Abstract
Viral diseases are major constraints to tomato, cucumber and mung bean production in most areas where these crops are grown. To identify the viruses on the crops in Tajikistan, a field survey was conducted in 2019. Samples of cucumber, mung bean and tomato with virus-like symptoms were collected and the viruses present were diagnosed by RT-PCR and PCR. Across all the samples, a very high proportion of the samples were infected with viruses from the genera Cucumovirus and Potyvirus. Cucumber mosaic virus (CMV; Cucumovirus) was very common in the collected samples of the three crops. As for Potyvirus, Potato virus Y (PVY) was detected in the collected tomato samples, Zucchini yellow mosaic virus (ZYMV) was identified in the collected cucumber samples, and Bean common mosaic virus (BCMV) was detected in 53% of the mung bean samples. Over 68% of the collected samples were infected with two or more viruses, suggesting that mixed infections are common for the three crops. Due to the results that the most identified viruses for the three crops are transmitted by aphids, the management of aphids is extremely important for the production of tomato, cucumber and mung bean in Tajikistan.
1. Introduction
Vegetables provide vitamins, minerals and other compounds which are required for good health. Consumption of vegetables in the daily diet not only prevents micronutrient deficiencies but also reduces the risk of heart disease, eye problems and diabetes. Due to the health benefits of vegetables, they are cultivated all over the world.
The Republic of Tajikistan is landlocked in Central Asia, bordered by China to the east, Afghanistan to the south, Uzbekistan to the west and Kyrgyzstan to the north. The cultivated area of Tajikistan is small, accounting for about 6% of the total land area; the remaining areas are mountainous and unsuitable for agriculture [1]. Although arable land is very limited, agriculture is important for Tajikistan. Agriculture not only supports Tajikistan’s economy, accounting for 19.77% of GDP [1], but also supports livelihoods and provides employment as more than 72% of Tajiks live in rural areas. The Fergana Valley in the north and the Vaksh and Panj River Valleys in the southwest are the major agricultural areas in Tajikistan, and Khatlon Province in the southwest is the principal area. Khatlon has a population of more than 2.5 million and holds about 33% of the agricultural area and 49% of the cropped area [2]. The most extensively grown crops in Tajikistan are cotton and wheat, though many vegetables are also produced, including tomato, cucumber and beans.
Diseases caused by bacteria, fungi and viruses often threaten the growth and development of vegetables, greatly reducing the yield and quality of the produce. Among the pathogens, viral infection is the most difficult to control since managing insect vectors is often inefficient and infected plants cannot be cured. Several genera of viruses that infect tomato and cucurbits have been reported, including Begomovirus [3,4], Tospovirus [5], Tobamovirus [6,7], Cucumovirus [8], Crinivirus [9,10], Polerovirus [11,12] and Potyvirus (Pepper mottle virus (PepMoV) [13], Pepper veinal mottle virus (PVMV) [14], PVY [15] and Chili veinal mottle virus (ChiVMV) [16] on tomato, and Papaya ringspot virus (PRSV) [17] and ZYMV on cucurbits [18]). Similarly, viruses that infect beans have also been reported, including begomoviruses [19], CMV [20], poleroviruses [21] and potyviruses [22]. Among the viruses, BCMV has been cited as a major constraint to mung bean yield worldwide [22].
In order to secure vegetable production, effective strategies to control viral diseases are important for farmers. One of the key components to develop a control strategy is to identify the type and distribution of viral diseases in vegetable crops. However, such information in Tajikistan is very limited because vegetable production is not a major industry and most vegetable farmers are smallholders. Limited field surveys near Dushanbe identified two distinct Potato virus Y (PVY; Potyvirus) strains, PVYO and PVYNTN, infecting potato [23] and Iris yellow spot virus (IYSV; Orthotospovirus) infecting onion [24], but as far as we are aware, these are the only reports in English identifying viral diseases infecting vegetables in Tajikistan. To better understand the incidence and potential threat of viral diseases in tomato, cucumber and mung bean, a field survey was conducted in the major production areas in Tajikistan. The current research not only uncovered the viral diseases in the three crops in major production areas in Tajikistan but also provided important information for developing integrated pest management (IPM) strategies to manage viral diseases while producing the crops, thus improving the yield and quality of the crops.
