Abstract
Plectranthus amboinicus (Lour.) Spreng is a perennial plant from the mint family with aromatic, succulent leaves and several health benefits. Multiple shoot regeneration was accomplished in vitro using nodal segments (NS) explants of P. amboinicus pretreated with 0, 0.5, 5, 25, 50, and 100 μM thidiazuron (TDZ) for 4 h, then transferred to a growth regulator-free media. After 8 weeks of growth, NS explants pre-treated with 25 μM TDZ for 4 h and then transferred to TDZ-free Murashige and Skoog (MS) media produced the greatest number of shoots (27.3 per NS) with the longest average shoot length (4.9 mm) in 97.2% of cultures. On the same medium, regeneration of roots in most of the P. amboinicus shoots occurred spontaneously. The in vitro-regenerated P. amboinicus plantlets were adequately hardened off and adapted to the ex-vitro environment with a 90% survival rate. Total phenolic, tannin, and flavonoid contents, as well as 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical-scavenging/antioxidant activity, were significantly higher in in vitro-regenerated plants than in ex vitro-plants. Flow cytometry (FCM) analysis validated the nuclear genome stability of the in vitro generated plants, which assessed their nuclear DNA content and found it to be comparable in genome size to that of the field-grown plants. The study found a quick and efficient method for in vitro multiplication of P. amboinicus which can aid to increased availability and accessibility of this plant species for various purposes. The genetic and phytochemical analysis of the in vitro propagated plants can also provide valuable insight into the plant’s properties and potential applications, which can further assist in its preservation and sustainable usage.
1. Introduction
Plectranthus amboinicus (Lour.) Spreng is a species of plant in the mint family, ‘Lamiaceae’, frequently known as ‘Country Borage’, ‘Mexican mint’ or ‘Spanish thyme’, and native to eastern and southern Africa, as well as naturally widespread throughout the tropical and warm regions of the globe [1]. In addition to its ornamental value, this plant has long been valued for its medicinal properties, particularly in the treatment of such conditions as a runny nose, cough, sore throat, bronchitis, diarrhea, and insect bites [2,3]. It has several useful pharmacological effects, including antimicrobial [4,5], antitumor, anti-inflammatory [6,7], anti-rheumatoid arthritis [8], anti-cancer [9], antioxidant [10,11,12], antithrombotic [3], antihyperglycemic and antihyperlipidemic [13] properties. The plethora of bioactive chemicals, including terpenoids, alkaloids, phenols, amino acids, glycosides, flavonoids, tannins, and sesquiterpenoids, in this plant is responsible for its numerous beneficial effects [2,3,14]. The Gas chromatography–mass spectrometry (GC-MS) analysis showed that the extract had 25 bioactive compounds. Total phenol (48 mg GAE/g) and total flavonoid (25 mg CE/g) content were found to be rather high in hexane leaf extracts of P. amboinicus in an analysis reported by Ashaari et al. [2]. In addition, phenolic content (146.77 g GAE/kg extract) and antioxidant activity (0.491, 0.396, and 0.643 mol TE/kg extract in 2,2-diphenyl-1-picrylhydrazyl (DPPH), ferric reducing antioxidant power (FRAP), and 2,2-azinobis (3-ethyl-benzothiazoline-6-sulfonic acid) (ABTS) tests, respectively) were detected in P. amboinicus methanolic leaf extract [12].
The practice of cultivating plant cells, tissues, and organs in vitro plays a crucial role in plant biotechnology and crop improvement, and it has proven to be a highly effective method in producing a wide array of rare, endangered, medicinal, and economically valuable plants, thereby ensuring a steady supply of vital raw materials. Clonal multiplication in vitro offers scalable options for large-scale plant propagation, conservation, and sustainable utilization [15,16]. Multiple investigations on the in vitro micropropagation of several species of Plectranthus, including P. amboinicus, have been reported by a number of researchers in the past [17,18,19,20,21,22,23,24,25,26,27]. To this day, there are no reports on the use of thidiazuron (TDZ) for in vitro proliferation and plant regeneration of P. amboinicus. TDZ (N-phenyl N-1,2,3-thiazol-5-yl urea) was initially developed as a cotton defoliant; it is a diphenylurea synthetic herbicide and plant growth regulator with strong cytokinin-like activities that has been found to be effective in promoting shoot formation, improving shoot quality, and increasing the number of shoots produced from explants. [28,29,30]. The significant effect of TDZ on tissue culture regeneration is related to endogenous cytokinin production, changes in cell membranes, nutritional absorption, metabolism, and better nutrient assimilation in plant tissues, continuing to make it distinguish out from other plant growth regulators (PGRs) [28,31,32]. The biological effect of TDZ is very variable and is determined by factors such as the concentration of the compound, the amount of time it is exposed for, the kind of explant that is utilized, and the genotype of the plant [33,34]. During the process of tissue culture regeneration, the impacts of numerous elements, including as light, elicitors, and PGRs, not only have an influence on the process of shoot regeneration and the production of biomass, but they also have an impact on the synthesis of a variety of bioactive compounds [35]. Plants are subjected to a variety of stresses during the processes of in vitro culture and acclimatization and, in order for the plant’s cells to cope with these stresses and maintain growth and development, they produce phytochemical compounds such as phenols, alkaloids, flavonoids, terpenoids, tannins, and many others. It has been shown that TDZ is effective for in vitro regeneration and synthesis of phytochemicals in a broad range of medicinal plants, including Merwilla plumbea [36], Dendrobium nobile [37], Crocus sativus [38], Tecoma stans [28], Linum usitatissimum [35,39] and Lagerstroemia speciosa [29].
Long-term exposure to TDZ has been found to have a negative impact on plant growth and development leading to the appearance of undesired shoot abnormalities such hyperhydricity, fasciation, shortening, and thickening. These abnormalities can then cause poor elongation and weak rooting capacity, ultimately affecting the overall health and productivity of the plant [40,41,42]. Regeneration of Arachis hypogaea [43] and Dianthus caryophyllus [44] in a continuous culture that contained media with TDZ resulted in the formation of unusual bud primordia that were unable to mature into fully developed plantlets. Kadota and Niimi [45] reported that TDZ significantly increased the occurrence of hyperhydric shoots in Pyrus pyrifolia by approximately ten times, compared benzyl adenine or kinetin. Ivanova and van Staden [46] suggested that TDZ should be used with caution in plant tissue culture, as its prolonged exposure can lead to the development of flattened shoot anomalies in Aloe polyphylla that ultimately failed to develop into plantlets. Furthermore, prolonged exposure to TDZ has been shown to negatively impact the rooting capacity of Solanum melongena, leading to weak and poorly developed root systems [47]. The possibility of TDZ to induce genetic changes in the regenerated plants is a major concern for its use in tissue culture and regeneration. The incidence of DNA polymorphism was found to increase in Phalaenopsis bellina plants grown in vitro after being treated with TDZ [48]. In this study, we develop an effective in vitro multiple shoot induction and user-friendly plant regeneration system for P. amboinicus using nodal explants exposed with TDZ for a very short period of time before being transfer to MS basal medium. In addition, for the first time, the nuclear DNA content, genome size, phenolic contents, antioxidant potential and GC-MS profiles of in vitro-regenerated plants were evaluated and compared to the ex-vitro donor-plant.
