Physicochemical Properties and Biological Characteristics of Sargassum fusiforme Polysaccharides Prepared through Fermentation of Lactobacillus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Bacterial Strains
2.3. Preparation of Fermented SFPs
2.4. Physicochemical Characteristics of SFPs
2.4.1. Chemical Characteristics
2.4.2. Molecular Weight (MW) Analysis
2.4.3. Fourier Transform Infrared (FT-IR) Spectroscopy
2.5. Antioxidant Activity Analysis
2.5.1. DPPH Free Radical Scavenging Rate Measurement
2.5.2. ABTS Free Radical Scavenging Rate Measurement
2.6. Prebiotic Activity Analysis
2.7. Antioxidant Activity Study Based on Zebrafish Model
2.7.1. Maintenance of Zebrafish
2.7.2. Polysaccharide and AAPH Treated Embryos
2.7.3. Estimation of AAPH-Induced Intracellular Reactive Oxygen Species (ROS) Production Rate and Cell Mortality
fluorescence intensity of the treatment group/fluorescence intensity of the control
group × 100
2.7.4. Antioxidant Enzyme Activity Assay
2.8. Study on the Laxative Function Activity Based on Zebrafish Model
polysaccharide-treated group/fluorescence intensity of model control group) × 100%
2.9. Statistical Analysis
3. Results
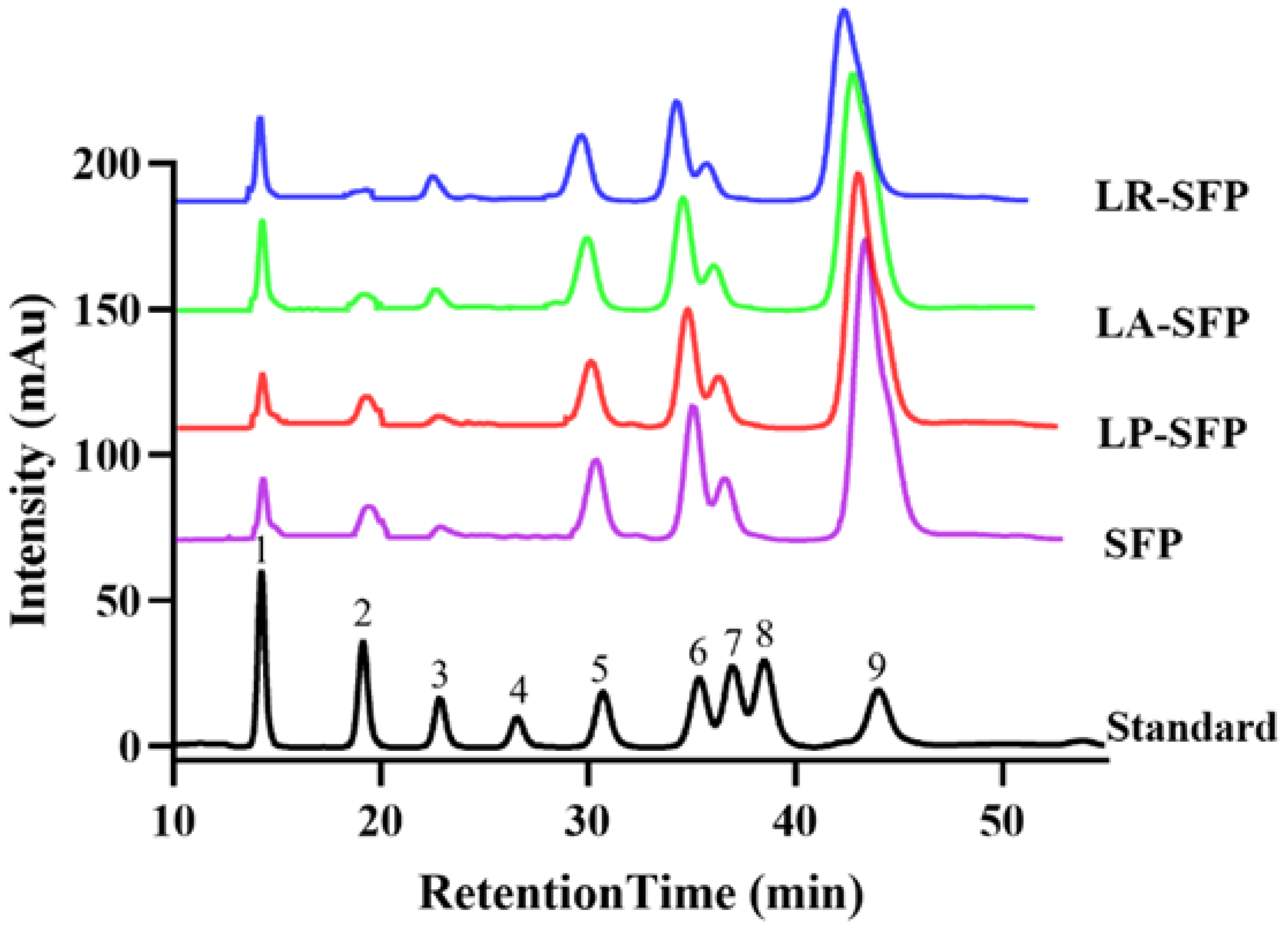
3.1. Preliminary Characterization of SFPs
3.2. Antioxidant Activity
3.2.1. In Vitro Antioxidant Activity
3.2.2. Antioxidant Activity in Zebrafish
3.3. Prebiotic Activity
3.4. Promotes Intestinal Motility Function
4. Discussion
4.1. Effect of Fermentation on the Physicochemical Properties of SFPs
4.2. Effect of Fermentation on the Activity of SFPs
4.2.1. Effect of Fermentation on the Antioxidant Activity of SFPs
4.2.2. Effect of Fermentation on the Prebiotic Activity of SFPs
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cong, Q.F.; Chen, H.J.; Liao, W.F.; Xiao, F.; Wang, P.P.; Qin, Y.; Dong, Q.; Ding, K. Structural characterization and effect on anti-angiogenic activity of a fucoidan from Sargassum fusiforme. Carbohydr. Polym. 2016, 136, 899–907. [Google Scholar] [CrossRef]
- Hu, P.; Li, Z.X.; Chen, M.C.; Sun, Z.L.; Ling, Y.; Jiang, J.; Huang, C.G. Structural elucidation and protective role of a polysaccharide from Sargassum fusiforme on ameliorating learning and memory deficiencies in mice. Carbohydr. Polym. 2016, 139, 150–158. [Google Scholar] [CrossRef]
- Chen, H.L.; Zhang, L.; Long, X.G.; Li, P.F.; Chen, S.C.; Kuang, W.; Guo, J.M. Sargassum fusiforme polysaccharides inhibit VEGF-A-related angiogenesis and proliferation of lung cancer in vitro and in vivo. Biomed. Pharmacother. 2017, 85, 22–27. [Google Scholar] [CrossRef]
- Liu, J.; Luthuli, S.; Yang, Y.; Cheng, Y.; Zhang, Y.; Wu, M.J.; Choi, J.I.; Tong, H.B. Therapeutic and nutraceutical potentials of a brown seaweed Sargassum fusiforme. Food Sci. Nutr. 2020, 8, 5195–5205. [Google Scholar] [CrossRef]
