Abstract
FODMAPs are fermentable oligo-, di-, monosaccharides, and polyols. The application of homofermentative lactic acid bacteria (LAB) has been investigated as a promising approach for producing low-FODMAP whole-wheat bread. The low-FODMAP diet is recommended to treat irritable bowel syndrome (IBS). Wheat flour is staple to many diets and is a significant source of fructans, which are considered FODMAPs. The reduction of fructans via sourdough fermentation, generally associated with heterofermentative lactic acid bacteria (LAB), often leads to the accumulation of other FODMAPs. A collection of 244 wild-type LAB strains was isolated from different environments and their specific FODMAP utilisation profiles established. Three homofermentative strains were selected for production of whole-wheat sourdough bread. These were Lactiplantibacillus plantarum FST1.7 (FST1.7), Lacticaseibacillus paracasei R3 (R3), and Pediococcus pentosaceus RYE106 (RYE106). Carbohydrate levels in flour, sourdoughs (before and after 48 h fermentation), and resulting breads were analysed via HPAEC-PAD and compared with whole-wheat bread leavened with baker’s yeast. While strain R3 was the most efficient in FODMAP reduction, breads produced with all three test strains had FODMAP content below cut-off levels that would trigger IBS symptoms. Results of this study highlighted the potential of homofermentative LAB in producing low-FODMAP whole-wheat bread.
1. Introduction
Cereal-based foods have a rich nutritional profile and are associated with a healthy diet [1,2]. Whole-grain sourdough bread with higher concentration of fibre, biogenic compounds, vitamins, minerals, and reduced levels of anti-nutritional factors and lower postprandial glycaemic response is associated with a reduced risk of non-communicable diseases [3,4,5,6]. Moreover, bread and other cereal-based foods have a rich nutritional profile and are associated with a healthy diet [1,2] and are a convenient staple food, widely consumed across the globe [3,4,5]. While a piece of general dietary advice is to increase the consumption of whole-grain products [6], their intake by individuals with irritable bowel syndrome (IBS) is restricted due to the high content of FODMAPs (fermentable oligo-, di-, monosaccharides, and polyols), namely, fructans and, to a lesser degree, galacto-oligosaccharides (GOS), polyols, and fructose in excess of glucose [7,8,9].
Reduced FODMAP consumption alleviates gastrointestinal symptoms of IBS. The most common IBS symptoms are bloating, flatulence, pain, or irregular bowel movement that influences the ability of the sufferers to partake in social interactions, reduces their work-related availability and productivity, therefore, their quality of life [10,11]. Considering that more than 4% of the global adult population suffers from IBS, the economic impact of this disorder is enormous [12,13]. Reported effectiveness of the low-FODMAP approach among IBS sufferers is 50% to 80% [14]. And the low-FODMAP diet has also been successfully investigated for the management of other gastrointestinal disorders: an exercise-induced gastrointestinal syndrome in athletes [15,16], inflammatory bowel disease [17], nonceliac gluten sensitivity [18], Crohn’s disease, ulcerative colitis [19], ileal pouch–anal anastomosis [20], and more [21].
FODMAPs are indigestible or poorly absorbed, osmotically active, and rapidly fermentable carbohydrates [22]. Recommended threshold values based on dietary studies classify the cereal product as low in FODMAP if one portion (i.e., 50 g of bread) contains less than 0.5 g of the sum of FODMAPs, with an individual upper limit of 0.3 g of oligosaccharides—fructans and galacto-oligosaccharides (GOS), 0.4 g of polyols (of which up to 0.2 g consist of sorbitol and mannitol), and 0.15 g of fructose in excess of glucose; the cut-off value for lactose is 1 g [23].
Low-FODMAP diets require replacing non-tolerated foods with alternatives low in FODMAPs, hence the need for low-FODMAP options. Breads low in FODMAP are scarce, primarily gluten-free, less nutritive, and more expensive breads [24,25,26]. It has also been reported that 41% of IBS patients identify wheat bread as a dietary trigger of IBS symptoms [27]. Therefore, there is an unquestionable demand for the development of bread which can satisfy the requirements for beneficial whole-grain cereal products with a low-FODMAP profile. In addition, implementation of standardised microbial processing, such as sourdough technology which has a proven capacity to reduce FODMAPs [28], should be considered.
The most abundant group of FODMAPs found in wheat are oligosaccharides, particularly fructans, present at considerably higher levels in whole wheat (~2% DM) compared with refined wheat flours (~1.2% DM) [7,8,9]. On the other hand, the raffinose family oligosaccharides (GOS) present in wheat at ~0.2%, have a minor contribution to total FODMAP levels in bread. While oligosaccharides are well-recognised prebiotics and dietary fibre, of which consumption is encouraged, they induce gastrointestinal symptoms in sensitive individuals [29]. The two main types of fructans are linear inulin and levan. Inulin comprises fructose monomers connected via β (2-1) fructosyl linkages, whereas fructose molecules in levan are connected via β (2-6) linkages, both with a terminal glucose residue. In wheat, two types of fructans are present: branched fructose polymers termed graminans, with both β (2-1) and β (2-6) linkages, and neo-type fructans that may contain β (2-1), β (2-6) or both types of linkages and internal glucose. The degree of polymerisation (DP) of wheat fructans varies between 3–19, with an average DP between 5 and 7 [9,30,31]. Shorter chain fructans (<DP 10) are referred to as fructooligosaccharides (FOS).
The degradation of fructans by microbes largely depends on the specificity of secreted enzymes belonging to the GH-32 or GH-68 family. The β-fructofuranosidases are exoinulinases (EC 3.2.1.80) hydrolysing terminal β (1-2) fructose bonds, and invertases (EC 3.2.1.26) and endoinulinases (EC 3.2.1.7) which act on the internal and external fructan bonds [32]. Degradation of levan-type fructans is facilitated by levanases (EC 2.4.1.10 and E C3.2.1.65), enabling hydrolysis of β (2-6) linkages [33]. Several pathways have been associated with fructan utilization in lactobacilli. For example, the fos operon is often associated with the Lacticaseibacillus group and consists of cell-wall-bound β-fructofuranosidase and a complex sugar phosphotransferase system (PTS) with broad substrate specificity [34,35]. This organization allows for the comprehensive transport of monosaccharides and shorter-chain fructans released by extracellular hydrolysis of longer inulin and levan-type fructans to the cytosol, where they can be utilized by intracellular enzymes. In contrast, a pts1BCA operon was observed to facilitate the degradation of short-chain fructans (FOS) with a DP < 4 [36]. The hydrolysis of longer-chain carbohydrates in homo-fermentative LAB is subject to catabolite regulation by glucose [34,36,37]. Therefore, only a careful and targeted selection of the isolates can result in sufficient FODMAPs reduction in bread.
Multiple studies on the reduction of FODMAPs in wheat and rye bread have been published, and there was a product in Finland commercially available, but it has been discontinued [26]. Research on reducing FODMAPs via sourdough fermentation has been primarily focused on spontaneously fermented sourdoughs, inoculation with heterofermentative LAB, mixed starter cultures, or a combination of food-grade fructan-degrading enzymes and lactobacilli [7,28,38,39,40,41,42,43]. The typical pattern emerging in the sourdough investigation is a significant reduction of fructans combined with a significant accumulation of polyols. Polyols in bread (especially mannitol) are primarily produced during hexose degradation by heterofermentative LAB that utilises fructose via the phosphogluconate pathway; this can result in high total FODMAP levels [44]. In fact, heterofermentative LAB have been investigated for a directed conversion of fructose to mannitol in food products [45,46]. In contrast, homofermentative LAB ferment hexoses via the Embden–Meyerhof pathway, where carbohydrates are almost exclusively reduced to lactic acid [28,44]. Therefore, among other targeted approaches, the use of homofermentative LAB (reducing fructose to lactic acid) in SD production could be used to avoid mannitol accumulation. A few studies investigated FODMAP degradation in bread with homofermentative LAB, and the findings are encouraging [47,48,49,50].
This study investigated the effect of type II sourdough processing with homofermentative LAB fermentation on the FODMAP reduction in whole-wheat bread. LAB were isolated from fruit, vegetables, and spontaneous sourdoughs based on various flours. Homofermentative isolates were screened for FODMAP utilisation and selected strains were applied as sourdough starters. Growth kinetics, acidification, and the influence of each strain on the bread carbohydrate profile were analysed. The range of FODMAPs and other carbohydrates in the flour, sourdoughs, and sourdough breads were compared to the bread leavened with commercial baker’s yeast (Saccharomyces cerevisiae) (RB). Additionally, the techno-functional bread quality parameters of the different sourdough breads samples were assessed and compared to RB.
2. Materials and Methods
2.1. Materials
Whole-wheat flour (Odlums; Dublin, Ireland) used in the study contained 63.9% carbohydrates, 2.2% fat, 9.0% fibre, and 14–15% protein; the falling number was >250 s (supplier’s specifications). Salt was supplied by Glacia British Salt Limited Ltd. (Northwich, UK), sugar (Siúcra, granulated sugar) by Nordzucker (Dublin, Ireland), and sunflower oil by Musgrave (Cork, Ireland). Instant dry baker’s yeast was obtained from Puratos (Bruggeman instant yeast; Gent, Belgium). Unless otherwise stated, the chemicals were obtained from Sigma-Aldrich (Arklow, Ireland).
2.2. Isolation and Characterisation of Lactic Acid Bacteria
2.2.1. Strains and Culture Conditions
LAB were isolated from various materials including sourdoughs based on flours (whole wheat, baker’s flour, white rice, brown rice, rye, oat, sorghum, tiger nut, lentils, and faba bean), and homogenates of high in FODMAP fennel, leek, and watermelon [51,52]. Other homofermentative LAB isolates were obtained from Munster Technological University, University College Cork, and the German Collection of Microorganisms and Cell Cultures GmbH (DSMZ). A list of the strains and their origins is provided in Supplementary Table S1. Strains selected for sourdough trials were Lactiplantibacillus plantarum FST1.7 (FST1.7), Lacticaseibacillus paracasei R3 (R3), and Pediococcus pentosaceus RYE106 (RYE106). All strains were routinely cultured on an MRS5 medium [53]. The medium’s acidity was adjusted with 2 M hydrochloric acid to pH 5.8 before autoclaving. Filter sterilised carbohydrate solution (10 g of maltose, 5 g of fructose, and 5 g of glucose) and 1 mL of vitamin mixture (cobalamin, folic acid, nicotinic acid amide, pantothenic acid, pyridoxal phosphate, and thiamine; 0.2 g/litre each) were added to a sterile medium cooled to 50 °C. Cycloheximide (0.1 g/L) was added to prevent fungal contamination; bromocresol green (0.04 g/L) was added to facilitate phenotypic identification of isolates. Strains were incubated at 30 °C in anaerobic jars using EZ Anaersystem Sachets (Analab, Dublin, Ireland) until adequate colony size was reached (48–72 h). All tests were initiated by inoculating 10 mL of MRS broth with one colony in a 15 mL screw-cap centrifuge tube (Sarstedt Ltd., Drinagh, Ireland). The suspension was incubated overnight at 30 °C, subcultured at 1% (v/v) in 10 mL MRS broth, and incubated under the same conditions for 16 h. All isolates were maintained at −80 °C in MRS or MRS5 broth containing 40% (v/v) glycerol. Where required, isolates were identified by partial 16 s rRNA fragments amplified using the universal primers fD1 (5′ AGAGTTTGATCCTGGCTCAG 3′) and rP2 (5′ ACGGCTACCTTGTTACGACTT 3′) [54]; consensus sequences were analysed via a basic local alignment tool (BLAST) [55]. Identification of the species of interest was later confirmed through whole-genome analysis where average nucleotide identity with a relevant type strain above 98% confirmed species assignment.
2.2.2. Isolation and Characterisation of LAB Isolates
Spontaneous sourdoughs were produced from 150 g of flour and 150 g of sterile distilled water by blending for 2 min in a stomacher (Lab-Blender 400), resting for 5 min, and mixed again for 2 min. LAB were isolated after each 24 h incubation interval at 30 °C for 24 h over four days. Daily backslopping was performed (1:4.5:4.5 ratio of sourdough:flour:water). For the isolation, 1 g of sourdough was homogenised with 9 mL of sterile ¼ strength Ringer’s solution and solid-glass beads (~2 mm), followed by further decimal dilutions using 9 mL of Ringer’s. The appropriate dilutions were plated on MRS5 agar and incubated in anaerobic conditions. Phenotypically distinctive colonies were picked from each plate, passed at least two times on the fresh MRS5 agar for purification and incubated anaerobically at 30 °C for 72 h. For the LAB isolation from watermelon, leek, and fennel, 10 g of plant material were homogenised in the stomacher with 90 mL of sterile 0.1% peptide water, and 100 µL of the suspension was plated on MRS5 agar. Cultivation and purification of isolates were performed as described above. The inverted Durham tube technique was used to characterise the homofermentative isolates. MRS5 medium without meat extract, with 2% glucose (w/v) as a sole carbohydrate source and bromocresol purple as a pH indicator, were used. A single colony from MRS5 agar was added to the 15 mL screw-cap conical tube containing 10 mL of the medium with submerged inverted Durham tube (1.5 mL) and incubated at 30 °C for 24 h. The absence of a gas bubble in the Durham tube after incubation indicated homofermentative fermentation; a gas bubble in the tube indicated heterofermentative hexose metabolism.
2.3. FODMAP Utilisation
2.3.1. Carbohydrate Solutions
Sucrose, fructose, lactose, sorbitol, xylitol, maltitol, mannitol, raffinose, fructooligosaccharides (FOS), and inulin fermentation by LAB were analysed (carbohydrates specification provided in Supplementary Table S2). Stock solutions of the carbohydrates were prepared with deionised water to contain 5% (w/v) of the individual carbohydrate. Complete solubilisation of inulin was achieved by sonication for 10 min at 80 °C and 100% ultrasonic power (Elma Transsonic Digital S, Singen, Germany). All carbohydrate solutions were filter-sterilised (0.2 µm, Filtropur, Sarstedt Inc. MS) and stored at −20 °C until used. The final sugar concentration in the test media was 0.5% (w/v).