2. Materials and Methods
2.1. Starting Materials and Aseptic Cultures
Young proliferating shoots were collected from a Plectranthus amboinicus (Lour.) Spreng plants growing in the growth room at the Botany & Microbiology Department, College of Science, King Saud University. The collected shoots underwent a thorough washing using the laboratory’s running tap water, after which they were subjected to a Tween-20 (0.1%, v/v), treatment for five minutes, and a subsequent rinsing in ultra-pure water (UPW) to remove any traces of dust and debris. The shoot samples, now cleaned, were further sterilized with 0.1% (w/v) aqueous mercury chloride (HgCl2, Riedel-de Haan AG, Seelze, Germany) for 3 min, and then thoroughly washed with sterile UPW five times to remove to the traces of mercury. To prepare the starting materials (nodal segments, NS) for the subsequent in vitro culture studies, the sterilized shoots were cut into pieces that were 0.5–0.7 cm in length containing axillary node.
2.2. Thidiazuron (TDZ) Treatment and Shoot Regeneration
In the experiments TDZ (CAS number 51707-55-2, Duchefa Biochemie B.V., Haarlem, The Netherlands) were used for the pulse treatment of the sterilized NS explants. The NS explants were pre-soaked in a solution of Murashige and Skoog (MS) medium [49] containing micro and macro salts including vitamins (plant cell culture tested, MDL number MFCD00240976, Sigma-Aldrich, St. Louis, MO, USA) supplied with 0.5, 5, 25, 50 and 100 μM of TDZ for 4 h while gently shaking on a rotatory shaker (Incu-Shaker™ Mini, Benchmark Scientific, Sayreville NJ, USA) at 100 rpm. The treated NS explants were blotted dry on sterile filter papers and then transferred to agar (0.7%, w/v, plant cell culture tested, CAS number 9002-18-0, Sigma-Aldrich, St. Louis, MO, USA) solidified MS basal medium for further growth and regeneration. The untreated NS explants cultured on MS basal medium serve as control. The pH of the culture medium was adjusted to 5.7 using 1 N aqueous NaOH (96.0%, WINLAB, Middlesex, UK) solution before autoclaving at 121 °C (1.05 kg cm−2) for 20 min. Cultures were initiated on Petri dishes and further maintained in 100 mL culture bottles at 23 ± 2 °C under 50 μmol m−2 s−1 cool white fluorescent light (Philips 39-Watt T5 linear fluorescent tubes, Philips, Pila, Poland) with a 16/8 h day/night photoperiod.
2.3. Effect of Basal Media on Shoot Proliferation
Different basal media was also evaluated, after the determination of the most suitable TDZ pre-soaking of NS for induction and proliferation of shoots. In addition to MS, the four other types of media that were evaluated were: Gamborg B5 [50] medium (B5, Product number G0209), Linsmaier and Skoog [51] medium (LS, Product number L0230) Nitsch [52] medium (N, Product number N0224); Schenk and Hildebrandt [53] medium (SH, Product number S0225), all of which were purchased from Duchefa Biochemie B.V. (Haarlem, The Netherlands). Once every three weeks, the explants were moved to new culture vials with fresh medium. The number of regenerated shoots per treated explant and the frequency with which this occurred were determined after 8 weeks of culture on the medium.
2.4. Rooting and Acclimatization
The majority of the P. amboinicus shoots developed roots concurrently on the same in vitro culture medium, and the shoots that were unable to do so were switched to ½MS media with 0.5 μM indole 3-butyric acid (IBA, CAS number 133-32-4, Duchefa Biochemie B.V., Haarlem, The Netherlands). After washing with sterile water to remove any remnants of the basal nutrient media, the TDZ-induced shoots that had already developed a root system were carefully removed from the culture vials and transplanted into 12 cm diameter pots filled with a soil mixture (Planta-Guard Germany). The plantlets were grown in a controlled environment (Plant Growth Chamber, Conviron Adaptis, Manitoba, Canada) at a temperature of 24 ± 2 °C under 16-h day and 8-h night photoperiod with light intensity of 50 µmol m−2 s−1. The plants were watered twice a week with a nutrient solution consisting of ½MS basal media, which only included micro and macro salts, applied through sprinkling from above. After 8 weeks, the survival rate of the plants was recorded, and the acclimated in vitro P. amboinicus plants were transferred to the growth room for continued growth and development.
2.5. Flow Cytometry (FCM) and Nuclear DNA Content
FCM analysis were carried out to determine the nuclear DNA (nDNA) content and genome size of P. amboinicus donor plants and in vitro regenerated ones using a Beckman Coulter XL-MCL flow cytometer system (Beckman Coulter, Inc. USA). From 100 mg of fresh leaf tissues, nuclei were extracted by chopping them using a scalpel and suspending the pieces in 1.0 mL of filtered Galbraith buffer [54] (pH 7.0) containing 20 mM MOPS (CAS number 1132-61-2), 45 mM MgCl2 (CAS number 7791-18-6, Loba Chemie, Mumbai India), 30 mM sodium citrate (CAS number 6132-04-3, Loba Chemie, Mumbai India) and 0.1% (v/v) Triton X-100 (CAS number 9036-19-5, Sigma-Aldrich, St. Louis, MO, USA). The leaf-homogenates were filtered through a 22 µm membrane using micro syringe after being chopped, and then they were mixed for 15 min with a 50 g/mL solution of propidium iodide (PI, CAS number 25535-16-4, Sigma-Aldrich, St. Louis, MO, USA). To avoid the staining of RNA, RNase (50 µ g/mL, DNase and protease-free, Catalog number EN0531, Thermo Scientific™, Massachusetts, USA) was also included in the samples. The relative nuclear DNA (nDNA, 2C) content of each leaf sample was determined by analyzing it three times using flow cytometry (FCM) with a green laser (532 nm). The G1 peak mean values were used to compute the nDNA content, as described by Doležel and Bartoš [55], and the values were expressed in picograms (pg).
2C nDNA content of sample = standard 2C value × (sample 2C mean peak value/standard 2C mean peak value)
The genome size of P. amboinicus samples was determined by applying the formula as follows: genome size (bps) = (0.978 × 109) × 1C DNA content. This calculation relied on the previously reported information that 1 pg of DNA is equivalent to 978 million base pairs, as stated by Doležel et al. [56].
2.6. Preparation of Plant Extracts
Leaf samples from both in vitro-established and ex vitro-grown P. amboinicus donor plants were oven-dried at 50 °C for 48 h. Using a laboratory grinder, the dried leaf samples were processed to a fine powder. After grinding the materials to a fine powder, they were extracted with 50 mL of methanol for 24 h while being shaken gradually at room temperature. Following this step, the methanolic extracts were filtered using filter paper (Whatman filter paper, Grade 1). The solvent was evaporated with the help of a rotary evaporator (IKA Werke GmbH & Co. KG, Staufen im Breisgau, Germany).
2.6.1. Determination of Total Phenolics
The total phenolic content of P. amboinicus leaf extracts from both in vitro and ex vitro-grown plants was determined using a modified version of the technique reported by Singleton et al. [57]. The 10% (v/v) Folin-Ciocalteu reagent was added to a test tube containing 3.2 mL of the plant extract, and the mixture was mixed thoroughly before being let to stand for 5–10 min. Following the addition of 600 µL of a 20% (w/v) sodium carbonate solution to the mixture, it was left to incubate at room temperature for one hour. The absorbance of the mixtures was measured using a UV-Visible spectrophotometer at a wavelength of 765 nm, and the total phenolic content in both the leaf samples was calculated from the calibration curve (y = 0.0021x + 0.0245) using gallic acid as the reference standard. The results were expressed in terms of mg of gallic acid equivalents (GAE) per g dry weight of extract.
