The Antioxidant Activity and Protection of Probiotic Bacteria in the In Vitro Gastrointestinal Digestion of a Blueberry Juice and Whey Protein Fermentation System
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Lactobacillus Strains
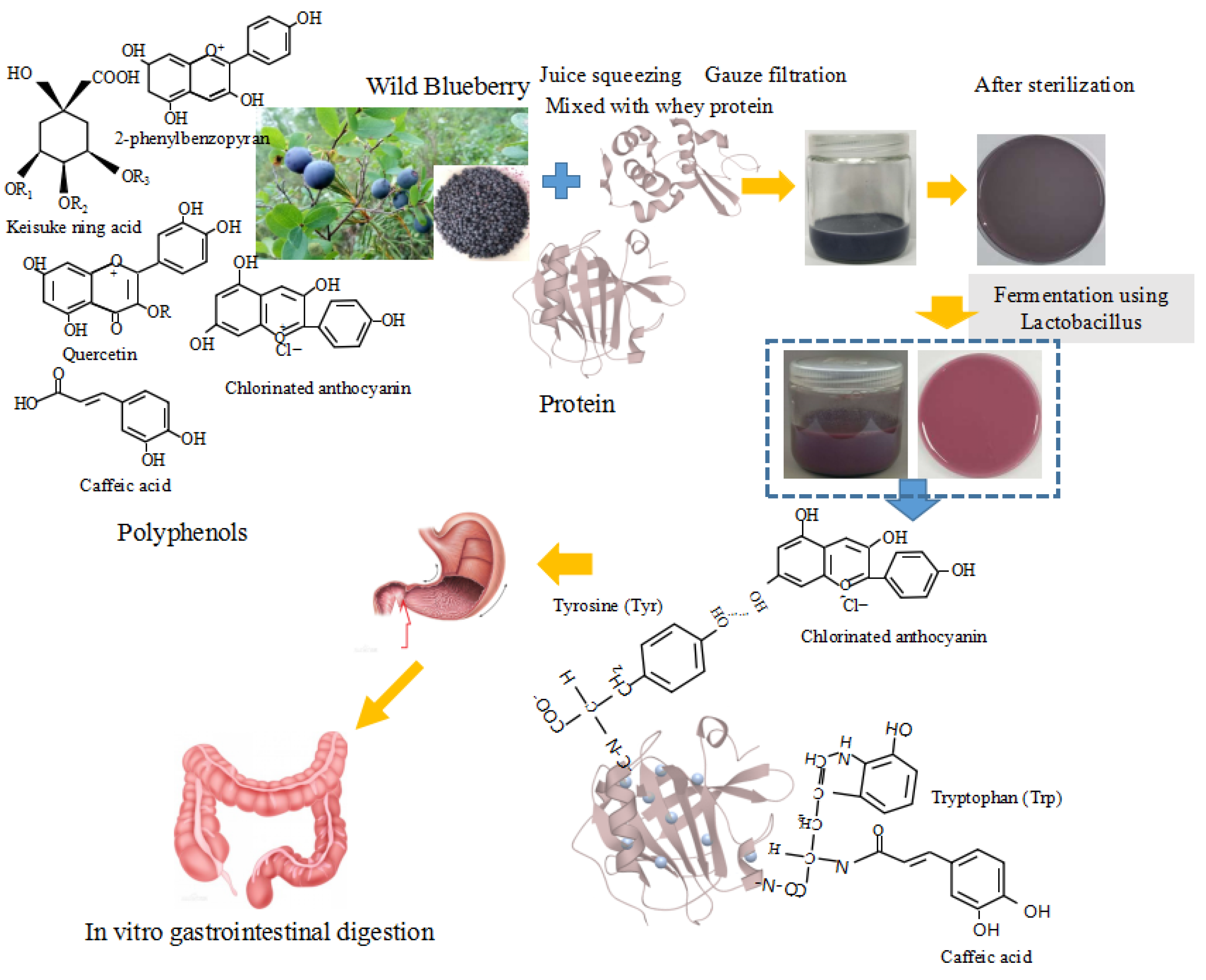
2.2. Fermented Beverage Preparation
2.3. Preparation of Separated Products through Ultrafiltration

2.4. Enumeration of the LAB
2.5. Determination of Total Phenolic Content (TPC)
2.6. Determination of the Anthocyanin Concentration (TAC)
2.7. Determination of Reducing Power
2.8. Determination of DPPH Radical-Scavenging Activity
2.9. In Vitro Gastrointestinal Digestion
2.10. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.11. Confocal-Laser-Scanning Microscopy (CLSM)
2.12. SDS-PAGE
2.13. Statistical Analysis
3. Results and Discussion
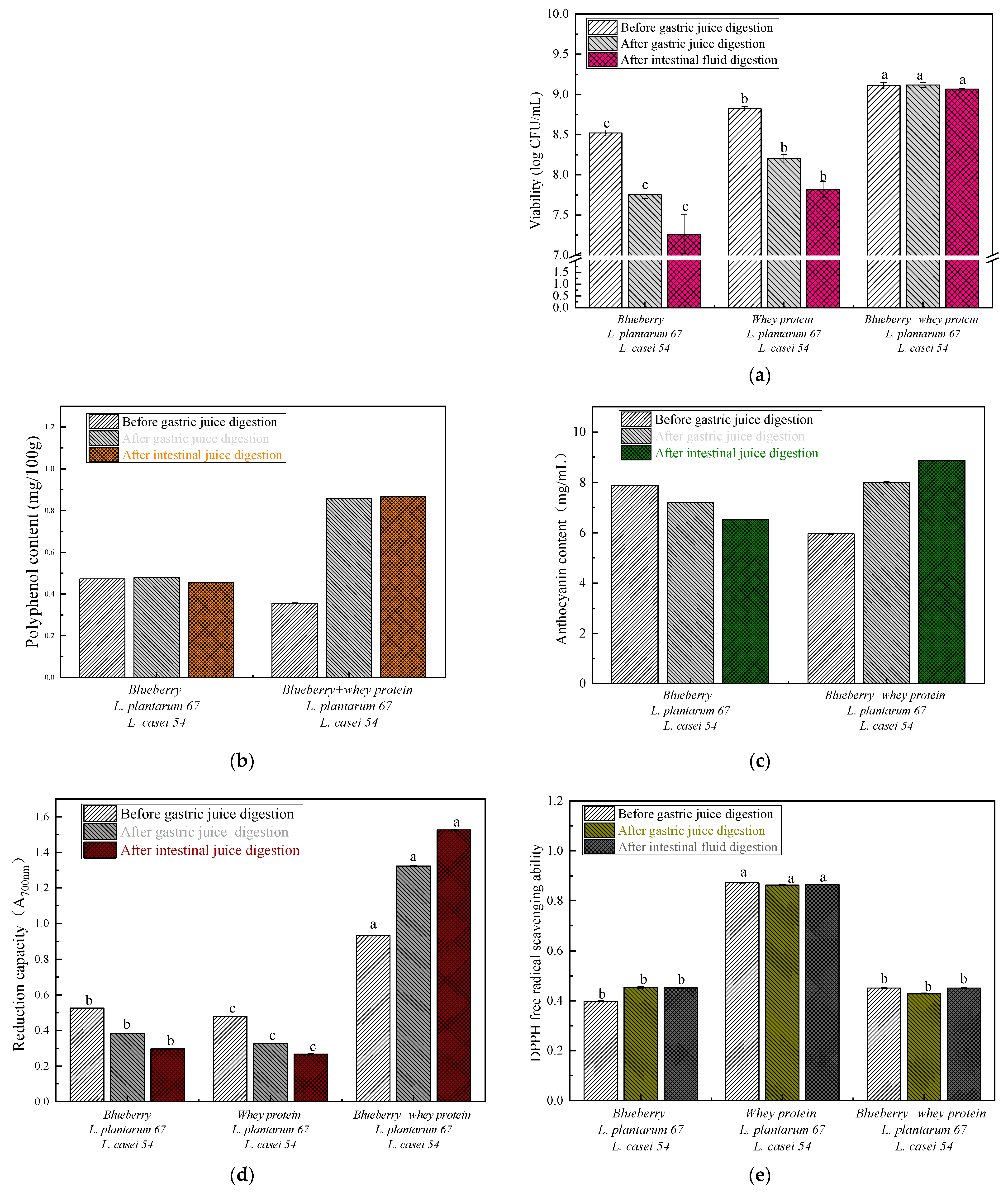
3.1. The Viable Probiotic Cell Count, Bioactive Compounds (TPC and TAC) and Antioxidant Activity (Reducing Power and DPPH) of the Fermented Samples after In Vitro Gastrointestinal Digestion
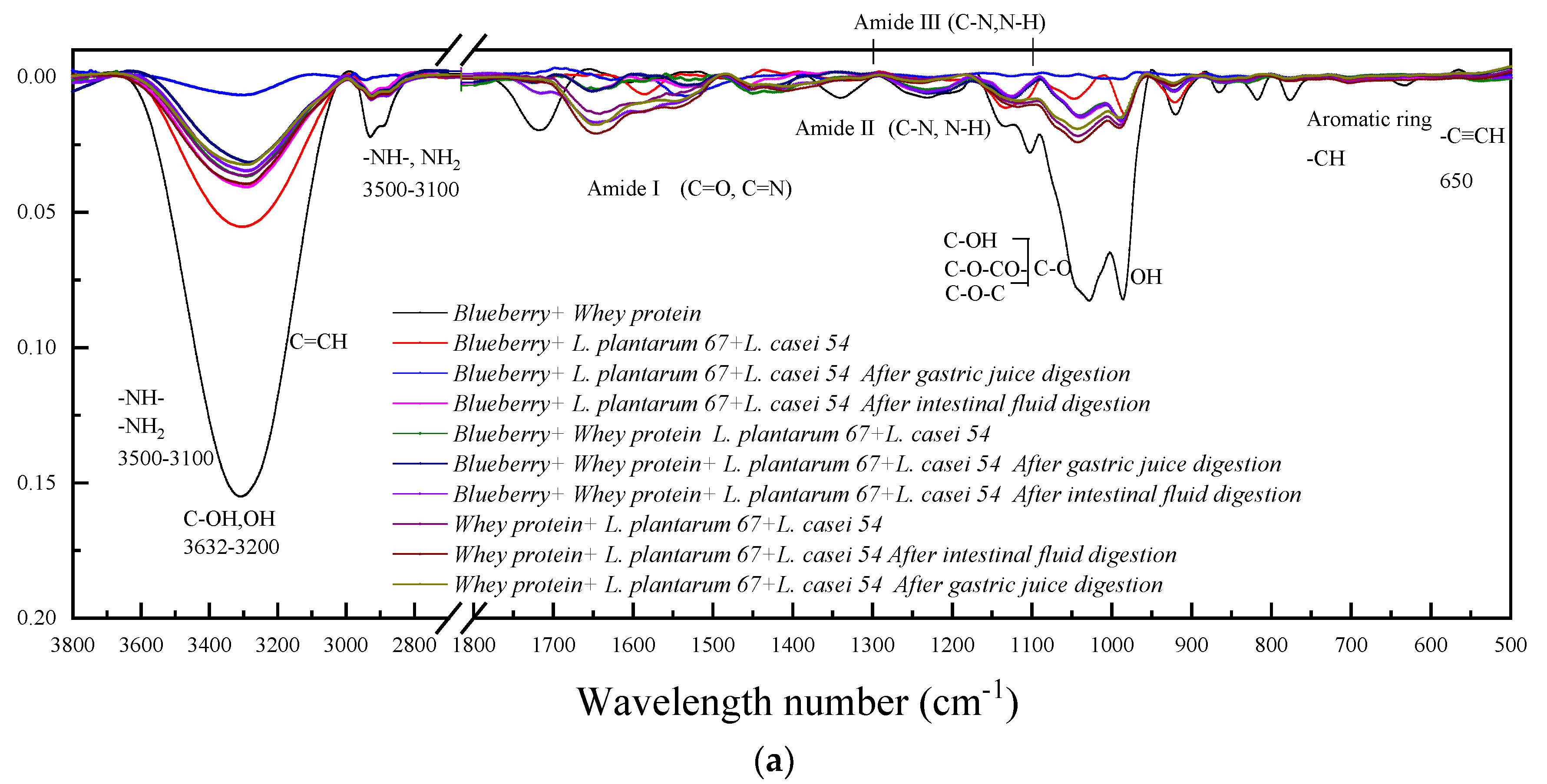
3.2. FTIR Spectrum Analysis
3.3. CLSM Images of the In Vitro Release of Anthocyanins and the Distribution of Probiotics
3.4. The Effect of Ultrafiltration Time on the Survival of Probiotics
3.5. The Total Phenolics Content and Antioxidant Activity (Reducing Power) in Products Separated through Ultrafiltration after In Vitro Gastrointestinal Digestion
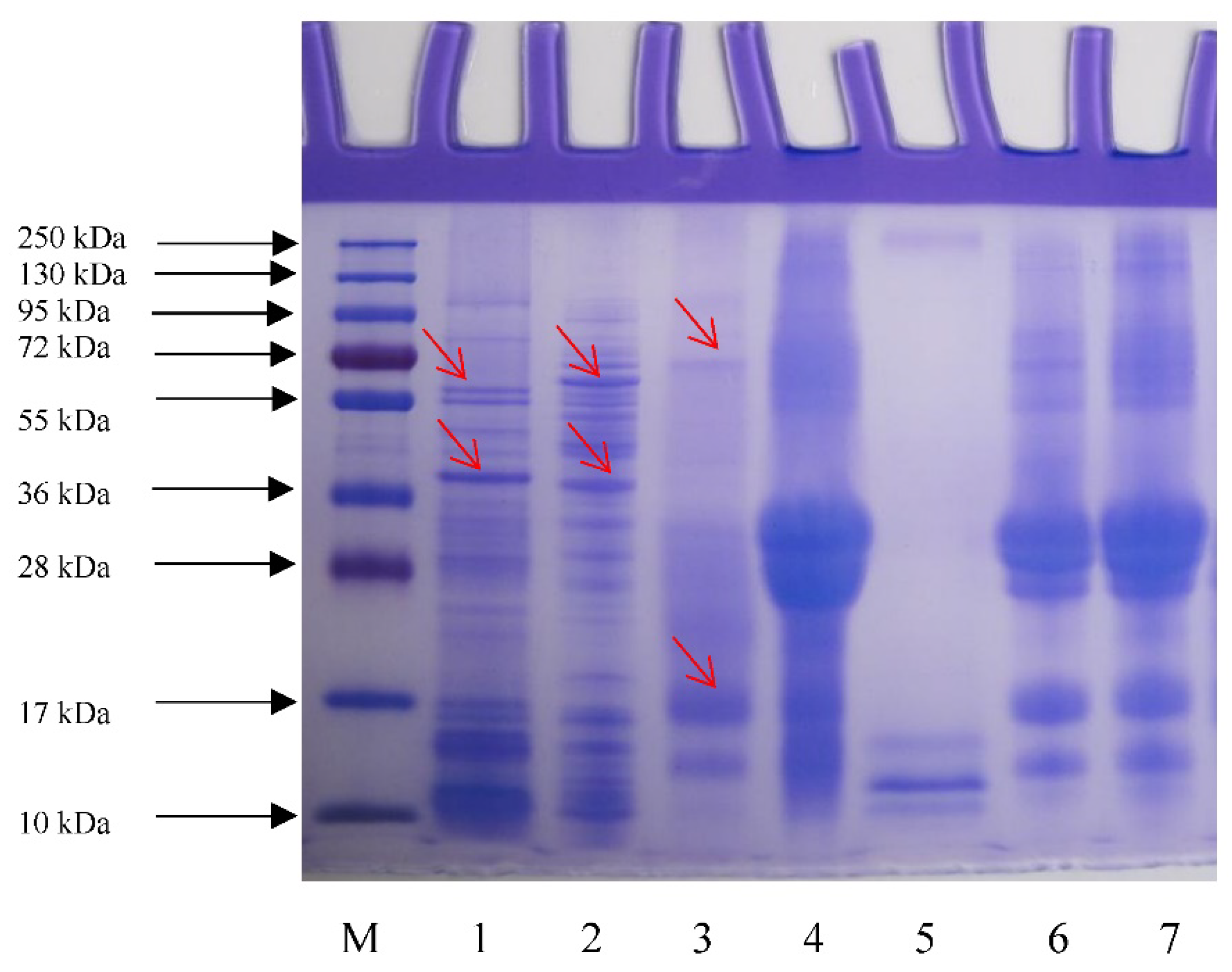
3.6. SDS-PAGE
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oancea, A.-M.; Hasan, M.; Vasile, A.M.; Barbu, V.; Enachi, E.; Bahrim, G.; Râpeanu, G.; Silvi, S.; Stănciuc, N. Functional evaluation of microencapsulated anthocyanins from sour cherries skins extract in whey proteins isolate. LWT-Food Sci. Technol. 2018, 95, 129–134. [Google Scholar] [CrossRef]