2. Materials and Methods
2.1. Survey and Sample Collection
A field survey of viral diseases in tomato, cucumber and mung bean was conducted in Fayzobod district in the east of the capital city, Dushanbe, and in five districts in the Khatlon region (Jomi, Jaloliddin Balkhi, Dusti, Kushoniyon and Vakhsh) in southwest Tajikistan in September, 2019. The symptoms on tomato, cucumber and mung bean plants showing virus-like symptoms were recorded and photographed, and leaf samples were collected, dried using anhydrous silica gel, and sent to The World Vegetable Center, Taiwan (using the appropriate export–import permissions) for virus diagnosis.
2.2. Detection and Identification of Plant Viruses
To test for the presence of begomovirus(es), DNA was extracted from each collected sample and separately subjected to PCR amplification using universal begomovirus primers (Table 1) using amplification conditions published previously [5]. For the detection of CMV, poleroviruses, potyviruses, tospoviruses, tobamoviruses and criniviruses, RNA was extracted from the samples and subjected to RT-PCR using primers specific for CMV or universal primers for the various virus genera (Table 1).

Table 1.
Primer sets for diagnosis of viral diseases in collected samples.
To identify the species of Potyvirus in the collected samples that tested positive for the Potyvirus genus universal primers, sample RNAs were also subjected to RT-PCR with primers specific for ZYMV, PVY, BCMV, PRSV, PVMV, PepMoV and ChiVMV (Table 1).
To identify the species of Polerovirus and Crinivirus present, the RT-PCR products from genus-universal primers were sent for sequencing and the obtained nucleotide sequences were analyzed.
3. Results
3.1. Viral Diseases in Cucumber
When the seven collected cucumber leaf samples showing virus-like symptoms were tested for the presence/absence of begomoviruses, tospoviruses, tobamoviruses, poleroviruses, criniviruses and CMV, all were positive for CMV, Potyvirus and Polerovirus, while one out of the seven was also positive for Crinivirus (Table 2). None of the cucumber samples tested positive for Begomovirus, Tospovirus, or Tobamovirus (Table 2). To identify the species of Potyvirus in the samples, their RNAs were subjected to RT-PCR with specific primers for PRSV and ZYMV, two of the most prevalent potyviruses in cucurbits worldwide [17,18]. These tests indicated that all the samples were infected with ZYMV but not PRSV, suggesting that ZYMV was the main Potyvirus in the collected cucumber samples in the survey area. To identify the Polerovirus species in the cucumber samples, five samples were selected for RT-PCR product sequencing. BLASTn searching of the GenBank database using the partial virus genome sequences revealed sequence identities of over 96% with Cucurbit aphid-borne yellows virus (CABYV; Polerovirus). The Polerovirus RT-PCR detection signal in the two remaining cucumber samples was too weak to perform sequence analysis for species identification. When the single-sample Crinivirus RT-PCR product was sequenced, the partial virus genome sequence obtained showed high sequence identity (over 97%) with GenBank Cucurbit chlorotic yellows virus (CCYV; Crinivirus), indicating that the plant was also infected with CCYV.

Table 2.
Summary of virus detection in vegetable samples collected in Tajikistan.