2.3.2. FODMAP Utilisation Assays
Assessment of FODMAPs utilisation was performed as previously described by Ispiryan et al. [56] with modifications. Briefly, the base medium used was a carbohydrate-free modified MRS (mMRS) prepared from first principle [57] with distilled water and 10 g of trypticase peptone, 2.5 g yeast extract, 3 g of tryptose, 3 g of potassium phosphate dibasic, 3 g of potassium phosphate monobasic, 2 g of tri-ammonium citrate, 2 g of pyruvic acid, 0.3 g of L-cysteine hydrochloride, 1 mL of Tween-80, 0.575 g of magnesium sulfate heptahydrate, 0.12 g of Manganese (II) sulfate tetrahydrate, and 0.034 g of iron (II) sulfate heptahydrate per 1 L of the media. For solid media, 15 g/L of biological agar (Neogen, Heywood, UK) and 0.04 g of bromocresol purple as pH indicator were added. The medium’s acidity was adjusted with 2 M hydrochloric acid to pH 6.8 before autoclaving. A sterile carbohydrate stock solution (5 mL) was mixed with the mMRS agar to achieve a 0.5% (w/v) concentration in a final volume of 50 mL and poured into a squared ventilated petri dish (120 mm × 120 mm, Grainer, London, UK); the control plate contained 50 mL of mMRS agar alone. A master plate was prepared by adding 200 μL of individual cell suspension to a 96-well plate (one strain every second well). The agar plates were inoculated with a microplate replicator (Boekel Scientific™) and incubated at 30 °C in anaerobic jars using EZ Anaersystem Sachets (Analab, Dublin, Ireand). The ability to ferment the carbohydrate was indicated by a yellow discolouration zone surrounding a colony after 72 h of incubation. A minimum of two biological replicates were analysed. Based on the agar assay, 10 strains were selected for a microtitre FODMAP fermentation analysis.
For the growth-curve analyses, cell suspensions were centrifuged at 6000 rpm for 10 min at 4 °C (Jouan MR23i, Saint Herblain, France) and washed twice in 10 mL of Ringer’s solution. Cell suspensions were adjusted to the optical density (OD) of 1 at 600 nm (Biochrom, Libra S12, Bath, UK) using Ringer’s solution as a diluent. Flat-bottom microtitre plates (400 μL; Sterilin™) were used. The control wells contained 2 µL of standardised cell suspension and 198 µL of mMRS. The test wells contained 2 µL of the cell suspension, 20 µL of individual carbohydrate solution, and 178 µL of mMRS. For the blanks, 20 μL of carbohydrate solution and 180 μL of mMRS were mixed. Plates were sealed with a sterile polyester film (SealPlate, Excel Scientific, Victorville, CA, USA.) before incubation at 30 °C. OD was automatically recorded every hour over 72 h at 595 nm wavelength (Multiscan FC plate reader; Thermo Fisher Scientific, Carrigaline, Ireland.). Generated data were analysed in Rstudio, version 1.4.1106 [58] using “Growthcurver” [59]. Growth-curve data were fitted to a standard form of the logistic equation:
where Nt is the number of the absorbance reading at time t, N0 is the initial absorbance reading, K is the carrying capacity (the maximum OD; ODmax), and r is the growth rate. ODmax in supplemented mMRS (ODFODMAP) and FODMAP-free mMRS (ODmMRS) were calculated. ODFODMAP were normalised against the ODmMRS (ODNORM = ODFODMAP/ODmMRS, Table A1). The assay was performed in three biological replicates. Based on the results, the best fructan fermenter from each genus was further assessed for reducing FODMAPs in sourdough.
2.4. Whole-Wheat Sourdough Preparation
Selected strains were cultivated on the MRS5 agar. A single colony was inoculated in 10 mL of MRS broth, incubated at 30 °C for 24 h, subcultured in 10 mL of MRS and further incubated at 30 °C for 16 h. Cells were harvested by centrifugation at 3500 rpm for 10 min (JOUAN C-412) and washed twice in 10 mL of sterile tap water, followed by resuspension in sterile tap water. Three replicate sourdoughs (SD; 1200 g) were produced with each strain, with a single-strain inoculum at a final cell density of 7 log CFU g−1 of SD and a dough yield of 250 (DY = 100 × (flour weight (g) + water weight (g))/flour weight (g)). The following sourdoughs were produced for bread-making: SD-FST1.7 produced with FST1.7, SD-RYE106 with RYE106, and SD-R3 with R3. Whole-wheat flour (480 g) and sterile tap water combined with cell suspension (720 g) were mixed for 1 min at speed 1, followed by 2 min at speed 3 in Kenwood KM330 classic chef mixer (Kenwood, Havant, UK). SD aliquots required for the analysis at different time points were divided into separate stomacher bags, airtightly sealed, and incubated at 30 °C for a maximum of 48 h.
2.4.1. Sourdough Fermentation Characteristics
The cell titre (CFU/g), pH, and total titratable acidity (TTA) were determined in the unfermented SD (T0, after mixing) and after 10, 24, and 48 h of fermentation as previously described [46] with some modifications. In short, to determine the cell density, two aliquots (1 g) of relevant SD were vortexed with 9 mL sterile Ringer’s solution with solid-glass beads (~2 mm) in separate test tubes. Further decimal dilutions were performed in Ringer’s, of which relevant dilutions were plated in duplicates on MRS5 agar plates. The cell titre of each SD (CFU/g) was established after 48 h of incubation at 30 °C. The pH and TTA were analysed following standard procedures [60]. Briefly, SD aliquots (10 g each) of SD were homogenised with 95 mL of distilled water and 5 mL of acetone on a stirring plate. After reading the pH (Edge, Hanna Instruments, Bedfordshire, UK), the homogenate was titrated with 0.1 M NaOH. TTA was expressed as the volume (mL) of 0.1 M NaOH required to reach a pH of 8.5. Sourdough samples were stored at 4 °C for up to 1 h before processing. Each sample was analysed in duplicate.
2.4.2. Quantification of Organic Acids
Extraction and quantification of organic acids in SD were performed as previously described [61] except that 2 g of sample was extracted instead of 1 g. Extracts were analysed on a Dionex Ultimate 3000 system (Thermo Fisher Scientific, Waltham, MA, USA) and ultraviolet light/diode array detection (UV/DAD, quantification at 210 nm; Thermo Fisher Scientific). The organic acids were separated with a Hi-Plex H column (7.7 mm × 300 mm); Agilent Technologies, Santa Clara, CA, USA) at 60 °C and isocratic elution with 5 mM sulfuric acid and a flow rate of 0.5 mL/min. Reference standards of lactic acid, succinic acid, and acetic acid were used for an external standard calibration in the range of 0.03–6 g/L. Extractions were carried out in duplicate for each of the two fermentation replicates. The fermentation quotient (FQ) was expressed as a lactic to acetic acid molar ratio.
2.5. Bread Production
For the bread production, mature SDs (incubated at 30 °C for 48 h; pH range 3.68–3.82; TTA range 17.95–21.45 mL of 1 M NaOH) were used following the recipe listed in Table 1. Straight dough reference bread (RB) was produced by mixing dry ingredients first, followed by adding yeast suspension and sunflower oil. Yeast suspension was prepared by adding commercial baker’s yeast to the water (25 °C) required for the recipe and activated for 10 min. After adding liquid ingredients, the dough was mixed at speed 1 for 1 min, followed by a second stage of mixing at speed 2 for 7 min (Kenwood chef mixer, Kenwood Ltd., New Hampshire, UK). In the sourdough bread (SB), 20% of the flour was replaced with mature SD, and the water level was corrected for water originating from the SD. The dough was left to rest for a 60 min bulk fermentation (35 °C and 75% relative humidity) in a proofer (KOMA SunRiser, Roermond, The Netherlands). After the bulk fermentation, the dough was divided into three 450 g pieces, moulded, transferred to greased tins, and proofed for 90 min at the same temperature and relative humidity. Breads were baked in a deck oven (MIWE Condo, Arnstein, Germany) for 35 min at 220/230 °C top/bottom temperature. Before loading, 400 mL of steam was injected into the oven, leaving the draft open throughout the baking. Bread loaves were cooled (60 min) before analysis. Each recipe outlined in Table 1 was baked and analysed in duplicate.

Table 1.
(A) Reference and (B) sourdough bread recipes expressed as respective dosage (g) and based on flour (%) dosage.
2.5.1. Bread Quality Analysis
Bread quality analyses were performed following the procedure described by Neylon et. al. [62] with minor modifications. Two bread loaves per baking replicate were analysed. The bake loss (water loss during baking) was determined gravimetrically by weighing the moulded dough pieces and the final baked bread loaves. Reported per cent values were calculated using following formulas:
Specific volume (SV, mL/g) was analysed using a Volscan Profiler (Stable Micro Systems, Surrey, UK). The crumb water activity was determined with a water activity meter (AquaLab series 3, Decagon Devices Inc., Pullman, WA, USA). Approximately 1 g of the bread crumb was placed in AquaLab sample containers and measured following 10 min of equilibration. Microbial shelf life was analysed using the mould environmental challenge introduced by [63], later modified by [62,64]. Briefly, both sides of four central slices (25 mm) from one loaf per batch were exposed to the environment for 5 min, then placed into individual sterile bags and heat-sealed. A sterile filter pipette tip was inserted in each bag for consistent gaseous exchange. Bread samples were stored at 20 °C and 50% relative humidity in a sterilised and temperature-controlled chamber (KOMA SunRiser, Roermond, The Netherlands) for 14 days. Visually assessed mould growth was rated daily as “mould free”, “mould growth < 10%”, “10–24% mould growth”, “25–49% mould growth”, and “mould growth > 50%”.
Crumb structure and texture parameters were analysed on two loaves per baking replicate (five slices (25 mm)/loaf). C-Cell imaging system was used (Calibre Control International Ltd., Warrington, UK) for slice area, number of cells, and cell diameter analysis. Crumb texture was determined using a TA-XT2i texture analyser (Stable Micro Systems, Surrey, UK) equipped with a 25 kg load cell. Two-compression test with a 35 mm cylindrical probe at 40% strain, with a test speed of 5 mm/s, a trigger force of 0.05 N, and a waiting time of 5 s between compressions, was used. Crumb hardness and crumb chewiness were evaluated. Crumb and crust colours were determined with a hand-held colourimeter (Minolta CR-331, Konica Minolta Holdings Inc., Osaka, Japan) using the CIE L*a*b* system.
2.5.2. Quantification of FODMAPs and Other Carbohydrates
The carbohydrates were extracted and quantified as previously described [9,65] without any modifications. Analyses were performed on a high-performance anion-exchange chromatography system coupled with pulsed amperometric detection (HPAEC-PAD) on a Dionex ICS-5000+ system (Sunnyvale, CA, USA). Fructans were quantified after enzymatic hydrolysis to the glucose and fructose monomers, while all other carbohydrates were quantified using reference standards. Extractions were carried out in duplicate for each of the two fermentation replicates. Carbohydrate changes in the flour, the SD, and the final bread are presented as per cent on a dry weight basis (% DM). The whole wheat was used for analysis as supplied. Frozen overnight samples (−80 °C) were lyophilised (3 days; VirTis BenchTop K freeze drier) and ground to a fine powder using a coffee grinder (sourdough; Wahl) or a Qiagen Tissue Lyser II (bread; Hilden, Germany). The dry matter (DM) of flour and freeze-dried SDs and breads was determined according to AACC 44-15.02. The two-stage method AACC 44-15.02 was used to determine the moisture content of the breads and used for calculations of FODMAP content on a fresh weight basis (g/100 g ‘as is’). The compliance of bread with low-FODMAP criteria described by Varney et al. [23] was assessed, with a serving of a bread estimated as 50 g [66].
2.6. Statistical Analyses
ODNORM values were used to classify the growth of each strain in different FODMAPs based on significant differences between growth in FODMAP and mMRS with Dunnett’s multiple comparison test in RStudio [58] using “DescTool” package [67]. ODNORM values were assessed for normality by the Kolmogorov–Smirnov test and found to not follow a Gaussian distribution. Growth of individual strains within FODMAP were compared using a Kruskal–Wallis test with Dunn’s pairwise comparisons in SPSS Statistic 28 (IBM Corp., Armonk, NY, USA). One-way ANOVA with Tukey post hoc testing was performed in SPSS to analyse sourdough fermentation and baking trial data. Results of all experiments were deemed to be significant at p < 0.05. Results are reported as the mean ± standard deviation.
3. Results and Discussion
The isolation of homofermentative lactic acid bacteria (LAB), screening for their abilities to ferment FODMAPs and an application of selected isolates in low-FODMAP whole-wheat sourdough bread production was investigated. The long history of LAB use in food production has resulted in a qualified presumption of safety (QPS) status, making them prime candidates for food-processing applications [68]. Furthermore, LAB fermentation in bread may allow production of good-quality, preservative-free, clean-label products with an extended shelf life [69,70,71], as desired by modern consumers [72].
3.1. Isolation and Hexose Fermentation Characterisation of LAB Isolates
Presumed LAB were isolated on MRS5 agar based on colony morphology. Isolates were further tested to select Gram-positive, non-spore-forming bacilli and cocci, and displaying homolactic hexose (glucose) fermentation. To ensure a diverse metabolic profile, isolation was performed starting at the early SD fermentation stages (day 1 to 5 of back slopping), before the heterogeneity of the microbial population was reduced and the dominance of heterofermentative species was established [73,74,75]. As expected, the number of colonies increased with each isolation step, while the diversity of colony morphology declined. The acidity of different fermentates varied. After 96 h of fermentation (with daily back slopping) the ΔpH value varied between 1.3 (faba bean flour) and 2.9 (sorghum flour), while ΔTTA values ranged from 1.99 mL of 1 M NaOH in rice flour to 22.4 mL of 0.1 M NaOH in faba bean flour fermentate, owing to the differences in buffering capacity of the flours used for isolation [76]. Viable cell count range recorded after 48 h of fermentation was between 7.8 (oat) and 9.9 log CFU/g of sourdough (faba bean), and similar cell titres were observed until the end of fermentation (96 h).
Cell titre of assumed LAB in fennel, leek, and watermelon matrixes was <4 log CFU/mL. Of the 220 Gram-positive, non-spore-forming, and catalase-negative isolates, 123 were homofermentative (Figure 1). Depending on the food material used for isolation, 18–100% of isolated strains tested positive for homofermentative hexose fermentation. Although heterofermentative strains are a distinct group of LAB in sourdoughs [77], selective isolation at the early fermentation stage led to obtaining 100% of homofermentative strains from whole-wheat and rye flour.
