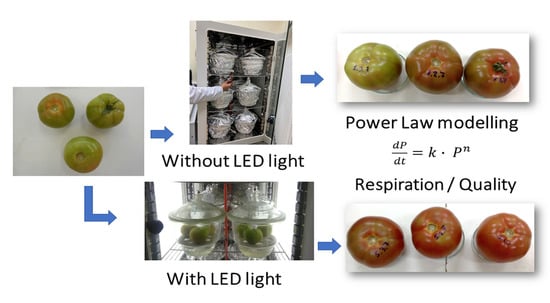
The Effect of Visible Light on the Postharvest Life of Tomatoes (Solanum lycopersicum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Storage Conditions
2.3. Gas Composition
2.4. Tomato Quality Attributes
2.5. Kinetics
2.6. Experimental Design and Statistical Analyses
3. Results and Discussion
3.1. Effect of Light on Preclimacteric Ripening of Tomato Fruit
3.2. Effect of Light on the Quality Attributes of Tomato Fruit
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhandari, R.; Neupane, N.; Adhikari, D.P. Climatic change and its impact on tomato (Lycopersicum esculentum L.) production in plain area of Nepal. Environ. Chall. 2021, 4, 100129. [Google Scholar] [CrossRef]
- Sipos, L.; Li, T.; Orbán, C.; Bálint, I.; Csambalik, L.; Divéky-Ertsey, A.; Gere, A. Colour parameters as indicators of lycopene and antioxidant activity traits of cherry tomatoes (Solanum lycopersicum L.). Eur. Food Res. Technol. 2017, 243, 1533–1543. [Google Scholar] [CrossRef]
- Raiola, A.; Pizzolongo, F.; Manzo, N.; Montefusco, I.; Spigno, P.; Romano, R.; Barone, A. A comparative study of the physico-chemical properties affecting the organoleptic quality of fresh and thermally treated yellow tomato ecotype fruit. Int. J. Food Sci. Technol. 2018, 53, 1219–1226. [Google Scholar] [CrossRef]
- Viskelis, P.; Radzevicius, A.; Urbonaviciene, D.; Viskelis, J.; Karkleliene, R.; Bobinas, C. Biochemical parameters in tomato fruits from different cultivars as functional foods for agricultural, industrial, and pharmaceutical uses. In Plants for the Future; El-Shemy, H., Ed.; IntechOpen Limited: London, UK, 2015; Volume 3, pp. 45–77. [Google Scholar] [CrossRef]
- Bhandari, G. An overview of agrochemicals and their effects on environment in Nepal. Appl. Ecol. Environ. Sci. 2014, 2, 66–73. [Google Scholar] [CrossRef]
- Patle, G.T.; Kharpude, S.N.; Dabral, P.P.; Kumar, V. Impact of organic farming on sustainable agriculture system and marketing potential: A review. Int. J. Environ. Clim. Chang. 2020, 10, 100–120. [Google Scholar] [CrossRef]
- Liu, J.; Stevens, C.; Khan, V.A.; Lu, J.Y.; Wilson, C.L.; Adeyeye, O.; Kabwe, M.K.; Pusey, P.L.; Chalutz, E.; Sultana, T.; et al. Application of ultraviolet-C light on storage rots and ripening of tomatoes. J. Food Prot. 1993, 56, 868–872. [Google Scholar] [CrossRef]
- FAO. Pérdida y Desperdicios de Alimentos en América Latina y el Caribe; Boletín No.4; Oficina Regional de la FAO para América Latina y el Caribe: Santiago, Chile, 2017; pp. 11–15. [Google Scholar]
- Recabarren, P.E.; Reyes, D.A. Pérdida y Desperdicio de Alimentos (PDA) en Chile: Avances y Desafíos; Oficina de Estudios y Políticas Agrarias-Odepa-Ministerio de Agricultura: Santiago, Chile, 2019; pp. 5–9. [Google Scholar]
- Saltveit, M.E. Effect of ethylene on quality of fresh fruits and vegetables. Postharvest Biol. Technol. 1999, 15, 279–292. [Google Scholar] [CrossRef]
- Domínguez, I.; Lafuente, M.T.; Hernández-Muñoz, P.; Gavarai, R. Influence of modified atmosphere and ethylene levels on quality attributes of fresh tomatoes (Lycopersicon esculentum Mill). Food Chem. 2016, 209, 211–219. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, D.; Wang, B.; Khan, I.; Ni, Y. Ethylene control technologies in extending postharvest shelf life of climacteric fruit. J. Agric. Food Chem. 2017, 65, 7308–7319. [Google Scholar] [CrossRef]
- Basso, A.; de Fátima Peralta Muniz Moreira, R.; José, H.J. Effect of operational conditions on photocatalytic ethylene degradation applied to control tomato ripening. J. Photochem. Photobiol. A Chem. 2018, 367, 294–301. [Google Scholar] [CrossRef]
- Pathak, N.; Caleb, O.J.; Rauh, C.; Mahajan, P.V. Efficacy of photocatalysis and photolysis systems for the removal of ethylene under different storage conditions. Postharvest Biol. Technol. 2019, 147, 68–77. [Google Scholar] [CrossRef]
- Verheul, M.J.; Slimestad, R.; Tjøstheim, I.H. From producer to consumer: Greenhouse tomato quality as affected by variety, maturity stage at harvest, transport conditions, and supermarket storage. J. Agric. Food Chem. 2015, 63, 5026–5034. [Google Scholar] [CrossRef] [PubMed]
- Quinet, M.; Angosto, T.; Juste-Lisbona, F.J.; Blanchard-Gros, R.; Bigot, S.; Martínez, J.P.; Lutts, S. Tomato fruit development and metabolism. Front. Plant Sci. 2019, 10, 1554. [Google Scholar] [CrossRef]
