Melatonin: An Eco-Friendly Preservative for Improving Post-Harvest Quality and Longevity of Cut Flowers
Abstract
1. Introduction
2. Effects and Mechanisms of Exogenous MT in Post-Harvest Management of Cut Flowers
2.1. Anthurium Flowers
2.2. Carnations
2.3. Chrysanthemums
2.4. Gerbera Flowers
2.5. Peony Flowers
2.6. Rose Flowers
2.7. Other Flowers
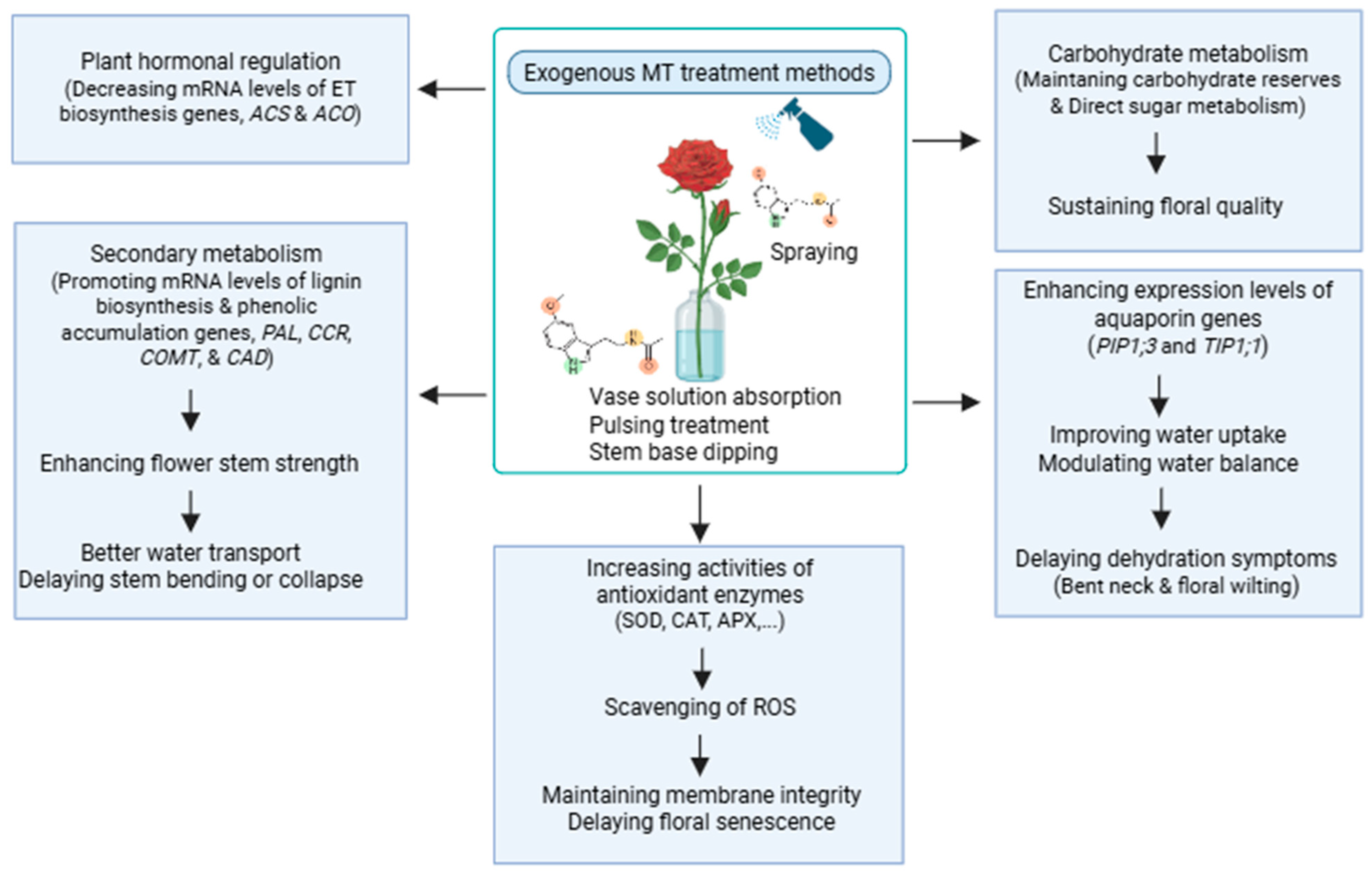
2.8. Mechanistic Synthesis of MT-Mediated Post-Harvest Regulation
3. Limitations and Challenges for Application of MT in the Cut Flower Industry
3.1. Standardization of Optimal Concentrations, Exposure Durations, and Application Methods
3.2. Interaction with Other Preservative Solutions
3.3. Flower Species-Specific Responses and Physiological Variability
3.4. Stability and Degradation of MT in Vase Solutions
3.5. Limited Field Validation and Large-Scale Cut Flower Industry Trials
3.6. Industrial-Scale Production and Cost Considerations
4. Future Prospects and Conclusions
- (i)
- Optimization of MT application strategies
- Establishing standardized MT concentrations, treatment durations, and application methods, such as pulsing, vase solutions, and foliar sprays tailored to different cut flower species.