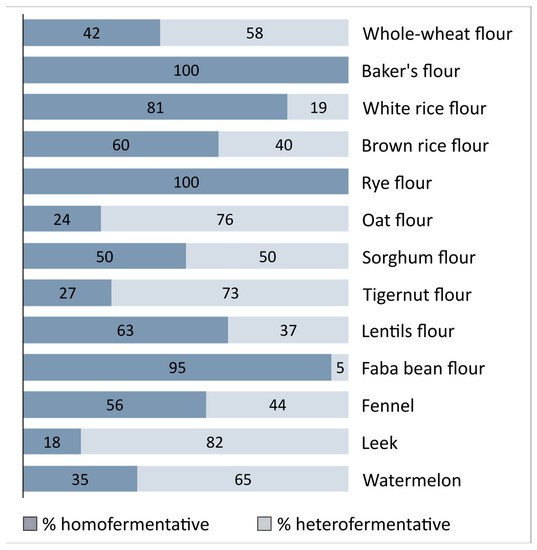
Figure 1.
Percentage of the homo- and hetero-fermentative LAB strains isolated from a variety of sourdoughs, fruit, and vegetables. Fermentation assay was performed via inverted Durham tube assay using MRS5 medium and 20% glucose as the sole carbon source.
3.2. FODMAP Utilisation Assays
3.2.1. Agar Plate FODMAP Utilisation Assay
High-throughput agar plate FODMAP utilisation assay was used to select the LAB able to grow in fructans (fructooligosaccharides (FOS) and inulin), and raffinose (galacto-oligosaccharides, GOS), disaccharides (sucrose and lactose), monosaccharide fructose, and polyol mannitol. Sucrose fermentation was also investigated because of its potential contribution to IBS symptoms in individuals with brush-border enzyme deficiencies [78,79] and its association with strains’ proliferative ability in SD [80]. Homofermentative isolates (n = 123) and strains retrieved from other culture collections (n = 121; Supplementary Table S1) were tested. Results showed rich carbohydrate utilisation profiles of isolates (Figure 2) concurring with the theory of inherent adaptability of lactobacilli to different environments [37,81,82,83]. Isolates fermenting all seven carbohydrates were the most numerous (40%). In contrast, 14% of the isolates were observed to utilise one carbohydrate only, namely fructose (Figure 2b). Such restricted carbohydrate fermentation was reported before and is thought to be associated with a small genome size such as in Fructilactobacillus sanfranciscensis [69] resulting from niche specialisation [84]. Ninety-nine per cent of tested strains utilised fructose and 82% fermented sucrose. Even though analyses of 38 genomes of Lactobacillus spp. suggested that most strains harboured at least two sucrose metabolic pathways [85], non-functional copies of carbon utilisation genes are common in LAB [86]. The remaining FODMAPs, including lactose, mannitol, raffinose, FOS, and inulin, were utilised by 50% to 58% of tested strains (Figure 2a). Identification of clonal isolates originating from the same material was not attempted.
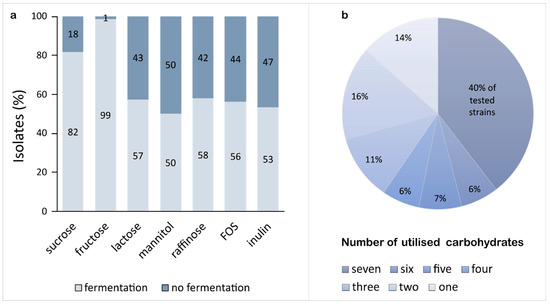
Figure 2.
FODMAP fermentation analysis of 244 homofermentative LAB isolates as determined on mMRS agar containing a pH indicator (bromocresol purple), supplemented with either sucrose, fructose, lactose, mannitol, raffinose, FOS, or inulin (each at 0.5% (w/v)); (a) LAB strains (%) established positive and negative for individual carbohydrate fermentation; and (b) strains (%) able to utilise a specific number of carbohydrates. Plates were inoculated at 30 °C for 72 h in anaerobic conditions. Ability to ferment specific carbohydrate was indicated by a yellow discolouration zone surrounding a colony.
3.2.2. Microtitre FODMAP Utilisation Assay
The growth parameters of 10 LAB isolates selected based on the agar plate FODMAP utilisation assay results were determined (Table 2). The following species were analysed: Lactiplantibacillus plantarum (L. plantarum; n = 2), Lacticaseibacillus paracasei (L. paracasei; n = 2), and Pediococcus pentosaceus (P. pentosaceus; n = 6). Data obtained from three biological replicates were fitted into a standard form logistic equation (Figure A1). Because of the fastidious nature of LAB and the inclusion of multiple genera in the analysis, mMRS medium was used as a carbohydrate-free base. The mMRS medium is rich in proteins, nucleic acids, and vitamins; therefore, although virtually carbohydrate-free, this allowed for a limited yet variable growth of different strains (ODmMRS range 0.23–0.67). Additionally, due to the difference in cell size and light scattering ratio, the comparison of ODmMRS alone could not be regarded as conclusive for distinguishing differences in the metabolic capacity between strains. Therefore, the ODFODMAP was normalised (ODNORM) against ODmMRS to establish a relative increase in cell density across carbohydrates tested for each strain individually (Table 2) and between strains in individual carbohydrates (Table A1).

Table 2.
FODMAP utilisation profile of selected lactic acid bacteria determined via microtitre FODMAP utilisation assay in mMRS supplemented with either sucrose, fructose, lactose, mannitol, raffinose, FOS, or inulin (each at 0.5%; w/v). Growth was measured as optical density (OD) at 595 nm. Strains were incubated at 30 °C for 72 h. Each strain was analysed in triplicate. The mean value of maximum optical density in mMRS supplemented with FODMAP (ODFODMAP) normalised against OD in unsupplemented mMRS (ODmMRS) was used for analysis.
Consistent with previous studies, strain-specificity for carbon utilisation of LAB was observed [75,87,88,89,90,91,92]. All strains vigorously utilised fructose. Robust growth of sucrose was observed for nine strains, while P. pentosaceus B1.7.1.8A did not grow well on sucrose. Although sucrose is highly favoured by lactobacilli carbohydrate, the ability towards sucrose utilisation may be diminished through environmental adaptation processes [85]. P. pentosaceus being unable to grow on sucrose was previously observed [93]. Good lactose fermentation was observed in L. plantarum FST1.7 and FB102 isolates. L. paracasei R3 and P. pentosaceus strains RYE100 and RYE102 did not grow on lactose, while other Pentosaceus strains could utilise lactose to varying degrees. P. pentosaceus strains could not utilise mannitol (or very limited growth was observed; Table S1), but it was an excellent carbohydrate source for L. plantarum and L. paracasei isolates. Eight strains grew well on short-chain fructans (FOS) but only five on inulin. Multiple comparisons of the strains within FODMAP are presented in Table A1. P. pentosaceus B1.7.1.8A, the least diverse in carbohydrate utilisation among tested isolates (Table 2), was one of the most potent FOS (ODNORM = 3.91 ± 1.16) and fructose (ODNORM = 3.38 ± 0.75) fermenters, while its growth on inulin was very weak (ODNORM = 1.26 ± 0.36) compared to other isolates fermenting this carbohydrate (Table A1).
In contrast, the RYE104, RYE106, and MZ2.6.1.5A Pediococcus isolates grew efficiently on both types of fructans (Table 2). While no significant differences were observed between L. paracasei R3 and P. pentosaceus isolates RYE104, MZ2.6.1.5A, and RYE106 (Table A1), the latter showed the highest biomass increase in inulin (ODNORM = 3.19 ± 0.17) and potent utilisation of FOS (ODNORM = 3.20 ± 0.17), fructose (ODNORM = 3.33 ± 0.19), and sucrose (ODNORM = 3.36 ± 0.39) (Table A1). While strain specificity to utilise short-chain fructans in P. pentosaceus has been described before [94], only limited information is available about inulin fermentation by the species. Good growth on both fructans was observed for L. paracasei strains. L. plantarum isolates had a limited growth on FOS and none on inulin (Table A1). Fermentation of fructans depends on the presence and type of secreted β-fructofuranosidases. While fructans with DP ≤ 4 can be transported and utilised within the cell cytoplasm, longer fructans chains require the expression of extracellular enzymes [28]. The fermentation of carbohydrates is often encoded on plasmids or in the “lifestyle adaptation regions” of the chromosome and contributes to intra-species variability [42,92,95,96,97,98]. Loss of ability to secrete specific enzymes, or expression of a less active enzyme homologue, often acquired via horizontal gene transfer, is common in LAB [86,98]. The same factors could lead to the diauxic growth patterns recorded for L. plantarum FST1.7 and FB102 isolates in raffinose, P. pentosaceus B1.7.8A in FOS, and P. pentosaceus RYE106 and RYE104 in sucrose (Figure A1). Bi-phasic growth is an adaptation mechanism that maximises population growth in multi-nutrient environments. When diauxic growth is observed, complex carbohydrates and metabolites are not co-fermented, which is advantageous in a complex matrix [99]. In commercial applications, however, it may extend the required fermentation time. Strains with strong fructans utilisation generally displayed little to no metabolism of raffinose. Correspondingly, isolates with a high capacity to grow on raffinose showed limited or no growth on fructans, especially inulin. A strain-specific adaptation mechanism, which may be associated with reduced competition for carbon sources in multispecies ecosystems [37], could explain the selectivity of the strains towards specific types of oligosaccharides observed in this and other studies [75,87,88].
Based on the microtitre assay results, two strains with robust growth on fructans were chosen for comparative analysis of the FODMAP reduction in whole-wheat sourdough (SD), namely, Lacticaseibacillus paracasei R3 (R3), a strain previously reported for its antifungal activity [100], and the newly isolated Pediococcus pentosaceus RYE106 (RYE106). Lactiplantibacillus plantarum FST1.7 (FST1.7), which was not able to utilise long-chain fructans (inulin), was also analysed. This strain displayed antifungal activities and has been successfully applied in wheat- and gluten-free SD bread production [63,101], while other members of the species showed efficient utilisation of fructans in wheat dough [49].
3.3. Determination of Cell Count, and Acidity in Sourdoughs
Viable cell count was determined to investigate the ability of three selected LAB strains (FST1.7; R3; and RYE106) to proliferate in whole wheat SD (Figure 3). Acidity is one of the main physical parameters used to evaluate the performance of LAB in SD. The pH of mature SDs ranges between 3.4 and 4.9 and TTA between 4.0 and 25 mL of 1 M NaOH. Higher ash content is associated with higher buffering capacity, therefore, a greater reduction of pH and higher TTA is achieved in whole-meal bread [102,103]. Decreased by LAB metabolism, the acidity of a SD adds to the complexity of the bread flavour profile. It positively influences the activity of endogenous cereal enzymes, and effectively the textural and physical properties of the bread, while limiting the colonisation of spoilage microorganisms [104,105]. Accordingly, the pH, TTA, and lactic and acetic acid content were analysed as the key determinants of SD quality. Acetone was used to ensure proper mitigation/extraction of ions into the water phase. It is a standard method used for cereal-related substrates [60]. With initial inoculum levels (T0) of 6.98 log CFU/g, 6.97 log CFU/g, and 7.34 log CFU/g in SD-FST1.7 (produced with FST1.7), SD-R3 (produced with R3), and SD-RYE106 (produced with RYE106), respectively, a typical mature sourdough cell count above 8 log CFU/g [77] was observed in the three SDs after 10, 24, and 48 h of fermentation (Figure 3a). A slow decline in cell titre has been observed in SD-FST1.7 between T10 and T48, while the pick cell concentration was reached at T24 in SD-RYE106 and T48 in SD-R3. A reduced cell number could indicate high-stress conditions and, possibly, a beginning of a death phase. Yet, the high cell titre established after 48 h of incubation combined with increased acidity between T24 and T48 suggested an active LAB metabolism in all three SD; therefore, there was a high survival rate in an acidic environment (pH < 4) at T48. A substantially reduced pH and increased TTA in SD-RYE106 and SD-FST1.7 during the first 10 h of fermentation was followed by a less pronounced pH reduction (Figure 3b). Nevertheless, the TTA increased at a similar rate during the stationary phase (T10–T48).
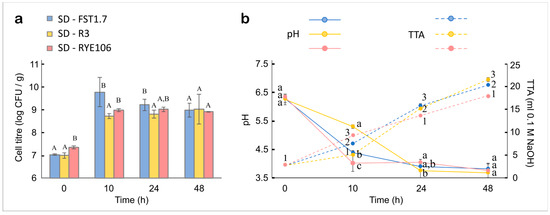
Figure 3.
(a) Microbial growth (log CFU/g of sourdough); and (b) pH (solid line) and total titratable acids TTA (ml of 0.1M NaOH; dashed line) of whole-wheat sourdoughs prepared with Lactiplantibacillus plantarum FST1.7 (SD-FST1.7; blue), Lacticaseibacillus paracasei R3 (SD-R3; yellow), and Pediococcus pentosaceus RYE106 (SD-RYE106; red). Error bars = standard deviation. Significant differences (p < 0.05) in cell titre of isolates at each time interval are indicated by different capital letters, in the pH by lower-case letters, and in the TTA by numbers.
In contrast, during the exponential growth of R3 (T0–T10), the changes in pH and TTA were three-fold slower compared to the later period between T10–T24 of SD-R3 fermentation. Even though a slower increase in TTA was recorded for SD-R3, it showed the highest acidity among the three SDs tested at T48. The differences observed in the changes to pH with respect to the TTA values can be explained by a buffering capacity of lactate and acetate, the main metabolites of LAB fermentation [106] as well as polycarboxylic acids, phosphate salts, fibre, and proteins profile [76]. The specificity of the metabolic activity of the starter culture, therefore, influences the buffering capacity of the SD [107].