- Lytovchenko, A.; Eickmeier, I.; Pons, C.; Osorio, S.; Szecowka, M.; Lehmberg, K.; Arrivault, S.; Tohge, T.; Pineda, B.; Anton, M.T.; et al. Tomato fruit photosynthesis is seemingly unimportant in primary metabolism and ripening but plays a considerable role in seed development. Plant Physiol. 2011, 157, 1650–1663. [Google Scholar] [CrossRef]
- Llorente, B.; D’Andrea, L.; Ruiz-sola, M.A.; Botterweg, E.; Pulido, P.; Andilla, J.; Botterweg, E.; Loza-Alvarez, P.; Rodriguez-Concepcion, M. Tomato fruit carotenoid biosynthesis is adjusted to actual ripening progression by a light-dependent mechanism. Plant J. 2016, 85, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.B.; Bianchetti, R.E.; Alves, F.R.R.; Purgatto, E.; Peres, L.E.P.; Rossi, M.; Freschi, L. Light, ethylene and auxin signaling interaction regulates carotenoid biosynthesis during tomato fruit ripening. Front. Plant Sci. 2018, 9, 1370. [Google Scholar] [CrossRef] [PubMed]
- López-Camelo, A.F.; Gómez, P.A. Comparison of color indexes for tomato ripening. Hortic. Bras. 2004, 22, 534–537. [Google Scholar] [CrossRef]
- A.O.A.C. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemist: Washington DC, USA, 1990; pp. 910–928. [Google Scholar]
- Fish, W.W.; Perkins-Veazie, P.; Collins, J.K. A quantitative assay for lycopene that utilizes reduced volumes of organic solvents. J. Food Compos. Anal. 2002, 15, 309–317. [Google Scholar] [CrossRef]
- De Bruijn, J.; Gómez, A.; Loyola, C.; Melín, P.; Solar, V.; Abreu, N.; Azzolina-Jury, F.; Valdés, H. Use of a copper- and zinc-modified natural zeolite to improve ethylene removal and postharvest quality of tomato fruit. Crystals 2020, 10, 471. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical Reaction Engineering, 3rd ed.; J. Wiley & Sons, Inc.: New York, NY, USA, 1999; pp. 63–67. [Google Scholar]
- Kuehl, R.O. Diseño de Experimentos, Principios Estadísticos de Diseño y Análisis de Investigación, 2nd ed.; Thompson Learning: Oaxaca, México, 2001; pp. 263–309. [Google Scholar]
- Canavos, G.C. Probabilidad y Estadística. Aplicaciones y Métodos; McGraw-Hill/Interamericano de México S.A.: Cuautitlán Izcalli, México, 1988; pp. 572–589. [Google Scholar]
- Smillie, R.M.; Hetherington, S.E.; Davies, W.J. Photosynthetic activity of the calyx, green shoulder, pericarp, and locular parenchyma of tomato fruit. J. Exp. Bot. 1999, 50, 707–718. [Google Scholar] [CrossRef]
- Yang, C.C.; Chinnan, M.S. Modeling the effect of O2 and CO2 on respiration and quality of stored tomatoes. Trans. Am. Soc. Agric. Eng. 1988, 31, 920–925. [Google Scholar] [CrossRef]
- Gong, S.; Corey, K.A. Predicting steady-state oxygen concentrations in modified-atmosphere packages of tomatoes. J. Am. Soc. Hortic. Sci. 1994, 119, 546–550. [Google Scholar] [CrossRef]
- Henig, Y.S.; Gilbert, S.G. Computer analysis of the variables affecting respiration and quality of produce packaged in polymeric films. J. Food Sci. 1975, 40, 1033–1035. [Google Scholar] [CrossRef]
- Castellanos, D.A.; Cerisuelo, J.P.; Hernandez-Muñoz, P.; Herrera, A.O.; Gavara, R. Modelling the evolution of O2 and CO2 concentrations in MAP of a fresh product: Application to tomato. J. Food Eng. 2016, 168, 85–95. [Google Scholar] [CrossRef]
- Barry, C.S.; Giovannoni, J.J. Ethylene and fruit ripening. J. Plant Growth Regul. 2007, 26, 143–159. [Google Scholar] [CrossRef]
- Shahidi, F.; Hossain, A. Role of lipids in food flavor generation. Molecules 2022, 27, 5014. [Google Scholar] [CrossRef]
- Daruwalla, A.; Kiser, P.D. Structural and mechanistic aspects of carotenoid cleavage dioxygenases (CCDs). Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158590. [Google Scholar] [CrossRef]
- Mirica, L.M.; Klinman, J.P. The nature of O2 activation by the ethylene-forming enzyme 1-aminocyclopropane-1-carboxylic acid oxidase. Proc. Natl. Acad. Sci. USA 2008, 105, 1814–1819. [Google Scholar] [CrossRef]
- Guyer, L.; Hofstetter, S.S.; Christ, B.; Lira, B.S.; Rossi, M.; Hörtensteiner, S. Different mechanisms are responsible for chlorophyll dephytylation during fruit ripening and leaf senescence in tomato. Plant Physiol. 2014, 166, 44–56. [Google Scholar] [CrossRef]
- Yang, M.; Zhu, S.; Jiao, B.; Duan, M.; Meng, Q.; Ma, N.; Lv, W. SISGRL, a tomato SGR-like protein, promotes chlorophyll degradation downstream of the ABA signaling pathway. Plant Physiol. Biochem. 2020, 157, 316–327. [Google Scholar] [CrossRef]