- Developing slow-release or encapsulated MT formulations to enhance stability and prolong their effectiveness under commercial storage and handling conditions.
- (ii)
- Mechanistic insights into MT’s role in post-harvest physiology
- Investigating the molecular and genetic mechanisms underlying MT-induced senescence delay, WU enhancement, and oxidative stress mitigation.
- Identifying key MT-responsive genes and signaling pathways involved in postharvest longevity to enhance understanding and optimize application methods.
- (iii)
- Integration with commercial post-harvest treatments
- Evaluating synergistic or antagonistic effects of MT with widely used post-harvest treatments, such as sucrose supplementation, ethylene inhibitors, and antimicrobial agents.
- Assessing its effectiveness under cold storage, modified atmosphere packaging, and long-distance transport conditions to ensure its practical applicability.
- (iv)
- Commercialization and large-scale validation
- Conducting extensive field trials to validate MT’s efficacy under real-world supply chain conditions, including storage, transport, and retail environments.
- Investigating cost-effective production methods to improve the affordability of MT-based preservatives and enhance their commercial viability.
- Addressing regulatory approvals and ensuring compliance with industry standards to facilitate MT’s adoption as a safe and sustainable post-harvest preservative. While MT is considered an eco-friendly preservative due to its natural occurrence in plants and relatively low toxicity, comprehensive life cycle assessments and comparative toxicity studies are still limited and warrant further investigation.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tripathi, S.K.; Tuteja, N. Integrated Signaling in Flower Senescence. Plant Signal. Behav. 2007, 2, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.S.; Jiang, C.-Z. Postharvest Biology and Technology of Cut Flowers and Potted Plants. Hortic. Rev. 2012, 4, 1–54. [Google Scholar]
- Fanourakis, D.; Pieruschka, R.; Savvides, A.; Macnish, A.J.; Sarlikioti, V.; Woltering, E.J. Sources of vase life variation in cut roses: A review. Postharvest Biol. Technol. 2013, 78, 1–15. [Google Scholar] [CrossRef]
- Shinde, S.P.; Chaudhari, S.R.; Matche, R.S. A way forward for a sustainable active packaging solution for prolonging the freshness and shelf life of Rosa hybrida L. cut flowers. Postharvest Biol. Technol. 2023, 204, 112475. [Google Scholar] [CrossRef]
- Sun, J.; Guo, H.; Tao, J. Effects of Harvest Stage, Storage, and Preservation Technology on Postharvest Ornamental Value of Cut Peony (Paeonia lactiflora) Flowers. Agronomy 2022, 12, 230. [Google Scholar] [CrossRef]
- Thakur, N. A review on the effect of storage methods and packaging material on the post-harvest longevity of cut flowers. Int. J. Chem. Stud. 2020, 8, 2375–2379. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Lim, J.H. Do Eco-Friendly Floral Preservative Solutions Prolong Vase Life Better than Chemical Solutions? Horticulturae 2021, 7, 415. [Google Scholar] [CrossRef]