2.6.2. Determination of Total Tannins
The total tannin content of in vitro and ex vitro grown plants of P. amboinicus leaf extracts was measured using a technique reported by Chandran and Indira [58], with minor changes. Following the addition of 200 µL of plant extract, 3.2 mL of 10% (v/v) Folin-Ciocalteu reagent containing and 600 µL of sodium carbonate solution containing 35% were mixed together in the test tube. The materials were well mixed and then incubated in the dark for a period of thirty minutes so that the reaction could take place. The absorbance of the mixture samples was measured using a UV-Visible spectrophotometer at a wavelength of 700 nm. The total tannin content in both the leaf extracts was determined by using tannic acid as a reference standard and the calibration curve (y = 0.0052x + 0.0171) to calculate the total tannin content. The findings were represented as mg of tannic acid equivalents (TAE) per g dry weight of extract.
2.6.3. Determination of Total Flavonoid
The total flavonoid content of P. amboinicus leaf extracts from both in vitro and ex vitro grown plants was determined using a method reported by Ordoñez et al. [59]. It was determined by mixing 2 mL of the extract with the same volume of 2% aluminum chloride solution in a test tube. After waiting 30 min for the reaction to take place at room temperature, the absorbance was taken at a wavelength of 420 nm using a UV-Visible spectrophotometer. Using quercetin as a standard, the total flavonoid content of both the leaf extracts was determined from the calibration curve (y = 0.0172x − 0.0657) and represented as mg of quercetin equivalents (QE) per g dry weight (DW) of extract.
2.6.4. Antioxidant and 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Assay
P. amboinicus extracts from both in vitro and ex vitro grown plants were tested for their capacity to scavenge free radicals using the DPPH radical scavenging assay [60]. A total of 0.5 mL of extract at several concentrations (100–500 g/mL) was mixed with 135 µM of DPPH solution in a test tube. After thoroughly mixing, the solution was permitted to rest in the dark for half an hour. Using a spectrophotometer, we measured the absorbance of the samples at 517 nm. By using the following formula: Antioxidant capacity = [(Absorbance of control solution—Absorbance of sample solution)/Absorbance of control solution] × 100, we were able to determine the sample’s antioxidant capacity.
2.6.5. Gas Chromatography-Mass Spectrometry (GC-MS)
The phytochemical analysis of the leaf extract of both in vitro and ex vitro grown plants of P. amboinicus was performed by using a GC-MS (Turbomass, PerkinElmer Inc., Waltham, MA, USA). Individually, the samples of the extracts were injected into the Elite-5MS column, which was 30 m long, 0.25 m thick, and 0.25 m in diameter. The carrier gas was helium gas, which flowed at a rate of 1 mL/min. Starting at 40 °C, the temperature was held for 2 min before gradually rising to 200 °C (at a rate of 5 °C per minute) and then being held there for 2 min. The heat was turned up to 300 °C at a rate of 5 °C per minute, and it stayed there for the next two minutes. The resulting mass spectra were cross-referenced to those in the Adams and Wiley databases.
2.7. Statistical Analysis
The data on the shoot regeneration frequency and number of shoots from NS explants of P. amboinicus were evaluated using one-way analysis of variance (ANOVA) in IBM SPSS software version 26 (SPSS Inc., Chicago, IL, USA), and the graphs were made using the Microsoft Excel for Macintosh (Microsoft Office suite 2019, Microsoft Corporation, Washington, DC, USA). The data were given as means and standard errors (M ± SE) of the sum total of the three repeated experiments, with a total of 10 explants used in each experiment. Tukey’s HSD significant difference test in the BM SPSS software was used to determine significant differences between the means using a p-value of 0.05.
3. Results and Discussion
3.1. Effect of TDZ Pre-Treatment and Shoot Regeneration
In compared to purine-based cytokinins, TDZ is the most efficient synthetic cytokinin for eliminating apical dominance in tissue culture, and it also fulfills the cytokinin and auxin needs of regeneration responses in several plants. In the present study, we evaluated the TDZ pulse treatment of P. amboinicus NS explants to minimize the deleterious effect of extended TDZ exposure on the growth of in vitro shoots. Pretreatment of explants with TDZ prior to culture initiation can improve the responsiveness of the explants to subsequent regeneration procedures, which may overcome the inhibitory effects of endogenous growth inhibitors, such as phenolic compounds, and can also help to break the dormancy of explants, allowing for more efficient regeneration. In this study, the NS explants pre-soaked with different concentrations (0, 0.5, 5, 25, 50 and 100 μM) of TDZ exhibited initial bud break after one week of transfer on MS basal medium (Figure 1A). Explants pre-treated with varying dosages of TDZ and subsequently transferred to MS media showed statistically significant differences in the percentage of explants that produced shoots (range: 23–97%) as well as the number of shoots (range: 7–27 shoot/explants) that were developed (Table 1).

Figure 1.
Shoot induction and plant regeneration from nodal segment explants of Plectranthus amboinicus pretreated with thidiazuron for 4 h. (A) Shoot induction after 2 weeks of culture on Murashige and Skoog basal medium from nodal segment explants pretreated with 25 μM TDZ. (B) Proliferated shoot with roots after 8 weeks of culture on MS medium. (C) An acclimated plants regenerated from nodal segment explants.

Table 1.
Effect of different concentrations of thidiazuron pretreatment on shoot regeneration from nodal segment explants of Plectranthus amboinicus after 8 weeks of transfer on MS basal medium.
Only 1-2 shoots were produced by the untreated NS explants cultured on MS basal media, whereas the number and growth of the shoots were inhibited when the NS explants were exposed to greater concentrations of TDZ (100 μM). After 8 weeks of growth on MS basal media, the NS explants that were pre-treated with 25 μM TDZ produced the highest number of 27.3 shoots per explant, with the longest average shoot length (4.9 mm) in 97.2% of cultures (Figure 1B). Similarly, TDZ pretreatment improves in vitro shoot induction and plant regeneration in a number of plant species including Rauvolfia tetraphylla [41] Curculigo orchioides [61], Begonia semperflorens and Begonia spp. [62], Nyctanthes arbor-tristis [63], Lepidium campestre [64], Mentha arvensis [65], Bacopa monnieri [66], Rhododendron mucronulatum [67]. It is suggested that TDZ may be needed as a trigger for initiating the proliferation of shoot meristems and further incubation on TDZ free MS medium led the explants to further development [68]. The potential of TDZ to sustain activity even after plant materials have been transferred to a new growth medium may account for the extended activity resulting from a brief TDZ treatment [69].