- Yang, W.; Guo, Y.; Liu, M.; Chen, X.; Xiao, X.; Wang, S.; Gong, P.; Ma, Y.; Chen, F. Structure and function of blueberry anthocyanins: A review of recent advances. J. Funct. Foods 2022, 88, 104864. [Google Scholar] [CrossRef]
- Anuyahong, T.; Chusak, C.; Adisakwattana, S. Incorporation of anthocyanin-rich riceberry rice in yogurts: Effect on physicochemical properties, antioxidant activity and in vitro gastrointestinal digestion. LWT-Food Sci. Technol. 2020, 129, 109571. [Google Scholar] [CrossRef]
- Afzaal, M.; Saeed, F.; Saeed, M.; Azam, M.; Hussain, S.; Mohamed, A.A.; Alamri, M.S.; Anjum, F.M. Survival and stability of free and encapsulated probiotic bacteria under simulated gastrointestinal and thermal conditions. LWT-Food Sci. Technol. 2020, 23, 1899–1912. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Bosnea, L.; Kanellaki, M.; Kopsahelis, N. Novel frozen yogurt production fortified with sea buckthorn berries and probiotics. LWT-Food Sci. Technol. 2019, 105, 242–249. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, L.; Ding, Z.; Yin, B.; Chen, D.; Guan, C.; Gu, R. New selective media for isolation and enumeration of Lactobacillus rhamnosus and Streptococcus thermophilus. J. Food Meas. Charact. 2019, 13, 1431–1439. [Google Scholar] [CrossRef]
- Shen, X.; Sun, X.; Xie, Q.; Liu, H.; Zhao, Y.; Pan, Y.; Hwang, C.-A.; Wu, V.C.H. Antimicrobial effect of blueberry (Vaccinium corymbosum L.) extracts against the growth of Listeria monocytogenes and Salmonella Enteritidis. Food Control. 2014, 35, 159–165. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, Y.; Li, B.; Tan, H.; Li, D.; Li, L.; Liu, X.; Han, J.; Meng, X. Comparative transcriptome analysis of genes involved in anthocyanin synthesis in blueberry. Plant Physiol. Bioch. 2018, 127, 561–572. [Google Scholar] [CrossRef]