- Chen, S. Review of Recent Advances in Chemical Preservatives for Cut Flowers. Br. J. Biol. Stud. 2024, 4, 1–8. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.-X.; Zhou, Z.; Cruz, M.H.C.; Fuentes-Broto, L.; Galano, A. Phytomelatonin: Assisting Plants to Survive and Thrive. Molecules 2015, 20, 7396–7437. [Google Scholar] [CrossRef]
- Liu, G.; Hu, Q.; Zhang, X.; Jiang, J.; Zhang, Y.; Zhang, Z. Melatonin biosynthesis and signal transduction in plants in response to environmental conditions. J. Exp. Bot. 2022, 73, 5818–5827. [Google Scholar] [CrossRef]
- Teng, Z.; Zheng, W.; Jiang, S.; Hong, S.-B.; Zhu, Z.; Zang, Y. Role of melatonin in promoting plant growth by regulating carbon assimilation and ATP accumulation. Plant Sci. 2022, 319, 111276. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Ali, S.; Manghwar, H.; Saqib, S.; Ullah, F.; Ayaz, A.; Zaman, W. Melatonin Function and Crosstalk with Other Phytohormones under Normal and Stressful Conditions. Genes 2022, 13, 1699. [Google Scholar] [CrossRef]
- Huang, S.; Jin, S. Melatonin Interaction with Other Phytohormones in the Regulation of Abiotic Stresses in Horticultural Plants. Antioxidants 2024, 13, 663. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Jia, M.; Wang, Y.; Lu, H.; Wang, X. The complexity of melatonin and other phytohormones crosstalk with other signaling molecules for drought tolerance in horticultural crops. Sci. Hortic. 2023, 321, 112348. [Google Scholar] [CrossRef]
- Ali, M.; Pan, Y.; Liu, H.; Cheng, Z. Melatonin interaction with abscisic acid in the regulation of abiotic stress in Solanaceae family plants. Front. Plant Sci. 2023, 14, 1271137. [Google Scholar] [CrossRef]
- Zhou, C.; Luo, L.; Miao, P.; Dong, Q.; Cheng, H.; Wang, Y.; Li, D.; Pan, C. A novel perspective to investigate how nanoselenium and melatonin lengthen the cut carnation vase shelf. Plant Physiol. Biochem. 2023, 196, 982–992. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, Y.; Wang, Y.; Zhang, L.; Li, L.; Looi, L.J.; Zhang, Z. The potential of melatonin and its crosstalk with other hormones in the fight against stress. Front. Plant Sci. 2024, 15, 1492036. [Google Scholar] [CrossRef]
- Yu, R.; Zuo, T.; Diao, P.; Fu, J.; Fan, Y.; Wang, Y.; Zhao, Q.; Ma, X.; Lu, W.; Li, A.; et al. Melatonin Enhances Seed Germination and Seedling Growth of Medicago sativa Under Salinity via a Putative Melatonin Receptor MsPMTR1. Front. Plant Sci. 2021, 12, 702875. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Jing, T.; Wang, Y.; Ai, X.; Bi, H. Melatonin delays leaf senescence and improves cucumber yield by modulating chlorophyll degradation and photoinhibition of PSII and PSI. Environ. Exp. Bot. 2022, 200, 104915. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin and its relationship to plant hormones. Ann. Bot. 2017, 121, 195–207. [Google Scholar] [CrossRef]
- Xu, T.; Chen, Y.; Kang, H. Melatonin Is a Potential Target for Improving Post-Harvest Preservation of Fruits and Vegetables. Front. Plant Sci. 2019, 10, 1388. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, X.; Sun, M.; Zhu, W.; Zheng, Y.; Zhu, S.; Chen, L.; Chen, X.; Teixeira da Silva, J.A.; Dong, G.; et al. Melatonin enhances vase life and alters physiological responses in peony (Paeonia lactiflora Pall.) cut flowers. Postharvest Biol. Technol. 2024, 212, 112896. [Google Scholar] [CrossRef]