Mixed infection with two or more viruses is a common phenomenon in samples collected from the field. Of the cucumber plant samples studied here, two plants were co-infected with CMV, ZYMV (Potyvirus) and an unidentified Polerovirus, four plants tested positive for CMV, ZYMV and CABYV (Polerovirus), and one plant was co-infected with four viruses (CMV, ZYMV, CABYV and CCYV) (Table 3). The plants infected with multiple viruses displayed diverse symptoms. One of the plants infected with CMV, ZYMV and CABYV showed mosaic and slightly curling (Figure 1A), while another plant apparently infected with the same virus combination displayed symptoms of mosaic and leaf deformation (Figure 1B). The plant infected with CMV, ZYMV and a Polerovirus at a titer too low to identify to species level was also recorded as displaying mosaic and leaf deformation (Figure 1C). The plant that tested positive for CMV, ZYMV, CABYV and CCYV showed mosaic and leaf deformation, and also some leaf curling (Figure 1D). The results show that relying on symptoms alone is insufficient for identifying the viruses present in a plant. Additionally, to increase the yield and quality of cucumber in this area of Tajikistan, strategies to manage multiple virus infections are required.

Table 3.
Diagnosis of viral diseases in vegetable samples collected in Tajikistan.
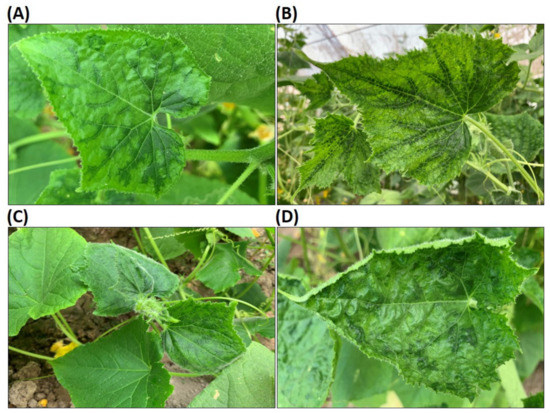
Figure 1.
Symptoms caused by viral diseases on cucumber samples. (A,B) are the plants infected with CMV, ZYMV and CABYV, (C) is a plant infected with CMV, ZYMV and an unidentified Polerovirus and (D) is the plant infected with CMV, ZYMV, CABYV and CCYV.
3.2. Viral Diseases in Mung Bean
Of the 32 mung bean leaf samples collected, 10 tested positive for presence of CMV alone, while 17 were positive for CMV and Potyvirus (Table 2). However, five samples with virus-like symptoms tested negative for the viruses tested (Table 2 and Table 3). When the samples testing positive for the universal Potyvirus primers were subjected to RT-PCR with BCMV-specific primers [29], all 17 samples tested positive, indicating that BCMV, which is common in beans worldwide [22], was also common and potentially harming mung bean production in the survey area in Tajikistan. This does not rule out that the Potyvirus-positive plants could have been infected by another Potyvirus as well as BCMV. The samples that tested positive for CMV alone were from plants with varied symptoms, including small leaves, yellow veins and blistering (Figure 2A), leaf deformation and mosaic (Figure 2B), and leaf yellowing and mosaic (Figure 2C). Similarly, various symptoms were recorded on the plants with mixed infection of CMV and BCMV. Symptoms on mung bean plants positive for both CMV and BCMV included leaf yellowing and mottling (Figure 2D), plant stunting with small leaves, leaf curling and mosaic on leaves (Figure 2E) and yellowing and distortion of leaves (Figure 2F). Thus, viral infection in mung bean plants caused diverse symptoms, and mixed-infection with CMV and BCMV did not always cause more severe symptoms compared to plants infected with CMV alone. The results also indicate that CMV and BCMV together constitute the important viral constraints to mung bean production in southwest Tajikistan.
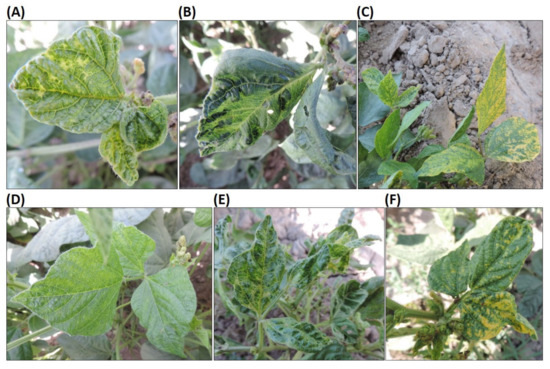
Figure 2.
