Enzymatic Hydrolysis of Kraft and Sulfite Pulps: What Is the Best Cellulosic Substrate for Industrial Saccharification?
Abstract
1. Introduction
2. Materials and Methods
2.1. Cellulosic Pulps
2.2. Enzymes
2.3. Enzymatic Hydrolysis of Pulp Substrates
2.4. Hydrolysate Analyses
2.4.1. Determination of Reducing Sugars, Glucose, and Minor Soluble Sugars
2.4.2. Gravimetrical Analysis of Non-Hydrolyzed Residue
2.5. Pulp Fiber Characterisation prior and after Enzymatic Hydrolysis
3. Results
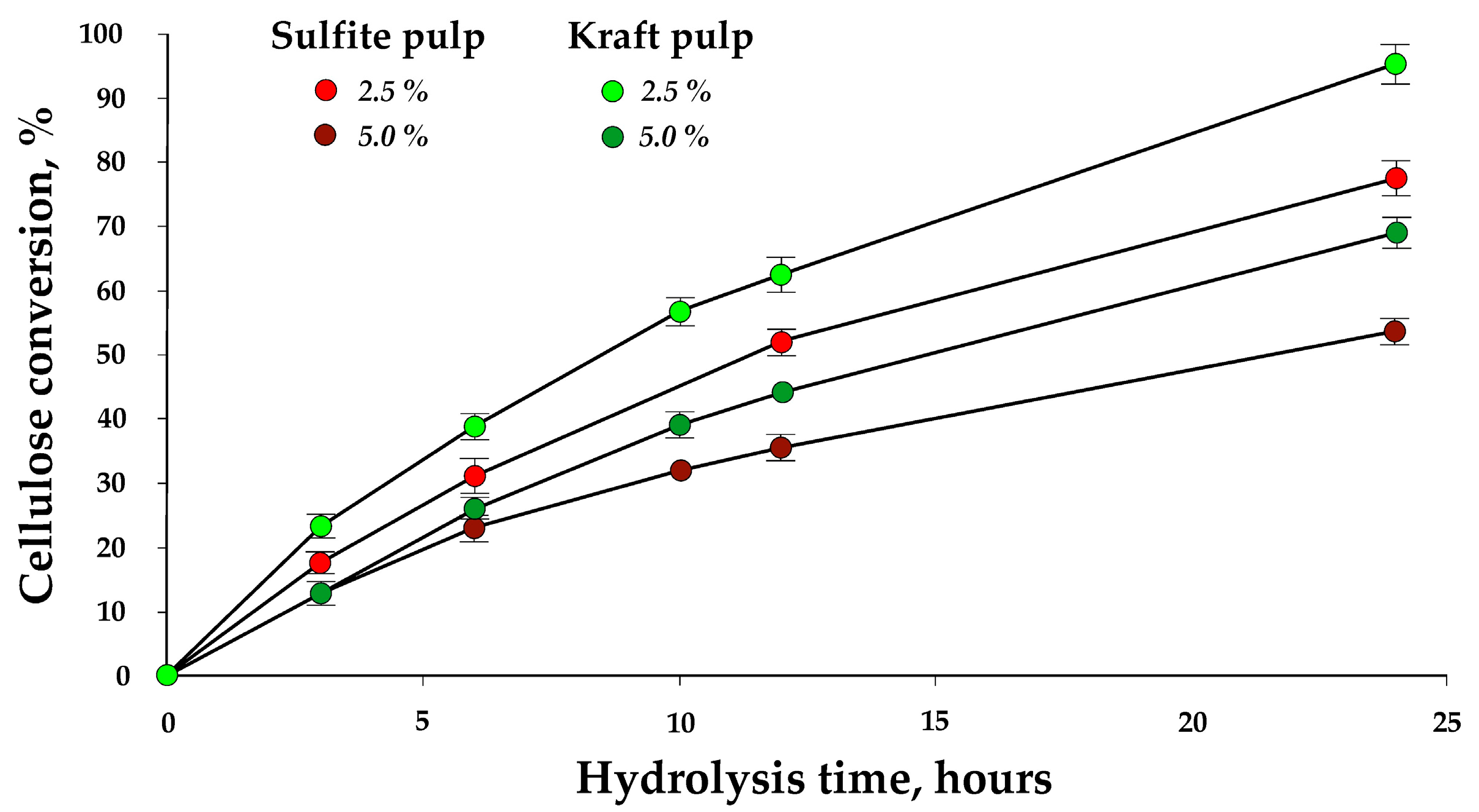
3.1. Enzymatic Hydrolysis at Low Pulp Concentrations
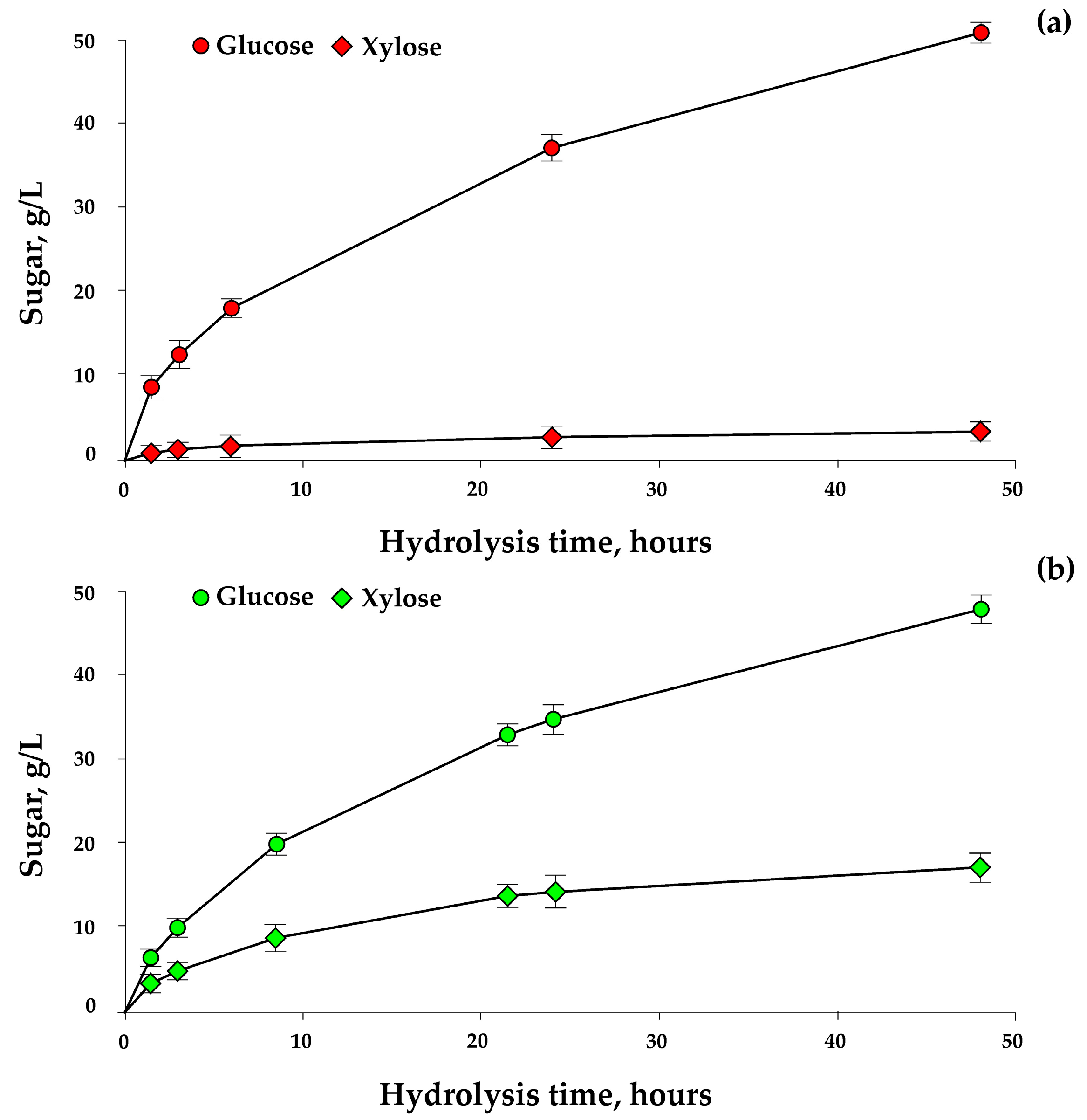
3.2. Enzymatic Hydrolysis at High Pulp Concentrations
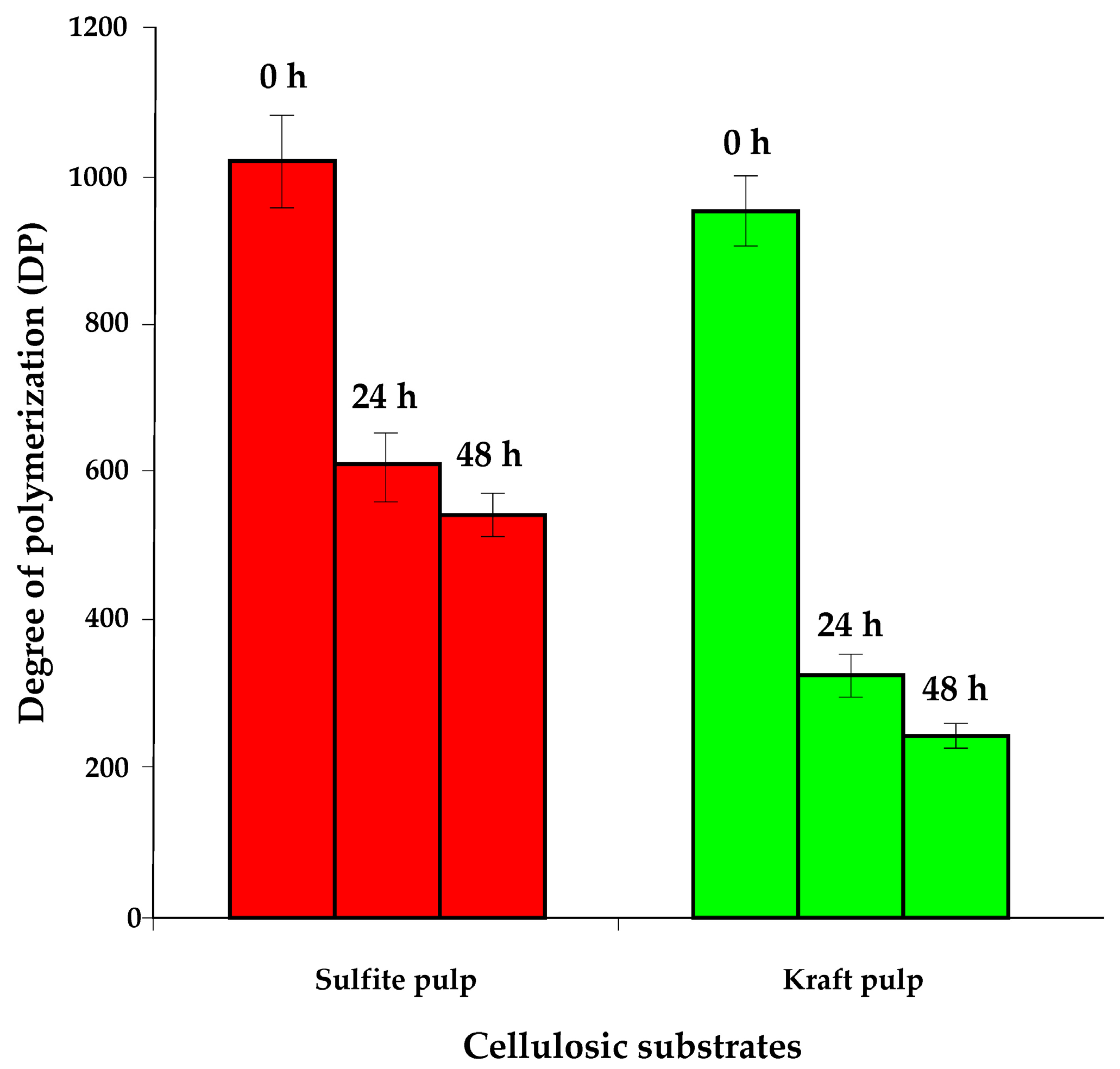
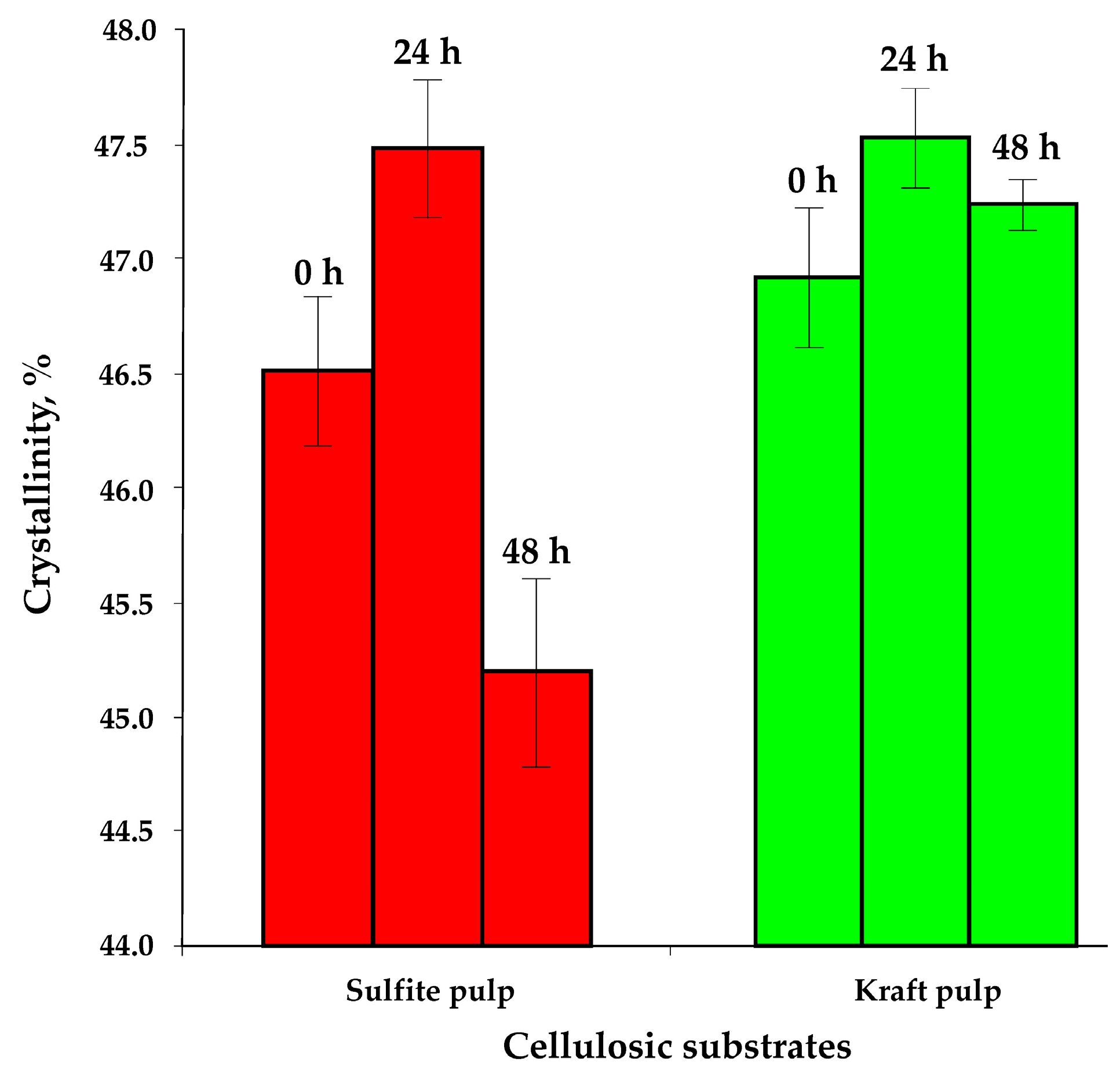
3.3. Transformation of Non-Hydrolysed Residue
4. Discussion
4.1. Feasibility of Industrial Pulping for Enzymatic Hydrolysis of Wood Polysaccharides
4.2. Characterization of Hydrolysis Products of Commercial Pulps and Their Potential Application
4.3. Complex Processing of Industrial Pulps Including Enzymatic Hydrolysis and Microbial Fermentation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, M.; Li, J.; Wang, S.; Zhao, F.; Zhang, C.; Zhang, C.; Chunhui, Z.; Han, S. Status and trends of enzyme cocktails for efficient and ecological production in the pulp and paper industry. J. Clean. Prod. 2023, 418, 138196. [Google Scholar] [CrossRef]
- Chatterjee, A.; Puri, S.; Sharma, P.K.; Deepa, P.R.; Chowdhury, S. Nature-inspired Enzyme engineering and sustainable catalysis: Biochemical clues from the world of plants and extremophiles. Front. Bioeng. Biotech 2023, 11, 1229300. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, C.; Reyes-Sosa, F.M.; Díez, B. Enzymatic hydrolysis of biomass from wood. Microb. Biotech. 2016, 9, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Zoghlami, A.; Paës, G. Lignocellulosic biomass: Understanding recalcitrance and predicting hydrolysis. Front. Chem. 2019, 7, 874. [Google Scholar] [CrossRef] [PubMed]
- Li, W.C.; Han, L.J.; Peng, T.B.; Xie, Y.Y.; Zou, Y.; Li, L.Z.; Jia, S.R.; Zhong, C. Structural and behavior changes of herbaceous and hardwood biomass during steam explosion pretreatment and enzymatic hydrolysis. BioResources 2020, 15, 691–705. [Google Scholar] [CrossRef]
- Mikulski, D.; Kłosowski, G. High-pressure microwave-assisted pretreatment of softwood, hardwood and non-wood biomass using different solvents in the production of cellulosic ethanol. Biotechnol. Biofuels 2023, 16, 19. [Google Scholar] [CrossRef] [PubMed]
- Lehto, J.T.; Alén, R.J. Chemical pretreatments of wood chips prior to alkaline pulping—A review of pretreatment alternatives, chemical aspects of the resulting liquors, and pulping outcomes. BioResources 2015, 10, 8604–8656. [Google Scholar] [CrossRef]