- Liu, J.; Wu, S.Y.; Chen, L.; Li, Q.J.; Shen, Y.Z.; Jin, L.; Zhang, X.; Chen, P.C.; Wu, M.J.; Choi, J.I.; et al. Different extraction methods bring about distinct physicochemical properties and antioxidant activities of Sargassum fusiforme fucoidans. Int. J. Biol. Macromol. 2020, 155, 1385–1392. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, X.X.; Tang, Y.X.; Mao, J.L. Composition, isolation, purification and biological activities of Sargassum fusiforme polysaccharides: A review. Carbohydr. Polym. 2020, 228, 115381. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Fan, S.T.; Huang, D.F.; Xiong, T.; Nie, S.P.; Xie, M.Y. Polysaccharides from fermented Asparagus officinalis with Lactobacillus plantarum NCU116 alleviated liver injury via modulation of glutathione homeostasis, bile acid metabolism, and SCFA production. Food Funct. 2020, 11, 7681–7695. [Google Scholar] [CrossRef]
- Tian, W.; Dai, L.; Lu, S.; Luo, Z.; Qiu, Z.; Li, J.; Li, P.; Du, B. Effect of Bacillus sp. DU-106 fermentation on Dendrobium officinale Chock for polysaccharide: Structure and immunoregulatory activities. Int. J. Biol. Macromol. 2019, 135, 1034–1042. [Google Scholar] [CrossRef]
- Kwaw, E.; Ma, Y.; Tchabo, W.; Apaliya, M.T.; Wu, M.; Sackey, A.S.; Xiao, L.; Tahir, H.E. Effect of lactobacillus strains on phenolic profile, color attributes and antioxidant activities of lactic-acid-fermented mulberry juice. Food Chem. 2018, 250, 148–154. [Google Scholar] [CrossRef]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef]
- Selhub, E.M.; Logan, A.C.; Bested, A.C. Fermented foods, microbiota, and mental health: Ancient practice meets nutritional psychiatry. J. Physiol. Anthropol. 2014, 33, 2. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Remize, F.; Poucheret, P. Fruits and vegetables, as a source of nutritional compounds and phytochemicals: Changes in bioactive compounds during lactic fermentation. Food Res. Int. 2018, 104, 86–99. [Google Scholar] [CrossRef]
- Moszak, M.; Szulinska, M.; Bogdanski, P. You Are What You Eat-The Relationship between Diet, Microbiota, and Metabolic Disorders—A Review. Nutrients 2020, 12, 1096. [Google Scholar] [CrossRef]
- Kolodziejczyk, A.A.; Zheng, D.; Elinav, E. Diet-microbiota interactions and personalized nutrition. Nat. Rev. Microbiol. 2019, 17, 742–753. [Google Scholar] [CrossRef]
- Kang, J.; Yin, S.J.; Liu, J.; Li, C.R.; Wang, N.F.; Sun, J.; Li, W.W.; He, J.; Guo, Q.B.; Cui, S.W. Fermentation models of dietary fibre in vitro and in vivo—A review. Food Hydrocolloid 2022, 131, 107685. [Google Scholar] [CrossRef]
- Huebner, J.; Wehling, R.L.; Parkhurst, A.; Hutkins, R.W. Effect of processing conditions on the prebiotic activity of commercial prebiotics. Int. Dairy J. 2008, 18, 287–293. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Shin, Z.U.; Mavlonov, G.T.; Abdurakhmonov, I.Y.; Yi, T.H. Solid-Phase Colorimetric Method for the Quantification of Fucoidan. Appl. Biochem. Biotechnol. 2012, 168, 1019–1024. [Google Scholar] [CrossRef]