Symptoms caused by viral diseases on mung bean samples. (A–C) show the sample infected with CMV, while (D–F) are the sample infected with CMV and BCMV.
3.3. Viral Diseases in Tomato
All the samples collected from 10 tomato plants showing virus-like symptoms in fields of southwest Tajikistan tested positive for CMV, while 6 out of the 10 samples were also positive by RT-PCR for the universal Potyvirus primers (Table 2). The samples positive for Potyvirus were then subjected to RT-PCR with specific primers to ChiVMV, PVMV, PepMoV, and PVY to identify virus species. None produced an amplification product specific for ChiVMV, PVMV or PepMoV, but all tested positive for PVY, indicating that PVY was the common Potyvirus detected in the collected tomato samples (Table 2). Four plants tested positive for CMV alone while the other six tested positive for both CMV and PVY (Table 3). Plants infected with CMV alone displayed yellowing and slight leaf curling (Figure 3A), while the plants with mixed infection of CMV with PVY showed symptoms of yellowing, leaf curling and chlorosis (Figure 3B) or yellowing and necrosis (Figure 3C). The results indicate that mixed infections were not rare in the survey area and that CMV and PVY are important virus challenges in tomato production in southwest Tajikistan.

Figure 3.
Symptoms caused by viral diseases in tomato samples. (A) shows a plant only infected with CMV, while (B,C) are different plants infected with CMV and PVY.
4. Discussion
Viral diseases can be major constraints to vegetable crop production, causing not only reduced yields but also affecting product quality. To ensure sustainable vegetable production, information on the important viral diseases is needed for the development of effective management strategies. A field survey was conducted on cucumber, mung bean and tomato in some major horticultural areas of Tajikistan to identify viral diseases. Viruses of the genera Cucumovirus, Crinivirus, Polerovirus and Potyvirus were detected in the collected samples of the three crops. The highest proportion of samples tested positive for CMV and some potyviruses. CMV has been reported to have a very broad host range, infecting more than 1200 plant species, including the crops under study [8]. Although infections of CMV alone were found in mung bean and tomato, a greater proportion of plants from both crops were diagnosed as co-infected with CMV and a Potyvirus. On the other hand, none of the cucumber plants tested positive for a single virus, and all of the samples showed mixed infection with CMV, Polerovirus and Potyvirus, and one sample also tested positive for the presence of a Crinivirus. Various potyviruses were found to infect the three crops, with cucumber samples infected with ZYMV, mung bean samples with BCMV, and tomato samples with PVY. Although the species of Potyvirus were different in the three crops, the identified viruses were transmitted by aphids, the same vector that transmits CMV [32]. The relatively high incidence of CMV and potyviruses in the three crops surveyed suggests that the cultivars being grown in Tajikistan are old and do not carry the resistance to CMV and the potyviruses that these crops carry in other countries where CMV and potyviruses are less prevalent. The results also show that, in the field, mixed infections of different virus species are common and that control strategies for multiple viruses are required. In addition, farmers should take care to manage aphids as they are vectors of the viral diseases detected in the three crops. This contrasts with the other countries to the southwest, south and southeast of Tajikistan, where the whitefly-vectored begomoviruses (not detected in this study) have emerged as predominant.
