Electrophysiological Mechanism and Identification of Effective Compounds of Ginger (Zingiber officinale Roscoe) Shoot Volatiles Against Aphis gossypii Glover (Hemiptera: Aphididae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Aphid Collection and Rearing
2.2. Plant Materials
2.3. Preparation and Separation of GE
2.3.1. Preparation of GE
2.3.2. Separation of GSE
2.4. Aphis gossypii Glover Repellent Assay
2.5. Column Chromatography and Thin Layer Chromatography
2.6. Electrophysiological Recordings
2.7. Gas Chromatography–Mass Spectrometry (GC–MS)
2.8. Electrophysiological and Behavioral Responses of A. gossypii to Potential Volatiles
2.9. Statistical Analysis
3. Results and Discussion
3.1. Repellency of GSE and 4 Extraction Phases Against A. gossypii
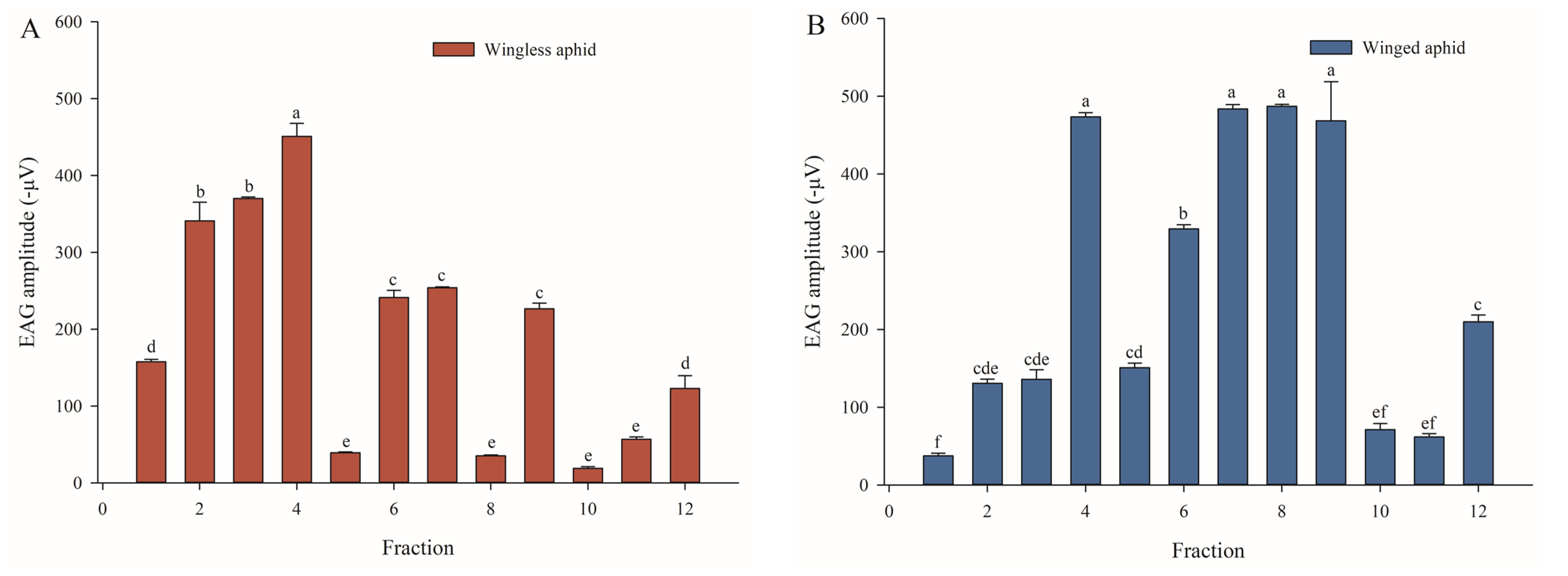
3.2. EAG Responses Induced by Major Components of Methanol Extraction Phase in Wingless and Winged Aphids
3.3. GC–MS Analysis of Fraction 4 Eliciting Strong EAG Responses of A. gossypii
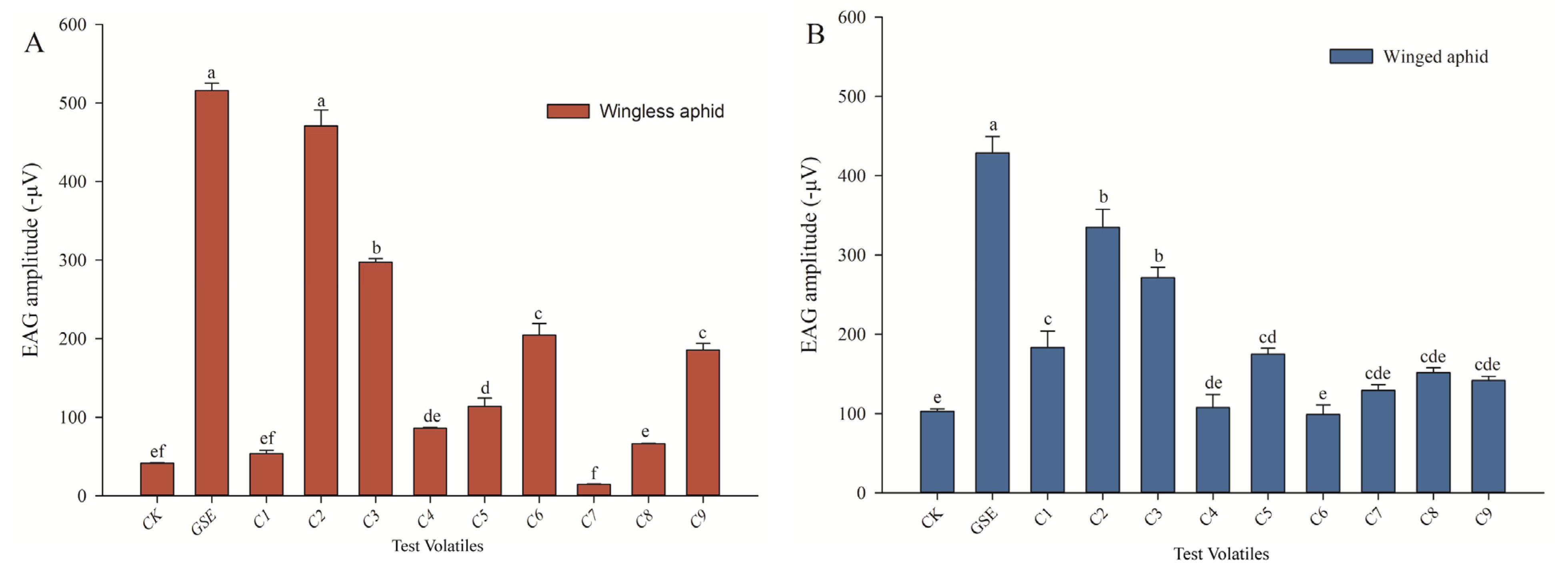
3.4. Nine Principal Volatiles That Elicited the EAG Responses in Wingless and Winged Aphids
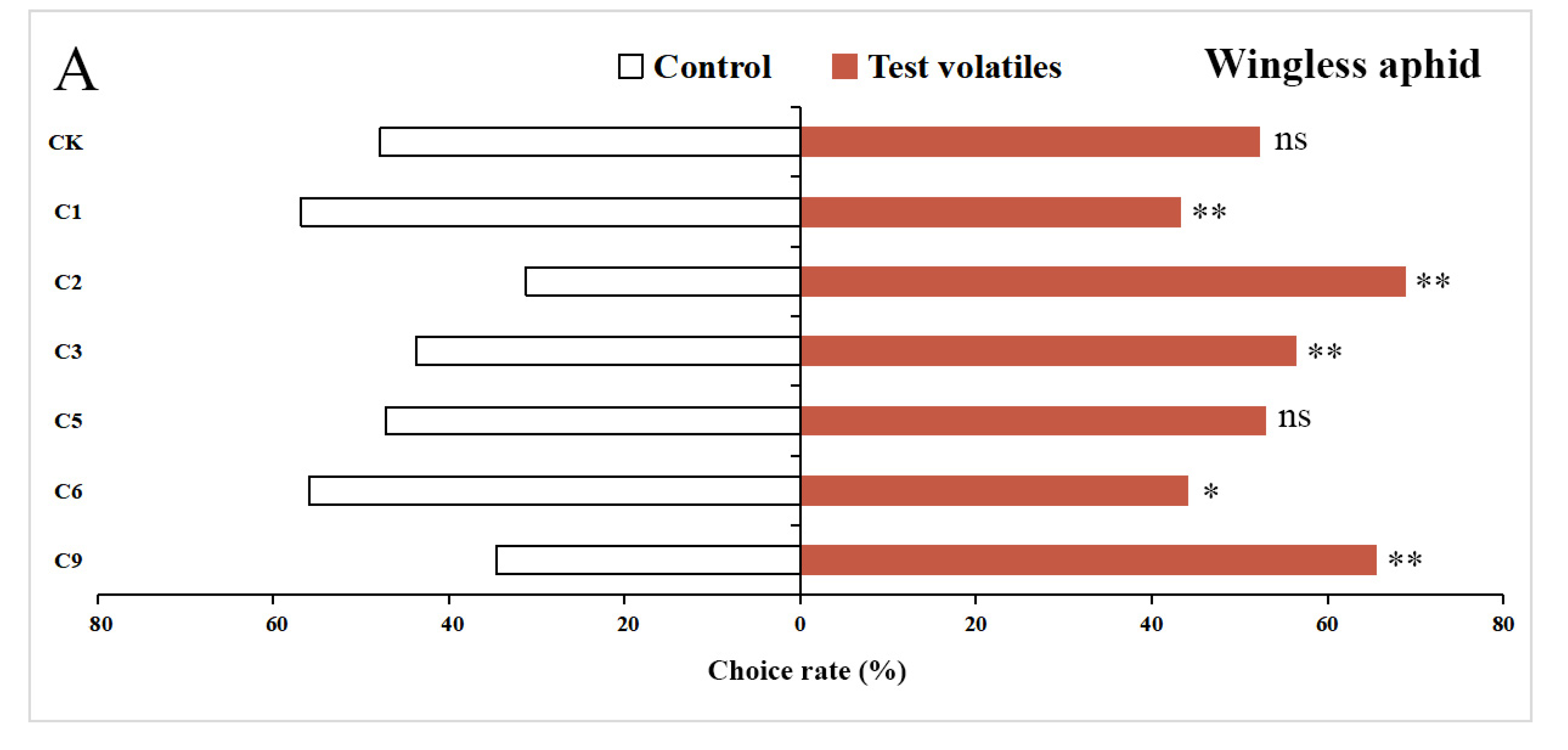
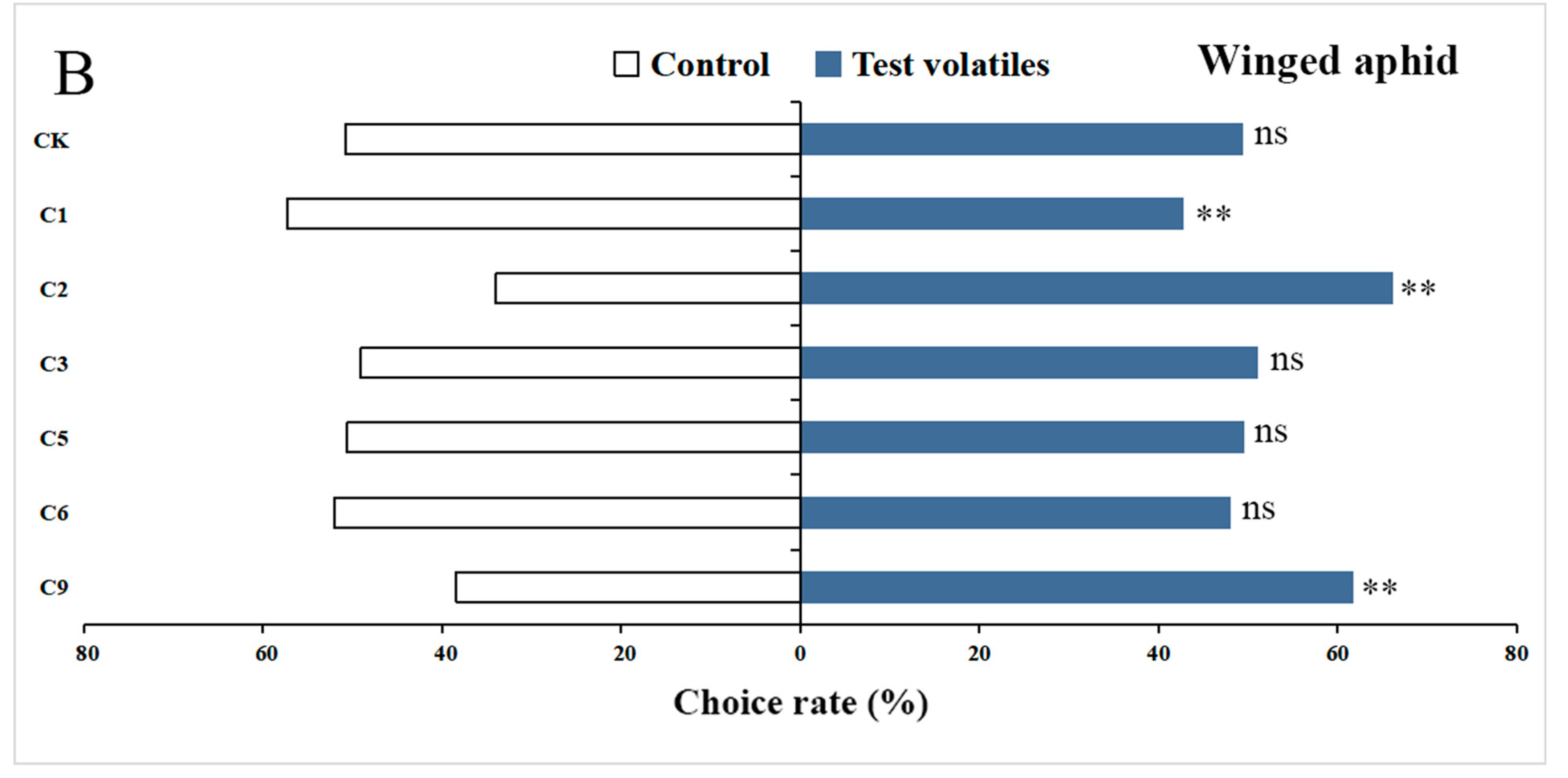
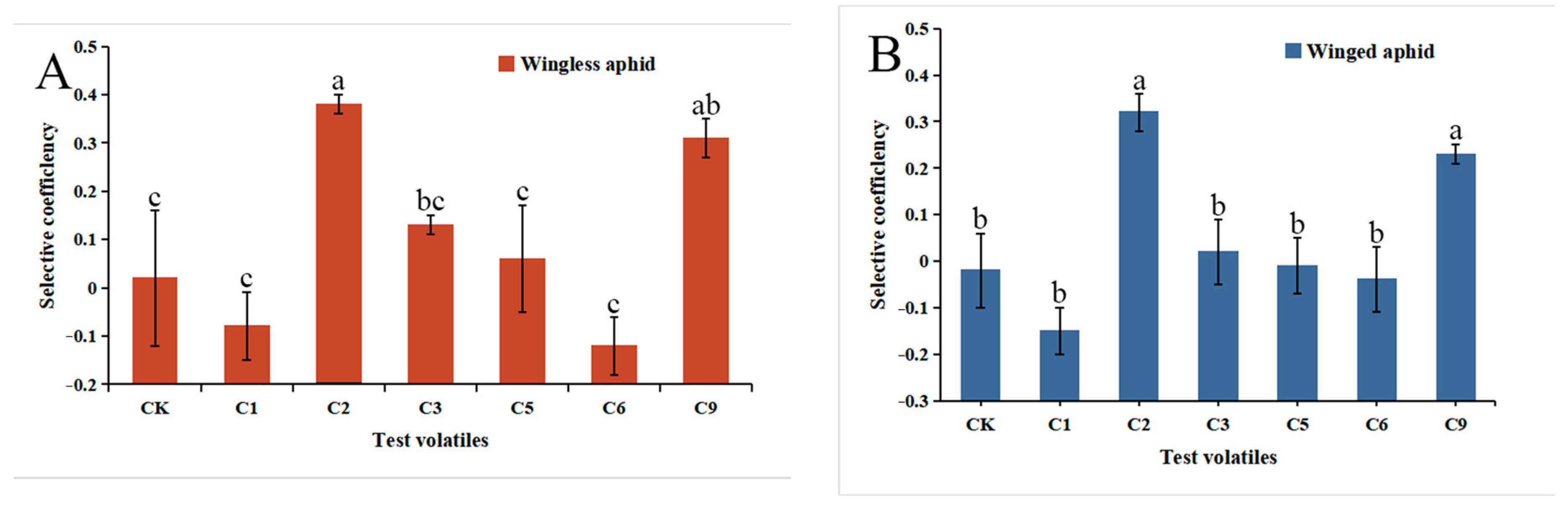
3.5. Behavioral Responses of Wingless and Winged Aphids to 6 Volatiles
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hullé, M.; Chaubet, B.; Turpeau, E.; Simon, J.C. Encyclop’Aphid: A website on aphids and their natural enemies. Entomol. Gen. 2019, 40, 97–101. [Google Scholar] [CrossRef]
- Sharma, A.; Shukla, A.; Attri, K.; Kumar, M.; Kumar, P.; Suttee, A.; Singh, G.; Barnwal, R.P.; Singla, N. Global trends in pesticides: A looming threat and viable alternatives. Ecotoxicol. Environ. Saf. 2020, 201, 110812. [Google Scholar] [CrossRef]
