1. Introduction
Blood oranges (
Citrus sinensis L. Osbeck) contain bioactive compounds including anthocyanins, flavonoid, polyphenols, hydroxycinnamic acids, and ascorbic acid. These compounds contribute to the antioxidant capacity and overall fruit quality [
1]. These bioactive compounds are beneficial for human health, helping to prevent heart disease (atherosclerosis), arteriosclerosis, cancer, diabetes, and other age-related diseases, and they exhibit antiviral activity [
2]. Furthermore, these compounds assist in scavenging free radicals and reactive oxygen species [
3].
Anthocyanin is an important internal quality marker for blood oranges. Their quality attributes are largely determined by anthocyanin levels, which are greatly linked to antioxidant capacity and health benefits [
1]. On the other hand, the deep red pulp color, associated with high concentrations of anthocyanin content, is indicative of high-quality blood orange fruit in the market. Several factors, including genotype, rootstock, cultural practices, nutrient availability, moisture content, climate conditions, maturity, and harvest timing can influence anthocyanin accumulation in blood oranges [
4,
5]. In addition, seasonal drought stress during the fruit expansion stage can significantly accelerate anthocyanin accumulation and biosynthesis in blood orange fruit by activating the expression of phenylalanine ammonia-lyase (
PAL3) and other structural genes in the anthocyanin biosynthesis pathway [
6].
Mediterranean climates are superior for the commercial cultivation of blood oranges due to their cooler night temperatures during the ripening period, which lead to optimum anthocyanin biosynthesis. Sufficiently low night temperatures to trigger anthocyanin accumulation are uncommon in subtropical and tropical regions [
7]. While blood oranges can be grown in subtropical/tropical climates, each region presents challenges for successful cultivation, often resulting in negligible anthocyanin levels and lower fruit quality at commercial maturity compared to those grown in Mediterranean climates [
8].
Cold storage is a simple method for prolonging the postharvest life of blood oranges after harvest by reducing fruit metabolism and other processes that lead to deterioration [
1]. Storing blood oranges at room temperature can greatly reduce their bioactive compounds and quickly reduce their shelf life due to water loss, senescence, and fungal decay. Furthermore, storing them below 6 °C can cause chilling injury (CI) [
1]. Blood oranges are cold-dependent, and unlike most non-climacteric fruits, their internal quality can be enhanced during postharvest cold storage by the production of anthocyanins. Low-temperature storage boosts anthocyanin levels and improves the fruit’s internal quality [
1]. Cold temperatures trigger the accumulation of anthocyanins, and this process is regulated by a gene called
Ruby, which is activated by cold stress [
7]. It is important to note that while low temperatures are beneficial for enhancing anthocyanin and antioxidant activity, prolonged low temperature storage can lead to a reduction in flavor and overall fruit quality [
9]. Therefore, finding the optimal storage temperature is key to maintaining the balance between enhancing beneficial compounds and preserving the overall quality of the fruit.
The effect of cold temperatures on anthocyanin production and accumulation in blood oranges after harvesting is well established. However, in previous studies, how chilling and moderate temperatures affect anthocyanin levels has typically been evaluated without focusing on more than two specific temperatures [
8,
9,
10,
11,
12]. Storing fruit at moderate temperatures after harvest can significantly enhance anthocyanin concentration and improve the internal quality of the fruit grown in subtropical and tropical regions [
1]. Suitable storage temperatures are crucial for enhancing anthocyanin concentration and improving the internal quality of blood oranges after harvest in a specific period. On the other hand, specific temperature strategies may vary depending on the desired result for anthocyanin levels and antioxidant activities. Therefore, careful management of storage temperatures is crucial for maintaining and enhancing the nutritional and antioxidant qualities of blood oranges after harvest [
1].
Non-chilling storage temperatures for blood orange storage are typically above the established threshold for the CI of 6 °C. Storing blood oranges at non-chilling temperatures can help maintain their quality without the risk of cold damage that can occur at lower temperatures. This practice is beneficial for short-term storage to preserve the fruit’s quality. Our previous research demonstrated that storing blood orange fruit at temperatures ranging from 6 to 12 °C showed that the higher temperatures (10 and 12 °C) within that range significantly enhanced anthocyanin accumulation and antioxidant activity compared to the lower temperatures (6 and 8 °C) [
1]. However, this study was limited to colder postharvest conditions and did not explore the effects of higher, potentially more economical storage temperatures. In addition, storing blood oranges at moderate temperatures can be cost-effective for storage expenses, including reduced electricity consumption. Moderate temperature storage can potentially increase the anthocyanin levels in blood oranges grown in subtropical and tropical regions, where high anthocyanin content does not typically develop on the tree. However, the impact of storage at various moderate, non-chilling temperatures on postharvest physiological and biochemical changes in blood oranges has not yet been reported.
To date, the effect of postharvest storage at temperatures above 12 °C has not been scientifically studied in the ‘Moro’ cultivar, which is commercially important in subtropical regions. The current study addresses this gap by evaluating key postharvest quality parameters of ‘Moro’ blood oranges including anthocyanin concentrations, total phenolic content, antioxidant capacity, juice quality, and changes in physical attributes during storage at 10, 15, and 20 °C. These findings offer practical insights for improving fruit quality in warmer climates where on-tree anthocyanin development is limited. This research further discovers how storage at non-chilling temperatures can be intentionally applied to enhance fruit coloration and biochemical properties. The findings contribute to advancing postharvest management strategies and enhancing the commercial value of blood oranges in subtropical supply chains.