3.2. Effect of Basal Media on Shoot Regeneration
The successful in vitro growth and differentiation of an explant is a complex process that is influenced by many factors, with the selection of a suitable nutrient-rich basal medium being a critical aspect. The right basal medium can provide the necessary nutrients and growth factors for the explant to thrive and develop into a mature plant. In this study, we attempted to find the most optimal culture medium for the in vitro growth of P. amboinicus explants. We compared the effectiveness of five different media, including MS, B5, LS, N and SH (as shown in Figure 2) in inducing in vitro shoot growth and plant regeneration from P. amboinicus explants that were pre-treated with 25 μM TDZ for 4 h. The results of this comparison will help to identify the most appropriate culture medium for future in vitro growth experiments with P. amboinicus and other medicinal plants. Number of shoots produced per explant varies significantly depending on the type of media used, with 27.3 shoots/explants on MS medium and 12.2 shoots/explants on LS medium, respectively (Figure 2). As a result of the comparison of various culture media, we found that MS medium was the most suitable for shoot regeneration from P. amboinicus NS explants that had been pre-treated with 25 μM TDZ. The use of MS medium, along with regular sub-culturing, was crucial in achieving successful shoot regeneration from P. amboinicus explants. The MS basal media contained a balanced mix of micro and macro salts, as well as essential vitamins, to provide the necessary nutrients for the continued proliferation of the shoots. This process ensured that the shoots received a consistent supply of nutrients, promoting their growth and development over time.
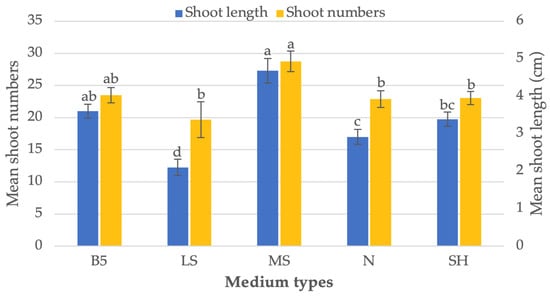
Figure 2.
Data presented in the form of bar-graph are mean and standard error (M ± SE) of three repeated experiments recorded after 8 weeks of transfer on different basal medium. Tukey’s HSD test with a p-value less than 0.05 indicates that bar with distinct letters indicate significant differences between medium.
3.3. Rooting and Acclimatization
In the present study, shoots that were regenerated from NS explants that had been pretreated with TDZ developed roots on their own in the same MS basal media, resulted in a reduction in the extra time and expenses required for the in vitro rooting of a microshoot. It has been estimated that the spontaneous rooting of microshoots may save costs from around 35 to 75 percent of the overall budget required for an in vitro regeneration [70]. The shoots that were not yet have roots were rooted in ½MS medium that contained 0.5 μM IBA. Eight weeks after being transferred to MS basal media, shoots that had been regenerated from NS explants that were pre-treated with optimal TDZ concentration (25 μM) produced an average of 4.3 roots which was 4.7 cm in length. The development of spontaneous roots system together with shoot on the same culture medium may be related to the endogenous amount of auxin as well as some other intrinsic rooting factors [71]. After 4 weeks of acclimatization in a growth chamber, the regenerated P. amboinicus micropropagated plants with robust shoot and root systems were successfully transplanted into an ex-vitro environment (Figure 1C), where they sustained a 90% survival rate.
3.4. Flow Cytometry (FCM) and Nuclear DNA Content
FCM is a fast, high-throughput technique for assessing a large number of cells in a short period of time, and it may be used to examine the genetic stability of in vitro regenerated plants by determining the DNA content and chromosome number of individual plant cells. The flow cytometer counts the number of chromosomes and the ploidy level by sorting cells based on the fluorescence intensity of DNA-specific dyes. This can help in figuring out whether there were any alterations to the DNA content throughout the in vitro regeneration process, which might have an effect on the stability and growth of the regenerated plants. In the present study, the acclimated plants that were regenerated from NS explants after being pre-treated with 25 μM TDZ were examined by FCM and compared with donor plants of P. amboinicus in order to make sure that the genetic stability of the plants was maintained. The histograms derived by the FCM analysis of both in vitro and ex vitro plants showed no significant variations in mean PI fluorescence peak, indicating that there was no difference nDNA content and ploidy level of the plants (Figure 3). Both the relative 2C nDNA content and the genome size of the P. amboinicus plants that were grown in vitro were determined to be 5.63 pg and 275.8 Mbs respectively (Figure 4). There were no discernible differences observed in either the amount of nDNA or the size of the genome among the donor plants and in vitro plants that were regenerated from NS explants pretreated with 25 μM TDZ. Similarly, the stability of nDNA content and genome size were also reported in the in vitro plants of Viola uliginosa [72], Bacopa monnieri [66] and Rhododendron mucronulatum [67].
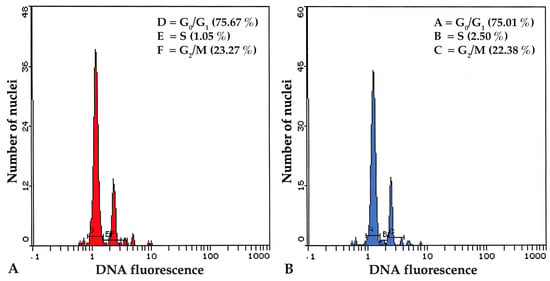
Figure 3.
FCM profiles of Plectranthus amboinicus showing the relative DNA fluorescence intensity). (A). In vitro plants; (B). Ex vitro donor plants. Letter A–F represents the phases of cell cycle.
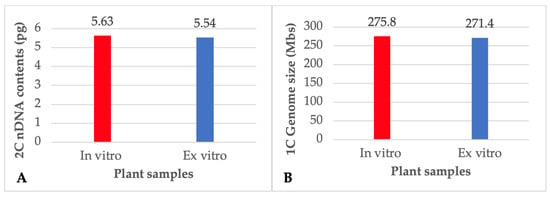
Figure 4.
Relative 2C nDNA contents (A) and genome size (B) of the in vitro regenerated and ex-vitro donor plants of Plectranthus amboinicus. pg = picogram; Mbs = Megabase pairs.
3.5. Phytochemical Analysis
Total phenolics, flavonoids, and tannin contents in leaf extracts of in vitro plants regenerated from NS explants pre-treated with 25 μM of TDZ were assessed and compared to those of ex vitro grown donor plants of P. amboinicus (Table 2). Phenolic compounds have emerged as potential targets for in vitro culture, which not only creates the optimal circumstances for their production but also encourages increased secondary metabolite accumulation and excretion by biosynthetic cells or tissues [73]. In the present study, higher concentrations of total phenolics and total tannins were detected in plant extracts grown in vitro compared to those of the ex-vitro grown donor plants (Table 2). While no significant difference was observed in the contents of total flavonoids between the donor plants and the in vitro plants that were regenerated from NS explants pre-treated with 25 μM of TDZ for 4 h (Table 2). Total phenolics, tannins, and flavonoid were measured to be 81.2, 55.6, and 42.7 mg GAE, TAE, and QE/g DW respectively, in the leaf extracts from in vitro regenerated P. amboinicus plants. It has been suggested that the discrepancy in the concentration of secondary metabolites in plants grown in vitro is regulated by the composition of the media and growth regulators in which the plants are grown [36,74,75].

Table 2.
Total phenolic compound, tannin and flavonoid contents of Plectranthus amboinicus plants grown in vitro and ex vitro.
