Quantifying Yield Losses in Canola (Brassica napus) Caused by Verticillium longisporum
Abstract
1. Introduction
2. Materials and Methods
2.1. Grain Inoculum Preparation
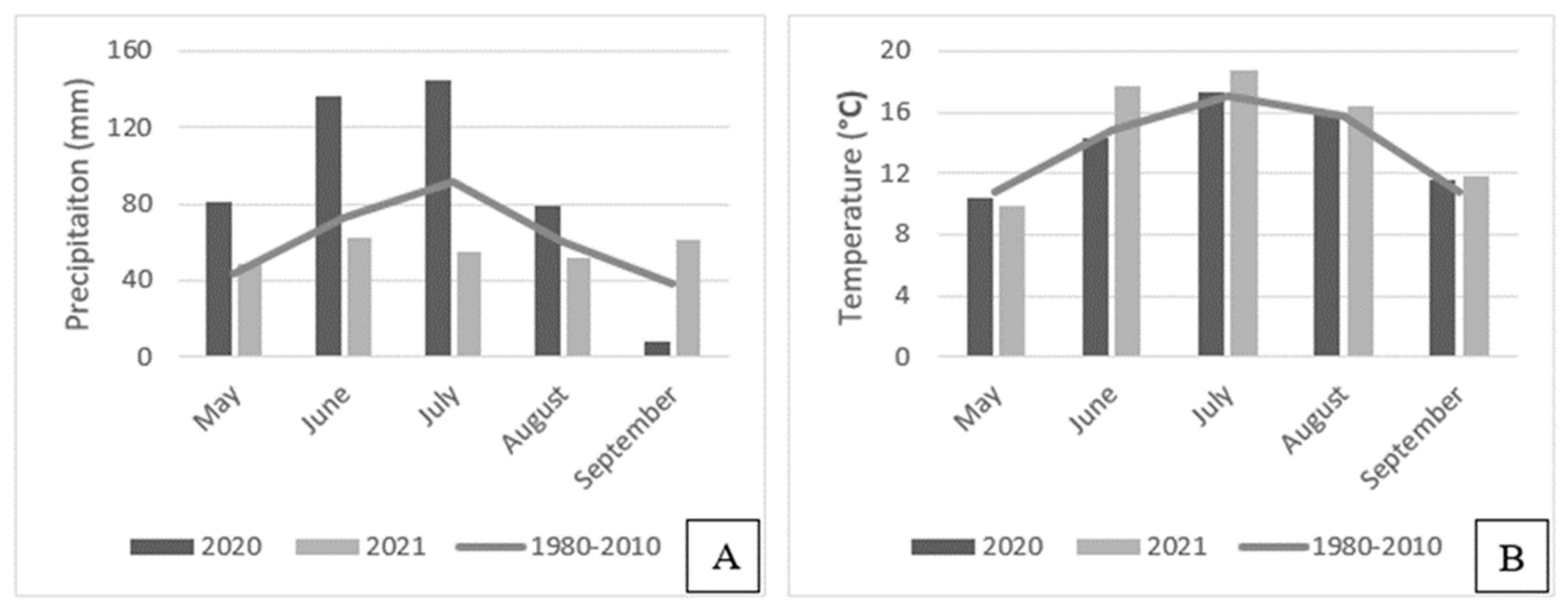
2.2. Field Experiments
2.3. Assessments of Disease Severity and Seed Yield
2.4. Statistical Analysis
3. Results
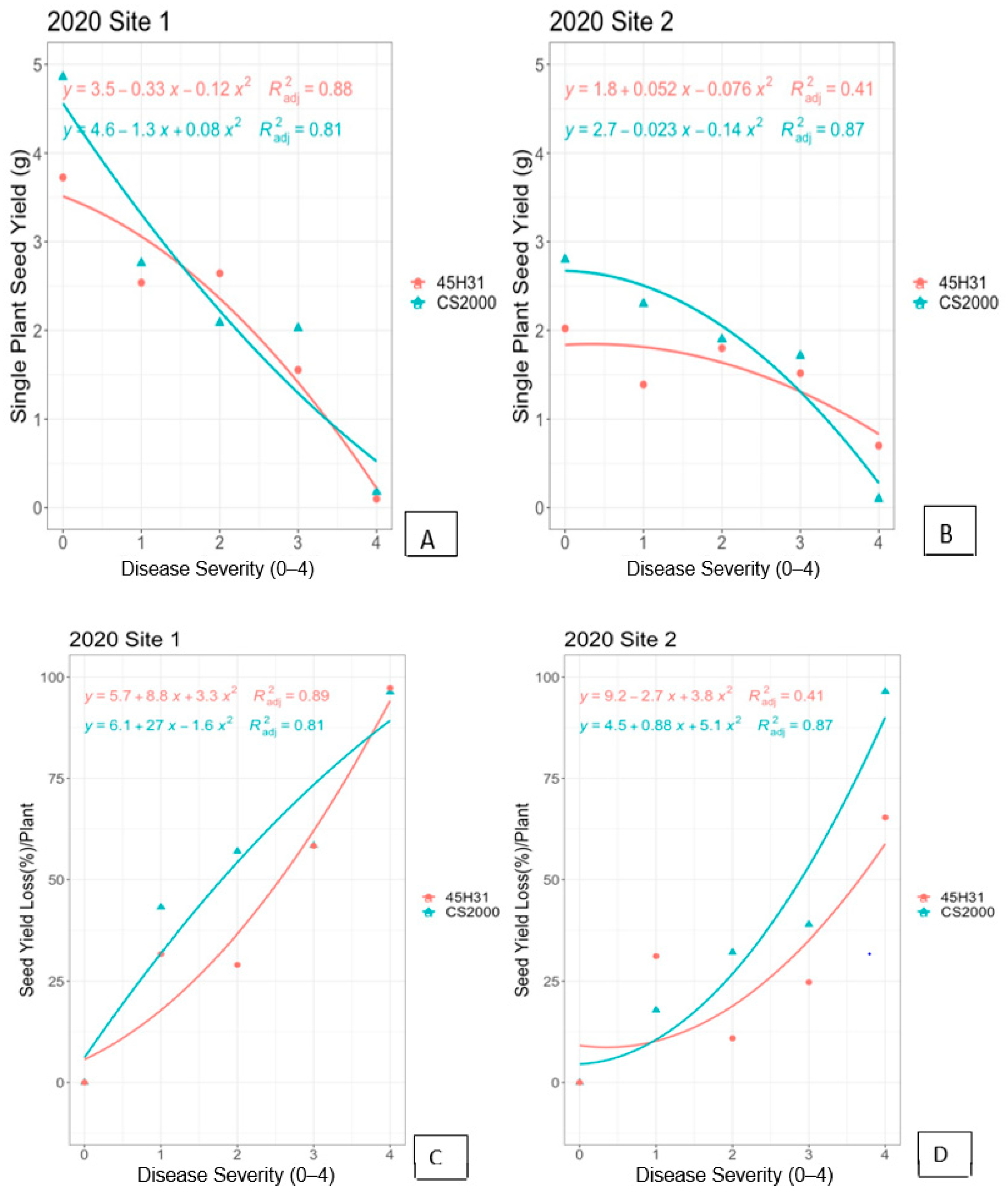
3.1. Field Experiments in 2020
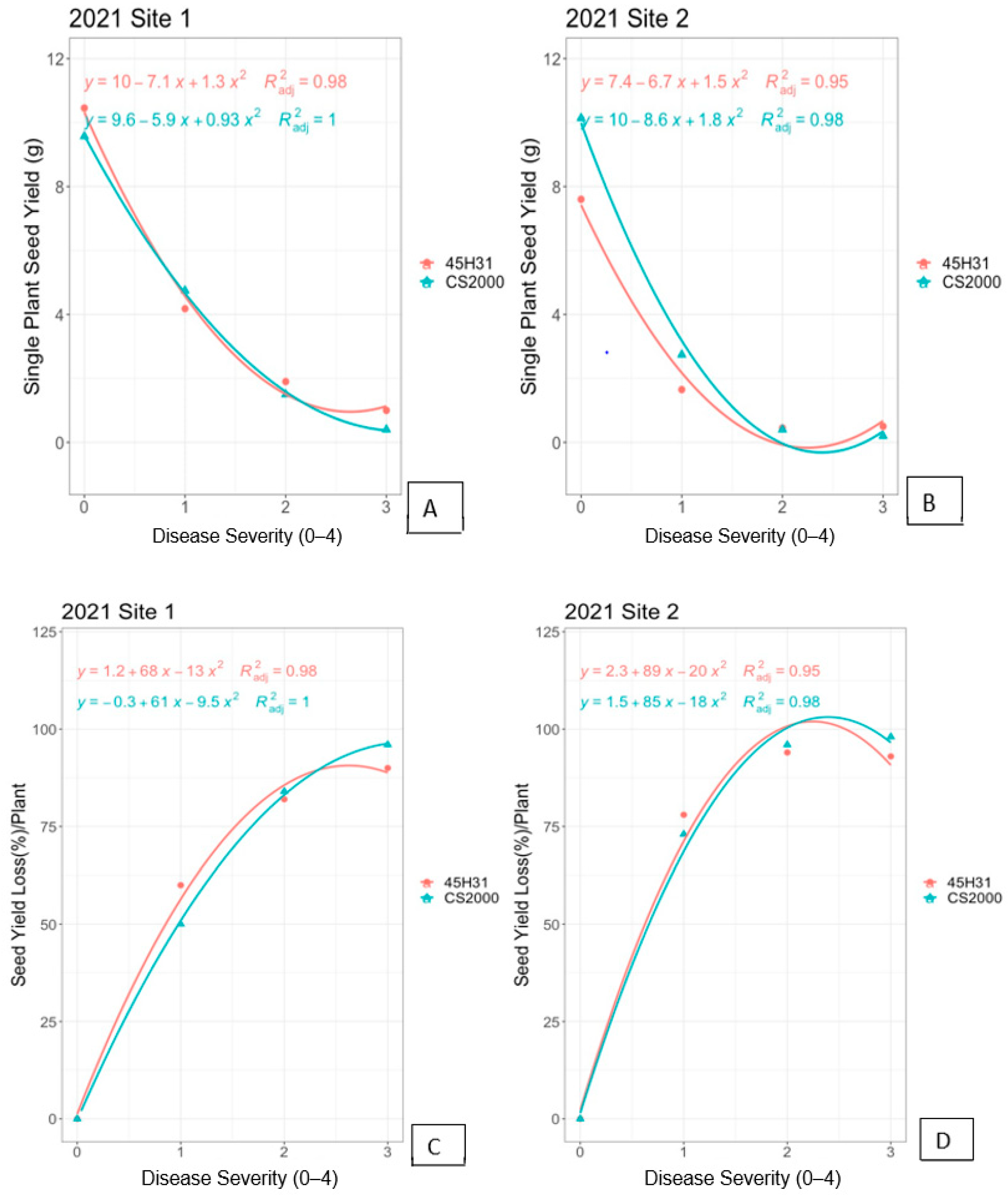
3.2. Field Experiments in 2021
3.3. Regression Models
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B

References
- Depotter, J.R.L.; Deketelaere, S.; Inderbitzin, P.; Tiedemann, A.V.; Höfte, M.; Subbarao, K.V.; Wood, T.A.; Thomma, B.P.H.J. Verticillium longisporum, the invisible threat to oilseed rape and other brassicaceous plant hosts. Mol. Plant Pathol. 2016, 17, 1004–1016. [Google Scholar] [CrossRef] [PubMed]