- Lu, A.; Wang, T.; Hui, H.; Wei, X.; Cui, W.; Zhou, C.; Li, H.; Wang, Z.; Guo, J.; Ma, D.; et al. Natural products for drug discovery: Discovery of gramines as novel agents against a plant virus. J. Agric. Food Chem. 2019, 67, 2148–2156. [Google Scholar] [CrossRef]
- Sidauruk, L.; Panjaitan, E.; Sipayung, P.; Hutauruk, S. Botanical pesticides, a potential ethnobotany Karo Regency to support food safety of the horticultural product. IOP Conf. Ser. Earth Environ. Sci. 2022, 1005, 012020. [Google Scholar] [CrossRef]
- Heydari, M.; Amirjani, A.; Bagheri, M.; Sharifian, I.; Sabahi, Q. Eco-friendly pesticide based on peppermint oil nanoemulsion: Preparation, physicochemical properties, and its aphicidal activity against cotton aphid. Environ. Sci. Pollut. Res. Int. 2020, 27, 6667–6679. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Han, C.; Zhang, C.; Kuchkarova, N.; Wei, C.; Zhang, C.; Shao, H. Allelopathic, phytotoxic, and insecticidal effects of Thymus proximus Serg. essential oil and its major constituents. Front. Plant Sci. 2021, 12, 689875. [Google Scholar] [CrossRef]
- Rodrigues, G.C.; dos Santos Maia, M.; Silva Cavalcanti, A.B.; de Sousa, N.F.; Scotti, M.T.; Scotti, L. In silico studies of lamiaceae diterpenes with bioinsecticide potential against Aphis gossypii and Drosophila melanogaster. Molecules 2021, 26, 766. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Tang, Q.; Liang, P.; Xia, J.; Zhang, B.; Gao, X. Toxicity and sublethal effects of two plant allelochemicals on the demographical traits of cotton aphid, Aphis gossypii Glover (Hemiptera: Aphididae). PLoS ONE 2019, 14, e0221646. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, G.; Huang, X.; Guo, H.; Su, X.; Han, L.; Zhang, Y.; Qi, Z.; Xiao, Y.; Cheng, H. Overexpression of the caryophyllene synthase gene GhTPS1 in cotton negatively affects multiple pests while attracting parasitoids. Pest Manag. Sci. 2020, 76, 1722–1730. [Google Scholar] [CrossRef]