4. Discussion
Storing blood oranges fruit at moderately low temperatures, as tested here, is beneficial for preserving their quality. It helps maintain the fruit’s firmness, reduces weight loss, and conserves sugar content. These factors are essential for preserving the overall quality, texture, and taste of the blood oranges during storage. However, blood oranges fruit are sensitive to lower, non-freezing temperatures in the range of 0 to 6 °C, which can induce CI [
1]. Non-chilling storage temperatures can minimize the risk of CI, which can lead to physiological disorders in blood oranges and negatively impact their marketability. By avoiding temperatures that cause CI, non-chilling storage can prolong the shelf life of blood oranges, making them more attractive to consumers. These benefits make non-chilling storage temperatures a compelling choice for maintaining the quality and enhancing the health benefits of blood oranges. However, studies on the effects of various non-chilling storage temperatures on postharvest quality in blood oranges are lacking. Further research was necessary to optimize these conditions for commercial application. In our research reported here, the effects of non-chilling storage temperatures (10, 15, and 20 °C) on physical attributes, bioactive compounds, and antioxidant activity in ‘Moro’ blood orange fruit were investigated.
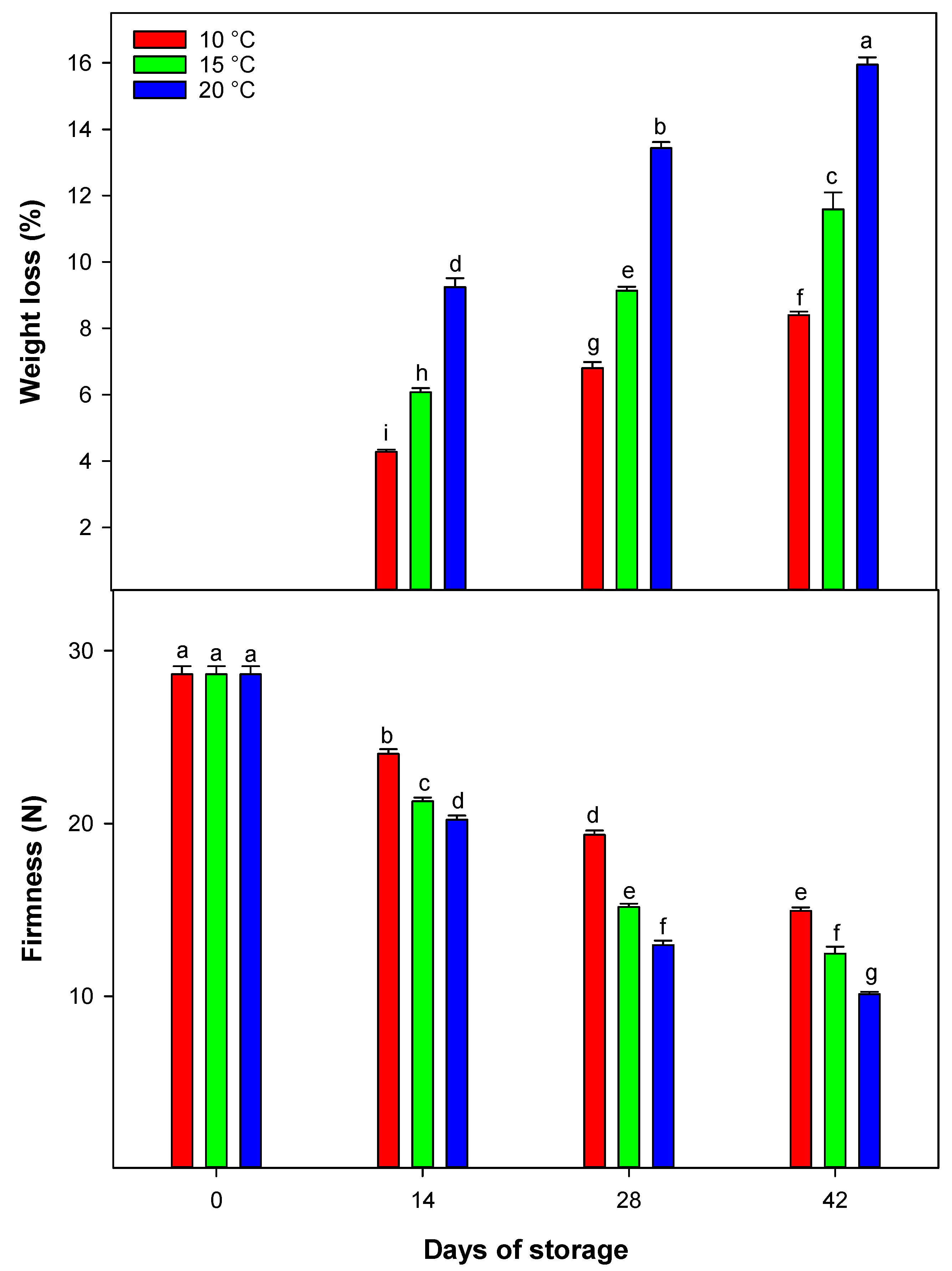
The results of the study on the WL of blood oranges during storage show that WL is significantly affected by both storage time and temperature. Higher storage temperatures significantly increase the rate of WL due to enhanced transpiration. Lower temperatures are more effective at preserving fruit weight over time by slowing down this process. Moreover, despite maintaining equal relative humidity (RH) levels across all storage temperatures, higher temperatures lead to greater water vapor pressure deficits, which further promote WL [
1]. In addition, at lower temperatures, the rate of respiration decreases, contributing to a smaller reduction in WL as less carbon is lost as carbon dioxide [