- Civzele, A.; Stipniece-Jekimova, A.A.; Mezule, L. Fungal ligninolytic enzymes and their application in biomass lignin pretreatment. J. Fungi 2023, 9, 780. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, S.; Sun, X.Y.; Yuan, T.Q. Biological treatment of poplar wood with white-rot fungus Trametes hirsuta C7784: Structural elucidation of the whole lignin in treated wood. BioResources 2016, 11, 7305–7321. [Google Scholar] [CrossRef][Green Version]
- Kim, S.M.; Dien, B.S.; Singh, V. Erratum to: Promise of combined hydrothermal/chemical and mechanical refining for pretreatment of woody and herbaceous biomass. Biotechnol. Biofuels 2016, 9, 263. [Google Scholar] [CrossRef]
- El Hage, M.; Louka, N.; Rezzoug, S.-A.; Maugard, T.; Sablé, S.; Koubaa, M.; Debs, E.; Maache-Rezzoug, Z. Bioethanol production from woody biomass: Recent advances on the effect of pretreatments on the bioconversion process and energy yield aspects. Energies 2023, 16, 5052. [Google Scholar] [CrossRef]
- Valamonfared, J.; Javanmard, A.S.; Ghaedi, M.; Bagherinasab, M. Bioethanol production using lignocellulosic materials and thermophilic microbial hydrolysis. Biomass Conv. Bioref. 2023, 1–13. [Google Scholar] [CrossRef]
- Novozhilov, E.V.; Sinelnikov, I.G.; Aksenov, A.S.; Chukchin, D.G.; Tyshkunova, I.V.; Rozhkova, A.M.; Osipov, D.O.; Zorov, I.N.; Sinitsyn, A.P. Biocatalytic conversion of kraft pulp using cellulase complex of Penicillium verruculosum. Catal. Ind. 2016, 8, 95–100. [Google Scholar] [CrossRef]
- Novozhilov, E.V.; Aksenov, A.S.; Demidov, M.L.; Chukhchin, D.G.; Dotsenko, G.S.; Osipov, D.O.; Sinitsyn, A.P. Application of complex biocatalysts based on recombinant Penicillium verruculosum enzyme preparations in the hydrolysis of semichemical hardwood pulp. Catal. Ind. 2014, 6, 348–354. [Google Scholar] [CrossRef]
- Aksenov, A.S.; Tyshkunova, I.V.; Poshina, D.N.; Guryanova, A.A.; Chukhchin, D.G.; Sinelnikov, I.G.; Terentyev, K.Y.; Skorik, Y.A.; Novozhilov, E.V.; Synitsyn, A.P. Biocatalysis of industrial kraft pulps: Similarities and differences between hardwood and softwood pulps in hydrolysis by enzyme complex of Penicillium verruculosum. Catalysts 2020, 10, 536. [Google Scholar] [CrossRef]
- Buzała, K.; Przybysz, P.; Rosicka-Kaczmarek, J.; Kalinowska, H. Production of glucose-rich enzymatic hydrolysates from cellulosic pulps. Cellulose 2015, 22, 663–674. [Google Scholar] [CrossRef]
- Przybysz Buzała, K.; Kalinowska, H.; Przybysz, P.; Malachowska, E. Conversion of various types of lignocellulosic biomass to fermentable sugars using kraft pulping and enzymatic hydrolysis. Wood Sci. Technol. 2017, 51, 873–885. [Google Scholar] [CrossRef]
- Kafle, K.; Shin, H.; Lee, C.M.; Park, S.; Kim, S.H. Progressive structural changes of Avicel, bleached softwood and bacterial cellulose during enzymatic hydrolysis. Sci. Rep. 2015, 5, 15102. [Google Scholar] [CrossRef]