- Gao, T.; Liu, X.; Tan, K.; Zhang, D.; Zhu, B.; Ma, F.; Li, C. Introducing melatonin to the horticultural industry: Physiological roles, potential applications, and challenges. Hortic. Res. 2022, 9, uhac094. [Google Scholar] [CrossRef] [PubMed]
- Jayarajan, S.; Sharma, R. Melatonin: A blooming biomolecule for postharvest management of perishable fruits and vegetables. Trends Food Sci. Technol. 2021, 116, 318–328. [Google Scholar] [CrossRef]
- Mandal, M.K.; Suren, H.; Ward, B.; Boroujerdi, A.; Kousik, C. Differential roles of melatonin in plant-host resistance and pathogen suppression in cucurbits. J. Pineal Res. 2018, 65, e12505. [Google Scholar] [CrossRef]
- Li, S.; Xu, Y.; Bi, Y.; Zhang, B.; Shen, S.; Jiang, T.; Zheng, X. Melatonin treatment inhibits gray mold and induces disease resistance in cherry tomato fruit during postharvest. Postharvest Biol. Technol. 2019, 157, 110962. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, Q.; Zhang, H.; Cao, Y.; Weeda, S.; Ren, S.; Guo, Y.-D. Melatonin improved tomato fruit quality by promoting ripening and reducing chilling injury. Postharvest Biol. Technol. 2016, 117, 68–75. [Google Scholar]
- Mazrou, R.M.; Hassan, S.; Yang, M.; Hassan, F.A.S. Melatonin Preserves the Postharvest Quality of Cut Roses through Enhancing the Antioxidant System. Plants 2022, 11, 2713. [Google Scholar] [CrossRef]
- Wang, B.; Huang, A.; Liu, L.; Li, Y.; Zhang, H.; Wang, L. Effects of an Exogenous Melatonin Treatment on the Physiological Indexes and Storage Duration of Cut Chrysanthemum Flowers. Hortic. Sci. Technol. 2024, 42, 533–548. [Google Scholar] [CrossRef]
- Trivellini, A.; Ferrante, A.; Mensuali, A.; Incrocci, L. Pulse-treatments with thidiazuron and melatonin improve quality and prolong vase-life of Ranunculus asiaticus L. cut flowers. Act. Hortic. 2024, 1397, 31–34. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Jannatizadeh, A.; Nojadeh, M.S.; Ebrahimzadeh, A. Exogenous melatonin ameliorates chilling injury in cut anthurium flowers during low temperature storage. Postharvest Biol. Technol. 2019, 148, 184–191. [Google Scholar] [CrossRef]
- Lezoul, N.E.H.; Serrano, M.; Ruiz-Aracil, M.C.; Belkadi, M.; Castillo, S.; Valero, D.; Guillén, F. Melatonin as a new postharvest treatment for increasing cut carnation (Dianthus caryophyllus L.) vase life. Postharvest Biol. Technol. 2022, 184, 111759. [Google Scholar] [CrossRef]
- Safaei Far, A.; Sadegh, M.-F.; Abdolhossein, R.N.; Feizollah, S.; Masoumeh, A.-M.; Fanourakis, D. Nano Silver and melatonin effectively delay the senescence of cut carnation flowers under simulated vibrational stress. J. Hortic. Sci. Biotechnol. 2024, 99, 597–608. [Google Scholar] [CrossRef]
- Yu, Z.; Li, S.; Hong, Y. Influence of Tea Polyphenols, Chitosan, and Melatonin as the Eco-Friendly Post-Harvest Treatments on the Vase Life of the Cut Chrysanthemum ‘Pingpong’ Group. Agriculture 2024, 14, 1507. [Google Scholar] [CrossRef]