- Hewitt, S.; Dhingra, A. Beyond ethylene: New insights regarding the role of alternative oxidase in the respiratory climacteric. Front. Plant Sci. 2020, 11, 543958. [Google Scholar] [CrossRef] [PubMed]
- Carrara, S.; Pardossi, A.; Soldatini, G.F.; Tognoni, F.; Guidi, L. Photosynthetic activity of ripening tomato fruit. Photosynthetica 2001, 39, 75–78. [Google Scholar] [CrossRef]
- Bravdo, B.A.; Palgi, A.; Lurie, S.; Frenkel, C. Changing ribulose diphosphate carboxylase/oxygenase activity in ripening tomato fruit. Plant Physiol. 1977, 60, 309–312. [Google Scholar] [CrossRef]
- Trong, L.V.; Tuong, L.Q.; Thinh, B.B.; Khoi, N.T.; Trong, V.T. Physiological and biochemical changes in tomato fruit (Solanum lycopersicum L.) during growth and ripening cultivated in Vietnam. Biosci. Res. 2019, 16, 1736–1744. [Google Scholar]
- Bouvier, F.; Backhaus, R.A.; Camara, B. Induction and control of chromoplast-specific carotenoid genes by oxidative stress. J. Biol. Chem. 1998, 273, 30651–30659. [Google Scholar] [CrossRef] [PubMed]
- Resende, E.C.O.; Martins, P.F.; Azevedo, R.A.; Jacomino, A.P.; Bron, I.U. Oxidative processes during “Golden” papaya fruit ripening. Braz. J. Plant Physiol. 2012, 24, 85–94. [Google Scholar] [CrossRef]
- Pesari, P.; Mizzotti, P.F.; Colombo, M.; Masiero, S. Genetic regulation and structural changes during tomato fruit development and ripening. Front. Plant Sci. 2014, 5, 124. [Google Scholar] [CrossRef]
- Colombié, S.; Beauvoit, B.; Nazaret, C.; Bénard, C.; Vercambre, G.; Le Gall, S.; Biais, B.; Cabasson, C.; Maucourt, M.; Bernillon, S.; et al. Respiration climacteric in tomato fruits elucidated by constraint-based modelling. New Phytol. 2017, 213, 1726–1739. [Google Scholar] [CrossRef]
- Nájera, C.; Guil-Guerrero, J.L.; Enríquez, L.J.; Álvaro, J.E.; Urrestarazu, M. LED-enhanced dietary and organoleptic qualities in postharvest tomato fruit. Postharvest Biol. Technol. 2018, 145, 151–156. [Google Scholar] [CrossRef]
- Egea, L.; Bian, W.; Barsan, C.; Jauneau, A.; Pech, J.C.; Latché, A.; Li, Z.; Chervin, C. Chloroplast to chromoplast transition in tomato fruit: Spectral confocal microscopy analyses of carotenoids and chlorophylls in isolated plastids and time-lapse recording on intact live tissue. Ann. Bot. 2011, 108, 291–297. [Google Scholar] [CrossRef]
- Bahrami, A.R.; Chen, Z.H.; Walker, R.P.; Leegood, R.C.; Gray, J.E. Ripening-related occurrence of phosphoenolpyruvate carboxykinase in tomato fruit. Plant Mol. Biol. 2001, 47, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.C. Photoassimilate distribution in plants and crops: Source-sink relationship. In Tomato; Zamski, E., Scheffer, A.A., Eds.; Marcel Dekker: New York, NY, USA, 1996; pp. 709–727. [Google Scholar]
- Davies, J.W.; Cocking, E.C. Changing in carbohydrates, proteins and nucleic acids during cellular development in tomato fruit locule tissue. Planta 1965, 67, 242–253. [Google Scholar] [CrossRef]
- Navarro-González, I.; Periago, M.J. El tomate, ¿alimento saludable y/o funcional? Rev. Española Nutr. Hum. Y Dietética 2016, 20, 323–335. [Google Scholar] [CrossRef][Green Version]
- Alba, R.; Cordonnier-Pratt, M.M.; Prat, L.H. Fruit-localized phytochromes regulate lycopene accumulation independently of ethylene production in tomato. Plant Physiol. 2000, 123, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Galdames, R.J. Estudio de los efectos de adsorción y fotocatálisis en la vida postcosecha de tomates (Solanum lycopersicum L.). Agricultural Civil. Engineering Thesis, Universidad de Concepción, Chillán, Chile, 2019. [Google Scholar]
- Shi, Y.; Pang, X.; Liu, W.; Wang, R.; Su, D.; Gao, Y.; Wu, M.; Deng, W.; Liu, Y.; Li, Z. SIZHD17 is involved in the control of chlorophyll and carotenoid metabolism in tomato fruit. Hortic. Res. 2021, 8, 259. [Google Scholar] [CrossRef]
- Wang, S.; Jin, N.; Xiao, X.; Hu, L.; Liu, Z.; Wu, Y.; Xie, Y.; Zhu, W.; Lyu, J.; Yu, J. Response of tomato fruit quality depends on period of LED supplementary light. Front. Nutr. 2022, 9, 833723. [Google Scholar] [CrossRef]
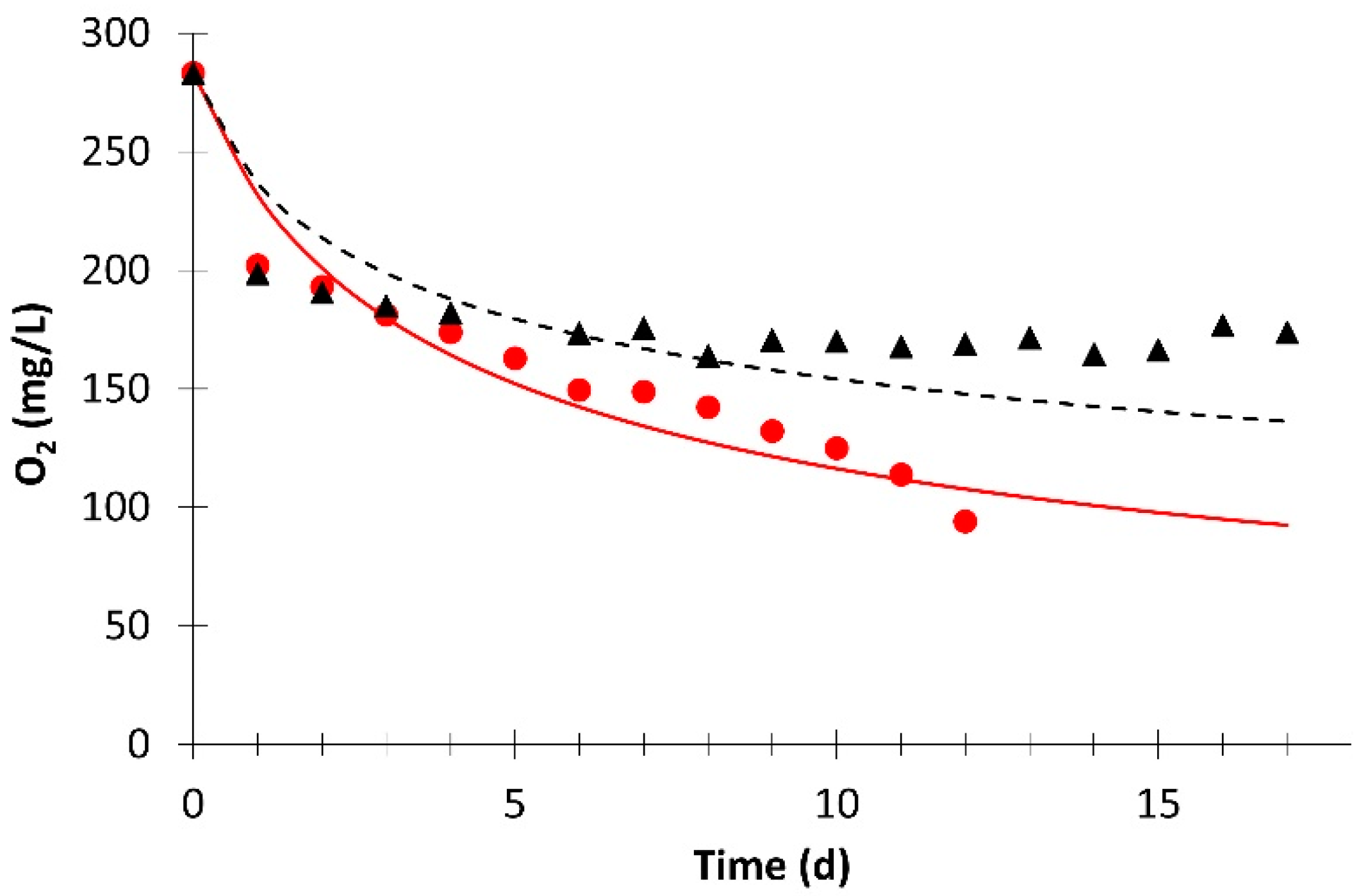
 ; simulation value:
; simulation value:  ) and without light (experimental value:
) and without light (experimental value:  ; simulation value:
; simulation value:  ).
).
 ; simulation value:
; simulation value:  ) and without light (experimental value:
) and without light (experimental value:  ; simulation value:
; simulation value:  ).
).