- Shang, D.; Ning, J.; Zhao, Y.; Zhai, Y.; Shu, B.; Sheng, X.; Guo, Y. Establishment of the determination on kelp alginate. Food Sci. Technol. 2011, 36, 252–254. [Google Scholar] [CrossRef]
- Wang, Z.; Cai, T.; He, X. Characterization, sulfated modification and bioactivity of a novel polysaccharide from Millettia dielsiana. Int. J. Biol. Macrogol. 2018, 117, 108–115. [Google Scholar] [CrossRef]
- Jin, W.H.; Zhang, W.J.; Wang, J.; Ren, S.M.; Song, N.; Duan, D.L.; Zhang, Q.B. Characterization of laminaran and a highly sulfated polysaccharide from Sargassum fusiforme. Carbohydr. Res. 2014, 385, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Hwang, J.; Lee, S.G.; Jo, H.Y.; Oh, M.J.; Liyanage, N.M.; Je, J.G.; An, H.J.; Jeon, Y.J. Structural characteristics of sulfated polysaccharides from Sargassum horneri and immune-enhancing activity of polysaccharides combined with lactic acid bacteria. Food Funct. 2022, 13, 8214–8227. [Google Scholar] [CrossRef] [PubMed]
- Bursal, E.; Aras, A.; Kilic, O.; Taslimi, P.; Goren, A.C.; Gulcin, I. Phytochemical content, antioxidant activity, and enzyme inhibition effect of Salvia eriophora Boiss. & Kotschy against acetylcholinesterase, alpha-amylase, butyrylcholinesterase, and alpha-glycosidase enzymes. J. Food Biochem. 2019, 43, e12776. [Google Scholar] [CrossRef]
- Wang, X.; Huang, M.Y.; Yang, F.; Sun, H.J.; Zhou, X.X.; Wang, X.L.; Zhang, M.L. Rapeseed polysaccharides as prebiotics on growth and acidifying activity of probiotics in vitro. Carbohydr. Polym. 2015, 125, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.A.; Lee, S.H.; Ko, C.I.; Cha, S.H.; Kang, M.C.; Kang, S.M.; Ko, S.C.; Lee, W.W.; Ko, J.Y.; Lee, J.H.; et al. Protective effect of fucoidan against AAPH-induced oxidative stress in zebrafish model. Carbohydr. Polym. 2014, 102, 185–191. [Google Scholar] [CrossRef]
- Oh, J.Y.; Kim, E.A.; Kang, S.I.; Yang, H.W.; Ryu, B.; Wang, L.; Lee, J.S.; Jeon, Y.J. Protective Effects of Fucoidan Isolated from Celluclast-Assisted Extract of Undaria pinnatifida Sporophylls against AAPH-Induced Oxidative Stress In Vitro and In Vivo Zebrafish Model. Molecules 2020, 25, 2361. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Le, H.D.; Kim, T.N.T.; The, H.P.; Nguyen, T.M.; Cornet, V.; Lambert, J.M.; Kestemont, P. Anti-Inflammatory and Antioxidant Properties of the Ethanol Extract of Clerodendrum cyrtophyllum Turcz in Copper Sulfate-Induced Inflammation in Zebrafish. Antioxidants 2020, 9, 192. [Google Scholar] [CrossRef]