- SeyedHajizadeh, H.; FarajiChelanolya, A.; Zahedi, S.M.; Moghadam, A.; Mahdavinia, G.; Kaya, O. Nanochitosan-encapsulated melatonin: An eco-friendly strategy to delay petal senescence in cut gerbera flowers. BMC Plant Biol. 2024, 24, 1024. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Tian, C.; Ren, X.; Zhang, X.; Xue, J.; Hao, R. A composite vase solution improved vase quality of cut peony after long-term cold storage by maintaining better physiological activities and enhancing the absorption of water. Postharvest Biol. Technol. 2024, 213, 112945. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Moosa, A.; Darras, A.; Nafees, M.; Ferrante, A.; Siddique, K.H.M. Preharvest melatonin foliar treatments enhance postharvest longevity of cut tuberose via altering physio-biochemical traits. Front. Plant Sci. 2023, 14, 1151722. [Google Scholar] [CrossRef]
- Xiang, D.; Nguyen, C.D.; Felter, L.; Clark, D.; Huo, H. The effects of preharvest LED light, melatonin and AVG treatments on the quality of postharvest snapdragon and vase life. J. Floric. Landsc. 2020, 6, 14–19. [Google Scholar] [CrossRef]
- Louredo de Brito, F.; Júnior, N.; Martins, L.; Martim, M.; Silva, L.; Guerra, T.; Simões, A. Application of melatonin and sucrose in prolonging the vase life of amaryllis cut flowers (Hippeastrum Hybridum Herb). Ornam. Hortic. 2023, 29, 489–499. [Google Scholar] [CrossRef]
- Zeinali Pour, N. Impact of Melatonin Treatment on Vase Life and Postharvest Quality of Eustomna grandiflorum cv. Grand white. In Proceedings of the First National Conference on Production and Postharvest Technology of Horticultural Plants, Birjand, Iran, 25 May 2022. [Google Scholar]
- Soleimani Aghdam, M.; Naderi, R.; Sarcheshmeh, M.A.A.; Babalar, M. Amelioration of postharvest chilling injury in anthurium cut flowers by γ-aminobutyric acid (GABA) treatments. Postharvest Biol. Technol. 2015, 110, 70–76. [Google Scholar] [CrossRef]
- Liang, L.; Deng, Y.; Sun, X.; Jia, X.; Su, J. Exogenous Nitric Oxide Pretreatment Enhances Chilling Tolerance of Anthurium. J. Amer Soc. Hort. Sci. 2018, 143, 3–13. [Google Scholar] [CrossRef]
- Ranjbar, A.; Ahmadi, N. Effects of 1-MCP and ethylene on antioxidant enzymes activity and postharvest physio-biochemical characterics of cut carnation flower cv. Fortune. Adv. Hortic. Sci. 2015, 29, 192–198. [Google Scholar]
- In, B.-C.; Ha, S.T.T.; Kim, Y.-T.; Lim, J.H. Relationship among floral scent intensity, ethylene sensitivity, and longevity of carnation flowers. Hortic. Environ. Biotechnol. 2021, 62, 907–916. [Google Scholar] [CrossRef]
- Yangkhamman, P.; Fukai, S.; Ichimura, K. Ethylene Production and Vase Life of Cut Carnation Flowers under High Temperature Conditions. Engei Gakkai Zasshi 2005, 74, 337–341. [Google Scholar] [CrossRef]
- Williams, M.H.; Nell, T.A.; Barrett, J.E. Investigation of proteins in petals of potted chrysanthemum as a potential indicator of longevity. Postharvest Biol. Technol. 1995, 5, 91–100. [Google Scholar] [CrossRef]
- Bartoli, C.G.; Simontacchi, M.; Montaldi, E.R.; Puntarulo, S. Oxidants and antioxidants during aging of chrysanthemum petals. Plant Sci. 1997, 129, 157–165. [Google Scholar] [CrossRef]
- Satoh, S.; Narumi, T.; Watanabe, M.; Aida, R.; Ohmiya, A. Reduced leaf senescence in chrysanthemum transformed with mutated ethylene receptor genes. Acta Hortic. 2007, 764, 89–94. [Google Scholar] [CrossRef]
- Liu, R.; Zuo, X.; Chen, Y.; Qian, Z.; Xu, C.; Wang, L.; Chen, S. Transcriptional Regulation in Leaves of Cut Chrysanthemum (Chrysanthemum morifolium) ‘FenDante’ in Response to Post-Harvest Ethylene Treatment. Horticulturae 2022, 8, 573. [Google Scholar] [CrossRef]
