Probiotics in the Sourdough Bread Fermentation: Current Status
Abstract
1. Introduction
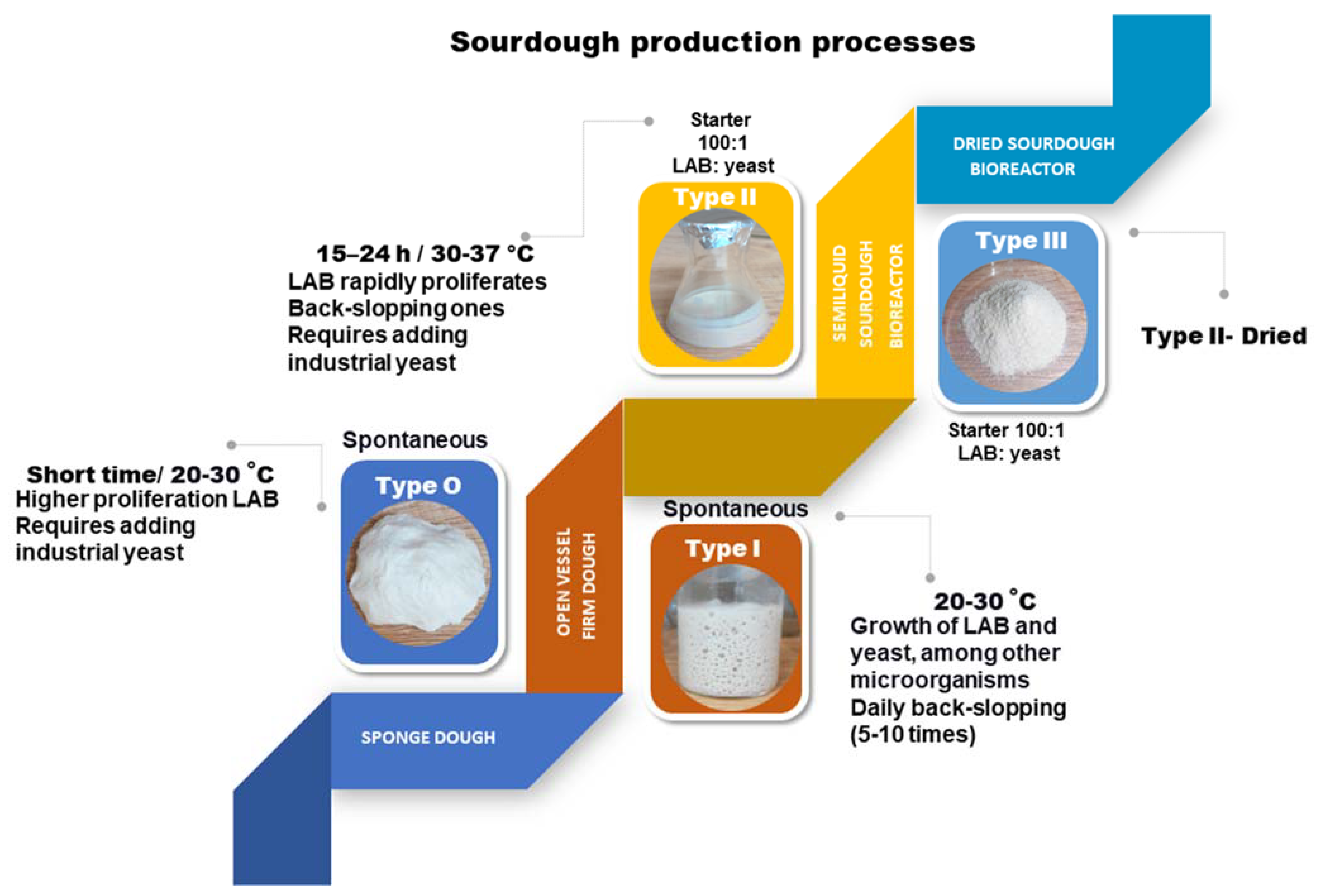
2. Sourdough Fermentation Types: Inoculum and Technology Processes
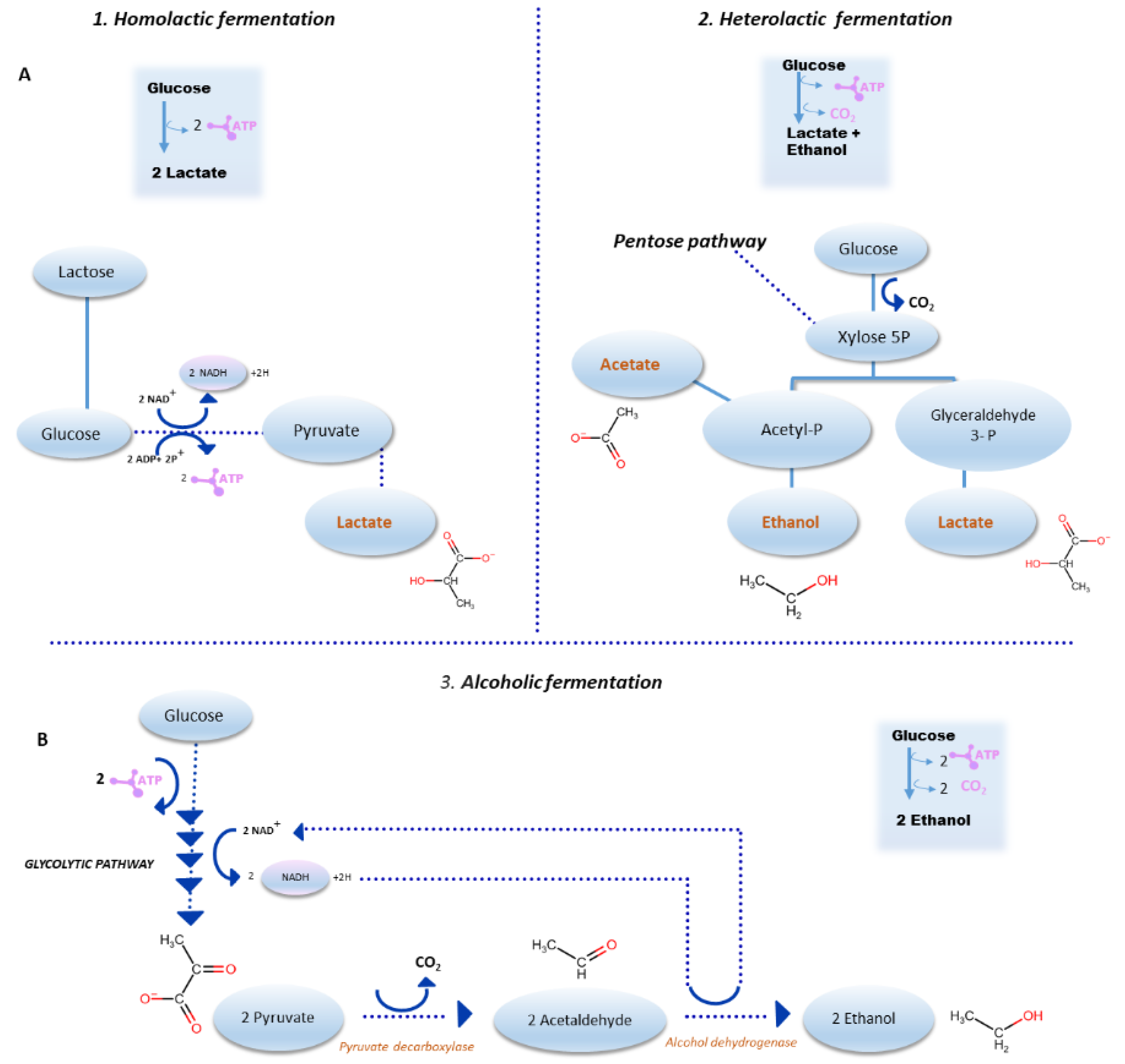
3. Sourdough Fermentation: Major Pathways
4. Probiotics and Postbiotics in Sourdough and the Impact on Human Health
| Microbiota | Reference | |
|---|---|---|
| Bacteria | Acetobacter lovaniensis spp. Acetobacter malorum ssp. Acetobacter pasteurianus/papaya Acetobacter tropicalis Enterecoccus durans Enterobacter hormaechei/cloacae Enterococcus faecalis Enterococcus faecium Enterococcus gilvus Enterococcus hirae Gluconobacter frateurii spp. Gluconobacter sphaericus spp. Komagataeibacter cluster 4 Lactobacillus brevis Lactobacillus coryniformis Lactobacillus curvatus Lactobacillus diolivorans Lactobacillus farciminis Lactobacillus fermentum Lactobacillus gallinarum Lactobacillus kimchii Lactobacillus otakiensis Lactobacillus parabrevis Lactobacillus paracasei Lactobacillus paralimentarius Lactobacillus plantarium Lactobacillus sakei Lactobacillus sanfranciscensis Lactobacillus xiangfangensis Lactococcus lactis Leuconostoc Leuconostoc citreum Pediococcus Pediococcus parvulus Pediococcus pentosaceus Psychrobacter Streptococcus Weissella | [1,34,53,54,60,61] |
| Yeasts | Cida glabrata Cida humilis Hanseniaspora uvarum Kazachstania humilis (synonym Cida humilis) Kazachstania servazzii Kazachstania unispora Kluyveromyces aestuarii Kluyveromyces lactis Kluyveromyces marxianus Pichia fermentans (synonym Cida lambica) Pichia kudriazevii Saccharomyces cerevisiae Saccharomyces uvarum Saccharomycestales sp. Torulaspora delbrueckii Wickerhamomyces anomalus Yarrowia keelungensis | [1,34,53,61,62,63] |
| Inoculum | Compound | Property | Reference |
|---|---|---|---|
| Lactobacillus sanfranciscensis and Lactobacillus reuteri | Glutaminase activity | Significantly influences wheat bread flavor | [64] |
| Lactobacillus diolivorans and Lactobacillus buchneri | Propionate | Increase antifungal property on bread | [65] |
| Weissella cibaria MG1 | Dextrans | Improved volume, crumb softness, and shelf-life | [66] |
| Lactobacillus hammessi | Monohydroxy fatty acid | Antifungal property improving the bread shelf-life | [67] |
| Lactobacillus paracasei RN5, Lactobacillus plantarum X2, Lactobacillus brevis LBRZ7, Lactobacillus fermentum LBRH10, Lactobacillus buchneri LBRZ6, and Propionibacterium frendenreichii ssp. Shermanii NBIMCC 327 | Antimicrobial | Prevent bacterial and mold spoilage | [68] |