- Lu, C.; Li, C.; Chen, B.; Shen, Y. Composition and antioxidant, antibacterial, and anti-HepG2 cell activities of polyphenols from seed coat of Amygdalus pedunculata Pall. Food Chem. 2018, 265, 111–119. [Google Scholar] [CrossRef]
- Zhou, L.; Xie, M.; Yang, F.; Liu, J. Antioxidant activity of high purity blueberry anthocyanins and the effects on human intestinal microbiota. LWT-Food Sci. Technol. 2020, 117, 108621. [Google Scholar] [CrossRef]
- Liao, M.; Ma, L.; Miao, S.; Hu, X.; Liao, X.; Chen, F.; Ji, J. The in-vitro digestion behaviors of milk proteins acting as wall materials in spray-dried microparticles: Effects on the release of loaded blueberry anthocyanins. Food Hydrocoll. 2021, 115, 106620. [Google Scholar] [CrossRef]
- Meng, J.; Wang, Y.-Y.; Hao, Y.-P. Protective function of surface layer protein from Lactobacillus casei fb05 against intestinal pathogens in vitro. Biochem. Biophys. Res. Commun. 2021, 546, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-Q.; Bao, Y.-H.; Chen, Y. Characteristics and antioxidant activity of water-soluble Maillard reaction products from interactions in a whey protein isolate and sugars system. Food Chem. 2013, 139, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Bambace, M.F.; Alvarez, M.V.; Moreira, M.R. Ready-to-eat blueberries as fruit-based alternative to deliver probiotic microorganisms and prebiotic compounds. LWT-Food Sci. Technol. 2021, 142, 111009. [Google Scholar] [CrossRef]