18]. Conversely, higher storage temperatures increase metabolic activity, leading to greater energy consumption and further WL. It is important to note that in our study, following each sampling time, the fruit were held for an additional 2 days at room temperature to simulate shelf-life conditions prior to WL evaluation. This additional period at ambient temperature likely accelerated moisture loss, as higher temperatures and lower RH at room conditions enhance transpiration from the fruit peel and the relatively high WL values observed in 20 °C [
1]. From a postharvest management perspective, WL can adversely affect fruit appearance, texture, and marketability, particularly when it leads to visible peel dehydration or loss of firmness. In this study, WL gradually increased with both temperature and storage duration and was most pronounced at 20 °C, indicating the unsuitability of such conditions for long-term storage. In contrast, fruit stored at 10 °C showed moderate WL values, suggesting that lower temperatures are more effective in limiting transpiration and preserving overall fruit quality. These results highlight the importance of maintaining low-temperature storage to reduce WL and extend the marketability of blood oranges during prolonged postharvest handling.
Fruit firmness is a key factor in marketability, directly influencing texture and consumer satisfaction [
1]. This study highlights the critical role of storage temperature in maintaining firmness over 42 days. Lower temperatures slowed firmness loss, consistent with the well-established understanding that cooler conditions inhibit enzymes responsible for cell wall degradation, such as polygalacturonase, pectin lyase, and pectin methylesterase. These enzymes contribute to fruit softening by breaking down pectin, a key structural component of the cell wall [