- Zhao, C.; Ma, C.; Luo, J.; Niu, L.; Hua, H.; Zhang, S.; Cui, J. Potential of cucurbitacin B and epigallocatechin gallate as biopesticides against Aphis gossypii. Insects 2021, 12, 32. [Google Scholar] [CrossRef]
- Mostafiz, M.M.; Hassan, E.; Shim, J.K.; Lee, K.Y. Insecticidal efficacy of three benzoate derivatives against Aphis gossypii and its predator Chrysoperla carnea. Ecotoxicol. Environ. Saf. 2019, 184, 109653. [Google Scholar] [CrossRef] [PubMed]
- Manjree, A.; Suresh, W.; Swaran, D.; Bhupinder, P.S.K. Insect growth inhibition, antifeedant and antifungal activity of compounds isolated/derived from Zingiber officinale Roscoe (ginger) rhizomes. Pest Manag. Sci. 2001, 57, 289–300. [Google Scholar]
- Liu, X.; Xi, K.; Wang, Y.; Ma, J.; Huang, X.; Liu, R.; Cai, X.; Zhu, Y.; Yin, J.; Jia, Q.; et al. Evaluation of the contact toxicity and physiological mechanisms of ginger (Zingiber officinale) shoot extract and selected major constituent compounds against Melanaphis sorghi Theobald. Horticulturae 2022, 8, 944. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, X.; Sun, C.; Li, G.; Yang, P.; Jia, Q.; Cai, X.; Zhu, Y.; Yin, J.; Liu, Y. Silica Nanoparticles Enhance the Disease Resistance of Ginger to Rhizome Rot during Postharvest Storage. Nanomaterials 2022, 12, 1418. [Google Scholar] [CrossRef]
- Li, G.; Ma, J.; Yin, J.; Guo, F.; Xi, K.; Yang, P.; Cai, X.; Jia, Q.; Li, L.; Liu, Y.; et al. Identification of Reference Genes for Reverse Transcription-Quantitative PCR Analysis of Ginger Under Abiotic Stress and for Postharvest Biology Studies. Front. Plant Sci. 2022, 13, 893495. [Google Scholar] [CrossRef]
- Peng, H.; Hu, H.; Xi, K.; Zhu, X.; Zhou, J.; Yin, J.; Guo, F.; Liu, Y.; Zhu, Y. Silicon Nanoparticles Enhance Ginger Rhizomes Tolerance to Postharvest Deterioration and Resistance to Fusarium solani. Front. Plant Sci. 2022, 13, 816143. [Google Scholar] [CrossRef]