| Lactobacillus curvatus 750(13), Pediococcus acidilactici EKO26, Pediococcus pentosaceus 1850(3), Lactobacillus coryniformis pA, Weissella cibaria EKO31, Pediococcus pentosaceus EKO23, Lactobacillus plantarum KKp 593/p, Lactobacillus helveticus 10, Lactobacillus plantarum W37/54, Lactobacillus sakei 750(20), and Lactobacillus rhamnosus Lr (23) | Proteolytic activity | Reducing allergenic proteins and improving the quality of bakery products | [69] |
| Lactobacillus curvatus MA2, Pediococcus pentosaceus OA2, and Pediococcus acidilactici O1A1 | Phytase and antioxidant activities | Improve textural and sensory features of bread | [70] |
| Weissella cibaria PON10030, Weissella cibaria PON10032, Lactobacillus citreum PON 10079, and Lactobacillus citreum PON10080 | Volatile organic compounds | Improve the taste of bread | [71] |
| Saccharomyces bayanus | Aromatic compounds | Improve the sensory profile of bread | [72] |
| Propionibacterium freudenreichii | B12 Vitamin | Potential improvement in nutritional and health value of bread | [73] |
| Torulaspora delbrueckii | Aromatic compounds | Improve the sensory profile of bread | [72] |
| Lactobacillus reuteri LTH5448 and Lactobacillus reuteri 100-23 | γ-glutamyl dipeptides | Influence in the salty taste | [74] |
| Lactobacillus sanfranciscensis, Candida milleri, and transglutaminase | Isodipeptide bonds, ketones, medium-chain fat acids, and alcohols | Positive effects on bread rheological features, shelf-life, and aroma profile | [75] |
| Lactobacillus acidophilus ATCC20552 and Bifidobacterium lactis Bb 12 | Antimicrobial | Inhibit rope-forming B. subtilus | [76] |
| Kluyveromyces marxianus and Saccharomyces cerevisiae | Inulinase | Reduction of fructans, consequently FODMAPs in dough prepared with whole wheat flour | [77] |
| Kluyveromyces marxianus | Inulinase, phytase | Reduction of FODMAPs Highest porosity and lowest hardness | [62,78] |
| Saccharomyces cerevisiae and Pediococcus pentosaceus | Phytase activity | Phytic acid decrease | [79] |
| Gluconobacter oxydans IMDO A845 | Higher amount of lactic acid | Positive aroma profile of sourdough bread | [80] |
| Leuconostoc citreum FDR241 | glycosyltransferase | dextran concentration in sourdough | [81] |
| Enterococcus mundtii QAUSD01 | Proteolytic activity | Gluten-degrading | [60] |