 ; simulation value:
; simulation value:  ) and without light (experimental value:
) and without light (experimental value:  ; simulation value:
; simulation value:  ).
).
 ; simulation value:
; simulation value:  ) and without light (experimental value:
) and without light (experimental value:  ; simulation value:
; simulation value:  ).
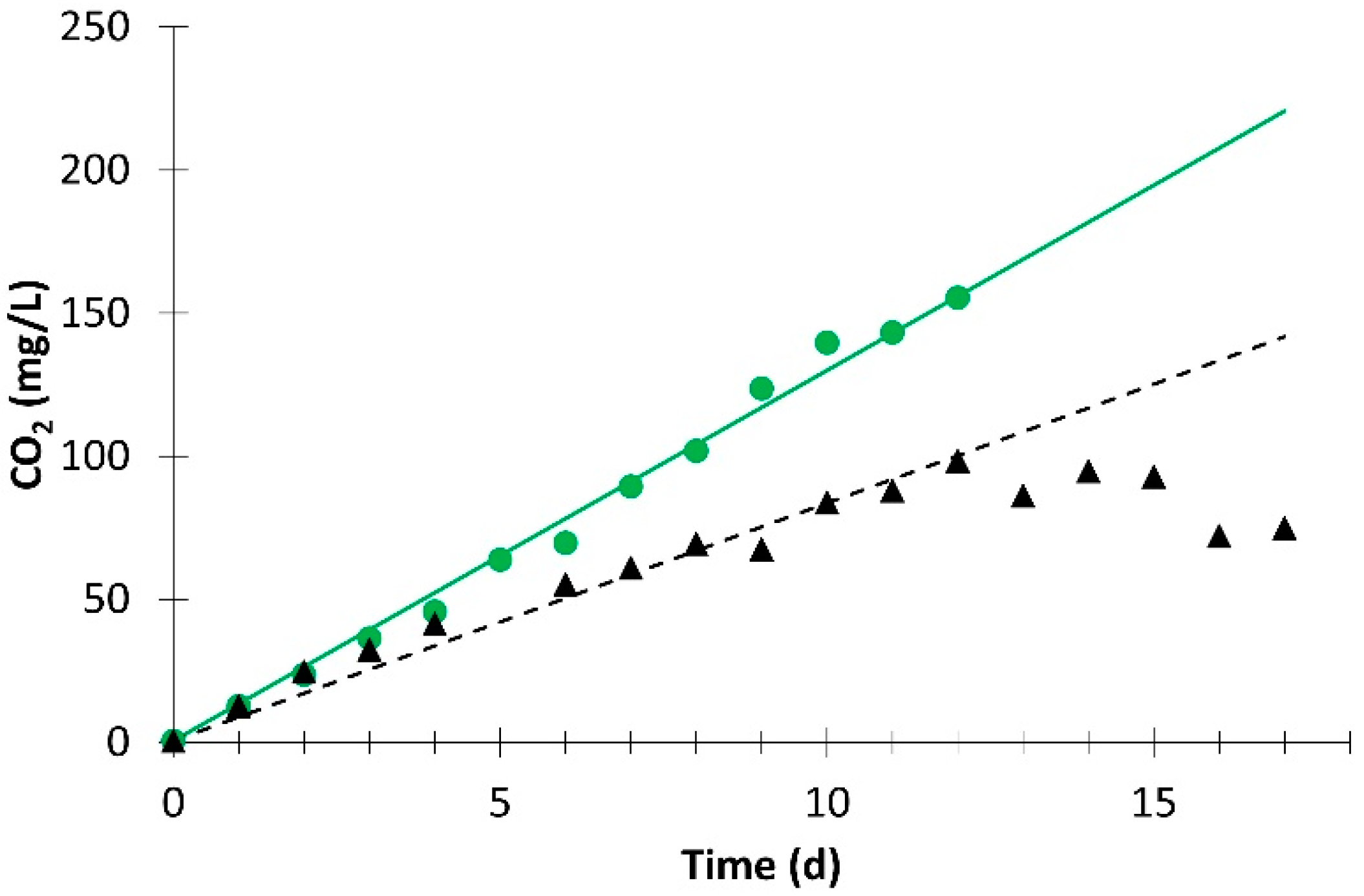
).
 ; simulation value:
; simulation value:  ) and without light (experimental value:
) and without light (experimental value:  ; simulation value:
; simulation value:  ).
).
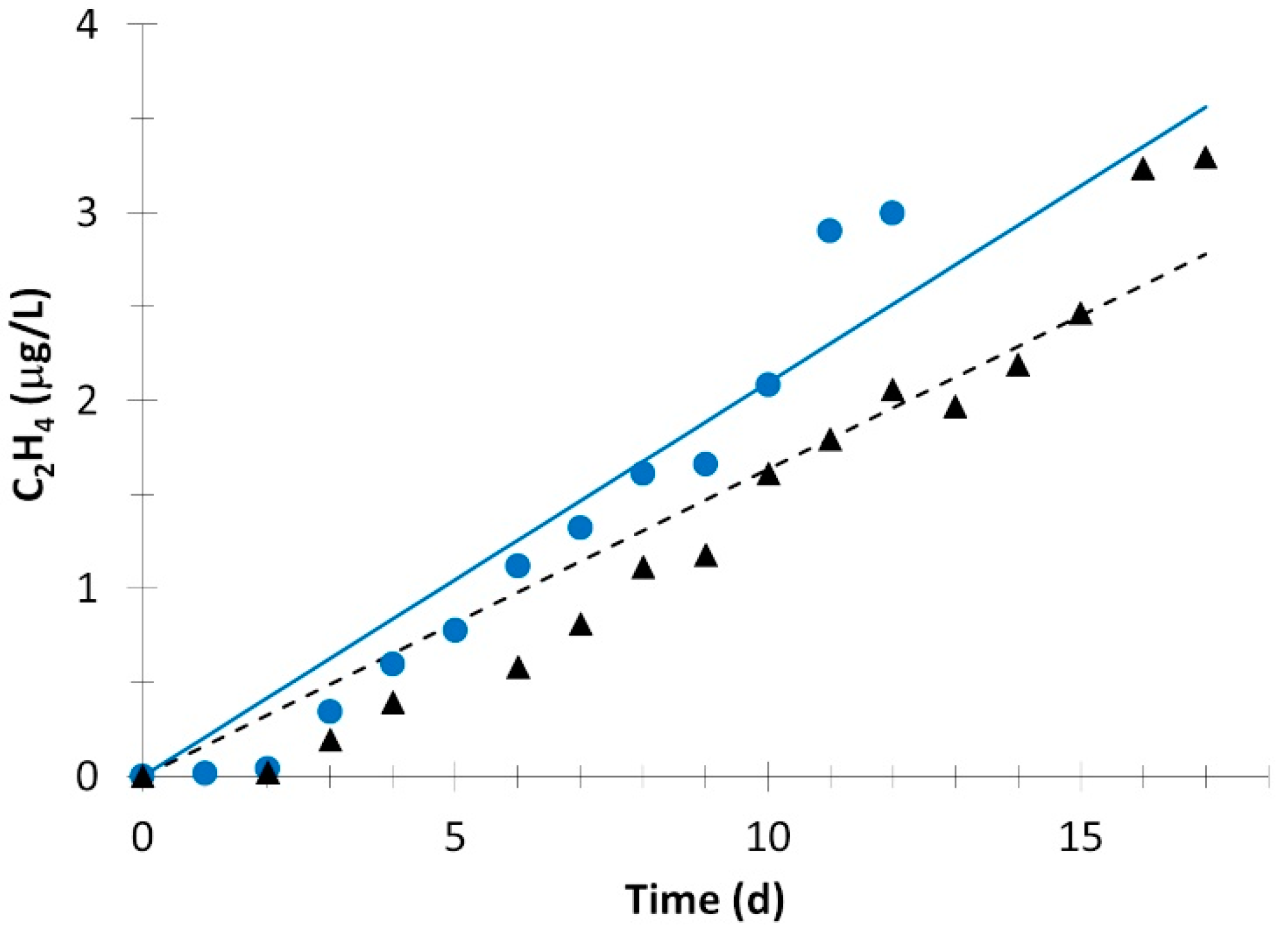
 ; simulation value:
; simulation value:  ) and without light (experimental value:
) and without light (experimental value:  ; simulation value:
; simulation value:  ).