- Annergren, G.; Germgard, U. Process aspects for sulfite pulping. Appita Technol. Innov. Manufact. Environ. 2014, 67, 270–276. [Google Scholar]
- Poshina, D.; Novozhilov, E. Modification of spruce sulphite pulp by cellulase treatment. Cellul. Chem. Technol 2015, 49, 187–194. [Google Scholar]
- Huang, C.; Li, R.; Tang, W.; Zheng, Y.; Meng, X. Improve Enzymatic Hydrolysis of Lignocellulosic Biomass by Modifying Lignin Structure via Sulfite Pretreatment and Using Lignin Blockers. Fermentation 2022, 8, 558. [Google Scholar] [CrossRef]
- Rozhkova, A.M.; Gusakov, A.V.; Dotsenko, A.S.; Sinitsyna, O.A.; Sinitsyn, A.P. Capabilities of the ascomycete fungus Penicillium verruculosum and its enzymes for conversion of cellulosic feedstock. In Liquid Biofuels: Bioethanol; Soccol, C.R., Pereira, G.A.G., Luciana, C.-G.D., Vandenberghe, P.S., Eds.; Springer International Publishing: Cham, Switzerland, 2022; Volume 12, pp. 243–266. [Google Scholar] [CrossRef]
- Ogunyewo, O.A.; Upadhyay, P.; Rajacharya, G.H.; Okereke, O.E.; Faas, L.; Gómez, L.D.; McQueen-Mason, S.J.; Yazdani, S.S. Accessory enzymes of hypercellulolytic Penicillium funiculosum facilitate complete saccharification of sugarcane bagasse. Biotechnol. Biofuels 2021, 14, 171. [Google Scholar] [CrossRef] [PubMed]
- Gusakov, A.V. Alternatives to Trichoderma reesei in biofuel production. Trends Biotech. 2011, 29, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Korotkova, O.G.; Rozhkova, A.M.; Rubtsova, E.A.; Sinitsyn, A.P. Cellulases from mycelial fungi Penicillium verruculosum as a real alternative to Trichoderma enzymes in industrial hydrolysis of cellulosic biomass. In From Biomass to Biobased Products; Jacob-Lopes, E., Queiroz Zepka, L., Rodrigues Dias, R., Eds.; IntechOpen: London, UK, 2023; p. 19. Available online: https://www.intechopen.com/online-first/87268 (accessed on 3 June 2023).
- Osipov, D.O.; Dotsenko, G.S.; Sinitsyna, O.A.; Kondratieva, E.G.; Zorov, I.N.; Shashkov, I.A.; Satrutdinov, A.D.; Sinitsyn, A.P. Comparative study of the convertibility of agricultural residues and other cellulose-containing materials in hydrolysis with Penicillium verruculosum cellulase complex. Agronomy 2020, 10, 1712. [Google Scholar] [CrossRef]
- Shevchenko, A.R.; Tyshkunova, I.V.; Chukhchin, D.G.; Malkov, A.V.; Toptunov, E.A.; Telitsin, V.D.; Rozhkova, A.M.; Sinitsyna, O.A.; Gofman, I.V.; Aksenov, A.S. Production of biomodified bleached kraft pulp by catalytic conversion using Penicillium verruculosum enzymes: Composition, properties, structure and application. Catalysts 2023, 13, 103. [Google Scholar] [CrossRef]
- Korotkova, O.G.; Rozhkova, A.M.; Kislitsin, V.Y.; Sinitsyna, O.A.; Denisenko, Y.A.; Marorochkina, M.A.; Zorov, I.N.; Shashkov, I.A.; Satrudinov, A.D.; Sinytsyn, A.P. New feed enzyme preparations for the destruction of nonstarch polysaccharides and phytates. Moscow Univ. Chem. Bull. 2023, 78, 63–68. [Google Scholar] [CrossRef]