| Wickerhamomyces anomalus QAUWA03 | Proteolytic activity | Gluten-degrading | [60] |
| Lactobacillus plantarum | Phenolic acid esterase, decarboxylases, reductase, and wide range of glycosil hydrolases | Its influence in bread quality needs study | [33] |
| Weissella cibaria VTT E-153485 | Peptidase | Increased proteolysis in faba bean dough | [82] |
| Weissella confusa VTT E-143403 | Dextran | Increased viscosity in faba bean dough | [82] |
| Pediococcus pentosaceus VTT E-153483 and Leuconostoc kimchi VTT E-153484 | Phytase | Reduction of phytic acid in faba bean dough | [82] |
| Lactobacillus amylovorus DSM19280 6% and Weisella cibaria MG1 18% | Organic acid and exopolysaccharide | Low-salt bread with desirable shelf-life, and high sensory quality (volume and crumb texture) | [83] |
| Lactobacillus brevis and Lactobacillus plantarum at 35 °C | Volatile compounds | Improve texture and aromatic properties of sourdough bread | [84] |
| Lactobacillus reuteri | Organic acid, saturated fatty acid, hydroxy fatty acid | Anti-aflatoxigenic capability and antifungal activity | [85] |
| Lactobacillus plantarum 29DAN and L. plantarum 98A | Polyphenol | Antioxidant activity | [86] |
| Lactobacillus plantarum NOS7315, Lactobacillus rossiae NOS7307, Lactobacillus brevis NOS7311, and Saccharomyces cerevisiae PS7314 | Synergistic fermentation | Improved bread sensory characteristic | [87] |
| Bacillus licheniformis | Exopolysaccharides (EPS) | Immunomodulatory potential | [88] |
| Lactobacillus paracasei K5 | Organic acid, higher the complexity of volatile compounds | Decrease spoilage, increase shelf-life, and improve sensory properties in sourdough bread | [89] |
| Enterococcus mundtii QAUSD01 and Wickerhamomyces anomalus QAUWA03 | Proteolytic activity | Toxic gliadin degraded in the sourdough fermentation | [90] |
| Lactobacillus plantarum CH1 | Antifungal compounds | Do not interfere in the sensory quality of bread | [91] |
| Streptococcus thermophilus | Glucosyltransferase B | Bread with lowly digestible starch and textural improvements | [92] |
| Pediococcus pentosaceus SP2 | Organic acid content | Reduce mold and rope spoilage | [93] |
| Enterococcus faecium and Kluyveromyces aestuarii | High phenolic and antioxidant capacity, respectively | Improve bread quality | [62] |
| Lactobacillus reuteri TMW1.656 | Reutericyclin | Inhibition of growth of rope-forming bacilli in bread | [94] |
| Wickerhamomyces anomalus P4 | Phytase | Decrease phytate and increase mineral solubilization in sourdough bread | [95] |