- Balthazar, C.F.; Santillo, A.; Guimaraes, J.T.; Capozzi, V.; Russo, P.; Caroprese, M.; Marino, R.; Esmerino, E.A.; Raices, R.S.L.; Silva, M.C.; et al. Novel milk-juice beverage with fermented sheep milk and strawberry (Fragaria x ananassa): Nutritional and functional characterization. J. Dairy Sci. 2019, 102, 10724–10736. [Google Scholar] [CrossRef]
- Zhang, C.; Quek, S.Y.; Fu, N.; Su, Y.; Kilmartin, P.A.; Chen, X.D. Storage stability and in vitro digestion of microencapsulated powder containing fermented noni juice and probiotics. Food Biosci. 2020, 37, 100740. [Google Scholar] [CrossRef]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein–phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Flores, F.P.; Singh, R.K.; Kerr, W.L.; Phillips, D.R.; Kong, F. In vitro release properties of encapsulated blueberry (Vaccinium ashei) extracts. Food Chem. 2015, 168, 225–232. [Google Scholar] [CrossRef]
- Zang, Z.; Chou, S.; Tian, J.; Lang, Y.; Shen, Y.; Ran, X.; Gao, N.; Li, B. Effect of whey protein isolate on the stability and antioxidant capacity of blueberry anthocyanins: A mechanistic and in vitro simulation study. Food Chem. 2021, 336, 127700. [Google Scholar] [CrossRef]
- Flores, F.P.; Singh, R.K.; Kerr, W.L.; Pegg, R.B.; Kong, F. Total phenolics content and antioxidant capacities of microencapsulated blueberry anthocyanins during in vitro digestion. Food Chem. 2014, 153, 272–278. [Google Scholar] [CrossRef]
- Hu, S.-M.; Liu, B.-H.; Sun, X.-Y.; Li, X.-D.; Wang, C.-C. The process and optimization of antioxidant peptide preparation by whey protein fermentation. China Dairy 2018, 4, 71–81. [Google Scholar] [CrossRef]