- Moreira, S.I.; Alvarenga, S.M.; Souza, T.W.; Dos, S.A.; Serrão, J.E.; José, V.Z.A.; Frederico, W.C.; Cola, Z.J.; Sigueyuki, S.C. Toxicity of essential oils to Diaphania hyalinata (Lepidoptera: Crambidae) and selectivity to its parasitoid Trichospilus pupivorus (Hymenoptera: Eulophidae). J. Econ. Econ. Entomol. Entomol. 2020, 113, 2399–2406. [Google Scholar] [CrossRef] [PubMed]
- Faheem, A.; Naeem, I.; Syed, M.Z.; Muhammad, K.Q.; Qamar, S.; Khalid, A.K.; Hamed, A.G.; Mohammad, J.A.; Waqar, J.; Muhammad, A.; et al. Comparative insecticidal activity of different plant materials from six common plant species against Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Saudi J. Biol. Sci. 2019, 26, 1804–1808. [Google Scholar]
- Zhang, W.; Heather, J.M.; David, J.S. Repellency of ginger oil to Bemisia argentifolii (Homoptera: Aleyrodidae) on tomato. J. Econ. Entomol. 2004, 97, 1310–1318. [Google Scholar] [CrossRef]
- Ukeh, D.A.; Birkett, M.A.; Bruce, T.J.A.; Allan, E.J.; Pickett, J.A.; Luntz, A.J.M. Behavioural responses of the maize weevil, Sitophilus zeamais, to host (stored-grain) and non-host plant volatiles. Pest Manag. Sci. 2010, 66, 44–50. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Zhang, Y. Research progress on chemical constituents of Zingiber officinale Roscoe. BioMed Res. Res. Int. Int. 2019, 2019, 5370823. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Guo, P.-F.; Nie, J.; Hu, X.; Wu, Y.-W.; Zhu, S.; Chen, B.-R.; Li, J.; Zeng, X.-A.; Xu, F.-Y. Inhibitory mechanism of xanthine oxidase by 6-, 8- and 10-gingerol: Enzyme kinetics, multi-spectroscopy and molecular simulations. J. Mol. Liq. 2024, 410, 125605. [Google Scholar] [CrossRef]
- Yang, Z.; Song, Z.; Tang, X.; Jie, L.; Cheng, Y.; Zang, J. Electrophysiological and olfactory behavioural responses of mature Stilpnotia candida to a mixture of volatiles from Populus × beijingensis. J. For. Res. 2025, 36, 5. [Google Scholar] [CrossRef]