- Wang, T.; Dai, M.Z.; Liu, F.S.; Cao, B.B.; Guo, J.; Shen, J.Q.; Li, C.Q. Probiotics Modulate Intestinal Motility and Inflammation in Zebrafish Models. Zebrafish 2020, 17, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Guo, S.Y.; Zhang, Y.; Li, C.Q. Human prokinetic drugs promote gastrointestinal motility in zebrafish. Neurogastroenterol. Motil. 2014, 26, 589–595. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Zhang, R.; Jia, X.; Dong, L.; Ma, Q.; Zhao, D.; Sun, Z.; Zhang, M.; Huang, F. Variation in characterization and probiotic activities of polysaccharides from litchi pulp fermented for different times. Front. Nutr. 2022, 9, 993828. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Hong, R.Y.; Zhang, R.F.; Yi, Y.; Dong, L.H.; Liu, L.; Jia, X.C.; Ma, Y.X.; Zhang, M.W. Physicochemical and biological properties of longan pulp polysaccharides modified by Lactobacillus fermentum fermentation. Int. J. Biol. Macromol. 2019, 125, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Samarakoon, K.W.; Lee, W.W.; Kim, H.S.; Kang, N.; Ranasinghe, P.; Lee, H.S.; Jeon, Y.J. A fucoidan fraction purified from Chnoospora minima; a potential inhibitor of LPS-induced inflammatory responses. Int. J. Biol. Macromol. 2017, 104, 1185–1193. [Google Scholar] [CrossRef]
- Wu, S.Y.; Zhang, X.; Liu, J.; Song, J.X.; Yu, P.; Chen, P.C.; Liao, Z.Y.; Wu, M.J.; Tong, H.B. Physicochemical characterization of Sargassum fusiforme fucoidan fractions and their antagonistic effect against P-selectin-mediated cell adhesion. Int. J. Biol. Macromol. 2019, 133, 656–662. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Fernando, I.P.S.; Lee, W.W.; Sanjeewa, K.K.A.; Kim, H.-S.; Lee, D.-S.; Jeon, Y.-J. Isolation and purification of fucoidan fraction in Turbinaria ornata from the Maldives; Inflammation inhibitory potential under LPS stimulated conditions in in-vitro and in-vivo models. Int. J. Biol. Macromol. 2019, 131, 614–623. [Google Scholar] [CrossRef]
- Chale-Dzul, J.; Moo-Puc, R.; Robledo, D.; Freile-Pelegrin, Y. Hepatoprotective effect of the fucoidan from the brown seaweed Turbinaria tricostata. J. Appl. Phycol. 2015, 27, 2123–2135. [Google Scholar] [CrossRef]
- Xie, L.M.; Shen, M.Y.; Wang, Z.J.; Xie, J.H. Structure, function and food applications of carboxymethylated polysaccharides: A comprehensive review. Trends Food Sci. Tech. 2021, 118, 539–557. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Wang, L.; Sanjeewa, K.K.A.; Kang, S.I.; Lee, J.S.; Jeon, Y.J. Antioxidant Potential of Sulfated Polysaccharides from Padina boryana; Protective Effect against Oxidative Stress in In Vitro and In Vivo Zebrafish Model. Mar. Drugs 2020, 18, 212. [Google Scholar] [CrossRef]
- Chen, P.L.; You, Q.X.; Li, X.; Chang, Q.; Zhang, Y.; Zheng, B.D.; Hu, X.K.; Zeng, H.L. Polysaccharide fractions from Fortunella margarita affect proliferation of Bifidobacterium adolescentis ATCC 15703 and undergo structural changes following fermentation. Int. J. Biol. Macromol. 