19]. Reduced enzyme activity at lower temperatures delays pectin degradation, thereby preserving firmness. As storage duration extended to 42 days, temperature-dependent effects became more pronounced. Fruit stored at 15 or 20 °C experienced greater firmness loss than those kept at 10 °C. This confirms that higher temperatures accelerate enzymatic activity and metabolic processes, leading to faster softening, while storage at 10 °C likely restricted these enzymatic actions, slowing pectin breakdown [
1].
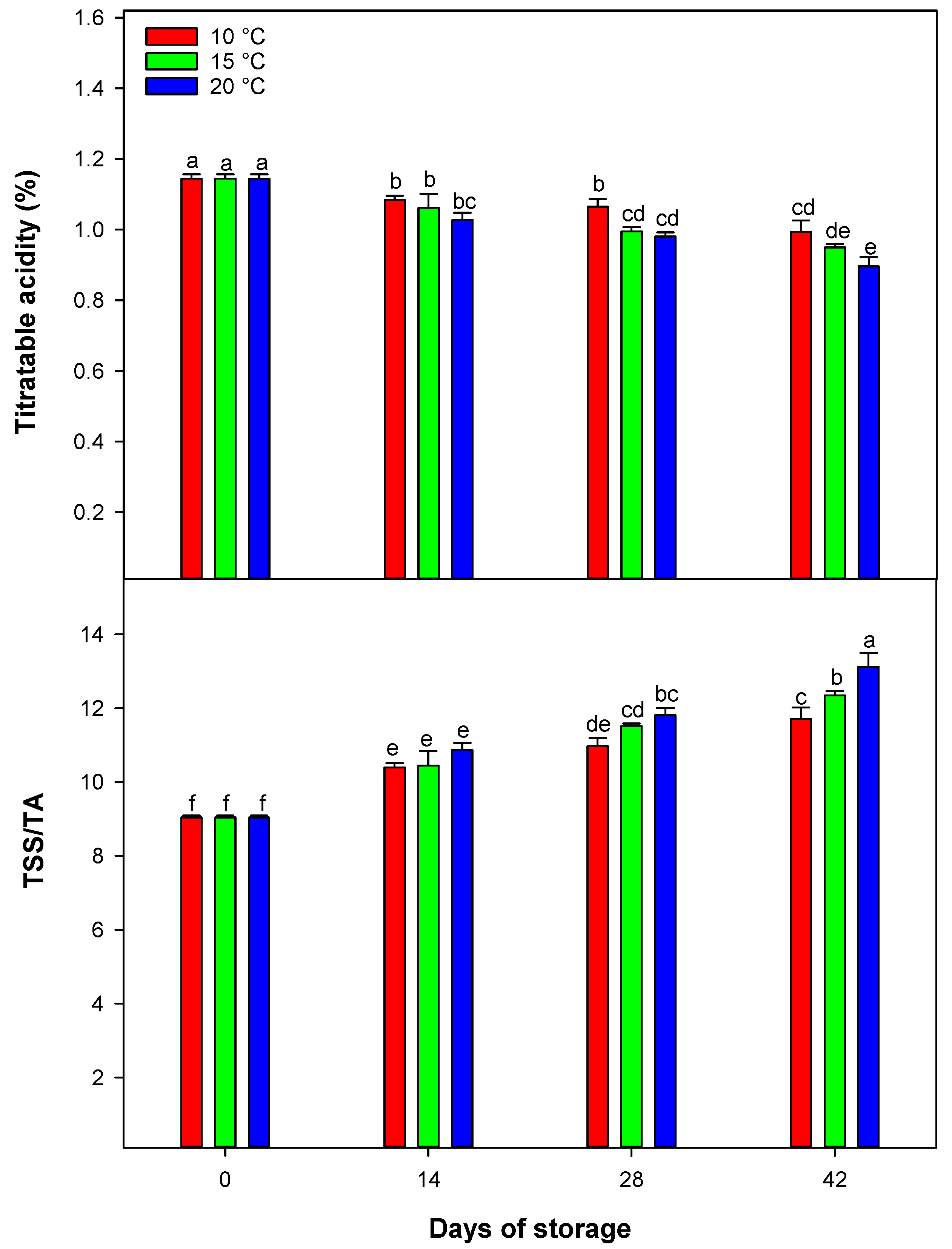
The changes in TSS observed in the juice of blood oranges during cold storage offer important insights into the fruit’s biochemical processes. TSS is an essential indicator for assessing the sugar levels in citrus fruit. Generally, sugars such as sucrose, glucose, and fructose make up the bulk of TSS, representing approximately 80%. The rest of the TSS consists of a mix of organic acids, vitamins, proteins, free amino acids, and glucosides, which together account for the remaining 20% [
20]. These constituents undergo various changes during cold storage as a result of continuous metabolic processes. In this study, a slight increase in TSS was observed in blood orange juice over the storage period, compared to levels at harvest. This increase implies that the cold storage might promote the conversion of organic acids into sugars through glycolytic pathways and the conversion of complex carbohydrate polymers into soluble [
19].
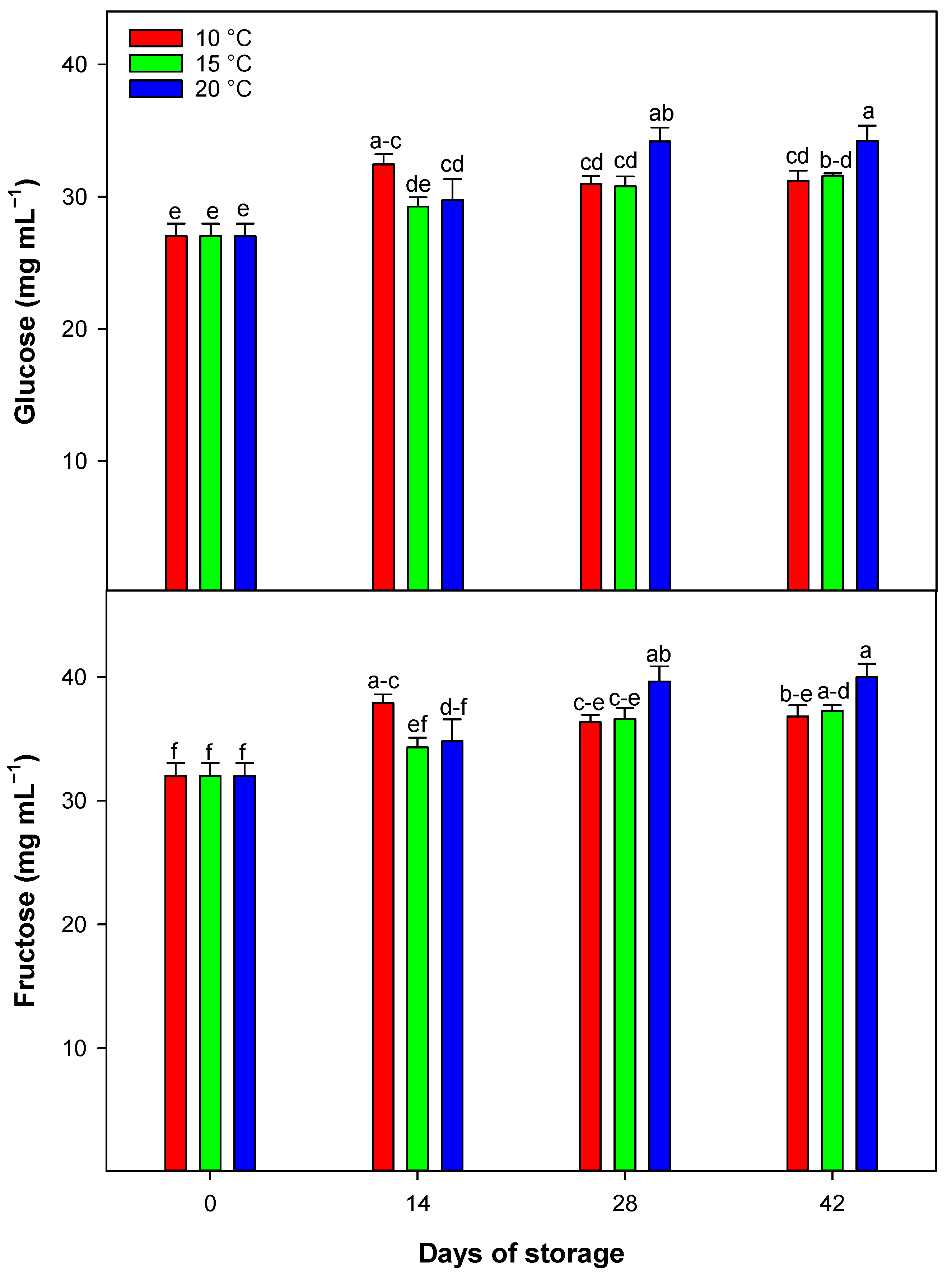
In citrus fruit, sucrose, glucose, and fructose represent the primary soluble sugars, and their content can change during the postharvest period. In the current research, we observed no changes in sucrose concentrations within the stored blood orange samples but, glucose and fructose concentrations both showed increasing trends [
1]. This pattern of increasing glucose and fructose suggests that cold storage may help the conversion of organic acids into these sugars through glycolytic enzymes. Temperature appears to have a catalytic effect on this enzymatic process, with the most significant increases in glucose and fructose occurring at 20 °C, highlighting the role of temperature in controlling postharvest sugar content [
1].