- Kamaruddin, M.S.; Chong, G.H.; Daud, N.M.; Putra, N.R.; Salleh, L.M.; Suleiman, N. Bioactivities and green advanced extraction technologies of ginger oleoresin extracts: A review. Food Res. Int. 2023, 164, 112283. [Google Scholar] [CrossRef]
- Akhtar, Y.; Pages, E.; Stevens, A.; Bradbury, R.; da Camara, C.A.G.; Isman, M.B. Effect of chemical complexity of essential oils on feeding deterrence in larvae of the cabbage looper. Physiol. Entomol. 2012, 37, 81–91. [Google Scholar] [CrossRef]
- da Camara, C.A.G.; Akhtar, Y.; Isman, M.B.; Seffrin, R.C.; Born, F.S. Repellent activity of essential oils from two species of Citrus against Tetranychus urticae in the laboratory and greenhouse. Crop Prot. 2015, 74, 110–115. [Google Scholar] [CrossRef]
- Li, X. Study on the Biological Activity and Antennal Potential (EAG) of Extracts from Persicaria capitata on Myzus persicae. Master’s Thesis, Shanxi Agricultural University, Shanxi, China, 2003. [Google Scholar]
- Du, Y.; Zhou, A.; Chen, J. Olfactory and behavioral responses of red imported fire ants, Solenopsis invicta, to ylang ylang oil and its components. J. Pest Sci. 2021, 94, 1031–1044. [Google Scholar] [CrossRef]
- Liu, F.; Lai, Y.; Wu, L.; Li, Q.; Lei, L.; Yin, W.; Zhang, Y.; Huang, Z.Y.; Zhao, H. AmelOBP4: An antenna-specific odor-binding protein gene required for olfactory behavior in the honey bee (Apis mellifera). Front. Zool. 2025, 22, 2. [Google Scholar] [CrossRef]
- Ngegba, P.M.; Cui, G.; Khalid, M.Z.; Zhong, G. Use of botanical pesticides in agriculture as an alternative to synthetic pesticides. Agriculture 2022, 12, 12050600. [Google Scholar] [CrossRef]