| Levilactobacillus brevis TMW 1.211, Pediococcus claussenii TMW 2.340 from breweries | O2-substituted (1,3)-β-D-glucan | Improving water binding capacity | [96] |
| Weissella confusa/cibaria 3MI3 from sourdough | Dextran | Technological properties of dough and bread, such as water absorption, rheology, stability in cold storage, bread staling, and syneresis of starch gels/avoided the resistant starch formation | [97] |
| Pediococcus lolii B72 and Lactiplantibacillus plantarum E75 from mature sourdough | Volatiles compounds | Improving sensorial acceptability | [98] |
| Microorganisms | Compound | Benefit | Reference |
|---|---|---|---|
| Probiotics Streptococcus thermophilus, Lactobacillus plantarum, L. acidophilus, L. casei, L. delbrueckii spp. bulgaricus, Bifidobacterium breve, B. longum, and B. infantis | Peptidase | Alfa-gliadin degradation, reduced wheat allergenic | [101] |
| LAB from sourdough | Gamma-aminobutyric acid (GABA) | ACE-inhibitory activity | [102] |
| LAB from sourdough | Multifactors | Low-glycemic index in the white wheat bread | [103] |
| Lactobacillus reuteri | Exopolysaccharide | Antiadhesive properties, inhibition enterotoxigenic Escherichia coli | [104] |
| Lactobacillus brevis with S. cerevisiae var. Chevalieri; L. Fermentum; L. Fermentum with phytase | Higher total phenolic and a lower molar ratio of lactic to acetic acid | Reduce glycemic index | [105] |
| L. curvatus SAL33 and L. Brevis AM7 | Peptide lunasin | Cancer preventive | [106] |
| Bifidobacterium strains | Phytase | Increase iron bioavailability in bread | [107] |
| Weissella ciabaria MG1; L. reuteri VIP, L. reuteri Y2 | Oligosaccharides | Improved nutritional quality of sorghum bread | [108] |
| L. brevis | Phytase | Decrease phytate levels, improve mineral bio-accessibility | [109] |
| L. Sakei KTU05-6 | Organic acids, bacteriocins | Bio-preservative | [110] |
| Weissella confusa LBAE C39-2 | Dextransucrase (glycoside hydrolase) | Alfa-glucans/ oligosaccharides or glycoconjugates | [111] |
| L. rossiae DSM 15814 from sourdough | Vitamin B12, folate, and riboflavin | Nutritional improvement | [112] |
| Lactobacillus amylovorus DSM 19280 and Weisella cibaria MG1 | Glutamate accumulation | NaCl reduction in bread | [83] |
| Traditional sourdough LAB starter culture | Essential and non-essential amino acids, flavonoids, antioxidant peptides | Nutritional improvement protects against oxidative stress and degenerative disease through phenolic compounds | [113] |