2019, 123, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Pardal, D.; Caro, M.; Tueros, I.; Barranco, A.; Navarro, V. Resveratrol and Piceid Metabolites and Their Fat-Reduction Effects in Zebrafish Larvae. Zebrafish 2014, 11, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Akbari-Alavijeh, S.; Soleimanian-Zad, S.; Sheikh-Zeinoddin, M.; Hashmi, S. Pistachio hull water-soluble polysaccharides as a novel prebiotic agent. Int. J. Biol. Macromol. 2018, 107, 808–816. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Wang, R.F.; Wang, Y.; An, X.P.; Liu, N.; Song, M.; Yang, Y.P.; Yin, N.; Qi, J.W. Characterization and antioxidant activity of wheat bran polysaccharides modified by Saccharomyces cerevisiae and Bacillus subtilis fermentation. J. Cereal Sci. 2021, 97, 103157. [Google Scholar] [CrossRef]
- Filannino, P.; Di Cagno, R.; Gobbetti, M. Metabolic and functional paths of lactic acid bacteria in plant foods: Get out of the labyrinth. Curr. Opin. Biotechnol. 2018, 49, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Hong, R.; Zhang, R.; Dong, L.; Bai, Y.; Liu, L.; Jia, X.; Wang, G.; Zhang, M. Dynamic variation in biochemical properties and prebiotic activities of polysaccharides from longan pulp during fermentation process. Int. J. Biol. Macromol. 2019, 132, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Cao, X.J.; Zhuang, X.H.; Han, W.; Guo, W.Q.; Xiong, J.; Zhang, X.L. Rice bran polysaccharides and oligosaccharides modified by Grifola frondosa fermentation: Antioxidant activities and effects on the production of NO. Food Chem. 2017, 223, 49–53. [Google Scholar] [CrossRef]
- Song, S.; Liu, X.Y.; Zhao, B.T.; Abubaker, M.A.; Huang, Y.L.; Zhang, J. Effects of Lactobacillus plantarum Fermentation on the Chemical Structure and Antioxidant Activity of Polysaccharides from Bulbs of Lanzhou Lily. ACS Omega 2021, 6, 29839–29851. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.Y.; Kim, Y.C.; Choi, I.; Kim, G.B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Calderon Santoyo, M.; Loiseau, G.; Rodriguez Sanoja, R.; Guyot, J.P. Study of starch fermentation at low pH by Lactobacillus fermentum Ogi E1 reveals uncoupling between growth and alpha-amylase production at pH 4.0. Int. J. Food Microbiol. 2003, 80, 77–87. [Google Scholar] [CrossRef]
- Cai, L.L.; Zou, S.S.; Liang, D.P.; Luan, L.B. Structural characterization, antioxidant and hepatoprotective activities of polysaccharides from Sophorae tonkinensis Radix. Carbohydr. Polym. 2018, 184, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Q.B.; Zhang, Z.S.; Song, H.F.; Li, P.C. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2010, 46, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Jahanbin, K.; Gohari, A.R.; Moini, S.; Emam-Djomeh, Z.; Masi, P. Isolation, structural characterization and antioxidant activity of a new water-soluble polysaccharide from Acanthophyllum bracteatum roots. Int. J. Biol. Macromol. 2011, 49, 567–572. [Google Scholar] [CrossRef]