- Tang, P.-L.; Hao, E.-W.; Deng, J.-G.; Hou, X.-T.; Zhang, Z.-H.; Xie, J.-L. Boost anti-oxidant activity of yogurt with extract and hydrolysate of cinnamon residues. Chin. Herb. Med. 2019, 11, 417–422. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; Qi, B.; Sui, X.; Jiang, L. Complexation of thermally-denatured soybean protein isolate with anthocyanins and its effect on the protein structure and in vitro digestibility. Food Res. Int. 2018, 106, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Liu, Y.; Li, L.; Qi, B.; Ju, M.; Xu, Y.; Zhang, Y.; Sui, X. Covalent conjugates of anthocyanins to soy protein: Unravelling their structure features and in vitro gastrointestinal digestion fate. Food Res. Int. 2019, 120, 603–609. [Google Scholar] [CrossRef]
- Quan, T.H.; Benjakul, S.; Sae-leaw, T.; Balange, A.K.; Maqsood, S. Protein–polyphenol conjugates: Antioxidant property, functionalities and their applications. Trends Food Sci. Technol. 2019, 91, 507–517. [Google Scholar] [CrossRef]
- Lan, W.; Zhang, R.; Ji, T.; E, S.D.; Saeed, A.; Qin, W.; Dai, J.; He, L.; Liu, Y. Improving nisin production by encapsulated Lactococcus lactis with starch/carboxymethyl cellulose edible films. Carbohydr. Polym. 2020, 251, 117062. [Google Scholar] [CrossRef]
- Gutiérrez, T.J.; Ponce, A.G.; Alvarez, V.A. Nano-clays from natural and modified montmorillonite with and without added blueberry extract for active and intelligent food nanopackaging materials. Mater. Chem. Phys. 2017, 194, 283–292. [Google Scholar] [CrossRef]
- Khalifa, I.; Nie, R.; Ge, Z.; Li, K.; Li, C. Understanding the shielding effects of whey protein on mulberry anthocyanins: Insights from multispectral and molecular modelling investigations. Int. J. Biol. Macromol. 2018, 119, 116–124. [Google Scholar] [CrossRef]