).
 ; simulation value:
; simulation value:  ) and without light (experimental value:
) and without light (experimental value:  ; simulation value:
; simulation value:  ). Different letters for the same day indicate significant difference at p ≤ 0.05.
). Different letters for the same day indicate significant difference at p ≤ 0.05.
 ; simulation value:
; simulation value:  ) and without light (experimental value:
) and without light (experimental value:  ; simulation value:
; simulation value:  ). Different letters for the same day indicate significant difference at p ≤ 0.05.
). Different letters for the same day indicate significant difference at p ≤ 0.05.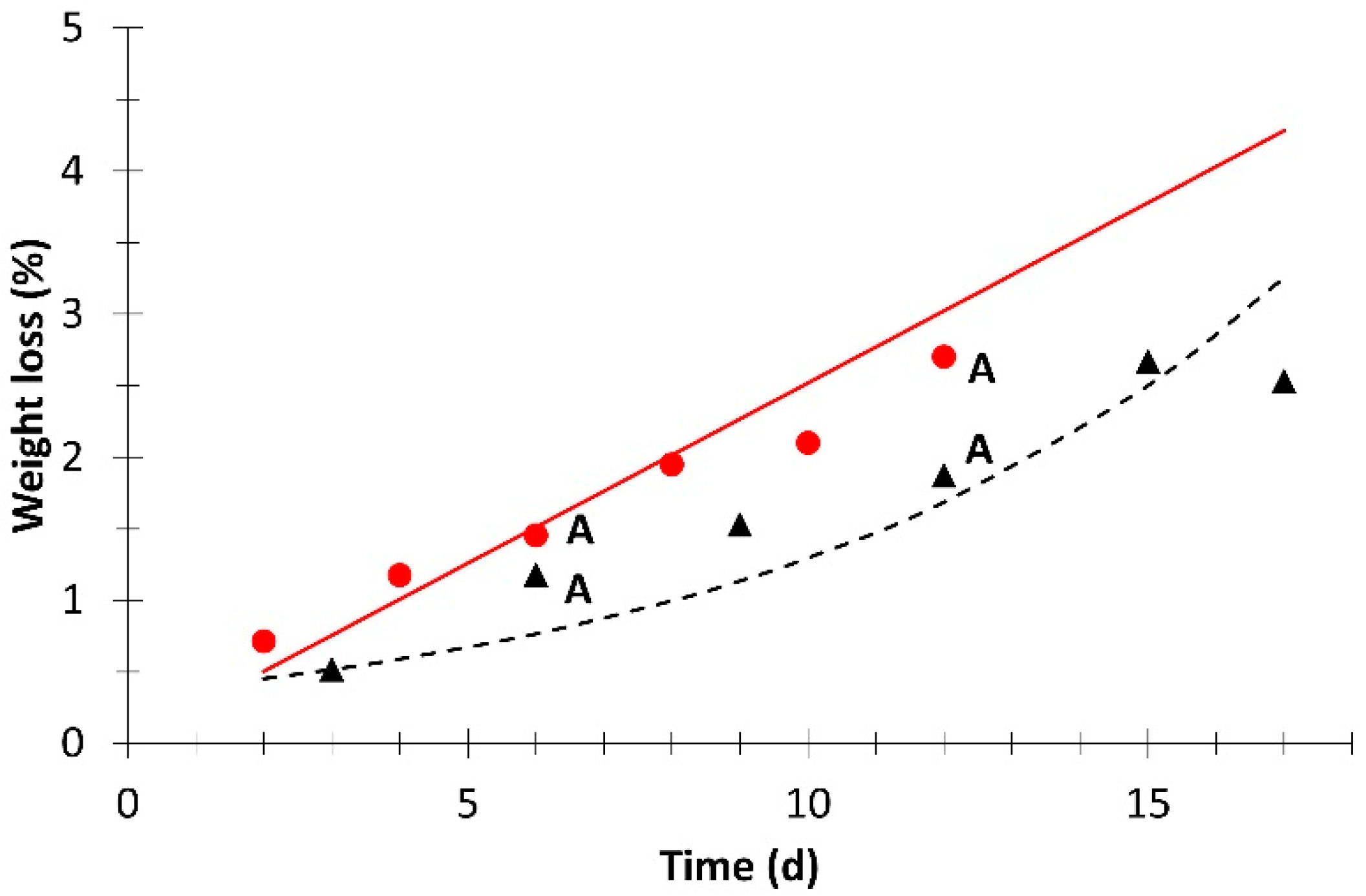
 ; simulation value:
; simulation value:  ) and without light (experimental value:
) and without light (experimental value:  ; simulation value:
; simulation value:  ). Different letters for the same day indicate significant difference at p ≤ 0.05.
). Different letters for the same day indicate significant difference at p ≤ 0.05.
 ; simulation value:
; simulation value:  ) and without light (experimental value:
) and without light (experimental value:  ; simulation value:
; simulation value: 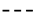 ). Different letters for the same day indicate significant difference at p ≤ 0.05.
). Different letters for the same day indicate significant difference at p ≤ 0.05.
 ; simulation value:
; simulation value:  ) and without light (experimental value:
) and without light (experimental value:  ; simulation value:
; simulation value:  ). Different letters for the same day indicate significant difference at p ≤ 0.05.
). Different letters for the same day indicate significant difference at p ≤ 0.05.
 ; simulation value:
; simulation value:  ) and without light (experimental value:
) and without light (experimental value:  ; simulation value:
; simulation value:  ). Different letters for the same day indicate significant difference at p ≤ 0.05.