- Sinitsyn, A.P.; Sinitsyna, O.A.; Zorov, I.N.; Rozhkova, A.M. Carbohydrases: 50 years of research at the department of chemical enzymology of Moscow state university—History and prospects. Moscow Univ. Chem. Bull. 2023, 78, 170–186. [Google Scholar] [CrossRef]
- Branco, R.H.R.; Amândio, M.S.T.; Serafim, L.S.; Xavier, A.M.R.B. Ethanol production from hydrolyzed kraft pulp by mono- and co-cultures of yeasts: The challenge of C6 and C5 sugars consumption. Energies 2020, 13, 744. [Google Scholar] [CrossRef]
- Kerssemakers, A.A.J.; Doménech, P.; Cassano, M.; Yamakawa, C.K.; Dragone, G.; Mussatto, S.I. Production of itaconic acid from cellulose pulp: Feedstock feasibility and process strategies for an efficient microbial performance. Energies 2020, 13, 1654. [Google Scholar] [CrossRef]
- Júnior, A.I.M.; Soccol, C.R.; Camara, M.C.; Aulestia, D.T.M.; de Souza Vandenberghe, L.P.; de Carvalho, J.C. Challenges in the production of second-generation organic acids (potential monomers for application in biopolymers). Biomass Bioenergy 2021, 149, 106092. [Google Scholar] [CrossRef]
- Shevchenko, A.; Aksenov, A.; Tyshkunova, I.; Falev, D.; Toptunov, E. Products of hydrolysis of bleached hardwood kraft pulp by carbohydrate-active enzymes. In The International Conference on Advances in Emerging Trends and Technologies; Springer: Cham, Switzerland, 2021; pp. 114–123. [Google Scholar] [CrossRef]
- Yang, T.; Li, X.; Xu, N.; Guo, Y.; Liu, G.; Zhao, J. Preparation of cellulose nanocrystals from commercial dissolving pulp using an engineered cellulose system. Bioresour. Bioprocess. 2023, 10, 42. [Google Scholar] [CrossRef]
- Sinitsyn, A.P.; Korotkova, O.G.; Rubtsova, E.A.; Sinitsyna, O.A.; Kondrat’eva, E.G.; Sereda, A.S.; Zorov, I.N.; Rozhkova, A.M. Construction of recombinant producers of enzyme preparations for feed production with an expression system based on Penicillium verruculosum fungus. Appl. Biochem. Microbiol. 2020, 56, 875–880. [Google Scholar] [CrossRef]
- Ghose, T.K. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Willför, S.; Pranovich, A.; Tamminen, T.; Puls, J.; Laine, C.; Suurnäkki, A.; Saake, B.; Uotila, K.; Simolin, H.; Hemming, J.; et al. Carbohydrate analysis of plant materials with uronic acid-containing polysaccharides—A comparison between different hydrolysis and subsequent chromatographic analytical techniques. Ind. Crops Prod. 2009, 29, 571–580. [Google Scholar] [CrossRef]
- Nelson, N. A photometric adaptation of the Somogyi method for the determination of glucose. J. Biol. Chem. 1944, 153, 375–380. [Google Scholar] [CrossRef]