| LAB from traditional Austrian sourdoughs | Fructose metabolized/antifungal and anti-bacillus properties | Decrease FODMAPS/ control molds | [114] |
| Lactobacillus plantarum ZJUFT17 from Chinese sourdough | In mice, decreased: the profile, insulin resistance, lipopolysaccharide, cytokines interleukin (IL)-1β, tumor necrosis factor (TNF)-α | Managing gut microbiota, decreasing pathogenic and pro-inflammatory microbes, and stimulating anti-obesity ones | [115] |
| Levilactobacillus brevis TMW 1.211, Pediococcus claussenii TMW 2.340 from breweries | O2-substituted (1,3)-β-D-glucan | Prebiotic effect in bread, improving water binding capacity | [96] |
| Lactobacillus plantarum ZJUFB2 from Chinese sourdough | Probiotic effect on gut microbiota | Prevent insulin resistance and modulate gut microbiota. | [116] |
| Levilactobacillus brevis TMW 1.2112, Pediococcus claussenii TMW 2.340 | Dietary fiber, short acid fat chain SCFA, butyrate, propionate,β-glucan | Healthy environment in the colon, chemopreventive | [117] |
| Pediococcus pentosaceus F01, Levilactobacillus brevis MRS4, Lactiplantibacillus plantarum H64, and C48 | Γ-aminobutyric acid (GABA) | Bread from surplus bread with high nutritional value | [118] |
| Weissella cibaria PDER21 | α-D-glucan | Antioxidant properties | [119] |
5. Enzymes in Sourdough
5.1. Transferases
5.2. Oxidoreductases
5.3. Lyases
5.4. Hydrolases
| Enzyme | Microorganisms | Reference |
|---|---|---|
| Xylanase | Sporotrichum thermophile BJAMDU5 | [184] |
| Pichia pastoris | [185] | |
| Bacillus subtilis | [186] | |
| Myceliophthora thermophila BJTLRMDU3 | [187] | |
| Phytase | Lactobacillus casei | [188] |
| Enterobacter sp. ACSS | [189] | |
| Sporotrichum thermophile | [190] | |
| Aspergillus niger NCIM 563 | [191] | |
| Amylase | Rhizopus oryzae | [192] |
| Bacillus subtilis US586 | [193] | |
| Bacillus subtilis M13 | [194] | |
| Streptomyces badiun DB-1 | [195] | |
| Glucose Oxidase | Aspergillus niger | [196] |
| Penicillium notatum | [197] | |
| Aspergillus niger | [198] | |
| Aspergillus niger | [199] | |
| Peptidase | Rhizopus oryzae | [192] |
| Bacillus subtilis PF1 | [200] | |
| Bacillus subtilis | [201] | |
| Bacillus pumilus SG2 | [202] | |
| Lipase | Aspergillus niger MTCC 872 | [203] |
| Pseudomonas fluorescens (NRLL B-2641) | [204] | |
| Bacillus subtilis I-4 | [205] | |
| Bacillus sp. MPTK 912 | [206] | |