In this study, we observed changes in TA in blood oranges during cold storage at different temperatures. TA, a vital indicator of citrus fruit juice acidity, is predominantly influenced by the concentrations of organic acids [
1]. We demonstrated that TA levels in blood orange juice significantly fluctuate depending on both storage temperature and duration. Throughout the storage period, a consistent decline in TA was noted across all samples. This reduction in acidity indicates the gradual breakdown and utilization of organic acids within the fruit. During cold storage, organic acids may be metabolized into sugars and ATP, which are essential for cellular processes [
21]. As a result, TA decreases, a trend confirmed by this study. The highest TA levels were maintained at 10 °C, while the lowest were recorded at the end of storage for fruit kept at 20 °C. These findings underscore the significant influence of temperature on organic acid metabolism, with lower temperatures preserving acidity and higher temperatures accelerating its loss [
1].
The TSS/TA ratio is a key indicator of citrus fruit maturity, offering insights into taste and flavor evolution by reflecting the balance between sugars and organic acids. This ratio significantly influences the sensory experience of fruit stored at low temperatures [
20]. In this study, the TSS/TA ratio consistently increased at all storage temperatures as storage progressed. This change is driven by metabolic pathways that regulate sugar and organic acid levels through synthesis and breakdown of molecules [
1]. As a result, sugar levels rise while organic acid concentrations decline, altering the TSS/TA ratio during cold storage. The lowest TSS/TA ratio (i.e., closest to the at-harvest ratio) was consistently recorded at 10 °C across all sampling times, indicating better preservation of organic acids relative to sugars at this temperature [
22].
Overall, these changes are commonly associated with enhanced sweetness perception and reduced sourness. This shift could potentially improve the sensory appeal of blood oranges over time, particularly under higher storage temperatures. Furthermore, the increase in glucose and fructose levels may further contribute to a sweeter flavor profile, despite the lack of change in sucrose content. Together, these biochemical changes suggest that storage conditions influence not only the physiological attributes of blood oranges fruit but also their organoleptic quality [
1].
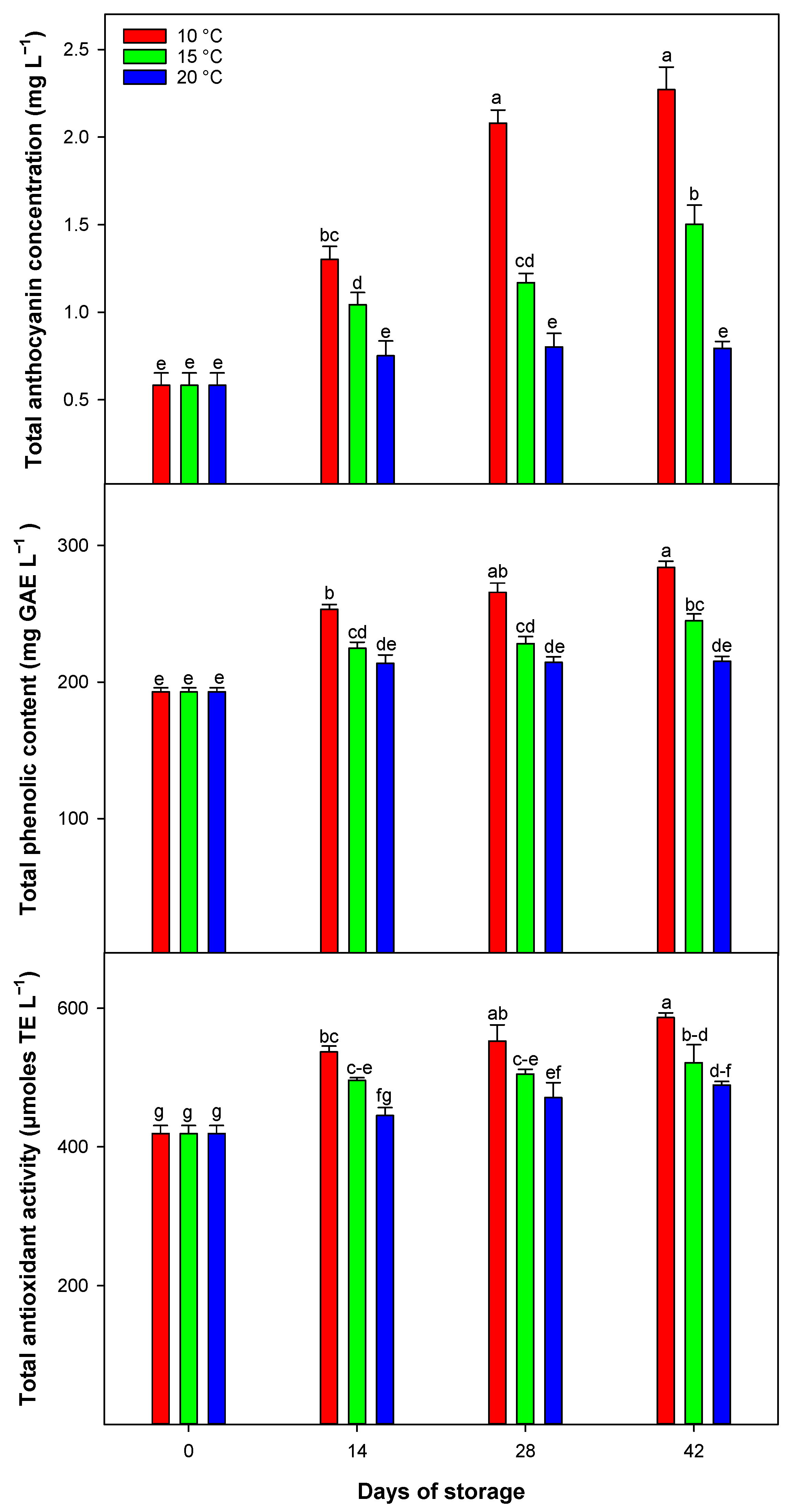
Blood oranges are unique among citrus fruit due to their anthocyanin content, which serves as a key quality indicator [
22]. This study highlights that the TAC in blood oranges significantly increased throughout the storage period, with variations in the rate of increase depending on storage temperatures. Notably, 10 °C was identified as the most effective temperature for enhancing TAC, establishing a clear relationship between storage temperatures and anthocyanin accumulation in blood oranges.
Anthocyanin synthesis occurs in the cytosol, with the resulting products subsequently transported to the vacuole to prevent oxidation and to function as pigments [