- Wei, W.; Wu, X.; Ren, Y.; Zhong, Y.; Wei, L.; Yang, G.; Liu, Y. Methyl jasmonate enabled maintained the postharvest flavor quality of ginger (Zingiber officinale Roscoe) by reducing the loss of terpene volatile compounds. Food Chem. 2025, 468, 14. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, S.; Sun, C.; Liang, H.; Ma, J.; Jia, Q.; Yin, J.; Zhu, Y.; Liu, Y. Chitosan boosts ginger disease resistance: Insights from transcriptomic and metabolomic analyses. LWT-Food Sci. Technol. 2024, 205, 116478. [Google Scholar] [CrossRef]
- Karmakar, A.; Barik, A. Solena amplexicaulis (Cucurbitaceae) flower surface wax influencing attraction of a generalist insect herbivore, Aulacophora foveicollis (Coleoptera: Chrysomelidae). Int. J. Trop. Insect Sci. 2016, 36, 70–81. [Google Scholar] [CrossRef]
- Poonsri, W.; Pluempanupat, W.; Chitchirachan, P.; Bullangpoti, V.; Koul, O. Insecticidal alkanes from Bauhinia scandens var. horsfieldii against Plutella xylostella L. (Lepidoptera: Plutellidae). Ind. Crops Prod. 2015, 65, 170–174. [Google Scholar]
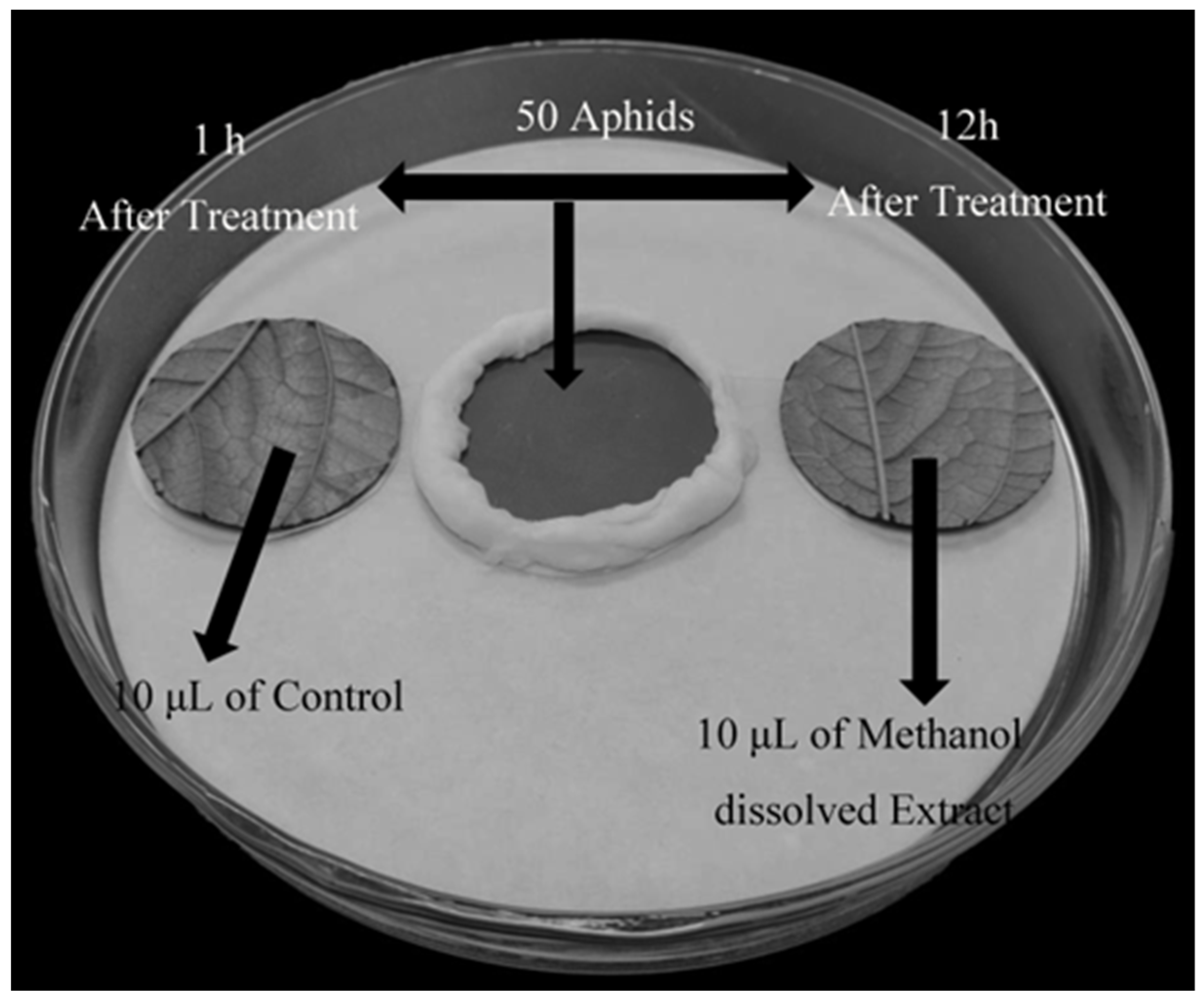

| Repellent Activity (%) | |||||
|---|---|---|---|---|---|
| 1 h | 3 h | 6 h | 9 h | 12 h | |
| Ginger Extract a | 42.22 ± 5.17 a | 50.95 ± 2.86 a | 64.82 ± 4.89 a | 50.95 ± 2.86 a | 51.28 ± 0.74 a |
| Petroleum ether a | 35.28 ± 0.13 a | 20.88 ± 0.31 c | 8.83 ± 0.26 d | 6.05 ± 1.08 d | 5.28 ± 1.06 d |
| Trichloromethane a | 18.68 ± 2.47 b | 20.40 ± 0.28 c | 26.71 ± 0.37 c | 38.52 ± 0.30 b | 35.74 ± 0.21 b |
| Ethyl acetate a | 16.81 ± 0.98 b | 20.23 ± 1.31 c | 21.22 ± 0.45 c | 13.69 ± 0.50 c | 10.36 ± 1.84 c |
| Methanol a | 32.86 ± 0.29 a | 39.84 ± 0.09 b | 47.37 ± 0.21 b | 56.99 ± 2.50 a | 55.19 ± 0.94 a |
| F | 7.51 | 37.96 | 41.72 | 72.42 | 208.31 |
| df | 4, 10 | 4, 10 | 4, 10 | 4, 10 | 4, 10 |
| p | 0.005 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Compound | RT (min) | MF a | MW b | PA(%) c | Cas d |
|---|---|---|---|---|---|
| octahydro-pentalene | 4.16 | C8H14 | 110.197 | 58.98% | 694-72-4 |
| (Z)-cyclooctene | 4.34 | C8H14 | 110.197 | 6.19% | 931-87-3 |
| ethyl-methyl-cyclohexane | 4.83 | C9H18 | 126.239 | 0.26% | 19489-10-2 |