- Fröschel, C. In-depth evaluation of root infection systems using the vascular fungus Verticillium longisporum as soil-borne model pathogen. Plant Methods 2021, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Koike, S.T.; Subbarao, K.V.; Davis, R.M.; Gordon, T.R.; Hubbard, J.C. Verticillum wilt of cauliflower in California. Plant Dis. 1994, 78, 1116–1121. [Google Scholar] [CrossRef]
- Depotter, J.R.L.; Thomma, B.P.H.J.; Wood, T.A. Measuring the impact of Verticillium longisporum on oilseed rape (Brassica napus) yield in field trials in the United Kingdom. Eur. J. Plant Pathol. 2019, 153, 321–326. [Google Scholar] [CrossRef]
- Heale, J.B.; Karapapa, V.K. The verticillium threat to Canada’s major oilseed crop: Canola. Can. J. Plant Pathol. 1999, 21, 1–7. [Google Scholar] [CrossRef]
- Zheng, X.; Pfordt, A.; Khatri, L.; Eseola, A.B.; Wilch, A.; Koopmann, B.; von Tiedemann, A. Contrasting Patterns of Colonization with Verticillium longisporum in Winter- and Spring-Type Oilseed Rape (Brassica napus) in the Field and Greenhouse and the Role of Soil Temperature. Plant Dis. 2019, 103, 2090–2099. [Google Scholar] [CrossRef] [PubMed]