The quantities of lactic and acetic acid produced during the 48 h of fermentation established in each SD corresponded with the TTA values (Table 3). The highest organic acid concentration at T48 was calculated for SD-R3, where the highest TTA was recorded. The lowest acid concentration was found in SD-RYE106, the SD with the lowest TTA reading. The lactic acid concentration at T48 varied between 3.94 and 2.88% DM; acetic acid concentration varied between 0.08 and 0.16% DM. As expected, the fermentation quotients (FQ) calculated as a molar ratio of lactic to acetic acid (Table 3) were higher than typically observed in spontaneous SD or SD inoculated with heterofermentative LAB. A high FQ is a known characteristic of homolactic fermentation [37,108], yet, lower FQ obtained with heterofermentative LAB fermentation is considered optimal [109]. Nevertheless, highly acidic flavour is not well accepted by many consumers [110]. A higher ratio of lactic acid corresponding with a milder than acetic acid flavour [111] could prove advantageous, especially in whole-wheat breads with increased phenolic compounds [112,113] as these are associated with an intense aftertaste and bitter flavour [114].

Table 3.
Organic acids concentration (% dry matter; % DM) and fermentation quotient (FQ) of sourdoughs inoculated with Lactiplantibacillus plantarum FST1.7 (SD-FST1.7), Lacticaseibacillus paracasei R3 (SD-R3), and Pediococcus pentosaceus RYE106 (SD-RYE106).
3.4. Impact of LAB and Yeast on Carbohydrate Profile
The FODMAP fermentation assays (discussed above) gave insight into the carbohydrate degradation capabilities of LAB in a single-sugar environment. However, illustrating the catabolic potential of the bacteria alone does not always translate to their behaviour in the complex and dynamic environment of bread dough. To elucidate the impact of selected LAB strains on the carbohydrates in SDs, the concentration of selected carbohydrates (glucose, fructans, maltose, fructose, sucrose, galactose, arabinose, maltotriose, raffinose, stachyose, melibiose, sorbitol, and mannitol) in whole-wheat flour, unfermented SD (directly after mixing; T0), mature SD (after 48 h of fermentation; T48), and the final breads with 20% SD addition (SB) were compared to a straight dough reference bread (RB; Figure 4). Carbohydrate levels were expressed as a per cent of dry matter (% DM).
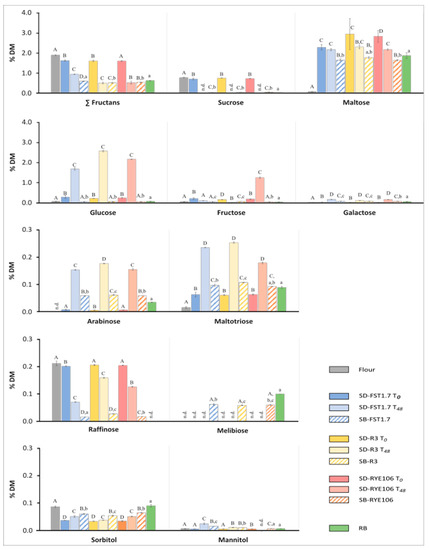
Figure 4.
Carbohydrate profile of whole-wheat flour (grey bars), sourdoughs inoculated with Lactiplantibacillus plantarum FST1.7 (blue bars), Lacticaseibacillus paracasei R3 (yellow bars), and Pediococcus pentosaceus RYE106 (red bars) directly after mixing (SD T0—dark bars), after 48 h of fermentation at 30 °C (SD T48—light bars), in breads with 20% sourdough and 2% yeast (SB—dashed bars) and reference bread (RB, green bar) produced with commercial baker’s yeast preparation (2%; Puratos, Belgium). Carbohydrate concentrations are expressed as a percentage of dry matter (%DM); n.d.—not detected or levels below 0.005% DM. Different capital letters above columns represent significant differences in the concentration of individual carbohydrates between flour, T0, T48, and SB of selected species; different lower-case letters represent significant difference in individual carbohydrate content between the different SBs and RB (p < 0.05).
A rapid increase in maltose concentration (from 0.1% DM in the flour to 2.3% in SD-FST1.7, 2.9% in SD-R3, and 2.8% DM in SD-RYE106) at T0, was in accordance with previous studies [115,116]. After 48 h of fermentation different maltose reduction was observed in individual SD: ~24% in SD-RYE106, ~22% in SD-R3, and ~5% in SD-FST1.7, although the final maltose concentrations in the three SDs (T48) were similar (between 2.16% in SD-FST1.7 and 2.29 in SD-R3). Maltose and maltotriose are released from the starch by flour amylases. It was shown that most of the maltose is produced during mixing while at later dough development stages maltose increase does not exceed 10% [115,116,117]. In the case of SD fermentation, plant amylase activities may be reduced due to increased acidity [118]. The amylolytic activity of LAB is strain-specific, and multiple enzymes were shown to hydrolyse both—starch and maltose [75,87,88,90,91,119]. Accordingly, changes in maltose concentration could be caused by synergistic hydrolysis of endogenous flour enzymes and LAB metabolic activities. Furthermore, the limited affinity of the LAB’s α-glucosidases towards maltotriose [120] could explain an increase in maltotriose levels during SD fermentation (from 0.02% DM in flour to 0.18% in SD-RYE106, 0.24% in SD-FST1.7, and 0.25% DM in SD-R3 at T48).
After 48 h of fermentation, fructans were reduced by 42%, 68%, and 69% in SD-FST1.7, SD-RYE106, and SD-R3, respectively. SD-RYE106 and SD-R3 fermentation resulted in significantly higher fructans reduction (1.08 and 1.10 g/100 g of DM utilised in SD-RYE106 and SD-R3, respectively) compared to SD-FST1.7 (0.69 g/100 g DM utilised). Pejcz et al. [49] recorded a similar reduction of fructans in refined wheat SDs fermented for 48 h with L. plantarum DSM 20174 and L. casei DSM20011 (0.84 and 1.04 g/100 g DM, respectively). To our knowledge, no literature is available on the reduction of fructans in SD by P. pentosaceus. Isolates from the Lacticaseibacillus group are known to secrete extracellular FosE—a very effective β-fructofuranosidase [35], while genes coding the less effective intracellular β-fructofuranosidase SacA were observed in L. plantarum and P. pentosaceus isolates [120,121]. Remarkably, 23% greater hydrolysis of fructans was observed in mature SD-RYE106 compared to SD-FST1.7. Additionally, RYE106 and R3 displayed similar fructans degradation even though L. paracasei R3 belongs to the L. casei group. Similarly, FST1.7 was the least effective at fermenting fructans in microtitre assay analysis (Figure A1, Table A1), while RYE106 showed the best growth in fructans between the three strains analysed in SD. The differences between the FST1.7 and the RYE106 strain could indicate a secretion of a more active homologue of β-fructofuranosidase by RYE106, possibly an extracellular enzyme encoded on a plasmid acquired via horizontal gene transfer (a common environmental adaptation phenomenon observed in lactobacilli, as discussed in Section 3.2).
Sucrose (present at 0.76% DM in the flour) was the only carbohydrate completely depleted during the 48 h of SD fermentation, despite potential carbon-catabolite repression influencing sucrose utilisation in homofermentative LAB [37,122]. Glucose content in wheat dough constantly changes as it is released from longer-chain carbohydrates such as sucrose, maltotriose, fructans, GOS, or starch. The same applies to fructose, a product of sucrose, fructans, and GOS metabolism [28]. Therefore, fluctuation in glucose and fructose concentrations in the whole-wheat matrix during fermentation does not directly reflect strains’ fermentative ability. Nevertheless, a much-increased quantity of fructose in mature SD-RYE106 may suggest a relatively low preference of the RYE106 towards fructose in a multi-carbohydrate environment. This would indicate glucose repression, most likely the cause of the diauxic growth pattern observed in sucrose in the microtitre assay for RYE106 (Figure A1). Catabolite repression by glucose is a known phenomenon in homofermentative LAB; similar fermentation patterns of glucose and fructose by P. pentosaceus were reported by Paramithiotis et al. [93]. It is understood that glucose repression plays a role in the competitiveness of LAB and the maintenance of ecosystem stability in SD [73,80,93]. Despite significantly higher fructose levels in SD-RYE106, excess of fructose in respect to glucose was not observed in either SD. The main GOS found in whole-wheat flour, raffinose, was present in small quantities prior to fermentation (0.21% DM) and was best utilised by the FST1.7 strain (0.07% DM at T48). After 48 h of incubation raffinose in SDs produced with R3 and RYE106 isolates constituted 0.13% and 0.16% DM, respectively. These results correlated with the microtitre FODMAP fermentation assay results. As expected, the application of homofermentative LAB strains limited an increase in polyol content; no xylitol and only very low levels of mannitol and sorbitol (below 0.06% DM) were detected in tested SDs.
Quantification of the carbohydrates in the SBs (produced with 20% of the respective mature SD and 2% baker’s yeast) and RB produced using a straight dough method (with 2% baker’s yeast), allowed us to observe the impact of a synergistic metabolism of LAB and baker’s yeast on the carbohydrate profile of whole-wheat bread (Figure 4). Similar maltose (1.85% DM in RB versus ~1.59%, 1.62%, and 1.76% DM in SD-R3, SD-FST1.7, and SD-RYE106, respectively) and maltotriose levels (~0.1% DM) were found in the different SBs and the RB. Sucrose was added to the final dough mixes to improve their technological qualities, allowing for the optimal production of CO2 by yeast [123]. Increasing the sucrose content of the dough could be controversial given that fructans are degraded with the same enzymes. Nevertheless, the addition of up to 6% of sucrose was shown not to influence the final fructan concentrations in bread after 140 min of fermentation [124]. Hence, with a fermentation time of 150 min in this study, the addition of 1% sucrose was not expected to impair the fructan fermentation rate. Subsequently, sucrose levels in the SBs and RB were below 0.02% DM, and the combined action of yeast and LAB resulted in greater reduction of fructans compared to the RB (0.6% DM). Similar to the mature SDs, slightly higher fructan levels in SB-FST1.7 (0.57% DM) than in the other two SBs (~0.5% DM) could be seen, although the differences in fructan concentration between the SBs were less pronounced than in SDs. These results corresponded with previous findings [115] which showed that baker’s yeast preferentially utilises fructans and fructan-derived metabolites, especially during the first hour of fermentation. Additionally, sourdough has been reported to increase yeast invertase activity by lowering the dough’s pH [125].
The addition of baker’s yeast to the SB formulations resulted in near-complete degradation of glucose and fructose. It also mitigated the differences in fructose concentration in SDs produced with different LAB; the significantly higher fructose concentration in SD-RYE106 compared to the other two SDs was not observed in SB-RYE106. No fructose was detected in RB. Compared to mature SDs (T48), much lower levels of raffinose were detected in SBs; no raffinose was found in RB. As expected, the reduction of raffinose by yeast paralleled with increased melibiose levels. Melibiose is produced during GOS hydrolysis by invertase, an enzyme that is also involved in fructans degradation [126,127]. No major impact of yeast on polyol concentrations was observed.
3.5. Impact of Sourdough Addition on FODMAPs Level in Whole-Wheat Bread
For the food to be considered low in FODMAPs the levels of individual groups of carbohydrates and the sum of all FODMAPs must be below established limits [23]. Commercial bread production processes may or may not lead to sufficient FODMAP reduction; it largely depends on processing parameters, type of flour used, and added ingredients [7,9,39,40]. As stated in the introduction, fructans are the main contributors when it comes to FODMAPs in wheat flour. As well, it is more difficult to comply with low-FODMAP criteria in bread baked from whole-wheat flour, which contains more fructans. The addition of sourdough produced with homofermentative strains reduced fructans by 69% in SB-FST1.7 and by ~73% in SB-R3 and SB-RYE106. Regarding the fresh weight of the breads, the lowest fructans levels of 0.256 ± 0.007 g and 0.257 ± 0.010 g per 100 g ‘as is’ were found in SB-R3 and SB-RYE106, respectively, and were significantly lower than 0.295 ± 0.010 g in SB-FST1.7 and 0.313 ± 0.008 g per 100 g ‘as is’ in RB (Table 4). The low-FODMAP guidance combines fructans and GOS, and a total content of these oligosaccharides combined should not exceed 0.3 g per serving size [23]. The average serving of bread is estimated to be 50 g [66]. Nearly complete degradation of raffinose was achieved in all three SBs (0.01 g/100 g ‘as is’) while an increase in melibiose was observed (0.03 g per 100 g ‘as is’). Similarly, complete degradation of raffinose with concomitant accumulation of melibiose (0.05 g/100 g ‘as is’) was detected in RB, characteristic for GOS hydrolysis via yeasts’ invertase [126,127]. The final reduction of GOS (considering newly produced melibiose) reached ~ 60% in SBs, and 53% in RB. Effectively, combined concentration of oligosaccharides (fructans and GOS) in breads were below the low-FODMAP threshold of 0.3 g per 50 g serving [23,66].

Table 4.
FODMAP concentrations (g/100 g ‘as is’) in RB, produced with commercial baker’s yeast preparation (2%; Puratos, Belgium) and with baker’s yeast and sourdough (20%) based on homofermentative fermentation by Lactiplantibacillus plantarum FST1.7 (SB—FST1.7), Lacticaseibacillus paracasei R3 (SB—R3), and Pediococcus pentosaceus RYE106 (SB—RYE106), and compliance with low-FODMAP cut-off levels per 50 g serving size.
Different to what is often observed in yeasted bread [40,128,129], the metabolism of homofermentative LAB and baker’s yeast in this study resulted in the final glucose concentration being higher or equal to fructose. Therefore, fructose did not contribute to the final FODMAP content in tested products (Table 4). As expected, only low levels of mannitol concentration, a polyol typically found in sourdough [40,43,50], were found in SB breads (0.007 g DM in SB-FST1.7, 0.005 g in SB-R3, and 0.003 g in SB-RYE106 per 100 g ‘as is’). Similarly, a negligible amount of sorbitol (≤0.03 g/100 g ‘as is’) was found in SBs and in the yeasted RB. Therefore, polyol contribution to the total FODMAP content in all breads analysed was far below recommended cut-off value of 0.4 g per serving, and the impact of polyols on the total FODMAP content was insignificant. At the same time, despite their substantial reduction, fructans remained the main contributor to the total FODMAP content (Figure 5). The effective reduction of fructose and oligosaccharides (GOS and fructans), combined with negligible production of polyols, produced low-FODMAP breads (Table 4). Therefore, the use of homofermentative SD proved to be an effective approach in the production of low-FODMAP whole-wheat bread. This approach provides the opportunity to produce breads that are associated with complex flavours, potential health benefits, an absence of preservatives, and an extended shelf life [69,70,71], which are desirable for modern consumers [72].