The current study used RT-PCR with specific primers to diagnose the collected leaf samples, and the predominant species of Potyvirus, Crinivirus and Polerovirus were identified. However, some samples from plants with virus-like symptoms tested negative in all the tests and these may have been infected with other viruses that could not be detected with the limited testing methods used. To detect all the viruses potentially present in the samples, more broad-based detection systems are needed. One of the more recently developed tools for this is next-generation sequencing (NGS) (also known as high-throughput sequencing, [HTS]), which has been used to detect and discover a wide diversity of known and previously undescribed viruses in crops, without detection system bias [33,34]. More samples of the three crops or other vegetables could be collected and subjected to NGS to produce a more complete picture of the diversity and distribution of the viruses present.
Despite the importance of agriculture to Tajikistan, to the best of our knowledge, there is very limited information on the disease threats to agricultural crops and specifically, the viral disease threats to vegetable crops. The lack of such information means effective disease and pest control strategies have not been developed. Without integrated pest control, farmers cannot obtain high-yield and high-quality vegetable products. Although the sample size of this study was relatively small, as far as we are aware, this is the first report (in English, at least) identifying viral diseases in three important vegetable crops in the major production areas of Tajikistan. Although the survey in this study was only conducted in 2019, the diversity and incidence of viral diseases in the three crops were determined, which will be explored in the future through multi-year field surveys to discover the epidemiology of viral diseases in the crops in Tajikistan. The current study not only identifies diseases caused by CMV and potyviruses in the three crops and pests (aphids) that need to be controlled but also provides breeding targets for the three vegetable crops that are from lines with high resistance to multiple viral diseases.
Author Contributions
Conceptualization, Y.-L.C. and L.K.; investigation, Y.-L.C. and N.S.; methodology, L.-M.L., F.-H.K. and S.-L.S.; project administration, Y.-L.C. and N.S.; writing—original draft, Y.-L.C.; writing—review and editing, L.K. All authors have read and agreed to the published version of the manuscript.
Funding
Funding for this research was provided by the US Agency for International Development (USAID) and long-term strategic donors to the World Vegetable Center, Taiwan; UK aid from the UK government; Australian Centre for International Agricultural Research (ACIAR); Germany; Thailand; Philippines; Korea; and Japan.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are available in a publicly accessible repository. All data collected during this experiment were deposited in the World Vegetable Center repository, HARVEST (https://worldveg.org/harvest, accessed on 5 May 2022) and are available to the public.
Acknowledgments
We acknowledge the help of the several Tajik vegetable growers who allowed us to access, survey and sample their crops.
Conflicts of Interest
The authors declare no conflict of interest.
References
- AQUASTAT. Tajikistan. 2018. Available online: https://www.fao.org/aquastat/statistics/query/results.html (accessed on 25 March 2022).
- Feed the Future FEEDBACK; Feed the Future Tajikistan Zone of Influence Baseline Report; Westat: Rockville, MD, USA, 2014.