3.6. Antioxidant and DPPH Radical Scavenging Assay
The antioxidant potential of methanol extracts of leaves of the ex vitro donor plants and the plants that were regenerated in vitro from NS explants P. amboinicus pretreated with 25 μM of TDZ was determined using DPPH free radicals scavenging assay. It was determined that there was a concentration-dependent antioxidant activity against DPPH, and that the activity was at its highest at 300 µg/mL. The strongest levels of antioxidant activity were found in in vitro regenerated plants of P. amboinicus (67.0%), compared to ex vitro donor plants (59.3%) (Figure 5). The results of the study revealed that the antioxidant capability of regenerated plants was regulated directly by the quantities of total phenolics and total tannins. Tannins are secondary antioxidants that have the capacity to bind metal ions like Fe(II), interfere with one of the reaction stages in the Fenton reaction, and slow down the oxidation process [76]. By binding to metal ions and inhibiting the Fenton reaction, tannins in regenerated plants effectively reduce the concentration of ROS and prevent the damage caused by in vitro-induced oxidative stress. In addition to their antioxidant properties, tannins have also been shown to possess anti-inflammatory, anti-tumor, and anti-microbial properties, making them a highly valuable component of plant defense mechanisms. Similarly, high antioxidant activity was reported in in vitro regenerated plants of Ceropegia santapaui [77], Coleonema pulchellum [75], Cucumis anguria [78], Angelica glauca [79] and Ruta chalepensis [80] all of which are in agreement with our findings.
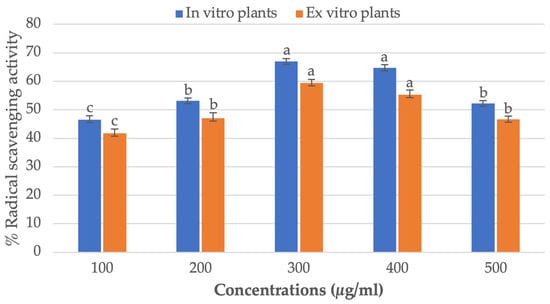
Figure 5.
Free radical-scavenging activity of Plectranthus amboinicus plants grown in vitro and ex vitro by the DPPH method. Tukey’s HSD test with a p-value less than 0.05 indicates that bar with distinct letters indicate significant differences between medium.
3.7. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
GC-MS is a widely used analytical technique for identifying and analyzing the chemical composition of plants, and it may offer useful information about the potential therapeutic effects of various chemicals such as alkaloids, flavonoids, amino acids, organic acids etc. The analysis can also be used to identify any changes in the metabolic profile of the in vitro regenerated plants compared to their wild-type counterparts, providing insight into the genetic and environmental factors that influence plant growth and development. In the present study, the chromatographic examination made it possible to identify 19 and 21, respectively, of the bioactive compounds that were present in ex vitro and in vitro P. amboinicus plants (Table 3).

Table 3.
Gas chromatography-mass spectrometry (GC-MS) analysis from leaf extracts of Plectranthus amboinicus plants grown in vitro and ex vitro.
The heatmap in Figure 6 depicts a cluster analysis based on the levels of phytochemicals in leaf extracts from in vitro and donor plants, as measured by GC-MS. The study found that both ex vitro and in vitro plants contained eleven chemicals that were chemically identical to one another. In both the ex vitro and in vitro leaf extracts of P. amboinicus plants, it was found that 5-methyl-2-(1-methylethyl)-phenol was the main chemical constituent, accounting for 91.38 and 86.69 percent of the total, respectively. Eleven phytocompounds were found in in vitro plants, but they were not present in ex vitro donor plants, some of which have biological activities such as octadecanoic acid (0.1%), caryophyllene oxide (0.19%), Alpha-humulene (0.22%), 5-(Hydroxymethyl)-2-furancarboxaldehyde (0.25%), 10-heneicosene (0.32%), aromadendrene (0.34%), hexadecanoic acid (0.51%), 9,12,15-octadecatrienoic acid (0.59%). The possible reasons for the increased number of phytochemical substances in in vitro propagated plantlets compared to the field grown plants could be influenced by various factors, including the tissue culture conditions, the supply of the nutrient media, the presence of growth regulators, genetic modifications and chemical and abiotic elicitors [80,81,82].
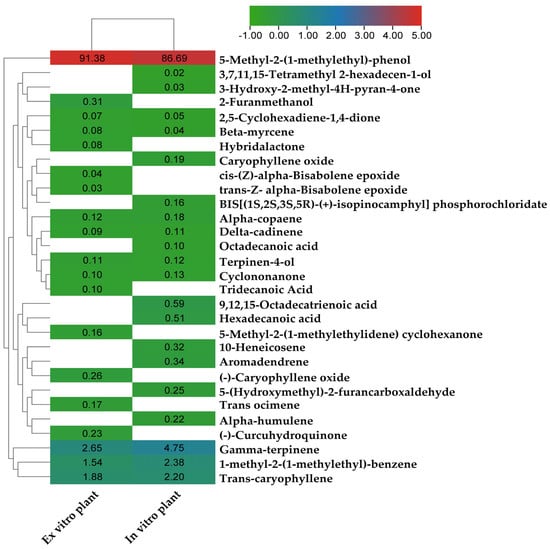
Figure 6.
Relative abundance of phytochemicals measured by GC-MS in leaf extracts of Plectranthus amboinicus plants grown in vitro and ex vitro.
4. Conclusions
This is the first report describing the TDZ induced plant regeneration, as well as total phenolics, total tannin, total flavonoid contents, DPHH radical-scavenging activity and GC-MS profiles of leaf extracts from in vitro and ex vitro growing plants of P. amboinicus. In this study a simple and efficient protocol for plant regeneration was established from NS explants that had been pretreated with 25 μM of TDZ followed by their transfer onto the MS basal media which offer an alternative approach for rapid in vitro propagation and mass multiplication of P. amboinicus. The application of TDZ pulse treatment for a short duration of 4h allows the production of healthy shoots with no abnormalities, as well as the development of spontaneous roots on the same medium, saving money and time in comparison to the technique of prolonged exposure to TDZ and other growth regulators. Moreover, the characteristics of the in vitro-regenerated plants were analyzed and compared to the ex-vitro donor plants with regards to the nuclear DNA content, genome size, phenolic content, antioxidant capacity, and GC-MS profiles. The results of the nuclear genome stability and biochemical analyses of the in vitro propagated P. amboinicus plants could lead to the development of conservation and sustainable use strategies for this valuable natural resource.