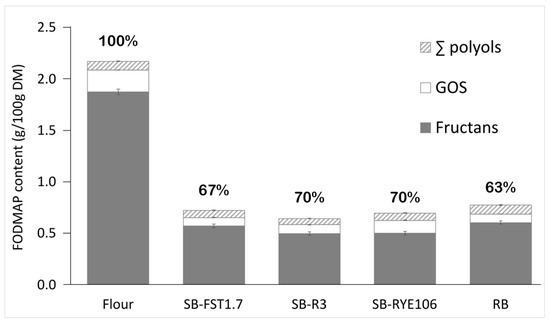
Figure 5.
Total FODMAP (fermentable oligo-, di-, and mono-saccharides and polyols) concentration (g/100 g DM) in whole-wheat flour, RB produced with commercial baker’s yeast preparation (2%; Puratos, Belgium) and with baker’s yeast and sourdough (20%) based on homofermentative fermentation by Lactiplantibacillus plantarum FST1.7 (SB-FST1.7), Lacticaseibacillus paracasei R3 (SB-R3), and Pediococcus pentosaceus RYE106 (SB-RYE106). The solid colour indicates fructans; no fill—GOS (raffinose + melibiose); diagonal straps—polyols (sorbitol + mannitol). No fructose in excess of glucose was detected. Values above the columns represent the degradation of total FODMAPs compared to the flour (%).
3.6. Bread Quality
Major benefits of commercial, defined starter cultures include reduced processing time and reproducibility in terms of stability and quality characteristics of the final product [130]. As mentioned earlier, LAB fermentation, contrary to alcoholic yeast fermentation, results in organic acids production, leading to much lower pH in bread dough [104,105]. A decrease in pH prevents mould and bacterial spoilage [80]; influences proteases/amylases activity which has an impact on gluten development and starch behaviour, and affects fibre solubilisation. This influences bread stability and texture [104,109]. Since texture and stability are pivotal sensory and quality properties of cereal products [131], the effect of sourdough on the technological qualities of whole-wheat bread was evaluated (Table 5).

Table 5.
Quality assessment of whole-wheat breads: RB, produced with commercial baker’s yeast preparation (2%; Puratos, Belgium) and with baker’s yeast and sourdough (20%) based on homofermentative fermentation by Lactiplantibacillus plantarum FST1.7 (SB—FST1.7), Lacticaseibacillus paracasei R3 (SB—R3), and Pediococcus pentosaceus RYE106 (SB—RYE106).
Water activity describes the amount of free water in the food matrix, which is used to predict the survival of microorganisms in food [132]. The incorporation of SD did not affect the bake loss or water activity of the breads (~13% and 0.98, respectively, in all products). SD incorporation did, however, extend the shelf life by one day. The antimicrobial properties of the SDs were most likely related to the metabolites produced by LAB during SD fermentation [101]. Specific volume (SV) is a parameter that reflects gluten network quality and the bread matrix’s capability to retain gas produced during the fermentation process. As the bran fraction in flour limits gluten hydration [133] and negatively interferes with the bonds required for gluten formation [134], a high volume in whole-grain bread is difficult to achieve [135,136,137]. With the addition of SDs, SV was reduced (by 13% in SB-RYE106, 18% in SB-FST1.7, and 21% in SD-R3) when compared to RB, with higher acid contents causing more pronounced effects (Table 3). Although the use of sourdough has often led to increases in SV [131,138,139,140], the higher acidification capacity of whole-grain flours [141] could result in a higher proteolytic activity, which could have a negative impact on the gluten proteins, possibly reducing the gas retention capability. Additionally, other LAB metabolites, including antimicrobials in SDs [75], may have interfered with yeast fermentation and CO2 production. The differences in SV were reflected in the hardness and chewiness of the crumb (HC), with lower specific volumes resulting in more dense crumbs and increasing crumb hardness. SB-R3 had the lowest SV and the hardest crumb (SV = 2.15 mg/mL, HC = 27.23 ± 2.48 N), followed by SB-FST1.7 (SV = 2.22 ± 0.06 mg ml; HC = 26.79 ± 2.74 N) and SB-RYE106 (SV = 2.38 ± 0.09 mg/mL, HC= 23.25 ± 2.49 N), while RB had the highest specific volume (SV = 2.72 ± 0.14 mg/mL) and, effectively, the softest crumb (HC= 16.05 ± 2.48 N). However, this did not appear to be a strain-dependent factor, as no significant differences were noted between SDs in crumb hardness. In line with the results of the SV and the HC, a reduction of the chewiness, slice area, and cell number were observed in SBs compared to the RB. Correspondingly, RB had the highest number of cells, while the average cell diameter did not differ significantly between the four breads. Tested products showed relatively small and uniform cells and no large voids (Table 5, Figure 6), which are desirable qualities in sourdough bread [142].

Figure 6.
Representative images of the whole-wheat breads: RB, produced with commercial baker’s yeast preparation (2%; Puratos, Belgium) and with baker’s yeast and sourdough (20%) based on homofermentative fermentation by Lactiplantibacillus plantarum FST1.7 (SB—FST1.7), Lacticaseibacillus paracasei R3 (SB—R3), and Pediococcus pentosaceus RYE106 (SB—RYE106).
The international colour space, CIE L*a*b* system, used for colour evaluation of the breads in this study determines the lightness of the colour (L*), colour red (a*), and yellow (b*), parameters associated with brown pigment intensity [143]. Maillard reaction (a non-enzymatic browning) is a series of reactions between carbonyl groups of reducing sugars and free amino groups of amino acids, peptides, or proteins resulting in multiple product formations, including melanoidins. Melanoidins, together with caramelisation processes (based on direct heating of carbohydrates), are responsible for the pleasant browning of bread crust [144,145]. Water availability plays a role in both processes [144,145]. However, as equal water activity and bake loss were determined in all four bread samples, this factor had no influence on the browning extent.
In SBs, no significant differences were observed in crust lightness, while redness and yellow colour values were generally higher than in RB explaining a warmer appearance of the breads (Figure 6). In contrast, the crumb lightness values decreased while the crumb’s redness and yellow values increased. Hence, the darker crumb was observed in SBs. While the colour of the crust is affected by Maillard and caramelisation reactions, due to a lower internal temperature of the bread during baking (≤100 °C), crumb colour was not affected by either reaction [144]. It can, therefore, be assumed, that the crumb colour was affected by the metabolism of the microbes used in dough fermentation, having an impact on the extent of protein denaturation and starch gelatinization, and the visual perception of the product [144]. Crust colour is the first sensory attribute assessed by consumers and is directly translated to flavour, texture, and satisfaction level based on previous consumer experience. Hence, dietary habits, and socio-demographic and environmental factors will influence an individual’s product perception [146,147,148,149]. Ultimately, even though some differences were determined with a colourimeter, colour variations between the breads were barely discernible under visual evaluation (Figure 6). The presence of SD in the recipe was the main factor influencing the sensory qualities of the bread, most likely due to assumed differences in protein composition. Overall, the impact of SD on the size, colour, and texture of the breads was minor.
4. Conclusions
In this study FODMAP utilisation profiles of 244 homofermentative lactic acid bacteria isolated from different environments were established. Based on growth analysis via agar and microtitre FODMAP fermentation assays, high variability in their utilisation of FODMAPs was established. Many isolates had the ability to ferment oligosaccharides, particularly short-chain fructooligosaccharides (FOS). Three homofermentative strains were selected to produce whole-wheat sourdough bread. These were Lactiplantibacillus plantarum FST1.7, Lacticaseibacillus paracasei R3, and Pediococcus pentosaceus RYE106. Their impact on FODMAP reduction and whole-wheat sourdough bread quality was investigated. To our knowledge, this is the first study to examine the reduction of FODMAPs in whole-wheat bread by Pediococcus pentosaceus. It is also one of a few focused on homofermentative LAB starters. Three selected strains showed similar acidification and FODMAP metabolism in sourdough, despite weaker growth of L. plantarum on fructans observed in single carbohydrate microtitre FODMAP utilisation analysis. Notwithstanding the relatively high fructans content in the whole-wheat flour, application of said homofermentative LAB isolates in sourdough breads led to a reduction of fructans below the recommended low-FODMAP threshold, while the content of polyols and galacto-oligosaccharides remained low and there was no excess of fructose over glucose. In conclusion, it is evident that sourdough processing with homofermentative Lactiplantibacillus plantarum FST1.7, Lacticaseibacillus paracasei R3, or Pediococcus pentosaceus RYE106 produced low-FODMAP whole-wheat bread with quality comparable to that of straight dough bread.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation9040336/s1, Table S1. Collection of 244 lactic acid bacteria used in the study; Table S2. Carbohydrates used in the study.
Author Contributions
Conceptualization, M.B., L.I., E.Z., E.K.A. and A.C.; methodology, M.B., L.I., E.N., A.W.S., C.P.M., E.K.A. and A.C.; validation, M.B., L.I. and E.N.; formal analysis, M.B., L.I. and C.P.M.; investigation, M.B. and L.I.; resources, E.Z., E.K.A. and A.C.; writing—original draft preparation, M.B.; writing—review and editing, M.B., L.I., E.N., A.W.S., C.P.M., E.Z., E.K.A. and A.C.; supervision, E.K.A. and A.C.; project administration, E.K.A. and A.C.; funding acquisition, E.Z., E.K.A. and A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Department of Agriculture, Food and the Marine, Ireland, TalentFood project code 15F602.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We would like to thank Linda Kerr, Elaine Lawton, and Henry O’Dea, the technical support staff at Muster Technological University.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
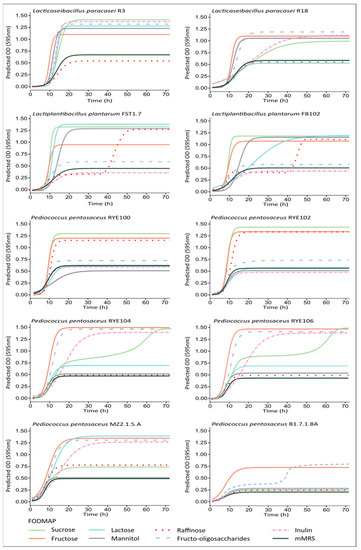
Figure A1.
Growth curves of selected homofermentative lactic acid bacteria in carbohydrate-free mMRS broth and mMRS broth supplemented with individual FODMAPs (0.5% w/v) based on microtitre FODMAPs fermentation assay (OD 595 nm, incubation at 30 °C over 72 h). Means of three independent trials were fitted into a standard form logistic equation. Growth on sucrose (green solid line), fructose (red solid line), lactose (blue solid line), mannitol (navy solid line), raffinose (dotted line), fructo-oligosaccharides (dashed line), and inulin (dash–dot line) and in mMRS (black) were analysed.

Table A1.
Normalised OD (ODNORM = ODFODMAP/ODmMRS) of selected homofermentative lactic acid bacteria in FODMAPs during 72 h incubation at 30 °C in microtitre FODMAP fermentation assay. Values represent means of three independent trials ± standard deviation.
Table A1.
Normalised OD (ODNORM = ODFODMAP/ODmMRS) of selected homofermentative lactic acid bacteria in FODMAPs during 72 h incubation at 30 °C in microtitre FODMAP fermentation assay. Values represent means of three independent trials ± standard deviation.
| Strain | Sucrose | Fructose | Lactose | Mannitol | Raffinose | FOS | Inulin |
|---|---|---|---|---|---|---|---|
| Lacticaseibacillus paracasei R3 | 2.11 ± 0.14 | 1.64 ± 0.13 E | 1.91 ± 0.07 A,C,D,E | 1.84 ± 0.12 A,B | 0.81 ± 0.09 D | 1.97 ± 0.12 | 2.05 ± 0.13 B,C,D |
| Lacticaseibacillus paracasei R18 | 1.88 ± 0.10 A,B | 1.89 ± 0.15 C,E | 0.90 ± 0.08 B | 1.81 ± 0.13 A,B,C | 0.95 ± 0.11 B,D | 2.04 ± 0.12 | 1.92 ± 0.24 |
| Lactiplantibacillus plantarum FST1.7 | 2.84 ± 0.08 B,C | 2.04 ± 0.11 C,D,E | 2.96 ± 0.03 D | 2.76 ± 0.11 A | 2.81 ± 0.09 A | 1.31 ± 0.07 B,C | 0.80 ± 0.05 A |
| Lactiplantibacillus plantarum FB102 | 2.35 ± 0.21 | 2.15 ± 0.21 A,C,D,E | 2.38 ± 0.01 A,D,E | 2.52 ± 0.10 A | 1.16 ± 0.05 A,E | 1.16 ± 0.05 B | 0.86 ± 0.07 A,B |
| Pediococcus pentosaceus RYE100 | 2.15 ± 0.05 | 1.99 ± 0.06 C,D,E | 1.00 ± 0.02 B,C | 0.85 ± 0.02 D | 1.91 ± 0.09 A,C,E | 1.20 ± 0.00 B | 0.98 ± 0.05 A,B,C |
| Pediococcus pentosaceus RYE102 | 2.63 ± 0.68 B,C | 2.46 ± 0.56 | 0.98 ± 0.15 B,C | 0.93 ± 0.18 C,D | 2.43 ± 0.59 A,E | 1.28 ± 0.55 B,C | 0.88 ± 0.27 A,B |
| Pediococcus pentosaceus RYE104 | 3.31 ± 0.27 C | 3.20 ± 0.22 A,B,D | 1.48 ± 0.02 A,B,C,E | 1.08 ± 0.08 B,C,D | 1.07 ± 0.08 B,C,D | 3.14 ± 0.21 A | 2.99 ± 0.22 D |
| Pediococcus pentosaceus RYE106 | 3.36 ± 0.39 C | 3.33 ± 0.19 B | 1.58 ± 0.06 | 1.24 ± 0.10 | 1.11 ± 0.05 B,C,D,E | 3.20 ± 0.17 A | 3.19 ± 0.17 D |
| Pediococcus pentosaceus MZ2.6.1.5A | 1.51 ± 0.09 A | 2.79 ± 0.18 A,B,C,D | 2.89 ± 0.05 D,E | 1.01 ± 0.06 B,C,D | 1.60 ± 0.08 A,B,C,E | 2.71 ± 0.17 A,C | 2.64 ± 0.26 C,D |
| Pediococcus pentosaceus B1.7.1.8A | 1.25 ± 0.45 A | 3.38 ± 0.75 A,B | 1.43 ± 0.53 A,B,C | 1.40 ± 0.58 | 1.21 ± 0.25 B,C,D,E | 3.91 ± 1.16 A | 1.26 ± 0.36 A,B,C |
Different capital letter within column indicates statistical difference (p < 0.05). If no letter was assigned, growth of the strain in a specific FODMAP was not significantly different to any other strain.