- Prasad, A.; Sharma, N.; Hari-Gowthem, G.; Muthamilarasan, M.; Prasad, M. Tomato yellow leaf curl virus: Impact, challenges, and management. Trends Plant Sci. 2020, 25, 897–911. [Google Scholar] [CrossRef]
- Ito, T.; Sharma, P.; Kittipakorn, K.; Ikegami, M. Complete nucleotide sequence of a new isolate of tomato leaf curl New Delhi virus infecting cucumber, bottle gourd and muskmelon in Thailand. Arch. Virol. 2008, 153, 611–613. [Google Scholar] [CrossRef]
- Parrella, G.; Gognalons, P.; Gebre-Selassiè, K.; Vovlas, C.; Marchoux, G. An update of the host range of Tomato spotted wilt virus. J. Plant Pathol. 2003, 85, 227–264. [Google Scholar]
- Blancard, D. (Ed.) 3—Principal characteristics of pathogenic agents and methods of control. In Tomato Diseases, 2nd ed.; Academic Press: San Diego, CA, USA, 2012; pp. 413–650. [Google Scholar]
- Dombrovsky, A.; Tran-Nguyen, L.T.T.; Jones, R.A.C. Cucumber green mottle mosaic virus: Rapidly increasing global distribution, etiology, epidemiology, and management. Annu. Rev. Phytopathol. 2017, 55, 231–256. [Google Scholar] [CrossRef] [PubMed]
- Jacquemond, M. Cucumber mosaic virus. Adv. Virus Res. 2012, 84, 439–504. [Google Scholar]
- Fiallo-Olivé, E.; Navas-Castillo, J. Tomato chlorosis virus, an emergent plant virus still expanding its geographical and host ranges. Mol. Plant Pathol. 2019, 20, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Okuda, M.; Okazaki, S.; Yamasaki, S.; Okuda, S.; Sugiyama, M. Host range and complete genome sequence of Cucurbit chlorotic yellows virus, a new member of the genus Crinivirus. Phytopathology 2010, 100, 560–566. [Google Scholar] [CrossRef]
- Tan, S.T.; Liu, F.; Lv, J.; Liu, Q.L.; Luo, H.M.; Xu, Y.; Ma, Y.; Chen, X.J.; Lan, P.X.; Chen, H.R.; et al. Identification of two novel poleroviruses and the occurrence of Tobacco bushy top disease causal agents in natural plants. Sci. Rep. 2021, 11, 21045. [Google Scholar] [CrossRef] [PubMed]
- Knierim, D.; Deng, T.C.; Tsai, W.S.; Green, S.K.; Kenyon, L. Molecular identification of three distinct Polerovirus species and a recombinant Cucurbit aphid-borne yellows virus strain infecting cucurbit crops in Taiwan. Plant Pathol. 2010, 59, 991–1002. [Google Scholar] [CrossRef]
- Fang, M.; Yu, J.; Kim, K.H. Pepper mottle virus and its host interactions: Current state of knowledge. Viruses. 2021, 13, 1930. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Wang, R.Y.; Chen, C.C.; Chang, C.A.; Jan, F.J. First report of Pepper veinal mottle virus in tomato and pepper in Taiwan. Plant Dis. 2009, 93, 107. [Google Scholar] [CrossRef]
- Torrance, L.; Talianksy, M.E. Potato virus Y emergence and evolution from the Andes of south America to become a major destructive pathogen of potato and other Solanaceous crops worldwide. Viruses 2020, 12, 1430. [Google Scholar] [CrossRef]
- Zhao, F.F.; Xi, D.H.; Liu, J.; Deng, X.G.; Lin, H.H. First report of Chilli veinal mottle virus infecting tomato (Solanum lycopersicum) in China. Plant Dis. 2014, 98, 1589. [Google Scholar] [CrossRef] [PubMed]
- Bateson, M.F.; Lines, R.E.; Revill, P.; Chaleeprom, W.; Ha, C.V.; Gibbs, A.J.; Dale, J.L. On the evolution and molecular epidemiology of the potyvirus Papaya ringspot virus. J. Gen. Virol. 2002, 83, 2575–2585. [Google Scholar] [CrossRef]