| methyl-decane | 7.55 | C11H24 | 156.308 | 2.05% | 13151-35-4 |
| dimethyl-nonane | 7.82 | C11H24 | 156.308 | 0.74% | 17302-28-2 |
| mesitylene | 8.04 | C9H12 | 120.192 | 0.17% | 108-67-8 |
| p-cymene | 9.96 | C10H14 | 134.218 | 0.25% | 99-87-6 |
| o-cymene | 10.18 | C10H14 | 134.218 | 0.27% | 527-84-4 |
| undecane | 10.54 | C11H24 | 156.308 | 1.32% | 1120-21-4 |
| dimethylstyrene | 12.24 | C10H12 | 132.202 | 0.22% | 2234-20-0 |
| ethyl-dimethyl-benzene | 12.74 | C10H14 | 134.218 | 1.55% | 1758-88-9 |
| cyclohexyldimethoxymethyl-silane | 13.21 | C9H20O2Si | 188.339 | 0.68% | 17865-32-6 |
| cyano-L-phenylalanine | 14.09 | C10H10N2O2 | 190.199 | 0.47% | 263396-42-5 |
| dodecane | 14.49 | C12H26 | 170.335 | 7.05% | 112-40-3 |
| tetramethyl-heptadecane | 17.11 | C21H44 | 296.574 | 0.76% | 18344-37-1 |
| tetrahydro-naphthalene | 17.34 | C11H14 | 146.229 | 1.02% | 1680-51-9 |
| trimethyl-octane | 17.48 | C11H24 | 156.308 | 0.24% | 62016-34-6 |
| hexyl pentadecyl ester sulfurous acid | 18.14 | C21H44O3S | 376.278 | 0.34% | NA |
| tetradecane | 22.72 | C14H30 | 198.388 | 2.30% | 629-59-4 |
| di-tert-butylphenol | 27.74 | C14H22O | 206.324 | 0.39% | 96-76-4 |
| hexadecane | 30.48 | C16H34 | 226.441 | 0.83% | 544-76-3 |
| nonadecane | 34.10 | C19H40 | 268.529 | 7.72% | 629-92-5 |
| methyl-octadecane | 35.95 | C19H40 | 268.521 | 0.38% | 1560-88-9 |
| methyl-eicosane | 42.90 | C21H44 | 296.574 | 0.62% | 1560-84-5 |
| dibutyl phthalate | 43.27 | C16H22O4 | 278.344 | 1.43% | 84-74-2 |
| heptacosane | 49.83 | C27H56 | 380.733 | 0.61% | 593-49-7 |
| methylenebis (tert-butyl-methylphenol) | 56.16 | C23H32O2 | 340.499 | 1.76% | 119-47-1 |
| Total | 98.60% |
| Code a | Compound b | Class I | RT | RSI c | SI c | Report |
|---|---|---|---|---|---|---|
| C1 | octahydro-pentalene | Terpenes | 4.16 | 920 | 906 | Yes |
| C2 | (Z)-cyclooctene | Terpenes | 4.34 | 905 | 880 | Yes |