References
- Fardet, A. New Hypotheses for the Health-Protective Mechanisms of Whole-Grain Cereals: What Is beyond Fibre? Nutr. Res. Rev. 2010, 23, 65–134. [Google Scholar] [CrossRef] [PubMed]
- Mobley, A.R.; Slavin, J.L.; Hornick, B.A. The Future of Recommendations on Grain Foods in Dietary Guidance. J. Nutr. 2013, 143, 1527S–1532S. [Google Scholar] [CrossRef]
- Kissock, K.R.; Warensjö Lemming, E.; Axelsson, C.; Neale, E.P.; Beck, E.J. Defining Whole-Grain Foods—Does It Change Estimations of Intakes and Associations with CVD Risk Factors: An Australian and Swedish Perspective. Br. J. Nutr. 2021, 126, 1725–1736. [Google Scholar] [CrossRef] [PubMed]
- Lockyer, S.; Spiro, A. The Role of Bread in the UK Diet: An Update. Nutr. Bull. 2020, 45, 133–164. [Google Scholar] [CrossRef]
- Laskowski, W.; Górska-Warsewicz, H.; Rejman, K.; Czeczotko, M.; Zwolińska, J. How Important Are Cereals and Cereal Products in the Average Polish Diet? Nutrients 2019, 11, 679. [Google Scholar] [CrossRef] [PubMed]
- Whole Grain Guidelines Worldwide. Available online: https://wholegrainscouncil.org/whole-grains-101/how-much-enough/whole-grain-guidelines-worldwide (accessed on 11 October 2022).
- Whelan, K.; Abrahmsohn, O.; David, G.J.P.; Staudacher, H.; Irving, P.; Lomer, M.C.E.; Ellis, P.R. Fructan Content of Commonly Consumed Wheat, Rye and Gluten-Free Breads. Int. J. Food Sci. Nutr. 2011, 62, 498–503. [Google Scholar] [CrossRef]
- Biesiekierski, J.R.; Rosella, O.; Rose, R.; Liels, K.; Barrett, J.S.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. Quantification of Fructans, Galacto-Oligosacharides and Other Short-Chain Carbohydrates in Processed Grains and Cereals. J. Hum. Nutr. Diet. 2011, 24, 154–176. [Google Scholar] [CrossRef]
- Ispiryan, L.; Zannini, E.; Arendt, E.K. Characterization of the FODMAP-Profile in Cereal-Product Ingredients. J. Cereal Sci. 2020, 92, 102916. [Google Scholar] [CrossRef]
- Corsetti, M.; Sabaté, J.-M.; Freemantle, N.; Tack, J. IBS Global Impact Report 2018; Canadian Society of Intestinal Research: Vancouver, BC, Canada, 2018. [Google Scholar]
- Canavan, C.; West, J.; Card, T. The Epidemiology of Irritable Bowel Syndrome. Clin. Epidemiol. 2014, 6, 71–80. [Google Scholar] [CrossRef]
- Palsson, O.S.; Whitehead, W.; Törnblom, H.; Sperber, A.D.; Simren, M. Prevalence of Rome IV Functional Bowel Disorders Among Adults in the United States, Canada, and the United Kingdom. Gastroenterology 2020, 158, 1262–1273.e3. [Google Scholar] [CrossRef]
- Sperber, A.D.; Bangdiwala, S.I.; Drossman, D.A.; Ghoshal, U.C.; Simren, M.; Tack, J.; Whitehead, W.E.; Dumitrascu, D.L.; Fang, X.; Fukudo, S.; et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology 2021, 160, 99–114.e3. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Whelan, K. The Low FODMAP Diet: Recent Advances in Understanding Its Mechanisms and Efficacy in IBS. Gut 2017, 66, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Lis, D.M. Exit Gluten-Free and Enter Low FODMAPs: A Novel Dietary Strategy to Reduce Gastrointestinal Symptoms in Athletes. Sport. Med. 2019, 49, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.J.S.; Gaskell, S.K.; McCubbin, A.J.; Snipe, R.M.J. Exertional-Heat Stress-Associated Gastrointestinal Perturbations during Olympic Sports: Management Strategies for Athletes Preparing and Competing in the 2020 Tokyo Olympic Games. Temperature 2020, 7, 58–88. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.A.; Zhan, Y.A.; Dai, S.X. Is a Low FODMAP Diet Beneficial for Patients with Inflammatory Bowel Disease? A Meta-Analysis and Systematic Review. Clin. Nutr. 2018, 37, 123–129. [Google Scholar] [CrossRef]
- Priyanka, P.; Gayam, S.; Kupec, J.T. The Role of a Low Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyol Diet in Nonceliac Gluten Sensitivity. Gastroenterol. Res. Prat. 2018, 2018, 1561476. [Google Scholar] [CrossRef]
- Malinowski, B.; Wiciński, M.; Sokołowska, M.M.; Hill, N.A.; Szambelan, M. The Rundown of Dietary Supplements and Their Effects on Inflammatory Bowel Disease—A Review. Nutrients 2020, 12, 1423. [Google Scholar] [CrossRef] [PubMed]
- Croagh, C.; Shepherd, S.J.; Berryman, M.; Muir, J.G.; Gibson, P.R. Pilot Study on the Effect of Reducing Dietary FODMAP Intake on Bowel Function in Patients without a Colon. Inflamm. Bowel Dis. 2007, 13, 1522–1528. [Google Scholar] [CrossRef]
- Biesiekierski, J.R.; Tuck, C.J. Low FODMAP Diet beyond IBS: Evidence for Use in Other Conditions. Curr. Opin. Pharm. 2022, 64, 102208. [Google Scholar] [CrossRef]
- Gibson, P.R.; Shepherd, S.J. Personal View: Food for Thought—Western Lifestyle and Susceptibility to Crohn’s Disease. The FODMAP Hypothesis. Aliment Pharm. 2005, 21, 1399–1409. [Google Scholar] [CrossRef]
- Varney, J.; Barrett, J.; Scarlata, K.; Catsos, P.; Gibson, P.R.; Muir, J.G. FODMAPs: Food Composition, Defining Cutoff Values and International Application. J. Gastroenterol. Hepatol. 2017, 32, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Moroni, A.V.; Dal Bello, F.; Arendt, E.K. Sourdough in Gluten-Free Bread-Making: An Ancient Technology to Solve a Novel Issue? Food Microbiol. 2009, 26, 676–684. [Google Scholar] [CrossRef]
- El Khoury, D.; Balfour-Ducharme, S.; Joye, I.J. A Review on the Gluten-Free Diet: Technological and Nutritional Challenges. Nutrients 2018, 10, 1410. [Google Scholar] [CrossRef] [PubMed]
- Ispiryan, L.; Zannini, E.; Arendt, E.K. FODMAP Modulation as a Dietary Therapy for IBS: Scientific and Market Perspective. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1491–1516. [Google Scholar] [CrossRef] [PubMed]
- Dimidi, E.; Whelan, K.; Lomer, M.C.E. FODMAP-Specific Mobile Application: Impact on Gut Symptoms in 11,689 People, and Dietary Triggers in 2053 People. Proc. Nutr. Soc. 2020, 79, 2020. [Google Scholar] [CrossRef]
- Loponen, J.; Gänzle, M.G. Use of Sourdough in Low FODMAP Baking. Foods 2018, 7, 96. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Verspreet, J.; Holmgaard Hansen, A.; Dornez, E.; Delcour, J.A.; Van den Ende, W.; Harrison, S.J.; Courtin, C.M. LC-MS Analysis Reveals the Presence of Graminan- and Neo-Type Fructans in Wheat Grains. J. Cereal Sci. 2015, 61, 133–138. [Google Scholar] [CrossRef]
- Van den Ende, W. Multifunctional Fructans and Raffinose Family Oligosaccharides. Front. Plant Sci. 2013, 4, 247. [Google Scholar] [CrossRef]
- Paludan-Müller, C.; Gram, L.; Rattray, F.P. Purification and Characterisation of an Extracellular Fructan β-Fructosidase from a Lactobacillus Pentosus Strain Isolated from Fermented Fish. Syst. Appl. Microbiol. 2002, 25, 13–20. [Google Scholar] [CrossRef]
- Menéndez, C.; Hernández, L.; Selman, G.; Mendoza, M.F.; Hevia, P.; Sotolongo, M.; Arrieta, J.G. Molecular Cloning and Expression in Escherichia Coli of an Exo-Levanase Gene from the Endophytic Bacterium Gluconacetobacter Diazotrophicus SRT4. Curr. Microbiol. 2002, 45, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.J.; Zhang, C.; Benson, A.K.; Schlegel, V.; Lee, J.H.; Hutkins, R.W. Identification of a Putative Operon Involved in Fructooligosaccharide Utilization by Lactobacillus Paracasei. Appl. Env. Microbiol. 2006, 72, 7518–7530. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.J.; Lee, J.-H.; Hutkins, R.W. Functional Analysis of the Fructooligosaccharide Utilization Operon in Lactobacillus Paracasei 1195. Appl. Env. Microbiol. 2007, 73, 5716–5724. [Google Scholar] [CrossRef]
- Saulnier, D.M.A.; Molenaar, D.; De Vos, W.M.; Gibson, G.R.; Kolida, S. Identification of Prebiotic Fructooligosaccharide Metabolism in Lactobacillus Plantarum WCFS1 through Microarrays. Appl. Env. Microbiol. 2007, 73, 1753–1765. [Google Scholar] [CrossRef]
- Gänzle, M.G. Lactic Metabolism Revisited: Metabolism of Lactic Acid Bacteria in Food Fermentations and Food Spoilage. Curr. Opin. Food Sci. 2015, 2, 106–117. [Google Scholar] [CrossRef]
- Acín Albiac, M.; di Cagno, R.; Filannino, P.; Cantatore, V.; Gobbetti, M. How Fructophilic Lactic Acid Bacteria May Reduce the FODMAPs Content in Wheat-Derived Baked Goods: A Proof of Concept. Microb. Cell Fact. 2020, 19, 182. [Google Scholar] [CrossRef] [PubMed]
- Pitsch, J.; Sandner, G.; Huemer, J.; Huemer, M.; Huemer, S.; Weghuber, J. FODMAP Fingerprinting of Bakery Products and Sourdoughs: Quantitative Assessment and Content Reduction through Fermentation. Foods 2021, 10, 894. [Google Scholar] [CrossRef]
- Schmidt, M.; Sciurba, E. Determination of FODMAP Contents of Common Wheat and Rye Breads and the Effects of Processing on the Final Contents. Eur. Food Res. Technol. 2021, 247, 395–410. [Google Scholar] [CrossRef]
- Menezes, L.; Molognoni, L.A.A.; de Sá Ploêncio, L.A.; Costa, F.B.M.; Daguer, H.; de Dea Lindner, J. Use of Sourdough Fermentation to Reducing FODMAPs in Breads. Eur. Food Res. Technol. 2019, 245, 1183–1195. [Google Scholar] [CrossRef]
- Menezes, L.A.A.; de Marco, I.; Neves Oliveira dos Santos, N.; Costa Nunes, C.; Leite Cartabiano, C.E.; Molognoni, L.; Pereira, G.V.d.M.; Daguer, H.; de Dea Lindner, J. Reducing FODMAPs and Improving Bread Quality Using Type II Sourdough with Selected Starter Cultures. Int. J. Food Sci. Nutr. 2021, 72, 912–922. [Google Scholar] [CrossRef]
- Shewry, P.R.; America, A.H.P.; Lovegrove, A.; Wood, A.J.; Plummer, A.; Evans, J.; van den Broeck, H.C.; Gilissen, L.; Mumm, R.; Ward, J.L.; et al. Comparative Compositions of Metabolites and Dietary Fibre Components in Doughs and Breads Produced from Bread Wheat, Emmer and Spelt and Using Yeast and Sourdough Processes. Food Chem. 2022, 374, 131710. [Google Scholar] [CrossRef] [PubMed]
- Wisselink, H.W.; Weusthuis, R.A.; Eggink, G.; Hugenholtz, J.; Grobben, G.J. Mannitol Production by Lactic Acid Bacteria: A Review. Int. Dairy J. 2002, 12, 151–161. [Google Scholar] [CrossRef]
- Rice, T.; Zannini, E.; Arendt, K.E.; Coffey, A. A Review of Polyols–Biotechnological Production, Food Applications, Regulation, Labeling and Health Effects. Crit. Rev. Food Sci. Nutr. 2020, 60, 2034–2051. [Google Scholar] [CrossRef] [PubMed]
- Sahin, A.W.; Rice, T.; Zannini, E.; Axel, C.; Coffey, A.; Lynch, K.M.; Arendt, E.K. Leuconostoc Citreum TR116: In-Situ Production of Mannitol in Sourdough and Its Application to Reduce Sugar in Burger Buns. Int. J. Food Microbiol. 2019, 302, 80–89. [Google Scholar] [CrossRef]
- Jasińska-Kuligowska, I.; Kuligowski, M.; Kołodziejczyk, P.; Michniewicz, J. Wpływ Procesów Fermentacji, Ekstruzji i Wypieku Na Zawartość Fruktanów w Produktach Żytnich. Żywność. Nauka. Technologia. Jakość 2013, 5, 129–141. [Google Scholar]
- Pejcz, E.; Spychaj, R.; Gil, Z. Technological Methods for Reducing the Content of Fructan in Rye Bread. Eur. Food Res. Technol. 2020, 246, 1839–1846. [Google Scholar] [CrossRef]
- Pejcz, E.; Lachowicz-Wiśniewska, S.; Nowicka, P.; Wojciechowicz-budzisz, A.; Spychaj, R.; Gil, Z. Effect of Inoculated Lactic Acid Fermentation on the Fermentable Saccharides and Polyols, Polyphenols and Antioxidant Activity Changes in Wheat Sourdough. Molecules 2021, 26, 4913. [Google Scholar] [CrossRef]
- Li, Q.; Loponen, J.; Gänzle, M.G. Characterization of the Extracellular Fructanase FruA in Lactobacillus Crispatus and Its Contribution to Fructan Hydrolysis in Breadmaking. J. Agric. Food Chem. 2020, 68, 8637–8647. [Google Scholar] [CrossRef]
- Liu, J.; Chey, W.D.; Haller, E.; Eswaran, S. Low-FODMAP Diet for Irritable Bowel Syndrome: What We Know and What We Have Yet to Learn. Annu. Rev. Med. 2020, 71, 303–314. [Google Scholar] [CrossRef]
- Muir, J.G.; Rose, R.; Rosella, O.; Liels, K.; Barrett, J.S.; Shepherd, S.J.; Gibson, P.R. Measurement of Short-Chain Carbohydrates in Common Australian Vegetables and Fruits by High-Performance Liquid Chromatography (HPLC). J. Agric. Food Chem. 2009, 57, 554–565. [Google Scholar] [CrossRef]
- Meroth, C.B.; Walter, J.; Hertel, C.; Brandt, M.J.; Hammes, W.P. Monitoring the Bacterial Population Dynamics in Sourdough Fermentation Processes by Using PCR-Denaturing Gradient Gel Electrophoresis. Appl. Env. Microbiol. 2003, 69, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S Ribosomal DNA Amplification for Phylogenetic Study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Ispiryan, L.; Borowska, M.; Sahin, A.W.; Zannini, E.; Coffey, A.; Arendt, E.K. Lachancea Fermentati FST 5.1: An Alternative to Baker’s Yeast to Produce Low FODMAP Whole Wheat Bread. Food Funct. 2021, 12, 11262–11277. [Google Scholar] [CrossRef] [PubMed]
- de Man, J.C.; Rogosa, M.; Sharpe, M.E. A Medium for the Cultivation of Lactobacilli. J. Appl. Bact. 1960, 23, 130–135. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2021; Available online: http://www.rstudio.com/ (accessed on 11 October 2022).