- Gbassi, G.K.; Vandamme, T.; Ennahar, S.; Marchioni, E. Microencapsulation of Lactobacillus plantarum spp. in an alginate matrix coated with whey proteins. Int. J. Biol. Macromol. 2009, 129, 103–105. [Google Scholar] [CrossRef]
- Gao, B.; Wang, J.; Wang, Y.; Xu, Z.; Li, B.; Meng, X.; Sun, X.; Zhu, J. Influence of fermentation by lactic acid bacteria and in vitro digestion on the biotransformations of blueberry juice phenolics. Food Control. 2022, 133, 1431–1439. [Google Scholar] [CrossRef]
- Correa-Betanzo, J.; Allen-Vercoe, E.; McDonald, J.; Schroeter, K.; Corredig, M.; Paliyath, G. Stability and biological activity of wild blueberry (Vaccinium angustifolium) polyphenols during simulated in vitro gastrointestinal digestion. Food Chem. 2014, 165, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, X.; Feng, S.; Liu, J.; Zhou, L.; Yuan, M.; Ding, C. Characteristics and bioactivities of different molecular weight polysaccharides from camellia seed cake. Int. J. Biol. Macromol. 2016, 91, 1025–1032. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Mujumdar, A.S. New technology to overcome defects in production of fermented plant products—A review. Trends Food Sci. Technol. 2021, 116, 829–841. [Google Scholar] [CrossRef]
- Xiao, Z.; Bin, H.; Jingyan, L.; Huibo, L.; Rongrong, L. Isolation, structure and properties of surface protein of lactic acid bacteria. Food Ferment. Ind. 2011, 37, 19–24. [Google Scholar] [CrossRef]
- Burgain, J.; Scher, J.; Francius, G.; Borges, F.; Corgneau, M.; Revol-Junelles, A.M.; Cailliez-Grimal, C.; Gaiani, C. Lactic acid bacteria in dairy food: Surface characterization and interactions with food matrix components. Adv. Colloid Interface Sci. 2014, 213, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Wakai, T.; Kano, C.; Karsens, H.; Kok, J.; Yamamoto, N. Functional role of surface layer proteins of Lactobacillus acidophilus L-92 in stress tolerance and binding to host cell proteins. Biosci. Microbiota Food Health 2021, 40, 33–42. [Google Scholar] [CrossRef]