- Karapapa, V.K.; Bainbridge, B.W.; Heale, J.B. Morphological and molecular characterization of Verticillium longisporum comb. nov., pathogenic to oilseed rape. Mycol. Res. 1997, 101, 1281–1294. [Google Scholar] [CrossRef]
- Daebeler, F.; Amelung, D.; Zeise, K. Verticillium-Welke an Winterraps—Auftreten und Bedeutung. Nachrichtenblatt Pflanzenschutz DDR 1988, 42, 71–73. [Google Scholar]
- Dunker, S.; Keunecke, H.; Steinbach, P.; von Tiedemann, A. Impact of Verticillium longisporum on Yield and Morphology of Winter Oilseed Rape (Brassica napus) in Relation to Systemic Spread in the Plant. J. Phytopathol. 2008, 156, 698–707. [Google Scholar] [CrossRef]
- Province of Manitoba-Agriculture. Verticillium Stripe of Canola Detected in Manitoba. Available online: https://www.gov.mb.ca/agriculture/ (accessed on 23 January 2022).
- Canadian Canadian Canola Growers Association. Canola: Growing Canadian Prosperity. Available online: https://www.ccga.ca/policy/Pages/AdvocacyPriorities.aspx (accessed on 19 April 2022).
- Hwang, S.F.; Ahmed, H.U.; Turnbull, G.D.; Gossen, B.D.; Strelkov, S.E. The effect of seed size, seed treatment, seeding date, and depth on Rhizoctonia seedling blight of canola. Can. J. Plant Sci. 2014, 94, 311–321. [Google Scholar] [CrossRef]
- Cui, J.; Strelkov, S.E.; Fredua-Agyeman, R.; Hwang, S.F. Development of optimized Verticillium longisporum inoculation techniques for canola (Brassica napus). Can. J. Plant Pathol. 2022, 45, 92–102. [Google Scholar] [CrossRef]
- Government of Canada CFIA. Verticillium Stripe—Verticillium longisporum. Available online: https://inspection.canada.ca/plant-health/invasive-species/plant-diseases/verticillium-stripe/eng/1420746212959/1420746213803 (accessed on 12 May 2022).
- Canola Council of Canada. Verticillium Stripe. Available online: https://www.canolacouncil.org/canola-encyclopedia/diseases/verticillium-stripe/?utm_source=chatgpt.com#footnote_3_1526 (accessed on 15 April 2005).