- Perik, R.R.J.; Razé, D.; Harkema, H.; Zhong, Y.; van Doorn, W.G. Bending in cut Gerbera jamesonii flowers relates to adverse water relations and lack of stem sclerenchyma development, not to expansion of the stem central cavity or stem elongation. Postharvest Biol. Technol. 2012, 74, 11–18. [Google Scholar] [CrossRef]
- Kamenetsky-Goldstein, R.; Yu, X. Cut peony industry: The first 30 years of research and new horizons. Acta Hortic. 2022, 9, uhac079. [Google Scholar] [CrossRef]
- Zhao, D.; Cheng, M.; Tang, W.; Liu, D.; Zhou, S.; Meng, J.; Tao, J. Nano-silver modifies the vase life of cut herbaceous peony (Paeonia lactiflora Pall.) flowers. Protoplasma 2018, 255, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yang, J.; Jeong, B.R. Synergistic Effects of Silicon and Preservative on Promoting Postharvest Performance of Cut Flowers of Peony (Paeonia lactiflora Pall.). Int. J. Mol. Sci. 2022, 23, 13211. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Luan, Y.; Shi, W.; Tang, Y.; Huang, X.; Tao, J. Melatonin enhances stem strength by increasing lignin content and secondary cell wall thickness in herbaceous peony. J. Exp. Bot. 2022, 73, 5974–5991. [Google Scholar] [CrossRef]
- Thi Ha, S.T.; Ham, J.Y.; Kim, Y.-T.; In, B.-C. Interaction between plant hormones in response to Botrytis cinerea infection in cut flowers with differential tissue sensitivity to ethylene. Postharvest Biol. Technol. 2025, 223, 113441. [Google Scholar] [CrossRef]
- Kumar, N.; Srivastava, G.; Dixit, K. Senescence in rose (Rosa hybrida L.): Role of the endogenous antioxidant system. J. Hortic. Sci. Biotechnol. 2008, 83, 125–131. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J.; Cano, A.; Reiter, R.J. Melatonin and Carbohydrate Metabolism in Plant Cells. Plants 2021, 10, 1917. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, N.; Wang, J.; Zhang, H.; Li, D.; Shi, J.; Li, R.; Weeda, S.; Zhao, B.; Ren, S.; et al. Melatonin promotes ripening and improves quality of tomato fruit during postharvest life. J. Exp. Bot. 2014, 66, 657–668. [Google Scholar] [CrossRef]
- Mubarok, S.; Suminar, E.; Abidat, A.H.; Setyawati, C.A.; Setiawan, E.; Buswar, A.S. Overview of Melatonin’s Impact on Postharvest Physiology and Quality of Fruits. Horticulturae 2023, 9, 586. [Google Scholar] [CrossRef]
- Samanta, S.; Roychoudhury, A. Crosstalk of melatonin with major phytohormones and growth regulators in mediating abiotic stress tolerance in plants. S. Afr. J. Bot. 2023, 163, 201–216. [Google Scholar] [CrossRef]
- Malakar, M.; Paiva, P.D.O.; Beruto, M.; da Cunha Neto, A.R. Review of recent advances in post-harvest techniques for tropical cut flowers and future prospects: Heliconia as a case-study. Front. Plant Sci. 2023, 14, 1221346. [Google Scholar] [CrossRef]
- Zeng, W.; Mostafa, S.; Lu, Z.; Jin, B. Melatonin-Mediated Abiotic Stress Tolerance in Plants. Front. Plant Sci. 2022, 13, 847175. [Google Scholar] [CrossRef] [PubMed]