Author Contributions
Conceptualization, M.F.; methodology, M.F. and A.A.Q.; validation, M.F. and A.A.A.; formal analysis, M.F., A.A.Q. and A.A.A.; investigation, M.F. and A.A.A.; resources, M.F. and A.A.A.; data curation, M.F.; writing—original draft preparation, M.F.; writing—review and editing, M.F., A.A.Q. and A.A.A.; project administration, M.F. and A.A.A.; funding acquisition, M.F. and A.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
The work was financially supported by the Researchers Supporting Project Number (RSP-2023R86), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Arumugam, G.; Swamy, M.K.; Sinniah, U.R. Plectranthus amboinicus (Lour.) Spreng: Botanical, Phytochemical, Pharmacological and Nutritional Significance. Molecules 2016, 21, 369. [Google Scholar] [CrossRef]
- Ashaari, N.S.; Mohamad, N.E.; Afzinizam, A.H.; Ab. Rahim, M.-H.; Lai, K.S.; Ong Abdullah, J. Chemical Composition of Hexane-Extracted Plectranthus amboinicus Leaf Essential Oil: Maximizing Contents on Harvested Plant Materials. Appl. Sci. 2021, 11, 10838. [Google Scholar] [CrossRef]
- Bhatt, P.; Joseph, G.S.; Negi, P.S.; Varadaraj, M.C. Chemical Composition and Nutraceutical Potential of Indian Borage (Plectranthus amboinicus) Stem Extract. J. Chem. 2013, 2013, 320329. [Google Scholar] [CrossRef]
- Vasconcelos, S.E.C.B.; Melo, H.M.; Cavalcante, T.T.A.; Júnior, F.E.A.C.; de Carvalho, M.G.; Menezes, F.G.R.; de Sousa, O.V.; Costa, R.A. Plectranthus amboinicus essential oil and carvacrol bioactive against planktonic and biofilm of oxacillin- and vancomycin-resistant Staphylococcus aureus. BMC Complement. Altern. Med. 2017, 17, 462. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, M.; Poonkothai, M. Phytochemical and antimicrobial analyses of Plectranthus amboinicus leaf extracts. Res. J. Pharm. Technol. 2021, 14, 6379–6384. [Google Scholar] [CrossRef]
- Gurgel, A.P.A.D.; da Silva, J.G.; Grangeiro, A.R.S.; Oliveira, D.C.; Lima, C.M.P.; da Silva, A.C.P.; Oliveira, R.A.G.; Souza, I.A. In vivo study of the anti-inflammatory and antitumor activities of leaves from Plectranthus amboinicus (Lour.) Spreng (Lamiaceae). J. Ethnopharmacol. 2009, 125, 361–363. [Google Scholar] [CrossRef]
- Akinbo, D.B.; Onyeaghala, A.A.; Emomidue, J.O.; Ogbhemhe, S.O.; Okpoli, H.C. Phytochemical and anti-inflammatory activities of aqueous leaf extract of Indian borage (oregano) on rats induced with inflammation. Cancer Biomark. 2018, 22, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-M.; Cheng, C.-M.; Hung, L.-M.; Chung, Y.-S.; Wu, R.-Y. Potential Use of Plectranthus amboinicus in the Treatment of Rheumatoid Arthritis. Evid.-Based Complement. Altern. Med. 2010, 7, 174726. [Google Scholar] [CrossRef]
- Manjamalai, A.; Grace, V.M.B. The Chemotherapeutic Effect of Essential Oil of Plectranthus amboinicus (Lour) on Lung Metastasis Developed by B16F-10 Cell Line in C57BL/6 Mice. Cancer Investig. 2013, 31, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, A.; Joel Karunakaran, R. Antioxidant and free radical scavenging activity of an aqueous extract of Coleus aromaticus. Food Chem. 2006, 97, 109–114. [Google Scholar] [CrossRef]
- Bhatt, P.; Negi, P.S. Antioxidant and antibacterial activities in the leaf extracts of Indian borage (Plectranthus amboinicus). Food Nutr. Sci. 2012, 2012, 146–152. [Google Scholar]
- Kozłowska, M.; Ścibisz, I.; Przybył, J.; Ziarno, M.; Żbikowska, A.; Majewska, E. Phenolic Contents and Antioxidant Activity of Extracts of Selected Fresh and Dried Herbal Materials. Pol. J. Food Nutr. Sci. 2021, 71, 269–278. [Google Scholar] [CrossRef]
- Viswanathaswamy, A.; Koti, B.; Gore, A.; Thippeswamy, A.; Kulkarni, R. Antihyperglycemic and antihyperlipidemic activity of Plectranthus amboinicus on normal and alloxan-induced diabetic rats. Indian J. Pharm. Sci. 2011, 73, 139. [Google Scholar] [PubMed]
- Patel, R.D.; Mahobia, N.K.; Singh, M.P.; Singh, A.; Sheikh, N.W.; Alam, G.; Singh, S.K. Antioxidant potential of leaves of Plectranthus amboinicus (Lour) Spreng. Pharm. Lett. 2010, 2, 240–245. [Google Scholar]
- Varshney, A.; Anis, M. Trees: Propagation and Conservation: Biotechnological Approaches for Propagation of a Multipurpose Tree, Balanites Aegyptiaca Del; Springer Science & Business Media: New Delhi, India, 2014. [Google Scholar]
- Ahmad, N.; Javed, S.B.; Khan, M.I.; Anis, M. Rapid plant regeneration and analysis of genetic fidelity in micropropagated plants of Vitex trifolia: An important medicinal plant. Acta Physiol. Plant. 2013, 35, 2493–2500. [Google Scholar] [CrossRef]
- Ab Rahman, Z.; Noor, E.S.M.; Ali, M.S.M.; Mirad, R.; Othman, A.N. In vitro micropropagation of a valuable medicinal plant, Plectranthus amboinicus. Am. J. Plant Sci. 2015, 6, 1091–1097. [Google Scholar] [CrossRef]
- Arumugam, G.; Sinniah, U.R.; Swamy, M.K.; Lynch, P.T. Micropropagation and essential oil characterization of Plectranthus amboinicus (Lour.) Sprengel, an aromatic medicinal plant. In Vitro Cell. Dev. Biol.-Plant 2020, 56, 491–503. [Google Scholar] [CrossRef]
- Faisal, M.; Alatar, A.A. Establishment of an Efficient In Vitro Propagation Method for a Sustainable Supply of Plectranthus amboinicus (Lour.) and Genetic Homogeneity Using Flow Cytometry and SPAR Markers. Horticulturae 2022, 8, 693. [Google Scholar] [CrossRef]
- Sreedevi, E.; Anuradha, M.; Pullaiah, T. Plant regeneration from leaf-derived callus in Plectranthus barbatus Andr. [Syn.: Coleus forskohlii (Wild.) Briq.]. Afr. J. Biotechnol. 2013, 12, 2441–2448. [Google Scholar]
- Tsegaw, M.; Feyissa, T. Micropropagation of Plectranthus edulis (Vatke) Agnew from meristem cultur. Afr. J. Biotechnol. 2014, 13, 3682–3688. [Google Scholar] [CrossRef]
- Kebede, B.; Abera, B. Micropropagation of Plectranthus edulis (Vatke) Agnew from shoot tip and nodal explants. Afr. J. Agric. Res. 2015, 10, 6–13. [Google Scholar] [CrossRef]
- Thaniarasu, R.; Kumar, T.S.; Rao, M. In vitro Propagation of Plectranthus bourneae Gamble-An Endemic Red Listed Plant. Plant Tissue Cult. Biotechnol. 2015, 25, 273–284. [Google Scholar] [CrossRef]
- Thaniarasu, R.; Senthil Kumar, T.; Rao, M.V. Mass propagation of Plectranthus bourneae Gamble through indirect organogenesis from leaf and internode explants. Physiol. Mol. Biol. Plants 2016, 22, 143–151. [Google Scholar] [CrossRef]