- Chen, H.; Shi, X.Q.; Cen, L.Y.; Zhang, L.; Dai, Y.F.; Qiu, S.Y.; Zeng, X.Y.; Wei, C.Y. Effect of Yeast Fermentation on the Physicochemical Properties and Bioactivities of Polysaccharides of Dendrobium officinale. Foods 2023, 12, 150. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Q.B.; Zhang, Z.S.; Li, Z. Antioxidant activity of sulfated polysaccharide fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2008, 42, 127–132. [Google Scholar] [CrossRef]
- Tao, Y.Z.; Zhang, L.; Cheung, P.C.K. Physicochemical properties and antitumor activities of water-soluble native and sulfated hyperbranched mushroom polysaccharides. Carbohydr. Res. 2006, 341, 2261–2269. [Google Scholar] [CrossRef]
- Falch, B.H.; Espevik, T.; Ryan, L.; Stokke, B.T. The cytokine stimulating activity of (1 fwdarw 3)-beta-D-glucans is dependent on the triple helix conformation. Carbohydr. Res. 2000, 329, 587–596. [Google Scholar] [CrossRef]
- Xing, R.E.; Liu, S.; Guo, Z.Y.; Yu, H.H.; Wang, P.B.; Li, C.P.; Li, Z.; Li, P.C. Relevance of molecular weight of chitosan and its derivatives and their antioxidant activities in vitro. Bioorganic Med. Chem. 2005, 13, 1573–1577. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H.; Liu, Q.; Feng, Y.; Wu, D.; Zhang, X.; Zhang, L.; Li, S.; Tang, F.; Liu, Q.; et al. Ultrasonic Treatment Enhances the Antioxidant and Immune-Stimulatory Properties of the Polysaccharide from Sinopodophyllum hexandrum Fruit. Foods 2023, 12, 910. [Google Scholar] [CrossRef]
- Leng, X.; Miao, W.; Li, J.; Liu, Y.; Zhao, W.; Mu, Q.; Li, Q. Physicochemical characteristics and biological activities of grape polysaccharides collected from different cultivars. Food Res. Int. 2023, 163, 112161. [Google Scholar] [CrossRef]
- Cheng, H.R.; Feng, S.L.; Jia, X.J.; Li, Q.Q.; Zhou, Y.H.; Ding, C.B. Structural characterization and antioxidant activities of polysaccharides extracted from Epimedium acuminatum. Carbohydr. Polym. 2013, 92, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.J.; Klaenhammer, T.R. Genetic Mechanisms of Prebiotic Oligosaccharide Metabolism in Probiotic Microbes. Annu. Rev. Food Sci. Technol. 2015, 6, 137–156. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Dong, S.Y.; Gao, J.; Jiang, C.Y. In vitro fermentation of sulfated polysaccharides from E. prolifera and L. japonica by human fecal microbiota. Int. J. Biol. Macromol. 2016, 91, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Steed, H.; Macfarlane, S. Bacterial metabolism and health-related effects of galacto-oligosaccharides and other prebiotics. J. Appl. Microbiol. 2008, 104, 305–344. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Y.; Dou, Z.M.; Chen, C.; Jiang, Y.M.; Yang, B.; Fu, X. Study on the Effect of Molecular Weight on the Gut Microbiota Fermentation Properties of Blackberry Polysaccharides In Vitro. J. Agric. Food Chem. 2022, 70, 11245–11257. [Google Scholar] [CrossRef]