- Kobylińska, A.; Borek, S.; Posmyk, M.M. Melatonin redirects carbohydrates metabolism during sugar starvation in plant cells. J. Pineal Res. 2018, 64, e12466. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Mehran, M.; Rehman, H.U.; Ullah, S.; Bakhsh, M.Z.M.; Tahira, M.; Maqsood, M.F.K.; Rauf, A.; Ghafar, S.; Haider, K.; et al. Mechanistic review of melatonin metabolism and signaling pathways in plants: Biosynthesis, regulation, and roles under abiotic stress. Plant Stress 2024, 14, 100685. [Google Scholar] [CrossRef]
- Wu, X.; Ren, J.; Huang, X.; Zheng, X.; Tian, Y.; Shi, L.; Dong, P.; Li, Z. Melatonin: Biosynthesis, content, and function in horticultural plants and potential application. Sci. Hortic. 2021, 288, 110392. [Google Scholar] [CrossRef]
- Pranil, T.; Moongngarm, A.; Loypimai, P. Influence of pH, temperature, and light on the stability of melatonin in aqueous solutions and fruit juices. Heliyon 2020, 6, e03648. [Google Scholar] [CrossRef]
- Canizo, B.V.; Jofré, M.F.; Mammana, S.B.; Dazat, R.E.; Silva, M.F.; Gomez, F.J.V. Stability of melatonin in eutectic systems: New avenues in therapeutic product development. J. Ion. Liq. 2024, 4, 100112. [Google Scholar] [CrossRef]
- Zhen, L.; Huang, Y.; Bi, X.; Gao, A.; Peng, L.; Chen, Y. Melatonin feeding changed the microbial diversity and metabolism of the broiler cecum. Front. Microbiol. 2024, 15, 1422272. [Google Scholar] [CrossRef]
| Cut Flowers | Variety | MT Treatment Concentration | Treatment Method and Duration | Effective of MT Treatment | Extended VL (days) | References |
|---|---|---|---|---|---|---|
| Anthurium (Anthurium andraeanum) | ‘Sirion’ | 1, 10, 100, and 100 μM | Dipping for 15 min at 20 °C and stored at 4 °C, 85–90% RH for 21 d | Reduced chilling injury symptoms by 11, 29, 51, and 31% at concentrations of 1, 10, 100, and 1000 μM | - | [31] |
| Carnations (Dianthus caryophyllus L.) | ‘Baltico’ | 0.01 mM | Vase solution | Delayed senescence and maintained initial levels of fresh weight, membrane stability index, and bioactive compounds and antioxidant activity for a longer time | 6.0 | [32] |
| 0.1 mM | 10.0 | |||||
| 1 mM | 2.0 | |||||
| - | 1 mg L−1 | Vase solution | Delayed senescence, enhanced the antioxidant ability, induced the biosynthesis of SA, JA, and ABA, and increased the amounts of key lignin biosynthesis pathway metabolites | 3.0 | [16] | |
| 1 mg L−1 MT + Nanoseleum 5 mg L−1 | 9.0 | |||||
| ‘White Liberty’ | 100 μM | Cut carnations were exposed to vibration (open and dark columns for 0 and 10 hz, respectively) for 10 min before holding in MT solution | Prolonged VL by both improving water balance, boosting antioxidant activity, and mitigating the negative effects of simulated vibrational stress | ~2.5–~4.0 | [33] | |
| Chrysanthemum (Chrysanthemum × morifolium) | ‘Yellow Pingpong’ | 0.02 mM 0.04 mM | Vase solution | MT maintained the best ornamental quality of the capitulum by decelerating fresh weight and flower diameter loss in terms of all varieties. | 3.0 4.2 | [34] |
| ‘White Pingpong’ | 0.02 mM 0.04 mM | 4.4 4.4 | ||||
| ‘Pink Pingpong’ | 0.02 mM 0.04 mM | 1.6 5.4 | ||||
| ‘Green Pingpong’ | 0.02 mM 0.04 mM | 0.6 1.2 | ||||
| Chrysanthemum morifolium R | 5 µM | Vase solution | Enhancing water balance, improving fresh weight and flower diameter, and regulating the antioxidant system. | 31.78% (~3.0 d) | [29] | |