- Sprouffske, K.; Wagner, A. Growthcurver: An R Package for Obtaining Interpretable Metrics from Microbial Growth Curves. BMC Bioinform. 2016, 17, 172. [Google Scholar] [CrossRef]
- Arbeitsgemeinschaft Getreideforschung e.V. Standard-Methoden Für Getreide, Mehl Und Brot; Verlag Moritz Schäfer: Detmold, Germany, 1954. [Google Scholar]
- Hoehnel, A.; Bez, J.; Sahin, A.W.; Coffey, A.; Arendt, E.K.; Zannini, E. Leuconostoc Citreum TR116 as a Microbial Cell Factory to Functionalise High-Protein Faba Bean Ingredients for Bakery Applications. Foods 2020, 9, 1706. [Google Scholar] [CrossRef]
- Neylon, E.; Arendt, E.K.; Zannini, E.; Sahin, A.W. Fermentation as a Tool to Revitalise Brewers’ Spent Grain and Elevate Techno-Functional Properties and Nutritional Value in High Fibre Bread. Foods 2021, 10, 1639. [Google Scholar] [CrossRef]
- Dal Bello, F.; Clarke, C.I.; Ryan, L.A.M.; Ulmer, H.; Schober, T.J.; Ström, K.; Sjögren, J.; van Sinderen, D.; Schnürer, J.; Arendt, E.K. Improvement of the Quality and Shelf Life of Wheat Bread by Fermentation with the Antifungal Strain Lactobacillus Plantarum FST 1.7. J. Cereal Sci. 2007, 45, 309–318. [Google Scholar] [CrossRef]
- Sahin, A.W.; Axel, C.; Arendt, E.K. Understanding the Function of Sugar in Burger Buns: A Fundamental Study. Eur. Food Res. Technol. 2017, 243, 1905–1915. [Google Scholar] [CrossRef]
- Ispiryan, L.; Heitmann, M.; Hoehnel, A.; Zannini, E.; Arendt, E.K. Optimization and Validation of an HPAEC-PAD Method for the Quantification of FODMAPs in Cereals and Cereal-Based Products. J. Agric. Food Chem. 2019, 67, 4384–4392. [Google Scholar] [CrossRef] [PubMed]
- FDA Reference Amounts Customarily Consumed: List of Products for Each Product Category: Guidance for Industry. Available online: https://www.fda.gov/food/new-nutrition-facts-label/serving-size-updates-new-nutrition-facts-label (accessed on 9 September 2022).
- Signorell, A.; Aho, K.; Alfons, A.; Anderegg, N.; Aragon, T.; Arachchige, C.; Arppe, A.; Baddeley, A.; Barton, K.; Bolker, B.; et al. DescTools: Tools for Descriptive Statistics, R Package Version 0.99.40. Available online: https://cran.r-project.org/package=DescTools (accessed on 11 October 2022).
- Bernardeau, M.; Guguen, M.; Vernoux, J.P. Beneficial Lactobacilli in Food and Feed: Long-Term Use, Biodiversity and Proposals for Specific and Realistic Safety Assessments. FEMS Microbiol. Rev. 2006, 30, 487–513. [Google Scholar] [CrossRef]
- Gänzle, M.G.; Zheng, J. Lifestyles of Sourdough Lactobacilli—Do They Matter for Microbial Ecology and Bread Quality? Int. J. Food Microbiol. 2019, 302, 15–23. [Google Scholar] [CrossRef]
- Katina, K.; Arendt, E.; Liukkonen, K.H.; Autio, K.; Flander, L.; Poutanen, K. Potential of Sourdough for Healthier Cereal Products. Trends Food Sci. Technol. 2005, 16, 104–112. [Google Scholar] [CrossRef]
- Zannini, E.; Gobbetti, M. The 7th International Symposium on Sourdough—“Sourdough for Health. ” Int. J. Food Microbiol. 2019, 302, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Aronson, G. An inside Look into the 2021 Global Consumer Health and Wellness Revolution. Available online: https://nielseniq.com/wp-content/uploads/sites/4/2022/05/NIQ_Global_Health_and_Wellness_Report_2021_1-1.pdf (accessed on 11 October 2022).
- van der Meulen, R.; Scheirlinck, I.; van Schoor, A.; Huys, G.; Vancanneyt, M.; Vandamme, P.; de Vuyst, L. Population Dynamics and Metabolite Target Analysis of Lactic Acid Bacteria during Laboratory Fermentations of Wheat and Spelt Sourdoughs. Appl. Env. Microbiol. 2007, 73, 4741–4750. [Google Scholar] [CrossRef]
- Gobbetti, M.; Minervini, F.; Pontonio, E.; Di Cagno, R.; De Angelis, M. Drivers for the Establishment and Composition of the Sourdough Lactic Acid Bacteria Biota. Int. J. Food Microbiol. 2016, 239, 3–18. [Google Scholar] [CrossRef]
- Bartkiene, E.; Lele, V.; Ruzauskas, M.; Domig, K.J.; Starkute, V.; Zavistanaviciute, P.; Bartkevics, V.; Pugajeva, I.; Klupsaite, D.; Juodeikiene, G.; et al. Lactic Acid Bacteria Isolation from Spontaneous Sourdough and Their Characterization Including Antimicrobial and Antifungal Properties Evaluation. Microorganisms 2020, 8, 64. [Google Scholar] [CrossRef]
- Al-dabbas, M.M.; Al-ismail, K.; Taleb, R.A.; Ibrahim, S. Acid-Base Buffering Properties of Five Legumes and Selected Food in Vitro. Am. J. Agric. Biol. Sci. 2010, 5, 154–160. [Google Scholar] [CrossRef]
- De Vuyst, L.; Neysens, P. The Sourdough Microflora: Biodiversity and Metabolic Interactions. Trends Food Sci. Technol. 2005, 16, 43–56. [Google Scholar] [CrossRef]
- Henström, M.; Diekmann, L.; Bonfiglio, F.; Hadizadeh, F.; Kuech, E.M.; Von Köckritz-Blickwede, M.; Thingholm, L.B.; Zheng, T.; Assadi, G.; DIerks, C.; et al. Functional Variants in the Sucrase-Isomaltase Gene Associate with Increased Risk of Irritable Bowel Syndrome. Gut 2016, 67, 263–270. [Google Scholar] [CrossRef]
- Halmos, E.P.; Gibson, P.R. Controversies and Reality of the FODMAP Diet for Patients with Irritable Bowel Syndrome. J. Gastroenterol. Hepatol. 2019, 34, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Gänzle, M.; Ripari, V. Composition and Function of Sourdough Microbiota: From Ecological Theory to Bread Quality. Int. J. Food Microbiol. 2016, 239, 19–25. [Google Scholar] [CrossRef]
- Vos, P.; Garrity, G.; Jones, D.; Krieg, N.R.; Ludwig, W.; Rainey, F.A.; Schleifer, K.-H.; Whitman, W. Bergey’s Manual of Systematic Bacteriology: Volume 3: The Fermicutes; Springer: New York, NY, USA, 2009; Volume 3, p. 480. [Google Scholar]
- Kandler, O. Carbohydrate Metabolism in Lactic Acid Bacteria. Antonie Leeuwenhoek 1983, 49, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Endo, A.; Dicks, L.M.T. Physiology of the LAB. In Lactic Acid Bacteria: Biodiversity and Taxonomy; Wiley-Blackwell: Chichester, UK, 2014; pp. 13–30. [Google Scholar]
- Leroy, F.; de Winter, T.; Adriany, T.; Neysens, P.; de Vuyst, L. Sugars Relevant for Sourdough Fermentation Stimulate Growth of and Bacteriocin Production by Lactobacillus Amylovorus DCE 471. Int. J. Food Microbiol. 2006, 112, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Gänzle, M.G.; Follador, R. Metabolism of Oligosaccharides and Starch in Lactobacilli: A Review. Front. Microbiol. 2012, 3, 340. [Google Scholar] [CrossRef]
- Wu, C.; Huang, J.; Zhou, R. Genomics of Lactic Acid Bacteria: Current Status and Potential Applications. Crit. Rev. Microbiol. 2017, 43, 393–404. [Google Scholar] [CrossRef]
- Bosma, E.F.; Forster, J.; Nielsen, A.T. Lactobacilli and Pediococci as Versatile Cell Factories—Evaluation of Strain Properties and Genetic Tools. Biotechnol. Adv. 2017, 35, 419–442. [Google Scholar] [CrossRef]
- Buron-Moles, G.; Chailyan, A.; Dolejs, I.; Forster, J.; Mikš, M.H. Uncovering Carbohydrate Metabolism through a Genotype-Phenotype Association Study of 56 Lactic Acid Bacteria Genomes. Appl. Microbiol. Biotechnol. 2019, 103, 3135–3152. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, H.P.; Motherway, M.O.C.; Lakshminarayanan, B.; Stanton, C.; Paul Ross, R.; Brulc, J.; Menon, R.; O’Toole, P.W.; van Sinderen, D. Carbohydrate Catabolic Diversity of Bifidobacteria and Lactobacilli of Human Origin. Int. J. Food Microbiol. 2015, 203, 109–121. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, C.J.; Kunz, B. Identification of Lactic Acid Bacteria Isolated from Kimchi and Studies on Their Suitability for Application as Starter Culture in the Production of Fermented Sausages. Meat Sci. 2006, 72, 437–445. [Google Scholar] [CrossRef]
- Hedberg, M.; Hasslöf, P.; Sjöström, I.; Twetman, S.; Stecksén-Blicks, C. Sugar Fermentation in Probiotic Bacteria—An in Vitro Study. Oral Microbiol. Immunol. 2008, 23, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Manini, F.; Casiraghi, M.C.; Poutanen, K.; Brasca, M.; Erba, D.; Plumed-Ferrer, C. Characterization of Lactic Acid Bacteria Isolated from Wheat Bran Sourdough. LWT-Food Sci. Technol. 2016, 66, 275–283. [Google Scholar] [CrossRef]
- Paramithiotis, S.; Sofou, A.; Tsakalidou, E.; Kalantzopoulos, G. Flour Carbohydrate Catabolism and Metabolite Production by Sourdough Lactic Acid Bacteria. World J. Microbiol. Biotechnol. 2007, 23, 1417–1423. [Google Scholar] [CrossRef]
- Jiang, J.; Yang, B.; Ross, R.P.; Stanton, C.; Zhao, J.; Zhang, H.; Chen, W. Comparative Genomics of Pediococcus Pentosaceus Isolated From Different Niches Reveals Genetic Diversity in Carbohydrate Metabolism and Immune System. Front. Microbiol. 2020, 11, 253. [Google Scholar] [CrossRef] [PubMed]
- Barrangou, R.; Altermann, E.; Hutkins, R.; Cano, R.; Klaenhammer, T.R. Functional and Comparative Genomic Analyses of an Operon Involved in Fructooligosaccharide Utilization by Lactobacillus Acidophilus. Proc. Natl. Acad. Sci. USA 2003, 100, 8957–8962. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, D.; Schuren, F.H.; Vos, W.M.d.; Siezen, R.J.; Kleerebezem, M. Exploring Lactobacillus Plantarum Genome Diversity by Using Microarrays. J. Bacteriol. 2005, 187, 6119–6127. [Google Scholar] [CrossRef]
- Siezen, R.J.; van Hylckama Vlieg, J.E. Genomic Diversity and Versatility of Lactobacillus Plantarum, a Natural Metabolic Engineer. Microb. Cell Fact. 2011, 10, S3. [Google Scholar] [CrossRef]
- Gonzalez, C.F.; Kunka, B.S. Evidence for Plasmid Linkage of Raffinose Utilization and Associated Alpha-Galactosidase and Sucrose Hydrolase Activity in Pediococcus Pentosaceus. Appl. Env. Microbiol. 1986, 51, 105–109. [Google Scholar] [CrossRef]
- Chu, D.; Barnes, D.J. The Lag-Phase during Diauxic Growth Is a Trade-off between Fast Adaptation and High Growth Rate. Sci. Rep. 2016, 6, 25191. [Google Scholar] [CrossRef]
- Pawlowska, A.M. “Green Preservatives”—Combating Fungi in the Food Industry by Applying Antifungal Lactic Acid Bacteria. Ph.D. Thesis, University College Cork, Cork, Ireland, 2013. [Google Scholar]
- Moore, M.M.; Dal Bello, F.; Arendt, E.K. Sourdough Fermented by Lactobacillus Plantarum FST 1.7 Improves the Quality and Shelf Life of Gluten-Free Bread. Eur. Food Res. Technol. 2008, 226, 1309–1316. [Google Scholar] [CrossRef]
- Decock, P.; Cappelle, S. Bread Technology and Sourdough Technology. Trends Food Sci Technol 2005, 16, 113–120. [Google Scholar] [CrossRef]
- Kim, Y.; Huang, W.; Zhu, H.; Rayas-Duarte, P. Spontaneous Sourdough Processing of Chinese Northern-Style Steamed Breads and Their Volatile Compounds. Food Chem. 2009, 114, 685–692. [Google Scholar] [CrossRef]