- Laine, J.; Buchert, J.; Viikari, L.; Stenius, P. Characterization of unbleached kraft pulps by enzymatic treatment, potentiometric titration and polyelectrolyte adsorption. Holzforschung 1996, 50, 208–214. [Google Scholar] [CrossRef]
- Vydrina, I.; Malkov, A.; Vashukova, K.; Tyshkunova, I.; Mayer, L.; Faleva, A.; Shestakov, S.; Novozhilov, E.; Chukhchin, D. A new method for determination of lignocellulose crystallinity from XRD data using NMR calibration. Carb. Pol. Technol. Appl. 2023, 5, 100305. [Google Scholar] [CrossRef]
- Huang, C.; Jiang, X.; Shen, X.; Hu, J.; Tang, W.; Wu, X.; Ragauskas, A.; Jameel, H.; Meng, X.; Yong, Q. Lignin-enzyme interaction: A roadblock for efficient enzymatic hydrolysis of lignocellulosics. Renew. Sustain. Energy Rev. 2022, 154, 111822. [Google Scholar] [CrossRef]
- Rahikainen, J.; Mikander, S.; Marjamaa, K.; Tamminen, T.; Lappas, A.; Viikari, L.; Kruus, K. Inhibition of enzymatic hydrolysis by residual lignins from softwood—Study of enzyme binding and inactivation on lignin-rich surface. Biotech. Bioeng. 2011, 108, 2823–2834. [Google Scholar] [CrossRef]
- Pan, X.; Gilkes, N.; Saddler, J.N. Effect of acetyl groups on enzymatic hydrolysis of cellulosic substrates. Holzforschung 2006, 60, 398–401. [Google Scholar] [CrossRef]
- Pažitný, A.; Russ, A.; Boháček, Š.; Stankovská, M.; Ihnát, V. Effect of steam explosion on enzymatic hydrolysis of various parts of poplar tree. Wood Res. 2020, 65, 579–590. [Google Scholar] [CrossRef]
- Mirmohamadsadeghi, S.; Karimi, K.; Horváth, I.S. Improvement of solid-state biogas production from wood by concentrated phosphoric acid pretreatment. BioResources 2016, 11, 3230–3243. [Google Scholar] [CrossRef]
- Wen, P.; Chen, Z.; Lian, Z.; Zhang, J. Efficient production of high concentration monosaccharides and ethanol from poplar wood after delignification and deacetylation. Bioresour. Technol. 2023, 385, 129459. [Google Scholar] [CrossRef] [PubMed]
- Doménech, P.; Manzanares, P.; Álvarez, C.; Ballesteros, M.; Duque, A. Comprehensive study on the effects of process parameters of alkaline thermal pretreatment followed by thermomechanical extrusion in sugar liberation from Eucalyptus grandis wood. Holzforschung 2021, 75, 250–259. [Google Scholar] [CrossRef]
- Chu, Q.; Tong, W.; Chen, J.; Wu, S.; Jin, Y.; Hu, J.; Song, K. Organosolv pretreatment assisted by carbocation scavenger to mitigate surface barrier effect of lignin for improving biomass saccharification and utilization. Biotech. Biofuels 2021, 14, 136. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.K.; Da Silva, S.S.; Singh, O.V. Detoxification of lignocellulose hydrolysates: Biochemical and metabolic engineering toward white biotechnology. BioEnergy Res. 2013, 6, 388–401. [Google Scholar] [CrossRef]
- Alriksson, B.; Horváth, I.S.; Sjöde, A.; Nilvebrant, N.O.; Jönsson, L.J. Ammonium hydroxide detoxification of spruce acid hydrolysates. In Twenty-Sixth Symposium on Biotechnology for Fuels and Chemicals; Humana Press: New York, NY, USA, 2005; pp. 911–922. [Google Scholar] [CrossRef]
