104, 13–21. [Google Scholar] [CrossRef]
- Badr-Elden, A.M.; Nower, A.A.; Ibrahim, I.A.; Ebrahim, M.K.; Abd Elaziem, T.M. Minimizing the hyperhydricity associated with in vitro growth and development of watermelon by modifying the culture conditions. Afr. J. Biotechnol. 2012, 11, 8705–8717. [Google Scholar] [CrossRef]
- Pence, V.C.; Finke, L.R.; Niedz, R.P. Evaluating a DOE screen to reduce hyperhydricity in the threatened plant, Cycladenia humilis var. jonesii. In Vitro Cell. Dev. Biol. Plant 2020, 56, 215–229. [Google Scholar] [CrossRef]
- Reyes-Vera, I.; Potenza, C.; Barrow, J. Hyperhydricity reversal and clonal propagation of four-wing saltbush (Atriplex canescens, Chenopodiaceae) cultivated in vitro. Aust. J. Bot. 2008, 56, 358–362. [Google Scholar] [CrossRef]
- Wu, H.; Yu, X.; Teixeira da Silva, J.A.; Lu, G. Direct shoot induction of Paeonia lactiflora ‘Zhong Sheng Fen’and rejuvenation of hyperhydric shoots. N. Zeal. J. Crop Hortic. Sci. 2011, 39, 271–278. [Google Scholar] [CrossRef]
- Bertsouklis, K.; Papafotiou, M.; Balotis, G. Effect of medium on in vitro growth and ex vitro establishment of Globularia alypum L. Acta Hortic. 2003, 616, 177–180. [Google Scholar] [CrossRef]
- Papafotiou, M.; Kalantzis, A. Studies on in vitro propagation of Lithodora zahnii. Acta Hortic. 2009, 813, 465–470. [Google Scholar] [CrossRef]
- Vlachou, G.; Papafotiou, M.; Bertsouklis, K.F. Studies on in vitro propagation of Anthyllis barba-jovis L. Acta Hortic. 2017, 1155, 317–320. [Google Scholar] [CrossRef]
- Trigka, M.; Papafotiou, M. In vitro propagation of Anthyllis barba-jovis from seedling tissues. Acta Hortic. 2017, 1189, 473–748. [Google Scholar] [CrossRef]
- Kataeva, N.V.; Alexandrova, I.G.; Butenko, R.G.; Dragavtceva, E.V. Effect of applied and internal hormones on vitrification and apical necrosis of different plants cultured in vitro. Plant Cell Tissue Organ Cult. (PCTOC) 1991, 27, 149–154. [Google Scholar] [CrossRef]
- Ravanfar, S.A.; Salim, S.; Aziz, M.A.; Abdullah, S.N.A.; Rashid, A.A. Influence of phenyl-urea and adenine-type cytokinins on direct adventitious shoot regeneration of cabbage (Brassica oleracea subsp. capitata) “KCross”. Biotechnology 2014, 31, 1–6. [Google Scholar] [CrossRef]
- Liu, M.; Jiang, F.; Kong, X.; Tian, J.; Wu, Z.; Wu, Z. Effects of multiple factors on hyperhydricity of Allium sativum L. Sci. Hortic. 2017, 217, 285–296. [Google Scholar] [CrossRef]
- Murashige, T. Principles of rapid propagation. In Propagation of Higher Plants through Tissue Culture: A Bridge between Research and Application; Hughes, K.W., Henke, R., Constantin, M., Eds.; Tech. Information Center, U.S. Dept. of Energy: Oak Ridge, TN, USA, 1979; pp. 14–24. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).