). Different letters for the same day indicate significant difference at p ≤ 0.05.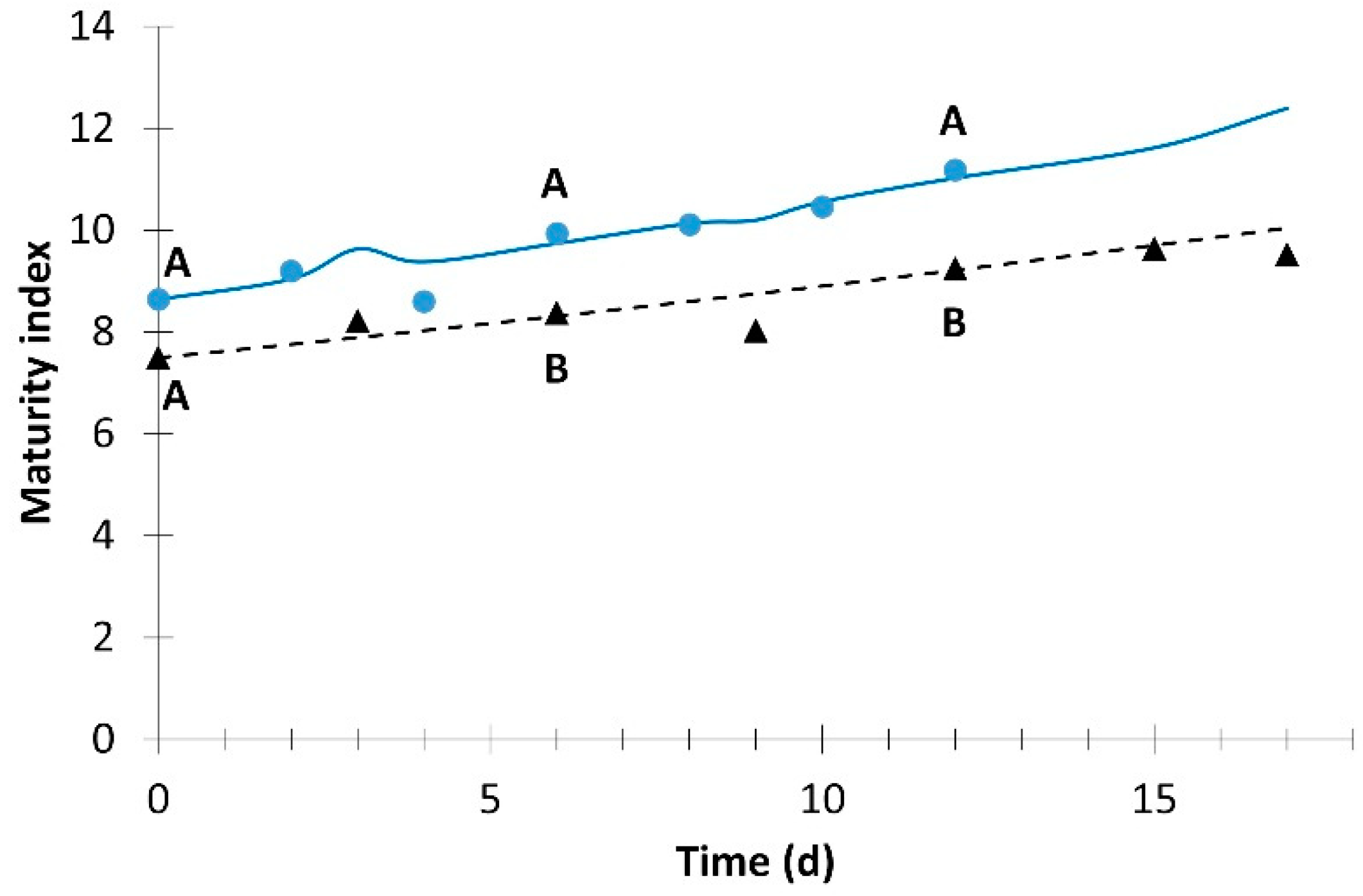
 ; simulation value:
; simulation value:  ) and without light (experimental value:
) and without light (experimental value:  ). Different letters for the same day indicate significant difference at p ≤ 0.05.
). Different letters for the same day indicate significant difference at p ≤ 0.05.
 ; simulation value:
; simulation value:  ) and without light (experimental value:
) and without light (experimental value:  ). Different letters for the same day indicate significant difference at p ≤ 0.05.
). Different letters for the same day indicate significant difference at p ≤ 0.05.
 ; simulation value:
; simulation value:  ) and without light (experimental value:
) and without light (experimental value:  ; simulation value:
; simulation value:  ). Different letters for the same day indicate significant difference at p ≤ 0.05.
). Different letters for the same day indicate significant difference at p ≤ 0.05.
 ; simulation value:
; simulation value:  ) and without light (experimental value:
) and without light (experimental value:  ; simulation value:
; simulation value:  ). Different letters for the same day indicate significant difference at p ≤ 0.05.