- Kononov, G.N.; Verevkin, A.N.; Serdyukova, Y.V.; Mironov, D.A. Wood as a chemical raw material. History and modernity. IV. Delignification of wood as a way to produce cellulose. Part I. For. Bull. 2022, 26, 97–113. [Google Scholar] [CrossRef]
- Nepenin, N.N. Production of sulfite pulp. In Pulp Technology; Nepenin, N.N., Nepenin, Y.N., Eds.; Forestry Industry: Moscow, Russia, 1976; Volume 1, p. 624. (In Russian) [Google Scholar]
- Rueda, C.; Calvo, P.A.; Moncalián, G.; Ruiz, G.; Coz, A. Biorefinery options to valorize the spent liquor from sulfite pulping. J. Chem. Tech. Biotech. 2015, 90, 2218–2226. [Google Scholar] [CrossRef]
- Asadollahzadeh, M.T.; Ghasemian, A.; Saraeian, A.R.; Resalati, H.; Lennartsson, P.R.; Taherzadeh, M.J. Ethanol and biomass production from spent sulfite liquor by filamentous fungi. Int. J. Biotech. Bioeng. 2017, 11, 718–724. [Google Scholar]
- Rahman, M.; Avelin, A.; Kyprianidis, K. A Review on the modeling, control and diagnostics of continuous pulp digesters. Processes 2020, 8, 1231. [Google Scholar] [CrossRef]
- Balkissoon, S.; Andrew, J.; Sithole, B. Dissolving wood pulp production: A review. Biomass Conv. Bioref. 2022, 1–18. [Google Scholar] [CrossRef]
- Ek, M.; Gellerstedt, G.; Henriksson, G. Pulping Chemistry and Technology. In Pulp and Paper Chemistry and Technology; Ek, M., Gellerstedt, G., Henriksson, G., Eds.; Walter de Gruyter GmbH & Co KG.: Berlin, Germany, 2009; Volume 2, p. 471. [Google Scholar]
- Li, M.; Yuan, Y.; Zhu, Y.; Jiang, B.; Wu, W.; Wu, S.; Jin, Y. Comparison of sulfomethylated lignin from poplar and masson pine on cellulase adsorption and the enzymatic hydrolysis of wheat straw. Bioresour. Technol. 2022, 343, 126142. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Yang, L.; Jameel, H.; Chang, H.M.; Phillips, R. Sodium sulfite–formaldehyde pretreatment of mixed hardwoods and its effect on enzymatic hydrolysis. Bioresour. Technol. 2013, 135, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Przybysz Buzała, K.; Kalinowska, H.; Małachowska, E.; Boruszewski, P.; Krajewski, K.; Przybysz, P. The effect of lignin content in birch and beech kraft cellulosic pulps on simple sugar yields from the enzymatic hydrolysis of cellulose. Energies 2019, 12, 2952. [Google Scholar] [CrossRef]
- Wang, C.; Lu, X.; Gao, J.; Li, X.; Zhao, J. Xylo-oligosaccharides inhibit enzymatic hydrolysis by influencing enzymatic activity of cellulase from Penicillium oxalicum. Energy Fuels 2018, 32, 9427–9437. [Google Scholar] [CrossRef]
- Kuenz, A.; Jäger, M.; Niemi, H.; Kallioinen, M.; Mänttäri, M.; Prüße, U. Conversion of xylose from birch hemicellulose hydrolysate to 2,3-butanediol with Bacillus vallismortis. Fermentation 2020, 6, 86. [Google Scholar] [CrossRef]