- Svoboda, J.; Leisova-Svobodova, L.; Amano, M. Evaluation of selected Cucurbitaceous vegetables for resistance to Zucchini yellow mosaic virus. Plant Dis. 2013, 97, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Mishra, G.P.; Dikshit, H.K.; Ramesh, S.V.; Tripathi, K.; Kumar, R.R.; Aski, M.; Singh, A.; Roy, A.; Kumari, N.; Dasgupta, U.; et al. Yellow mosaic disease (YMD) of Mungbean (Vigna radiata (L.) Wilczek): Current status and management opportunities. Front. Plant Sci. 2020, 11, 918. [Google Scholar] [CrossRef]
- Deng, T.C.; Tsai, C.H.; Tsai, H.L.; Liao, J.Y.; Huang, W.C. First report of Cucumber mosaic virus on Vigna marina in Taiwan. Plant Dis. 2010, 94, 1267. [Google Scholar] [CrossRef]
- Makkouk, K.M.; Kumari, S.G. Epidemiology and integrated management of persistently transmitted aphid-borne viruses of legume and cereal crops in West Asia and North Africa. Virus Res. 2009, 141, 209–218. [Google Scholar] [CrossRef]
- Worrall, E.A.; Wamonje, F.O.; Mukeshimana, G.; Harvey, J.J.; Carr, J.P.; Mitter, N. Bean common mosaic virus and Bean common mosaic necrosis virus: Relationships, biology, and prospects for control. Adv. Virus Res. 2015, 93, 1–46. [Google Scholar] [PubMed]
- Alabi, O.J.; Crosslin, J.M.; Saidov, N.; Naidu, R.A. First Report of Potato virus Y in Potato in Tajikistan. Plant Dis. 2012, 96, 1074. [Google Scholar] [CrossRef]
- Alabi, O.J.; Saidov, N.; Muniappan, R.; Naidu, R.A. First report of Iris yellow spot virus in onion in Tajikistan. New Dis. Rep. 2012, 26, 28. [Google Scholar] [CrossRef]
- Tsai, W.S.; Shih, S.L.; Kenyon, L.; Green, S.K.; Jan, F.J. Temporal distribution and pathogenicity of the predominant tomato-infecting begomoviruses in Taiwan. Plant Pathol. 2011, 60, 787–799. [Google Scholar] [CrossRef]
- Menzel, W.; Knierim, D.; Winter, S.; Hamacher, J.; Heupel, M. First report of tomato brown rugose fruit virus infecting tomato in Germany. New Dis. Rep. 2019, 39, 1. [Google Scholar] [CrossRef]
- Chen, T.C.; Li, J.T.; Lin, Y.P.; Yeh, Y.C.; Kang, Y.C.; Huang, L.H.; Yeh, S.D. Genomic characterization of Calla lily chlorotic spot virus and design of broad-spectrum primers for detection of tospoviruses. Plant Pathol. 2012, 61, 183–194. [Google Scholar] [CrossRef]
- Ha, C.; Coombs, S.; Revill, P.A.; Harding, R.M.; Vu, M.; Dale, J.L. Design and application of two novel degenerate primer pairs for the detection and complete genomic characterization of potyviruses. Arch. Virol. 2008, 153, 25–36. [Google Scholar] [CrossRef]
- Melgarejo, T.A.; Lehtonen, M.T.; Fribourg, C.E.; Rännäli, M.; Valkonen, J.P.T. Strains of BCMV and BCMNV characterized from lima bean plants affected by deforming mosaic disease in Peru. Arch. Virol. 2007, 152, 1941–1949. [Google Scholar] [CrossRef] [PubMed]
- Chikh-Ali, M.; Naidu, R.A.; Karasev, A.V. First report of Potato virus Y (PVY) strain PVYC associated with a tomato disease in Kenya. Plant Dis. 2016, 100, 864–865. [Google Scholar] [CrossRef]
- Golnaz, N.; Jafarpour, B.; Rastegar, M.F.; Sabokkhiz, M.A. Detection of Cucumber mosaic virus and typing using serological and molecular methods in Razavi Khorasan Province. Pak. J. Biol. Sci. 2009, 12, 657–659. [Google Scholar] [CrossRef][Green Version]
- Brault, V.; Uzest, M.; Monsion, B.; Jacquot, E.; Blanc, S. Aphids as transport devices for plant viruses. C. R. Biol. 2010, 333, 524–538. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.; Tzanetakis, I.E. Development of a virus detection and discovery pipeline using next generation sequencing. Virology 2014, 471–473, 54–60. [Google Scholar] [CrossRef]
- Maree, H.J.; Fox, A.; Rwahnih, M.A.; Boonham, N.; Candresse, T. Application of HTS for routine plant virus diagnostics: State of the art and challenges. Front. Plant Sci. 2018, 9, 1082. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).