- Arendt, E.K.; Ryan, L.A.M.; Dal Bello, F. Impact of Sourdough on the Texture of Bread. Food Microbiol. 2007, 24, 165–174. [Google Scholar] [CrossRef]
- Corsetti, A. Technology of Sourdough Fermentation and Sourdough Applications. In Handbook on Sourdough Biotechnology; Gobbetti, M., Gänzle, M., Eds.; Springer Science + Business Media: New York, NY, USA, 2013; Volume 1, pp. 85–103. ISBN 9781461454250. [Google Scholar]
- Piard, J.C.; Desmazeaud, M. Inhibiting Factors Produced by Lactic Acid Bacteria. 1. Oxygen Metabolites and Catabolism End-Products. Lait 1991, 71, 525–541. [Google Scholar] [CrossRef]
- Hansen, Å.; Hansen, B. Influence of Wheat Flour Type on the Production of Flavour Compounds in Wheat Sourdoughs. J. Cereal Sci. 1994, 19, 185–190. [Google Scholar] [CrossRef]
- Novotni, D.; Čukelj, N.; Smerdel, B.; Ćurić, D. Quality Attributes and Firming Kinetics of Partially Baked Frozen Wholewheat Bread with Sourdough. Int. J. Food Sci. Technol. 2013, 48, 2133–2142. [Google Scholar] [CrossRef]
- Hammes, W.P.; Gänzle, M.G. Sourdough Breads and Related Products. In Microbiology of Fermented Foods Wood; Springer: New York, NY, USA; London, UK, 1998; pp. 199–216. ISBN 978-0-7514-0216-2. [Google Scholar]
- Catzeddu, P. Sourdough Breads. In Flour and Breads and their Fortification in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Elsevier Inc.: London, UK, 2011; pp. 37–46. ISBN 9780123808868. [Google Scholar]
- Kröckel, L. The Role of Lactic Acid Bacteria in Safety and Flavour Development of Meat and Meat Products. In Lactic Acid Bacteria—R & D for Food, Health and Livestock Purposes; Kongo, J.M., Ed.; IntechOpen: Rijeka, Croatia, 2013; pp. 129–151. ISBN 978-953-51-0955-6. [Google Scholar]
- van Hung, P.; Maeda, T.; Miyatake, K.; Morita, N. Total Phenolic Compounds and Antioxidant Capacity of Wheat Graded Flours by Polishing Method. Food Res. Int. 2009, 42, 185–190. [Google Scholar] [CrossRef]
- Vaher, M.; Matso, K.; Levandi, T.; Helmja, K.; Kaljurand, M. Phenolic Compounds and the Antioxidant Activity of the Bran, Flour and Whole Grain of Different Wheat Varieties. Procedia Chem. 2010, 2, 76–82. [Google Scholar] [CrossRef]
- Heiniö, R.L.; Liukkonen, K.H.; Myllymäki, O.; Pihlava, J.M.; Adlercreutz, H.; Heinonen, S.M.; Poutanen, K. Quantities of Phenolic Compounds and Their Impacts on the Perceived Flavour Attributes of Rye Grain. J. Cereal Sci. 2008, 47, 566–575. [Google Scholar] [CrossRef]
- Struyf, N.; Laurent, J.; Lefevere, B.; Verspreet, J.; Verstrepen, K.J.; Courtin, C.M. Establishing the Relative Importance of Damaged Starch and Fructan as Sources of Fermentable Sugars in Wheat Flour and Whole Meal Bread Dough Fermentations. Food Chem. 2017, 218, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Hackenberg, S.; Leitner, T.; Jekle, M.; Becker, T. Maltose Formation in Wheat Dough Depending on Mechanical Starch Modification and Dough Hydration. Carbohydr. Polym. 2018, 185, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Potus, J.; Poiffait, A.; Drapron, R. Influence of Dough-Making Conditions on the Concentration of Individual Sugars and Their Utilization during Fermentation. Cereal Chem. 1994, 71, 505–508. [Google Scholar]
- Hartikainen, K.; Katina, K. Improving the Quality of High-Fibre Breads. In Breadmaking: Improving Quality; Cauvain, S.P., Ed.; Woodhead Publishing: Sawston, UK, 2012; pp. 736–753. ISBN 9780857090607. [Google Scholar]
- Petrova, P.; Petrov, K. Direct Starch Conversion into L-(+)-Lactic Acid by a Novel Amylolytic Strain of Lactobacillus Paracasei B41. Starch/Staerke 2012, 64, 10–17. [Google Scholar] [CrossRef]
- Zúñiga, M.; Yebra, M.J.; Monedero, V. Complex Oligosaccharide Utilization Pathways in Lactobacillus. Curr. Issues Mol. Biol. 2020, 40, 49–80. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, F.; Ren, J.; Ai, L.; Dong, Y.; Wu, Z.; Liu, Z.; Chen, W.; Guo, B. Cloning, Expression and Functional Validation of a β-Fructofuranosidase from Lactobacillus Plantarum. Process Biochem. 2014, 49, 758–767. [Google Scholar] [CrossRef]
- Naumoff, D.G.; Livshits, V.A. Molecular Structure of the Lactobacittus Plantarum Sucrose Utilization Locus: A Comparison with Pediococcus Pentosaceus. Mol. Biol. 2001, 35, 19–27. [Google Scholar] [CrossRef]
- Struyf, N.; Laurent, J.; Verspreet, J.; Verstrepen, K.J.; Courtin, C.M. Substrate-Limited Saccharomyces Cerevisiae Yeast Strains Allow Control of Fermentation during Bread Making. J. Agric. Food Chem. 2017, 65, 3368–3377. [Google Scholar] [CrossRef]
- Verspreet, J.; Hemdane, S.; Dornez, E.; Cuyvers, S.; Delcour, J.A.; Courtin, C.M. Maximizing the Concentrations of Wheat Grain Fructans in Bread by Exploring Strategies to Prevent Their Yeast (Saccharomyces Cerevisiae)-Mediated Degradation. J. Agric. Food Chem. 2013, 61, 1397–1404. [Google Scholar] [CrossRef]
- Nilsson, U.; Öste, R.; Jägerstad, M. Cereal Fructans: Hydrolysis by Yeast Invertase, in Vitro and during Fermentation. J. Cereal Sci. 1987, 6, 53–60. [Google Scholar] [CrossRef]
- Vincent, S.F.; Bell, P.J.L.; Bissinger, P.; Nevalainen, K.M.H. Comparison of Melibiose Utilizing Baker’s Yeast Strains Produced by Genetic Engineering and Classical Breeding. Lett. Appl. Microbiol. 1999, 28, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Silvestroni, A.; Connes, C.; Sesma, F.; De Giori, G.S.; Piard, J.C. Characterization of the MelA Locus for α-Galactosidase in Lactobacillus Plantarum. Appl. Env. Microbiol. 2002, 68, 5464–5471. [Google Scholar] [CrossRef] [PubMed]
- Longin, C.F.H.; Beck, H.; Gütler, A.; Gütler, H.; Heilig, W.; Zimmermann, J.; Bischoff, S.C.; Würschum, T. Influence of Wheat Variety and Dough Preparation on FODMAP Content in Yeast-Leavened Wheat Breads. J. Cereal Sci. 2020, 95, 103021. [Google Scholar] [CrossRef]
- Laurent, J.; Struyf, N.; Bautil, A.; Bakeeva, A.; Chmielarz, M.; Lyly, M.; Herrera-Malaver, B.; Passoth, V.; Verstrepen, K.J.; Courtin, C.M. The Potential of Kluyveromyces Marxianus to Produce Low-FODMAP Straight-Dough and Sourdough Bread: A Pilot-Scale Study. Food Bioprocess Technol. 2021, 14, 1920–1935. [Google Scholar] [CrossRef]
- Brandt, M.J. Sourdough Products for Convenient Use in Baking. Food Microbiol. 2007, 24, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Crowley, P.; Schober, T.J.; Clarke, C.I.; Arendt, E.K. The Effect of Storage Time on Textural and Crumb Grain Characteristics of Sourdough Wheat Bread. Eur. Food Res. Technol. 2002, 214, 489–496. [Google Scholar] [CrossRef]
- Tapia, M.S.; Alzamora, S.M.; Chirife, J. Effects of Water Activity (Aw) on Microbial Stability: As a Hurdle in Food Preservation. In Water Activity in Foods: Fundamentals and Applications; Barbosa-Cánovas, G.V., Fontana, A.J., Schmidt, S.J., Labuza, T.P., Eds.; Blackwell Publishing: Oxford, UK, 2008; pp. 239–271. ISBN 0813824087. [Google Scholar]
- Bock, J.E.; Damodaran, S. Bran-Induced Changes in Water Structure and Gluten Conformation in Model Gluten Dough Studied by Fourier Transform Infrared Spectroscopy. Food Hydrocoll. 2013, 31, 146–155. [Google Scholar] [CrossRef]
- Noort, M.W.J.; van Haaster, D.; Hemery, Y.; Schols, H.A.; Hamer, R.J. The Effect of Particle Size of Wheat Bran Fractions on Bread Quality—Evidence for Fibre-Protein Interactions. J. Cereal Sci. 2010, 52, 59–64. [Google Scholar] [CrossRef]
- Gobbetti, M.; Gänzle, M. Handbook on Sourdough Biotechnology; Springer Science & Business Media: New York, NY, USA, 2013; pp. 1–298. [Google Scholar] [CrossRef]
- Heiniö, R.L.; Noort, M.W.J.; Katina, K.; Alam, S.A.; Sozer, N.; de Kock, H.L.; Hersleth, M.; Poutanen, K. Sensory Characteristics of Wholegrain and Bran-Rich Cereal Foods—A Review. Trends Food Sci. Technol. 2016, 47, 25–38. [Google Scholar] [CrossRef]
- Sanz-Penella, J.M.; Tamayo-Ramos, J.A.; Haros, M. Application of Bifidobacteria as Starter Culture in Whole Wheat Sourdough Breadmaking. Food Bioprocess Technol. 2012, 5, 2370–2380. [Google Scholar] [CrossRef]
- Moroni, A.V.; Zannini, E.; Sensidoni, G.; Arendt, E.K. Exploitation of Buckwheat Sourdough for the Production of Wheat Bread. Eur. Food Res. Technol. 2012, 235, 659–668. [Google Scholar] [CrossRef]
- Axel, C.; Röcker, B.; Brosnan, B.; Zannini, E.; Furey, A.; Coffey, A.; Arendt, E.K. Application of Lactobacillus Amylovorus DSM19280 in Gluten-Free Sourdough Bread to Improve the Microbial Shelf Life. Food Microbiol. 2015, 47, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Clément, H.; Prost, C.; Chiron, H.; Ducasse, M.B.; Della Valle, G.; Courcoux, P.; Onno, B. The Effect of Organic Wheat Flour By-Products on Sourdough Performances Assessed by a Multi-Criteria Approach. Food Res. Int. 2018, 106, 974–981. [Google Scholar] [CrossRef]
- Armero, E.; Collar, C. Antistaling Additives, Flour Type and Sourdough Process Effects on Functionality of Wheat Doughs. J. Food Sci. 1996, 61, 299–303. [Google Scholar] [CrossRef]
- Clarke, C.I.; Schober, T.J.; Arendt, E.K. Effect of Single Strain and Traditional Mixed Strain Starter Cultures on Rheological Properties of Wheat Dough and on Bread Quality. Cereal Chem. 2002, 79, 640–647. [Google Scholar] [CrossRef]
- Starowicz, M.; Zieliński, H. How Maillard Reaction Influences Sensorial Properties (Color, Flavor and Texture) of Food Products? Food Rev. Int. 2019, 35, 707–725. [Google Scholar] [CrossRef]
- Purlis, E. Browning Development in Bakery Products—A Review. J. Food Eng. 2010, 99, 239–249. [Google Scholar] [CrossRef]
- Ames, J.M. Control of the Maillard Reaction in Food Systems. Trends Food Sci. Technol. 1990, 1, 150–154. [Google Scholar] [CrossRef]
- Gellynck, X.; Kühne, B.; van Bockstaele, F.; van de Walle, D.; Dewettinck, K. Consumer Perception of Bread Quality. Appetite 2009, 53, 16–23. [Google Scholar] [CrossRef]
- Dewettinck, K.; van Bockstaele, F.; Kühne, B.; van de Walle, D.; Courtens, T.M.; Gellynck, X. Nutritional Value of Bread: Influence of Processing, Food Interaction and Consumer Perception. J. Cereal Sci. 2008, 48, 243–257. [Google Scholar] [CrossRef]
- Hobbs, D.A.; Ashouri, A.; George, T.W.; Lovegrove, J.A.; Methven, L. The Consumer Acceptance of Novel Vegetable-Enriched Bread Products as a Potential Vehicle to Increase Vegetable Consumption. Food Res. Int. 2014, 58, 15–22. [Google Scholar] [CrossRef]
- Torbica, A.; Škrobot, D.; Janić Hajnal, E.; Belović, M.; Zhang, N. Sensory and Physico-Chemical Properties of Wholegrain Wheat Bread Prepared with Selected Food by-Products. LWT-Food Sci. Technol. 2019, 114, 108414. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).