- Puentes, J.G.; Mateo, S.; Sánchez, S.; Roberto, I.C.; Moya, A.J. Bioconversion study of olive tree biomass hemicellulosic hydrolysates by Candida guilliermondii at different scales for ethanol and xylitol production. Fermentation 2023, 9, 553. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Hassan, S.E.-D.; Alrefaey, H.M.A.; Elsakhawy, T. Efficient co-utilization of biomass-derived mixed sugars for lactic acid production by Bacillus coagulans Azu-10. Fermentation 2021, 7, 28. [Google Scholar] [CrossRef]
- Procópio, D.P.; Kendrick, E.; Goldbeck, R.; Damasio, A.R.D.L.; Franco, T.T.; Leak, D.J.; Jin, Y.S.; Basso, T.O. Xylo-oligosaccharide utilization by engineered Saccharomyces cerevisiae to produce ethanol. Front. Bioeng. Biotech. 2022, 10, 825981. [Google Scholar] [CrossRef]
- Novy, V.; Krahulec, S.; Longus, K.; Klimacek, M.; Nidetzky, B. Co-fermentation of hexose and pentose sugars in a spent sulfite liquor matrix with genetically modified Saccharomyces cerevisiae. Bioresour. Technol. 2013, 130, 439–448. [Google Scholar] [CrossRef]
- Cheng, L.; Huang, R.; Ying, J.; Fu, Y.; Zhou, X.; Jiang, K. Sequential bioprocess with Gluconobacter oxydans and Candida tropicalis for gluconic acid and single-cell protein production from enzymatic hydrolysate. Fermentation 2023, 9, 562. [Google Scholar] [CrossRef]
- Hama, S.; Mizuno, S.; Kihara, M.; Tanaka, T.; Ogino, C.; Noda, H.; Kondo, A. Production of D-lactic acid from hardwood pulp by mechanical milling followed by simultaneous saccharification and fermentation using metabolically engineered Lactobacillus plantarum. Bioresour. Technol. 2015, 187, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Pérez-García, F.; Burgardt, A.; Kallman, D.R.; Wendisch, V.F.; Bar, N. Dynamic co-cultivation process of Corynebacterium glutamicum strains for the fermentative production of riboflavin. Fermentation 2021, 7, 11. [Google Scholar] [CrossRef]
- Osorio-González, C.S.; Saini, R.; Hegde, K.; Brar, S.K.; Lefebvre, A.; Avalos Ramírez, A. Crabtree effect on Rhodosporidium toruloides using wood hydrolysate as a culture media. Fermentation 2023, 9, 11. [Google Scholar] [CrossRef]
- Zhong, C.; Ukowitz, C.; Domig, K.J.; Nidetzky, B. Short-chain cello-oligosaccharides: Intensification and scale-up of their enzymatic production and selective growth promotion among probiotic bacteria. J. Agric. Food Chem. 2020, 68, 8557–8567. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Yun, J.; Gu, Y.; Yan, B.; Yin, B.; Huang, C. Evaluating the in vitro and in vivo prebiotic effects of different xylo-oligosaccharides obtained from bamboo shoots by hydrothermal pretreatment combined with endo-xylanase hydrolysis. Int. J. Mol. Sci. 2023, 24, 13422. [Google Scholar] [CrossRef] [PubMed]