| C3 | dimethylstyrene | Terpenes | 12.24 | 881 | 839 | Yes |
| C4 | ethyl-dimethyl-benzene | Aromatics | 12.74 | 896 | 881 | Yes |
| C5 | tetramethyl-heptadecane | Alkanes | 17.11 | 869 | 847 | Yes |
| C6 | tetrahydro-naphthalene | Terpenes | 17.34 | 896 | 889 | Yes |
| C7 | di-tert-butylphenol | Phenols | 27.74 | 878 | 870 | Yes |
| C8 | dibutyl phthalate | Esters | 43.27 | 938 | 927 | Yes |
| C9 | heptacosane | Alkanes | 49.83 | 899 | 885 | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Tian, Y.; Liu, X.; Fang, S.; Sun, C.; Yin, J.; Zhu, Y.; Liu, Y. Electrophysiological Mechanism and Identification of Effective Compounds of Ginger (Zingiber officinale Roscoe) Shoot Volatiles Against Aphis gossypii Glover (Hemiptera: Aphididae). Horticulturae 2025, 11, 490. https://doi.org/10.3390/horticulturae11050490
Ma J, Tian Y, Liu X, Fang S, Sun C, Yin J, Zhu Y, Liu Y. Electrophysiological Mechanism and Identification of Effective Compounds of Ginger (Zingiber officinale Roscoe) Shoot Volatiles Against Aphis gossypii Glover (Hemiptera: Aphididae). Horticulturae. 2025; 11(5):490. https://doi.org/10.3390/horticulturae11050490
Chicago/Turabian StyleMa, Jiawei, Ye Tian, Xuli Liu, Shengyou Fang, Chong Sun, Junliang Yin, Yongxing Zhu, and Yiqing Liu. 2025. "Electrophysiological Mechanism and Identification of Effective Compounds of Ginger (Zingiber officinale Roscoe) Shoot Volatiles Against Aphis gossypii Glover (Hemiptera: Aphididae)" Horticulturae 11, no. 5: 490. https://doi.org/10.3390/horticulturae11050490
APA StyleMa, J., Tian, Y., Liu, X., Fang, S., Sun, C., Yin, J., Zhu, Y., & Liu, Y. (2025). Electrophysiological Mechanism and Identification of Effective Compounds of Ginger (Zingiber officinale Roscoe) Shoot Volatiles Against Aphis gossypii Glover (Hemiptera: Aphididae). Horticulturae, 11(5), 490. https://doi.org/10.3390/horticulturae11050490