- Fonseka, D.; Wickramaarachchi, W.; Situge, C. Mass production of Plectranthus zeylanicus-A valuable medicinal and aromatic plant with a future value. Int. J. Minor Fruits Med. Aromat. Plants 2019, 5, 15–20. [Google Scholar]
- Mahmoud, D.S.; Sayed, L.M.; Diab, M.; Fahmy, E.M. In vitro propagation of Plectranthus barbatus andrews as important medicinal plant. Arab. Univ. J. Agric. Sci. 2019, 27, 511–517. [Google Scholar] [CrossRef]
- Kujeke, G.T.; Chitendera, T.C.; Masekesa, R.T.; Mazarura, U.; Ngadze, E.; Rugare, J.T.; Matikiti, A. Micropropagation of Livingstone Potato (Plectranthus esculentus N.E.Br). Adv. Agric. 2020, 2020, 8364153. [Google Scholar] [CrossRef]
- Hussain, S.A.; Ahmad, N.; Anis, M.; Hakeem, K.R. Development of an efficient micropropagation system for Tecoma stans (L.) Juss. ex Kunth using thidiazuron and effects on phytochemical constitution. In Vitro Cell. Dev. Biol.-Plant 2019, 55, 442–453. [Google Scholar] [CrossRef]
- Ahmad, N.; Faisal, M.; Ahmad, A.; Alatar, A.A.; Qahtan, A.A.; Alok, A. Thidiazuron Induced In Vitro Clonal Propagation of Lagerstroemia speciosa (L.) Pers.—An Important Avenue Tree. Horticulturae 2022, 8, 359. [Google Scholar] [CrossRef]
- Gharari, Z.; Bagheri, K.; Sharafi, A.; Danafar, H. Thidiazuron induced efficient in vitro organogenesis and regeneration of Scutellaria bornmuelleri: An important medicinal plant. In Vitro Cell. Dev. Biol.-Plant 2019, 55, 133–138. [Google Scholar] [CrossRef]
- Mok, M.; Mok, D.; Armstrong, D.; Shudo, K.; Isogai, Y.; Okamoto, T. Cytokinin activity of N-phenyl-N′-1, 2, 3-thiadiazol-5-ylurea (thidiazuron). Phytochemistry 1982, 21, 1509–1511. [Google Scholar] [CrossRef]
- Murthy, B.N.S.; Murch, S.J.; Saxena, P.K. Thidiazuron: A potent regulator of in vitro plant morphogenesis. In Vitro Cell. Dev. Biol.-Plant 1998, 34, 267. [Google Scholar] [CrossRef]
- Ċosiċ, T.; Motyka, V.; Raspor, M.; Savić, J.; Cingel, A.; Vinterhalter, B.; Vinterhalter, D.; Trávníčková, A.; Dobrev, P.I.; Bohanec, B.; et al. In vitro shoot organogenesis and comparative analysis of endogenous phytohormones in kohlrabi (Brassica oleracea var. gongylodes): Effects of genotype, explant type and applied cytokinins. Plant Cell Tissue Organ Cult. 2015, 121, 741–760. [Google Scholar] [CrossRef]
- Guo, B.; Abbasi, B.H.; Zeb, A.; Xu, L.; Wei, Y. Thidiazuron: A multi-dimensional plant growth regulator. Afr. J. Biotechnol. 2011, 10, 8984–9000. [Google Scholar] [CrossRef]
- Khan, I.; Khan, M.A.; Shehzad, M.A.; Ali, A.; Mohammad, S.; Ali, H.; Alyemeni, M.N.; Ahmad, P. Micropropagation and Production of Health Promoting Lignans in Linum usitatissimum. Plants 2020, 9, 728. [Google Scholar] [PubMed]
- Baskaran, P.; Ncube, B.; Van Staden, J. In vitro propagation and secondary product production by Merwilla plumbea (Lindl.) Speta. Plant Growth Regul. 2012, 67, 235–245. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Kumaria, S.; Diengdoh, R.; Tandon, P. Genetic stability and phytochemical analysis of the in vitro regenerated plants of Dendrobium nobile Lindl., an endangered medicinal orchid. Meta Gene 2014, 2, 489–504. [Google Scholar] [CrossRef]
- Moradi, A.; Zarinkamar, F.; Caretto, S.; Azadi, P. Influence of thidiazuron on callus induction and crocin production in corm and style explants of Crocus sativus L. Acta Physiol. Plant. 2018, 40, 185. [Google Scholar] [CrossRef]
- Anjum, S.; Abbasi, B.H. Thidiazuron-enhanced biosynthesis and antimicrobial efficacy of silver nanoparticles via improving phytochemical reducing potential in callus culture of Linum usitatissimum L. Int. J. Nanomed. 2016, 11, 715. [Google Scholar] [CrossRef]
- Huetteman, C.A.; Preece, J.E. Thidiazuron: A potent cytokinin for woody plant tissue culture. Plant Cell Tissue Organ Cult. 1993, 33, 105–119. [Google Scholar] [CrossRef]
- Faisal, M.; Ahmad, N.; Anis, M. Shoot multiplication in Rauvolfia tetraphylla L. using thidiazuron. Plant Cell Tissue Organ Cult. 2005, 80, 187–190. [Google Scholar] [CrossRef]
- Dewir, Y.H.; Nurmansyah; Naidoo, Y.; Teixeira da Silva, J.A. Thidiazuron-induced abnormalities in plant tissue cultures. Plant Cell Rep. 2018, 37, 1451–1470. [Google Scholar] [CrossRef]
- Akasaka, Y.; Daimon, H.; Mii, M. Improved plant regeneration from cultured leaf segments in peanut (Arachis hypogaea L.) by limited exposure to thidiazuron. Plant Sci. 2000, 156, 169–175. [Google Scholar] [CrossRef]
- Ahmad, N.; Srivastava, R.; Anis, M. Improvement in carnation shoot multiplication using thidiazuron in vitro. Propag. Ornam. Plants 2006, 6, 109–113. [Google Scholar]
- Kadota, M.; Niimi, Y. Effects of cytokinin types and their concentrations on shoot proliferation and hyperhydricity in in vitro pear cultivar shoots. Plant Cell Tissue Organ Cult. 2003, 72, 261–265. [Google Scholar] [CrossRef]
- Ivanova, M.; van Staden, J. Effect of ammonium ions and cytokinins on hyperhydricity and multiplication rate of in vitro regenerated shoots of Aloe polyphylla. Plant Cell Tissue Organ Cult. 2008, 92, 227–231. [Google Scholar] [CrossRef]
- Magioli, C.; Rocha, A.P.M.; de Oliveira, D.E.; Mansur, E. Efficient shoot organogenesis of eggplant (Solanum melongena L.) induced by thidiazuron. Plant Cell Rep. 1998, 17, 661–663. [Google Scholar] [CrossRef] [PubMed]
- Khoddamzadeh, A.A.; Sinniah, U.R.; Kadir, M.A.; Kadzimin, S.; Mahmood, M.; Sreeramanan, S. Detection of somaclonal variation by random amplified polymorphic DNA analysis during micropropagation of Phalaenopsis bellina (Rchb. f.) Christenson. Afr. J. Biotechnol. 2010, 9, 6632–6639. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar]
- Linsmaier, E.M.; Skoog, F. Organic growth factor requirements of tobacco tissue cultures. Physiol. Plant. 1965, 18, 100–127. [Google Scholar]
- Nitsch, J.P.; Nitsch, C. Haploid Plants from Pollen Grains. Science 1969, 163, 85–87. [Google Scholar] [CrossRef]
- Schenk, R.U.; Hildebrandt, A. Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can. J. Bot. 1972, 50, 199–204. [Google Scholar] [CrossRef]
- Galbraith, D.W.; Harkins, K.R.; Maddox, J.M.; Ayres, N.M.; Sharma, D.P.; Firoozabady, E. Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science 1983, 220, 1049–1051. [Google Scholar] [CrossRef]