- Mayorova, K.; Aksenov, A.; Shevchenko, A. Wood microcrystalline cellulose: Preparation and pharmaceutical applications. In Proceedings of the International Conference on Advances in Emerging Trends and Technologies, Stavropol, Russia, 5–6 October 2023. [Google Scholar]
- Tyshkunova, I.V.; Poshina, D.N.; Skorik, Y.A. Cellulose cryogels as promising materials for biomedical applications. Int. J. Mol. Sci. 2022, 23, 2037. [Google Scholar] [CrossRef]
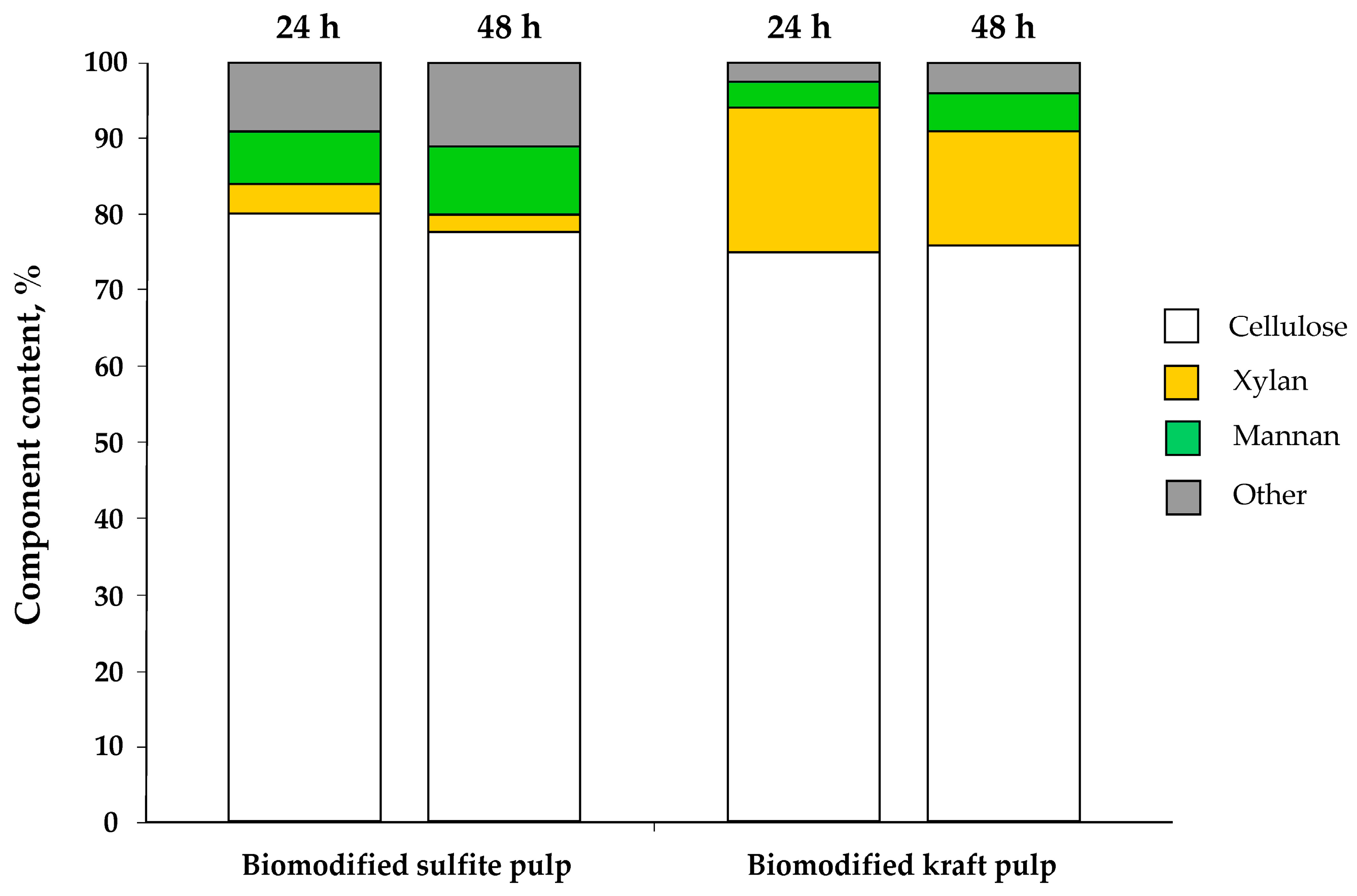
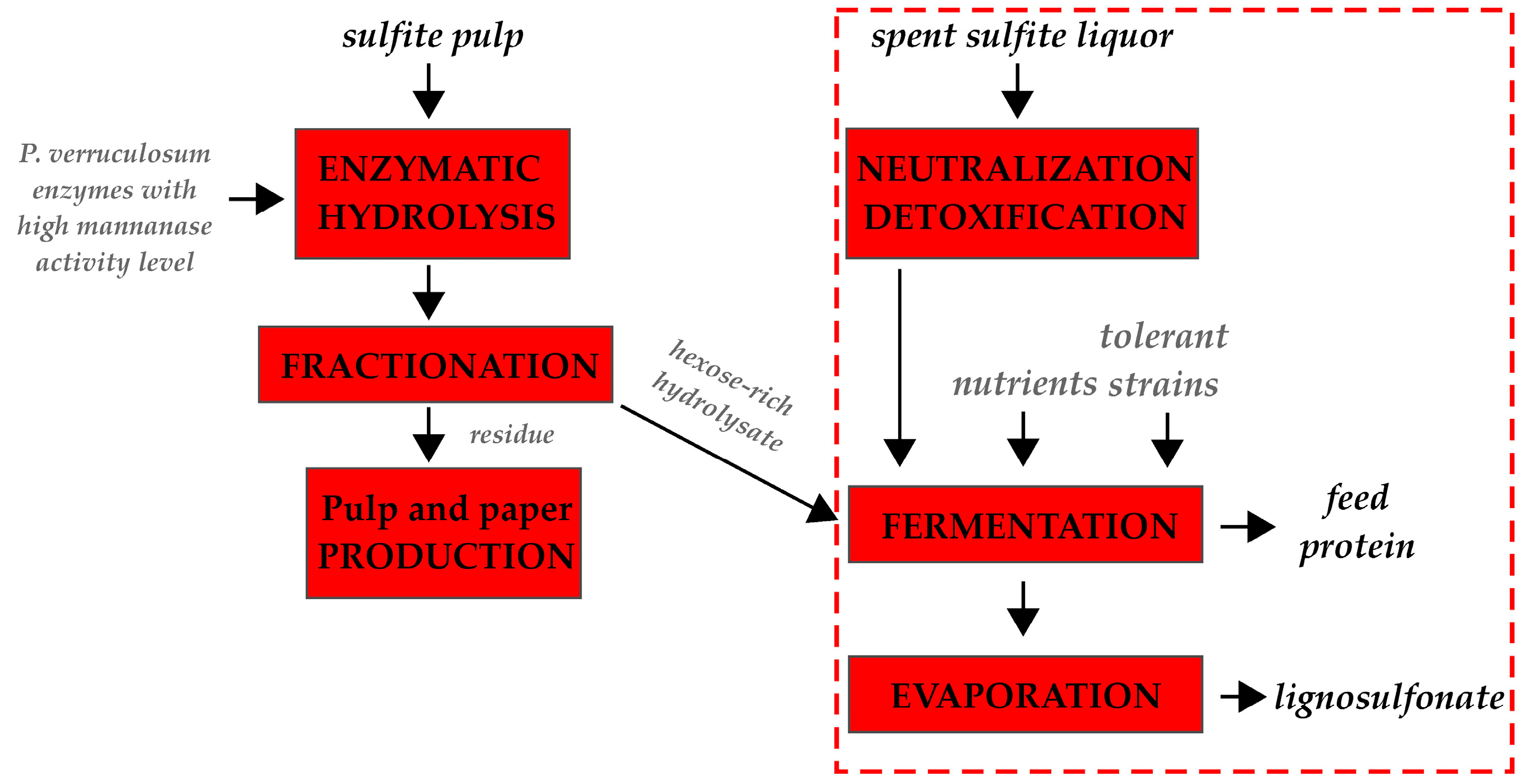
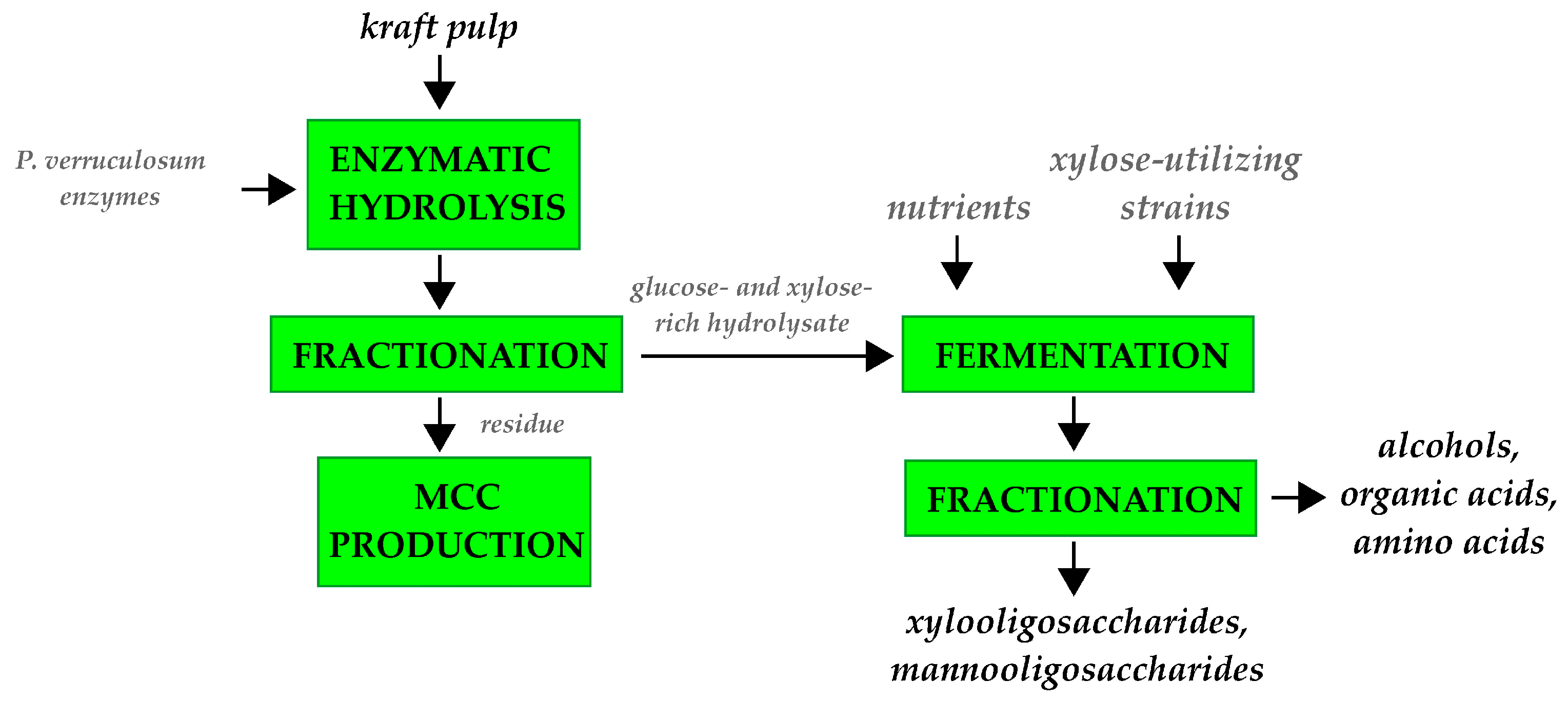
| Component | Kraft Pulp, % | Sulfite Pulp, % |
|---|---|---|
| Cellulose | 72.0 ± 1.4 | 78.9 ± 1.3 |
| Xylan | 23.7 ± 1.1 | 7.0 ± 0.5 |
| Mannan | 3.0 ± 0.6 | 9.1 ± 0.5 |
| Others | 1.3 ± 0.4 | 5.0 ± 1.5 |
| CMC-ase | MCC-ase | Xylanase | Mannanase | β-Glucosidase | Cellobiase |
|---|---|---|---|---|---|
| 286 ± 1.94 | 19.8 ± 0.12 | 2326 ± 6.2 | 29.1 ± 0.15 | 26.4 ± 0.12 | 10.9 ± 0.08 |
| Pulp | Hydrolysis Time | Cellobiose | Monosaccharides from Hemicelluloses Oligomers after Acid Inversion | |
|---|---|---|---|---|
| Xylose | Mannose | |||
| Sulfite | 24 | 3.7 | 3.4 | 6.5 |
| 48 | 3.3 | 4.2 | 6.8 | |
| Kraft | 24 | 4.2 | 4.6 | 1.7 |
| 48 | 4.7 | 5.3 | 2.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shevchenko, A.R.; Mayorova, K.A.; Chukhchin, D.G.; Malkov, A.V.; Toptunov, E.A.; Telitsin, V.D.; Rozhkova, A.M.; Zorov, I.N.; Rodicheva, M.A.; Plakhin, V.A.; et al. Enzymatic Hydrolysis of Kraft and Sulfite Pulps: What Is the Best Cellulosic Substrate for Industrial Saccharification? Fermentation 2023, 9, 936. https://doi.org/10.3390/fermentation9110936
Shevchenko AR, Mayorova KA, Chukhchin DG, Malkov AV, Toptunov EA, Telitsin VD, Rozhkova AM, Zorov IN, Rodicheva MA, Plakhin VA, et al. Enzymatic Hydrolysis of Kraft and Sulfite Pulps: What Is the Best Cellulosic Substrate for Industrial Saccharification? Fermentation. 2023; 9(11):936. https://doi.org/10.3390/fermentation9110936
Chicago/Turabian StyleShevchenko, Aleksandr R., Ksenia A. Mayorova, Dmitry G. Chukhchin, Alexey V. Malkov, Evgeniy A. Toptunov, Vadim D. Telitsin, Aleksandra M. Rozhkova, Ivan N. Zorov, Maria A. Rodicheva, Vadim A. Plakhin, and et al. 2023. "Enzymatic Hydrolysis of Kraft and Sulfite Pulps: What Is the Best Cellulosic Substrate for Industrial Saccharification?" Fermentation 9, no. 11: 936. https://doi.org/10.3390/fermentation9110936
APA StyleShevchenko, A. R., Mayorova, K. A., Chukhchin, D. G., Malkov, A. V., Toptunov, E. A., Telitsin, V. D., Rozhkova, A. M., Zorov, I. N., Rodicheva, M. A., Plakhin, V. A., Akishin, D. A., Poshina, D. N., Semenova, M. V., Aksenov, A. S., & Sinitsyn, A. P. (2023). Enzymatic Hydrolysis of Kraft and Sulfite Pulps: What Is the Best Cellulosic Substrate for Industrial Saccharification? Fermentation, 9(11), 936. https://doi.org/10.3390/fermentation9110936