- Soesanto, L.; Termorshuizen, A.J. Effect of Temperature on the Formation of Microsclerotia of Verticillium dahliae. J. Phytopathol. 2001, 149, 685–691. [Google Scholar] [CrossRef]
- Siebold, M.; von Tiedemann, A. Effects of experimental warming on fungal disease progress in oilseed rape. Glob. Change Biol. 2013, 19, 1736–1747. [Google Scholar] [CrossRef] [PubMed]
- Shirtliffe, S.; Hartman, M. Determining the Economic Plant Density in Canola; Saskatchewan Canola Development Council: 2009. Available online: https://www.canolacouncil.org/wp-content/uploads/2021/03/Shirtliffe-2009-Determining-the-economic-plant-density-in-canola-CARP-2006.08.pdf (accessed on 12 May 2022).
- Yu, H.; Chang, K.-F.; Hwang, S.-F.; Strelkov, S.E. Characterization of the Virulence and Yield Impact of Fusarium Species on Canola (Brassica napus). Plants 2023, 12, 3020. [Google Scholar] [CrossRef] [PubMed]
- Eynck, C.; Koopmann, B.; Grunewaldt-Stoecker, G.; Karlovsky, P.; von Tiedemann, A. Differential interactions of Verticillium longisporum and V. dahliae with Brassica napus detected with molecular and histological techniques. Eur. J. Plant Pathol. 2007, 118, 259–274. [Google Scholar] [CrossRef]
- Pullman, G.S.; DeVay, J.E. Epidemiology of Verticillium wilt of cotton: Effects of disease development on plant phenology and lint yield. Phytopathology 1982, 72, 554–559. [Google Scholar] [CrossRef]
| Hybrid | Treatment | Disease Severity | Mean Single Plant Yield (g) | Mean Plot Yield (kg/ha) | |||
|---|---|---|---|---|---|---|---|
| 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | ||
| ‘45H31’ | Control | 0.0 A | 0.1 a | 4.2 AB | 6.0 a | 83.5 A | 86.0 a |
| Low | 0.3 AB | 0.3 a | 2.8 ABC | 7.5 a | 70.8 A | 71.1 a | |
| Medium-Low | 0.7 ABC | 0.4 a | 2.1 BC | 8.9 a | 64.0 A | 75.3 a | |
| Medium | 1.1 BCD | 0.1 a | 1.7 C | 9.1 ab | 66.2 A | 90.7 a | |
| High | 1.4 CD | 0.1 a | 1.8 C | 9.3 ab | 71.8 A | 88.4 a | |
| ‘CS2000’ | Control | 0.0 A | 0.0 a | 4.7 A | 6.2 a | 62.7 A | 90.2 a |
| Low | 0.8 ABCD | 0.0 a | 2.4 ABC | 8.0 a | 88.5 A | 80.0 a | |
| Medium-Low | 1.3 BCD | 0.3 a | 2.2 BC | 8.7 a | 86.9 A | 100.9 a | |
| Medium | 1.9 D | 0.1 a | 1.6 C | 9.6 ab | 73.5 A | 78.4 a | |
| High | 1.5 CD | 0.1 a | 2.3 BC | 13.2 b | 76.7 A | 107.8 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, J.; Strelkov, S.E.; Hwang, S.-F. Quantifying Yield Losses in Canola (Brassica napus) Caused by Verticillium longisporum. Horticulturae 2025, 11, 494. https://doi.org/10.3390/horticulturae11050494
Cui J, Strelkov SE, Hwang S-F. Quantifying Yield Losses in Canola (Brassica napus) Caused by Verticillium longisporum. Horticulturae. 2025; 11(5):494. https://doi.org/10.3390/horticulturae11050494
Chicago/Turabian StyleCui, Ji, Stephen E. Strelkov, and Sheau-Fang Hwang. 2025. "Quantifying Yield Losses in Canola (Brassica napus) Caused by Verticillium longisporum" Horticulturae 11, no. 5: 494. https://doi.org/10.3390/horticulturae11050494
APA StyleCui, J., Strelkov, S. E., & Hwang, S.-F. (2025). Quantifying Yield Losses in Canola (Brassica napus) Caused by Verticillium longisporum. Horticulturae, 11(5), 494. https://doi.org/10.3390/horticulturae11050494