| Gerbera (Gerbera jamesonii) | ‘Terra kalina’ | 0.1 mM | Vase solution | Extending VL and maintaining flower quality through enhanced preservation of cellular integrity, antioxidant activity, and biochemical parameters | 5.0 | [35] |
| 0.5 mM | 3.0 | |||||
| nCS-MT 0.1 mM | 8.7 | |||||
| nCS-MT 0.5 mM | 5.3 | |||||
| Peonies (Paeonia lactiflora Pall.) | ‘Qi Hua Lu Shang’ | 25 μM | Vase solution | Delaying senescence, improving water balance and flower diameter, reducing relative electrical conductivity and MDA content, and enhancing the activities of two antioxidant enzymes, SOD and CAT | 1.0 | [22] |
| ‘Da Fu Gui’ | 50 μM | 1.2 | ||||
| ‘Qi Hua Lu Shang’ | 1.6 | |||||
| ‘Da Fu Gui’ | 75 μM | 0.8 | ||||
| ‘Sarah’ | 0.1 mM | Addition of MT to the vase solution containing sucrose 0.5% | Enhanced the absorption of water by inducing the expression of PlPIP1;3 and PlTIP1;1. Delayed senescence by inhibiting the PlSAG39-like gene. Alleviated the damage to cell membranes and enhanced the antioxidant capacity | 1.6 | [36] | |
| 0.5 mM | 2.0 | |||||
| Cut roses (Rosa hybrida L.) | ‘First Red’ | 0.1 mM | Pulsing treatment for 30 min | Enhancing the antioxidant system and improving water content | ~3.0 | [28] |
| 0.2 mM | ~5.8 | |||||
| 0.3 mM | ~4.8 | |||||
| Tuberose cut flowers (Polianthes tuberosa L.) | ‘Single’ | MT 100 µM + Arsenic 50 µM | The treatment plan was implemented after twenty days of sprouting of tuberose bulbs. | Stimulating oxidative defense-related antioxidants and prolonging VL | 60.0% (~3.7) | [37] |
| Snapdragon (Antirrhinum majus L.) | ‘Snapshot White’ | 150 µM | After budding, snapdragons were sprayed with MT solutions | Increasing numbers and size of florets and extending VL | 1.3 | [38] |
| 200 µM | 2.3 | |||||
| Cut ranunculus flowers (Ranunculus asiaticus L.) | - | 100 µM | Pulse treatments for 24 h | Delaying senescence processes and reducing the ET biosynthesis or action | 8.0–9.0 | [30] |
| Amaryllis bulbs (Hippeastrum hybridum Herb) | ‘Minerva’ | 0.01 mM | Vase solution | Delaying the degradation of anthocyanin and flavonoid pigments and maintaining the stability of phenolic compounds and total carbohydrates | 2.0 | [39] |
| MT 0.01 mM + Sucrose 0.4% | 2.0 | |||||
| Lisianthus (Eustoma grandiflorum) | ‘Grand White’ | 1.5 µM | Spraying | Increasing VL and more relative weight gain and catalase enzyme activity compared to the control | 14 | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.; Ha, S. Melatonin: An Eco-Friendly Preservative for Improving Post-Harvest Quality and Longevity of Cut Flowers. Horticulturae 2025, 11, 574. https://doi.org/10.3390/horticulturae11060574
Nguyen T, Ha S. Melatonin: An Eco-Friendly Preservative for Improving Post-Harvest Quality and Longevity of Cut Flowers. Horticulturae. 2025; 11(6):574. https://doi.org/10.3390/horticulturae11060574
Chicago/Turabian StyleNguyen, Toan, and Suong Ha. 2025. "Melatonin: An Eco-Friendly Preservative for Improving Post-Harvest Quality and Longevity of Cut Flowers" Horticulturae 11, no. 6: 574. https://doi.org/10.3390/horticulturae11060574
APA StyleNguyen, T., & Ha, S. (2025). Melatonin: An Eco-Friendly Preservative for Improving Post-Harvest Quality and Longevity of Cut Flowers. Horticulturae, 11(6), 574. https://doi.org/10.3390/horticulturae11060574