). Different letters for the same day indicate significant difference at p ≤ 0.05.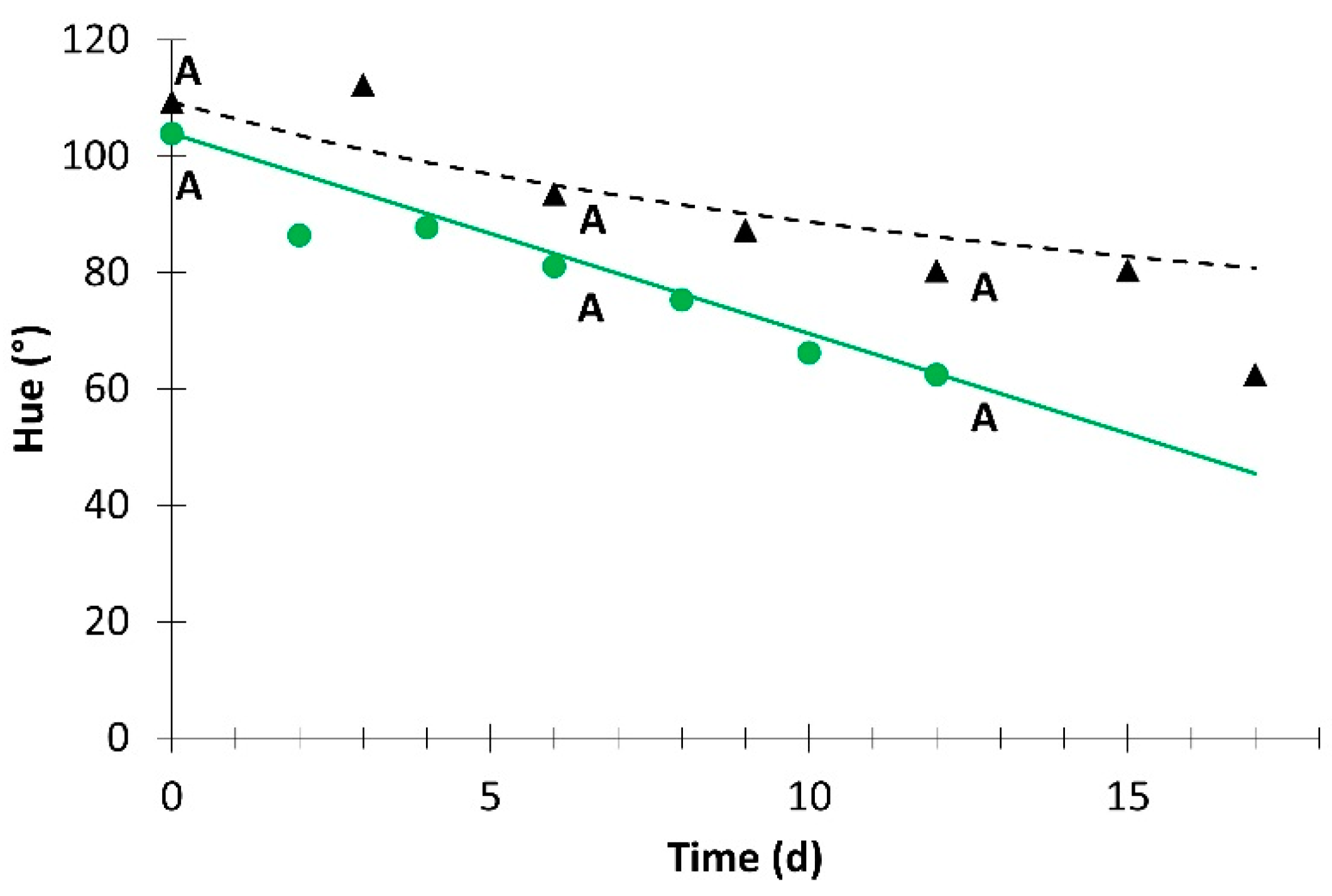
 ; simulation value:
; simulation value:  ) and without light (experimental value:
) and without light (experimental value:  ). Different letters for the same day indicate significant difference at p ≤ 0.05.
). Different letters for the same day indicate significant difference at p ≤ 0.05.
 ; simulation value:
; simulation value:  ) and without light (experimental value:
) and without light (experimental value:  ). Different letters for the same day indicate significant difference at p ≤ 0.05.
). Different letters for the same day indicate significant difference at p ≤ 0.05.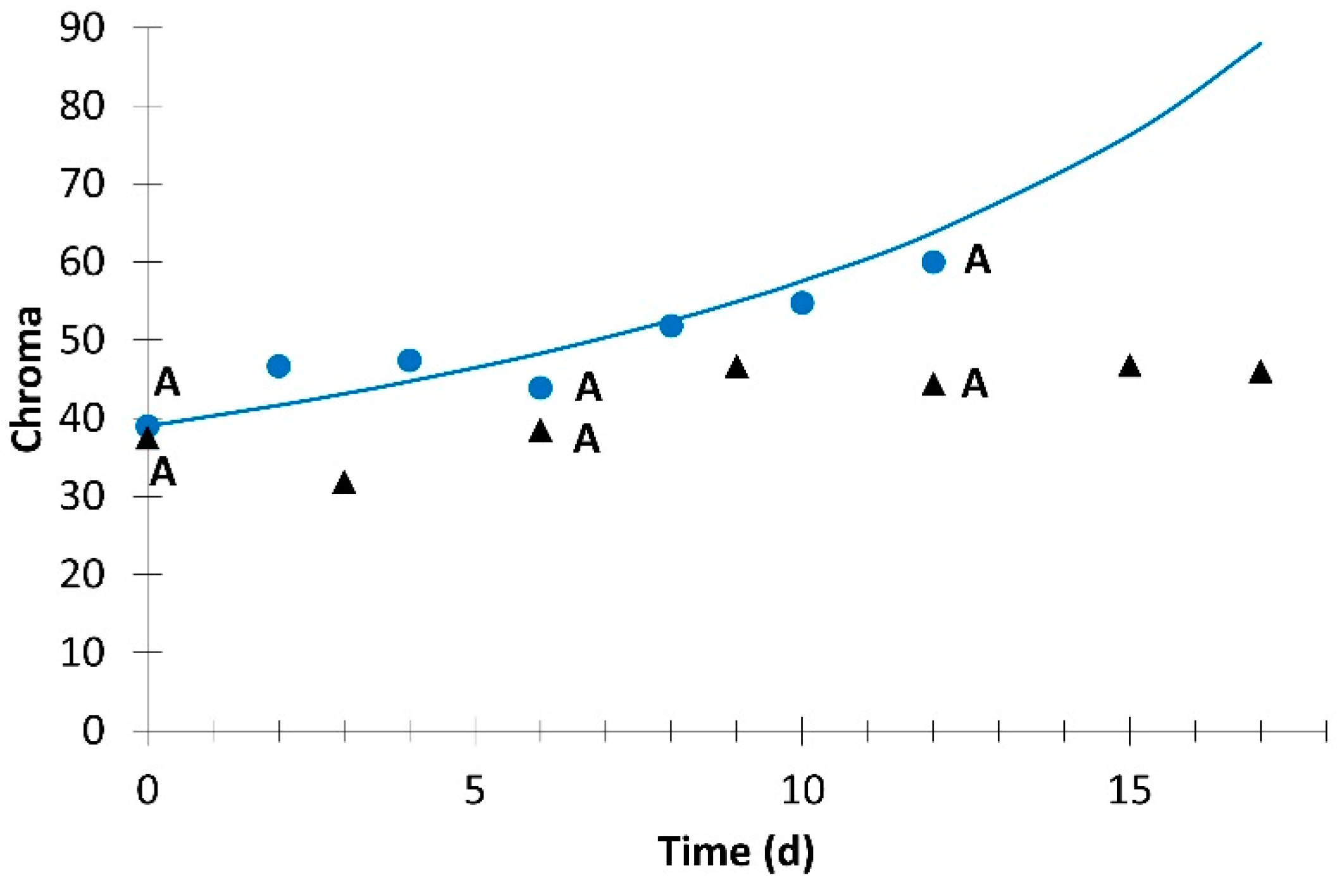
 ; simulation value:
; simulation value:  ) and without light (experimental value:
) and without light (experimental value:  ; simulation value:
; simulation value:  ). Different letters for the same day indicate significant difference at p ≤ 0.05.
). Different letters for the same day indicate significant difference at p ≤ 0.05.
 ; simulation value:
; simulation value:  ) and without light (experimental value:
) and without light (experimental value:  ; simulation value:
; simulation value:  ). Different letters for the same day indicate significant difference at p ≤ 0.05.