4]. This synthesis involves several enzymes that catalyze each step from phenylalanine deamination to production of anthocyanidins, which are then modified into various anthocyanin structures. Cold temperatures can regulate the activity of these enzymes by enhancing their expression or stability [
8]. Additionally, the
Ruby gene, encoding a transcription factor crucial for anthocyanin biosynthesis, is activated by cold stress due to a retrotransposon in its promoter region. This activation leads to increased
Ruby expression and, consequently, anthocyanin biosynthesis in blood oranges [
7]. These processes result in higher anthocyanin levels in the juice vesicles, imparting the characteristic color and associated health benefits to blood oranges postharvest.
Storage temperature influences the metabolic pathways that synthesize anthocyanins, with significant differences observed among various cultivars. Extremely low temperatures can reduce cellular metabolism, thereby downregulating transcripts involved in phenylpropanoid biosynthesis, a secondary metabolic pathway [
10]. This downregulation may inhibit anthocyanin biosynthesis and accumulation at lower temperatures by influencing the expression of structural genes within the phenylpropanoid pathway [
11]. Consequently, the effectiveness of moderate temperatures in enhancing anthocyanin accumulation is more pronounced than at either higher or lower temperatures, with the maximum enhancement observed at 10 °C across different postharvest storage times.
Phenolic compounds, a diverse group of secondary metabolites that include anthocyanins, are known for their antioxidant properties. In this study, TPC increased in certain treatments during storage, with temperature significantly influencing the rate of accumulation. The highest TPC levels were consistently recorded at 10 °C, whereas the lowest occurred at 20 °C across all storage durations. These results indicate that lower storage temperatures may promote the biosynthesis and accumulation of phenolic compounds in blood oranges by stimulating the phenylpropanoid pathway [
11]. In blood oranges, TAC and TPC exhibit a positive correlation [
1], likely influenced by temperature-dependent enzymatic and metabolic activities that regulate the synthesis and accumulation of phenolic compounds [
8]. This study highlights the importance of storage temperature in modulating the phenolic content, thereby impacting the overall quality and health benefits of the blood orange fruit.
In this study, TAA was observed to increase during the storage period. The findings indicate that lower storage temperatures, particularly around 10 °C, can enhance the antioxidant activity in blood oranges. This enhancement is attributed to the increased synthesis and accumulation of antioxidant compounds, such as anthocyanins and other phenolic compounds, at these temperatures. These antioxidants function by donating electrons or hydrogen atoms, neutralizing free radicals, and mitigating oxidative damage within the fruit [
22].