- Doležel, J.; Bartoš, J. Plant DNA flow cytometry and estimation of nuclear genome size. Ann. Bot. 2005, 95, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Doležel, J.; Bartoš, J.; Voglmayr, H.; Greilhuber, J. Nuclear DNA Content and Genome Size of Trout and Human. Cytom. Part A 2003, 51, 127–128. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Chandran, K.C.; Indira, G. Quantitative estimation of total phenolic, flavonoids, tannin and chlorophyll content of leaves of Strobilanthes Kunthiana (Neelakurinji). J. Med. Plants Stud. 2016, 4, 282–286. [Google Scholar]
- Ordoñez, A.A.L.; Gomez, J.D.; Vattuone, M.A.; lsla, M.I. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem. 2006, 97, 452–458. [Google Scholar] [CrossRef]
- Liyana-Pathirana, C.M.; Shahidi, F. Antioxidant activity of commercial soft and hard wheat (Triticum aestivum L.) as affected by gastric pH conditions. J. Agric. Food Chem. 2005, 53, 2433–2440. [Google Scholar] [PubMed]
- Thomas, T.D. Pretreatment in thidiazuron improves the in vitro shoot induction from leaves in Curculigo orchioides Gaertn., an endangered medicinal plant. Acta Physiol. Plant. 2007, 29, 455–461. [Google Scholar] [CrossRef]
- Kereša, S.; Mihovilović, A.; Barić, M.; Jerčić, I.H. Efficient plant regeneration of Begonia semperflorens and Begonia spp. from petiole and leaf explants. J. Food Agric. Environ. 2011, 9, 240–244. [Google Scholar]
- Jahan, A.A.; Anis, M.; Aref, I.M. Preconditioning of Axillary Buds in Thidiazuron-Supplemented Liquid Media Improves In Vitro Shoot Multiplication in Nyctanthes arbor-tristis L. Appl. Biochem. Biotechnol. 2011, 163, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Ivarson, E.; Ahlman, A.; Li, X.; Zhu, L.-H. Development of an efficient regeneration and transformation method for the new potential oilseed crop Lepidium campestre. BMC Plant Biol. 2013, 13, 115. [Google Scholar] [CrossRef] [PubMed]
- Faisal, M.; Alatar, A.A.; Hegazy, A.K.; Alharbi, S.A.; El-Sheikh, M.; Okla, M.K. Thidiazuron induced in vitro multiplication of Mentha arvensis and evaluation of genetic stability by flow cytometry and molecular markers. Ind. Crops Prod. 2014, 62, 100–106. [Google Scholar] [CrossRef]
- Faisal, M.; Alatar, A.A.; El-Sheikh, M.A.; Abdel-Salam, E.M.; Qahtan, A.A. Thidiazuron induced in vitro morphogenesis for sustainable supply of genetically true quality plantlets of Brahmi. Ind. Crops Prod. 2018, 118, 173–179. [Google Scholar] [CrossRef]
- Novikova, T.I.; Asbaganov, S.V.; Ambros, E.V.; Zaytseva, Y.G. TDZ-induced axillary shoot proliferation of Rhododendron mucronulatum Turcz and assessment of clonal fidelity using DNA-based markers and flow cytometry. In Vitro Cell. Dev. Biol.-Plant 2020, 56, 307–317. [Google Scholar] [CrossRef]
- Khalafalla, M.M.; Hattori, K. A combination of thidiazuron and benzyladenine promotes multiple shoot production from cotyledonary node explants of faba bean (Vicia faba L.). Plant Growth Regul. 1999, 27, 145–148. [Google Scholar] [CrossRef]
- Capelle, S.C.; Mok, D.W.; Kirchner, S.C.; Mok, M.C. Effects of thidiazuron on cytokinin autonomy and the metabolism of N 6-(Δ2-isopentenyl)[8-14C] adenosine in callus tissues of Phaseolus lunatus L. Plant Physiol. 1983, 73, 796–802. [Google Scholar] [CrossRef]
- Debergh, P.C.; Maene, L.J. A scheme for commercial propagation of ornamental plants by tissue culture. Sci. Hortic. 1981, 14, 335–345. [Google Scholar] [CrossRef]
- Agretious, T.K.; Martin, K.; Hariharan, M. In vitro clonal multiplication of Alpinia calcarata Rosc. Phytomorphology 1996, 46, 133–138. [Google Scholar]
- Slazak, B.; Sliwinska, E.; Saługa, M.; Ronikier, M.; Bujak, J.; Słomka, A.; Göransson, U.; Kuta, E. Micropropagation of Viola uliginosa (Violaceae) for endangered species conservation and for somaclonal variation-enhanced cyclotide biosynthesis. Plant Cell Tissue Organ Cult. 2015, 120, 179–190. [Google Scholar] [CrossRef]
- Matkowski, A. Plant in vitro culture for the production of antioxidants—A review. Biotechnol. Adv. 2008, 26, 548–560. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Khan, M.A.; Mahmood, T.; Ahmad, M.; Chaudhary, M.F.; Khan, M.A. Shoot regeneration and free-radical scavenging activity in Silybum marianum L. Plant Cell Tissue Organ Cult. 2010, 101, 371–376. [Google Scholar] [CrossRef]
- Baskaran, P.; Moyo, M.; Van Staden, J. In vitro plant regeneration, phenolic compound production and pharmacological activities of Coleonema pulchellum. S. Afr. J. Bot. 2014, 90, 74–79. [Google Scholar] [CrossRef]
- Karamać, M. Chelation of Cu(II), Zn(II), and Fe(II) by Tannin Constituents of Selected Edible Nuts. Int. J. Mol. Sci. 2009, 10, 5485–5497. [Google Scholar] [CrossRef] [PubMed]
- Chavan, J.J.; Gaikwad, N.B.; Umdale, S.D.; Kshirsagar, P.R.; Bhat, K.V.; Yadav, S.R. Efficiency of direct and indirect shoot organogenesis, molecular profiling, secondary metabolite production and antioxidant activity of micropropagated Ceropegia santapaui. Plant Growth Regul. 2014, 72, 1–15. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Chung, I.-M. Phenolic compound production and biological activities from in vitro regenerated plants of gherkin (Cucumis anguria L.). Electron. J. Biotechnol. 2015, 18, 295–301. [Google Scholar] [CrossRef]
- Rawat, J.M.; Bhandari, A.; Mishra, S.; Rawat, B.; Dhakad, A.K.; Thakur, A.; Chandra, A. Genetic stability and phytochemical profiling of the in vitro regenerated plants of Angelica glauca Edgew.: An endangered medicinal plant of Himalaya. Plant Cell Tissue Organ Cult. 2018, 135, 111–118. [Google Scholar] [CrossRef]
- Qahtan, A.A.; Faisal, M.; Alatar, A.A.; Abdel-Salam, E.M. Callus-Mediated High-Frequency Plant Regeneration, Phytochemical Profiling, Antioxidant Activity and Genetic Stability in Ruta chalepensis L. Plants 2022, 11, 1614. [Google Scholar] [CrossRef]
- López-Laredo, A.R.; Ramírez-Flores, F.D.; Sepúlveda-Jiménez, G.; Trejo-Tapia, G. Comparison of metabolite levels in callus of Tecoma stans (L.) Juss. ex Kunth. cultured in photoperiod and darkness. In Vitro Cell. Dev. Biol. Plant 2009, 45, 550–558. [Google Scholar] [CrossRef]
- Upadhyay, A.; Shahzad, A.; Ahmad, Z.; Alatar, A.A.; Guerriero, G.; Faisal, M. High frequency direct organogenesis, genetic homogeneity, chemical characterization and leaf ultra-structural study of regenerants in Diplocyclos palmatus (L.) C. Jeffrey. Agronomy 2021, 11, 2164. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).