). Different letters for the same day indicate significant difference at p ≤ 0.05.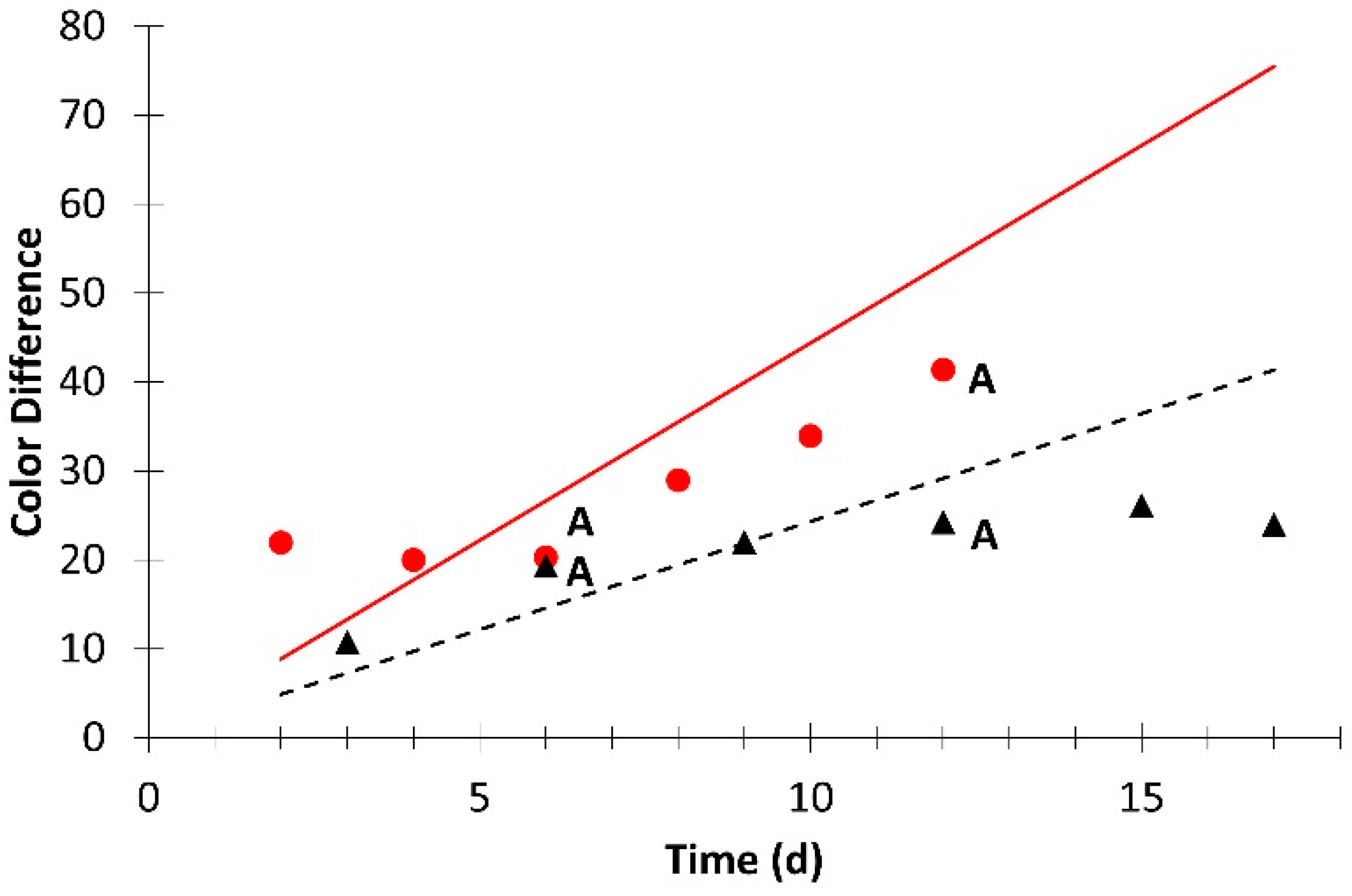
| Compound | With Light | Without Light | ||||
|---|---|---|---|---|---|---|
| k | n | R2 | k | n | R2 | |
| O2 | 1.3 × 10−7 | 3 | 0.990 | 1.7 × 10−12 | 5 | 0.985 |
| CO2 | 5.3 × 10−1 | 0 | 0.987 | 3.5 × 10−1 | 0 | 0.878 |
| C2H4 | 8.7 × 10−6 | 0 | 0.930 | 6.8 × 10−6 | 0 | 0.941 |
| Compound | With Light | Without Light | ||||
|---|---|---|---|---|---|---|
| k | n | R2 | k | n | R2 | |
| Weight loss | 1.1 × 10−2 | 0 | 0.914 | 5.5 × 10−3 | 1 | 0.747 |
| Titratable acidity | 2.5 × 10−5 | 0 | 0.968 | 6.3 × 10−4 | 1 | 0.962 |
| Maturity index | 8.1 × 10−5 | 2 | 0.965 | 7.2 × 10−4 | 1 | 0.960 |
| Lycopene | 6.9 × 10−2 | 0 | 0.812 | |||
| Hue | 1.4 × 10−1 | 0 | 0.946 | 9.2 × 10−10 | 4 | 0.970 |
| Chroma | 3.3 × 10−5 | 2 | 0.944 | |||
| Color difference | 1.9 × 10−1 | 0 | 0.813 | 1.0 × 10−1 | 0 | 0.753 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Bruijn, J.; Fuentes, N.; Solar, V.; Valdebenito, A.; Vidal, L.; Melín, P.; Fagundes, F.; Valdés, H. The Effect of Visible Light on the Postharvest Life of Tomatoes (Solanum lycopersicum L.). Horticulturae 2023, 9, 94. https://doi.org/10.3390/horticulturae9010094
de Bruijn J, Fuentes N, Solar V, Valdebenito A, Vidal L, Melín P, Fagundes F, Valdés H. The Effect of Visible Light on the Postharvest Life of Tomatoes (Solanum lycopersicum L.). Horticulturae. 2023; 9(1):94. https://doi.org/10.3390/horticulturae9010094
Chicago/Turabian Stylede Bruijn, Johannes, Nicole Fuentes, Víctor Solar, Ana Valdebenito, Leslie Vidal, Pedro Melín, Francis Fagundes, and Héctor Valdés. 2023. "The Effect of Visible Light on the Postharvest Life of Tomatoes (Solanum lycopersicum L.)" Horticulturae 9, no. 1: 94. https://doi.org/10.3390/horticulturae9010094
APA Stylede Bruijn, J., Fuentes, N., Solar, V., Valdebenito, A., Vidal, L., Melín, P., Fagundes, F., & Valdés, H. (2023). The Effect of Visible Light on the Postharvest Life of Tomatoes (Solanum lycopersicum L.). Horticulturae, 9(1), 94. https://doi.org/10.3390/horticulturae9010094