The color of citrus fruit peel is a critical quality attribute that significantly affects consumer preference. Peel color is primarily influenced by the presence of chlorophylls, carotenoids, and anthocyanins. Chlorophylls are responsible for the green hue in unripe citrus fruit, whereas carotenoids and anthocyanins are crucial in developing the final peel color [
20]. To objectively describe and quantify these color characteristics, parameters including
L*,
a*,
b*,
h°,
C* values, and the
CCI are used [
1].
The
L* value measures the lightness of an object, ranging from 0 (black) to 100 (white). In citrus fruit,
L* measures how light or dark the fruit’s peel or flesh or juice color appears. A higher
L* value indicates a lighter color, while a lower value suggests a darker color. The
a* value ranges from green (−) to red (+). Citrus fruit with a positive
a* value have more red or orange tendencies, while those with a negative
a* value may appear more green [
1]. The
b* value ranges from positive to negative values with positive values (+) indicating the presence of yellow hues, while negative values (−) indicate the presence of blue hues. Citrus fruit with a positive
b* value have more yellow coloration. The
h° or hue value ranges from 0 to 360°, progressing from red (0°) to yellow (90°), green (180°), and blue (270°). The
C* or chroma or color saturation index quantifies the purity, intensity or saturation of color. Citrus fruit with higher
C* values have more vibrant and vivid colors. Colors produced by a single, predominant pigment have higher
C* values than those resulting from a mixture of pigments. The