- Fu, M.; Mao, K.; Gao, J.; Wang, X.; Sadiq, F.A.; Li, J.; Sang, Y. Characteristics of surface layer protein from Lactobacillus kefiri HBA20 and the role in mediating interactions with Saccharomyces cerevisiae Y8. Int. J. Biol. Macromol. 2022, 201, 254–261. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Q.; Wang, W.; Liu, X.; Shen, W.; Gu, R.; Tang, C. The Antioxidant Activity and Protection of Probiotic Bacteria in the In Vitro Gastrointestinal Digestion of a Blueberry Juice and Whey Protein Fermentation System. Fermentation 2023, 9, 335. https://doi.org/10.3390/fermentation9040335
Yu Q, Wang W, Liu X, Shen W, Gu R, Tang C. The Antioxidant Activity and Protection of Probiotic Bacteria in the In Vitro Gastrointestinal Digestion of a Blueberry Juice and Whey Protein Fermentation System. Fermentation. 2023; 9(4):335. https://doi.org/10.3390/fermentation9040335
Chicago/Turabian StyleYu, Qian, Wenqiong Wang, Xian Liu, Wenwen Shen, Ruixia Gu, and Congcong Tang. 2023. "The Antioxidant Activity and Protection of Probiotic Bacteria in the In Vitro Gastrointestinal Digestion of a Blueberry Juice and Whey Protein Fermentation System" Fermentation 9, no. 4: 335. https://doi.org/10.3390/fermentation9040335
APA StyleYu, Q., Wang, W., Liu, X., Shen, W., Gu, R., & Tang, C. (2023). The Antioxidant Activity and Protection of Probiotic Bacteria in the In Vitro Gastrointestinal Digestion of a Blueberry Juice and Whey Protein Fermentation System. Fermentation, 9(4), 335. https://doi.org/10.3390/fermentation9040335