- Chen, L.; Guo, Y.; Hu, C.; Lam, P.K.S.; Lam, J.C.W.; Zhou, B. Dysbiosis of gut microbiota by chronic coexposure to titanium dioxide nanoparticles and bisphenol A: Implications for host health in zebrafish. Environ. Pollut. 2018, 234, 307–317. [Google Scholar] [CrossRef]
- Mantegazza, C.; Molinari, P.; D’Auria, E.; Sonnino, M.; Morelli, L.; Zuccotti, G.V. Probiotics and antibiotic-associated diarrhea in children: A review and new evidence on Lactobacillus rhamnosus GG during and after antibiotic treatment. Pharmacol. Res. 2018, 128, 63–72. [Google Scholar] [CrossRef]






| Sample | Total Sugar (%) | Sulphate (%) | Uronic Acid (%) | Alginate (%) | Fucoidan (%) | Protein (%) | MW (KDa) |
|---|---|---|---|---|---|---|---|
| SFP | 21.23 ± 0.35 a | 5.61 ± 0.06 bc | 13.41 ± 0.22 c | 22.69 ± 0.14 a | 11.11 ± 0.29 d | 0.31 ± 0.08 b | 27.21 ± 0.03 a |
| LP-SFP | 21.04 ± 0.48 a | 5.79 ± 0.28 b | 16.07 ± 1.27 b | 18.22 ± 0.15 c | 12.73 ± 0.60 c | 0.23 ± 0.16 b | 26.64 ± 0.11 c |
| LA-SFP | 14.31 ± 2.66 c | 5.45 ± 0.06 c | 15.90 ± 0.54 b | 18.83 ± 0.10 b | 16.92 ± 0.44 b | 0.93 ± 0.07 a | 26.86 ± 0.09 b |
| LR-SFP | 17.78 ± 1.65 b | 6.75 ± 0.16 a | 22.14 ± 0.88 a | 18.80 ± 0.08 b | 19.02 ± 0.44 a | 0.79 ± 0.04 a | 27.08 ± 0.04 a |
| Sample | Monosaccharide Composition (%) | ||||||
|---|---|---|---|---|---|---|---|
| Mannose | Rhamnose | Glucuronic Acid | Glucose | Galactose | Xylose | Fucose | |
| SFP | 2.88 | 2.51 | 0.81 | 8.79 | 16.25 | 8.25 | 60.51 |
| LP-SFP | 2.85 | 2.72 | 0.93 | 8.15 | 16.63 | 7.95 | 60.78 |
| LA-SFP | 6.07 | 1.22 | 2.14 | 10.76 | 15.62 | 6.79 | 59.86 |
| LR-SFP | 7.14 | 0.93 | 3.59 | 14.04 | 21.29 | 8.69 | 61.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Ouyang, D.; Song, J.; Chen, C.; Yin, C.; Su, L.; Wu, M. Physicochemical Properties and Biological Characteristics of Sargassum fusiforme Polysaccharides Prepared through Fermentation of Lactobacillus. Fermentation 2023, 9, 835. https://doi.org/10.3390/fermentation9090835
Yang Y, Ouyang D, Song J, Chen C, Yin C, Su L, Wu M. Physicochemical Properties and Biological Characteristics of Sargassum fusiforme Polysaccharides Prepared through Fermentation of Lactobacillus. Fermentation. 2023; 9(9):835. https://doi.org/10.3390/fermentation9090835
Chicago/Turabian StyleYang, Ying, Dan Ouyang, Jiayao Song, Chunyang Chen, Chenjing Yin, Laijin Su, and Mingjiang Wu. 2023. "Physicochemical Properties and Biological Characteristics of Sargassum fusiforme Polysaccharides Prepared through Fermentation of Lactobacillus" Fermentation 9, no. 9: 835. https://doi.org/10.3390/fermentation9090835
APA StyleYang, Y., Ouyang, D., Song, J., Chen, C., Yin, C., Su, L., & Wu, M. (2023). Physicochemical Properties and Biological Characteristics of Sargassum fusiforme Polysaccharides Prepared through Fermentation of Lactobacillus. Fermentation, 9(9), 835. https://doi.org/10.3390/fermentation9090835