CCI is a metric specifically designed to assess the color of citrus fruit. Being derived from the Hunter
L,
a, and
b values, the
CCI is essentially an indicator of how light or dark and deep orange the peel color appears. It is often used in the citrus industry to evaluate fruit maturity and quality, with higher values typically indicating better (i.e., more orange) coloration [
22].
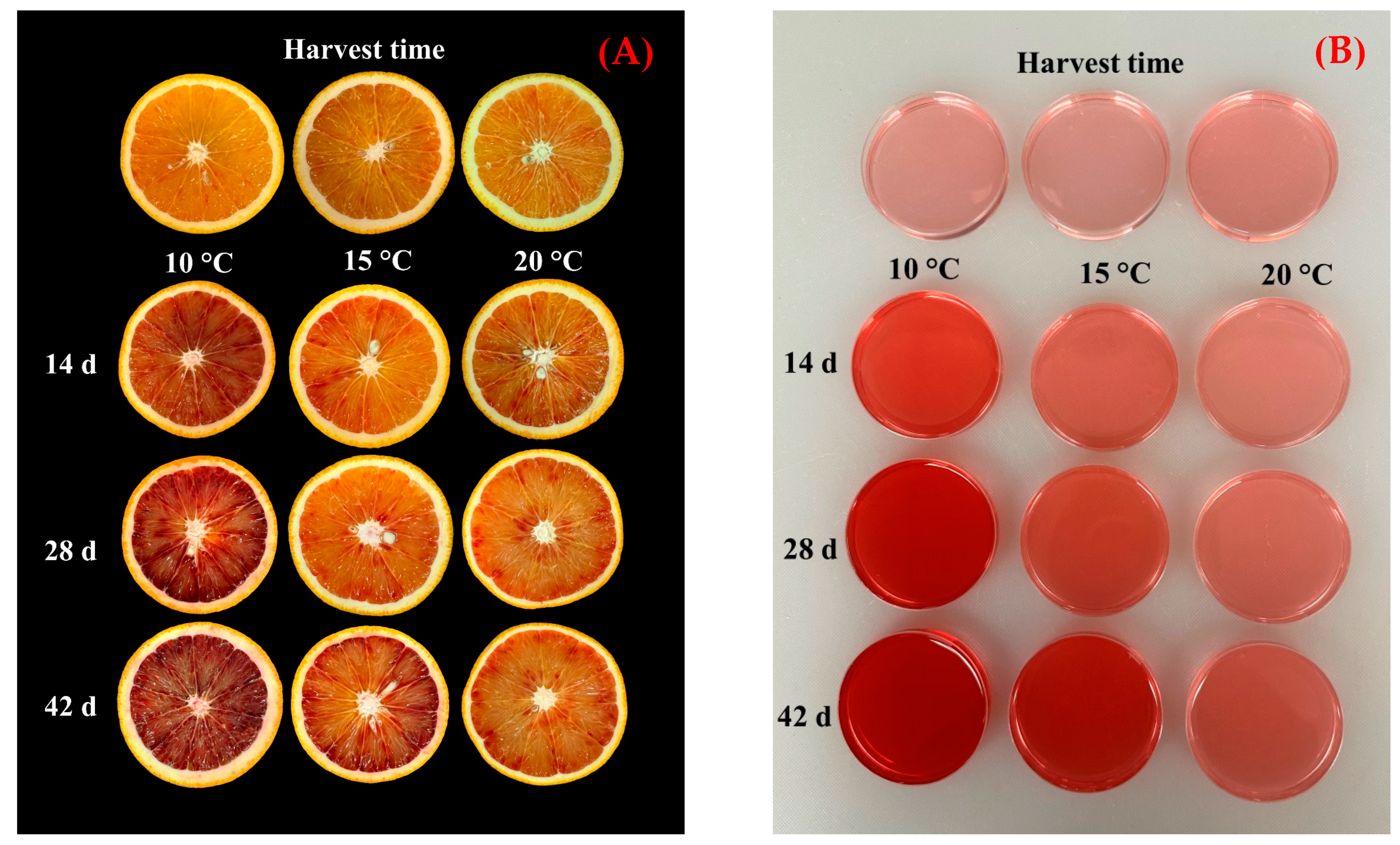
In this study, the peel color parameters
L*,
b*, and
h° all decreased across different storage temperatures, indicating the peel became darker and exhibited less yellow tones. Conversely, the
a* and
CCI values increased, suggesting a higher red component in the peel color. The
C* values changed little during storage, probably indicating maintenance of a somewhat complex pigment mixture. The rise in the red component (+
a*) was particularly notable, corresponding to the change from a more yellow to a more orange peel color. During the period of cold storage, the observed increase in
L* values can be attributed to the breakdown of carotenoids, which occurs through oxidative reactions and isomerization. Such transformations are known to result in notable alterations in the coloration of the peel [
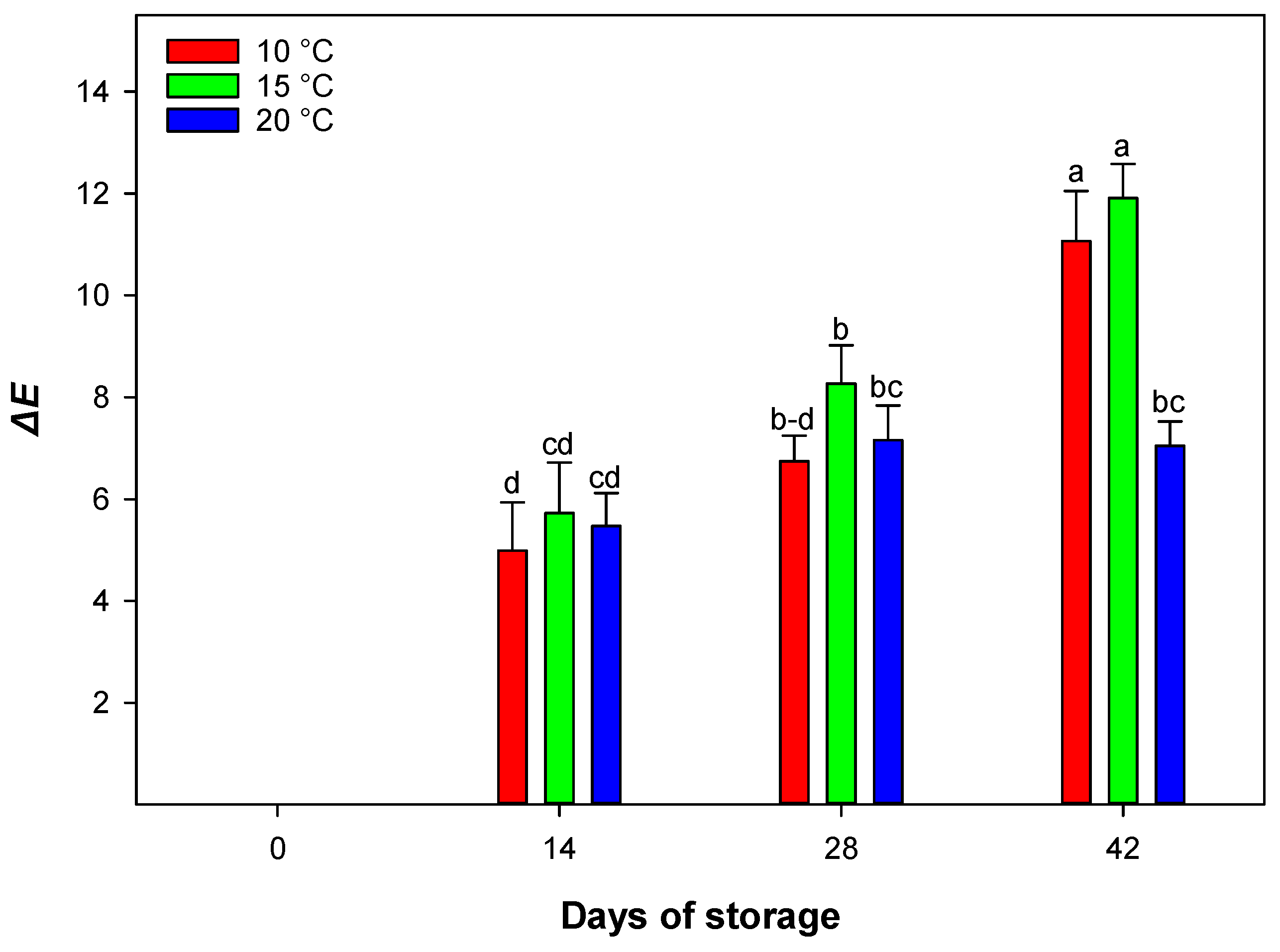
23]. The increase in Δ
E at 15 °C suggests that moderate temperatures may accelerate physiological and biochemical processes involved in pigment metabolism. In contrast, higher temperatures like 20 °C might slow these processes or maintain pigment stability, resulting in lower Δ
E values over time [
17].