| Cellulase | Sporotrichum thermophile BJAMDU5 | [203] |
| Streptomyces strain C188 | [207] | |
| Cellulomonas uda | [208] | |
| Trichoderma reesei NCIM 1186 | [209] |
5.4.1. Amylase, Inulinase, and Their Impact on Bread Structure and Properties
5.4.2. Cellulase, Phytase, and Xylanase for Mineral Bio-Accessibility Improvement in Bread
5.4.3. Lipase and the Baking Technology
5.4.4. Peptidase and Implications for the Gluten Network
6. General Regulation for Microbes Used in Sourdough Bread
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arora, K.; Ameur, H.; Polo, A.; Di Cagno, R.; Rizzello, C.G.; Gobbetti, M. Thirty Years of Knowledge on Sourdough Fermentation: A Systematic Review. Trends Food Sci. Technol. 2021, 108, 71–83. [Google Scholar] [CrossRef]
- De Vuyst, L.; Neysens, P. The Sourdough Microflora: Biodiversity and Metabolic Interactions. Trends Food Sci. Technol. 2005, 16, 43–56. [Google Scholar] [CrossRef]
- Gänzle, M.G. Enzymatic and Bacterial Conversions during Sourdough Fermentation. Food Microbiol. 2014, 37, 2–10. [Google Scholar] [CrossRef]
- Bartkiene, E.; Özogul, F.; Rocha, J.M. Bread Sourdough Lactic Acid Bacteria—Technological, Antimicrobial, Toxin-Degrading, Immune System-, and Faecal Microbiota-Modelling Biological Agents for the Preparation of Food, Nutraceuticals and Feed. Foods 2022, 11, 452. [Google Scholar] [CrossRef] [PubMed]
- Reale, A.; Di Renzo, T.; Preziuso, M.; Panfili, G.; Cipriano, L.; Messia, M.C. Stabilization of Sourdough Starter by Spray Drying Technique: New Breadmaking Perspective. LWT 2019, 99, 468–475. [Google Scholar] [CrossRef]
- Erten, H.; Tanguler, H.; Canbaş, A. A Traditional Turkish Lactic Acid Fermented Beverage: Shalgam (Salgam). Food Rev. Int. 2008, 24, 352–359. [Google Scholar] [CrossRef]
- Fois, S.; Piu, P.P.; Sanna, M.; Roggio, T.; Catzeddu, P. In Vivo and In Vitro Starch Digestibility of Fresh Pasta Produced Using Semolina-Based or Wholemeal Semolina-Based Liquid Sourdough. Foods 2021, 10, 2507. [Google Scholar] [CrossRef]
- Sahin, A.W.; Rice, T.; Zannini, E.; Lynch, K.M.; Coffey, A.; Arendt, E.K. The Incorporation of Sourdough in Sugar-Reduced Biscuits: A Promising Strategy to Improve Techno-Functional and Sensory Properties. Eur. Food Res. Technol. 2019, 245, 1841–1854. [Google Scholar] [CrossRef]
- Venturi, M.; Guerrini, S.; Vincenzini, M. Stable and Non-Competitive Association of Saccharomyces cerevisiae, Candida Milleri and Lactobacillus Sanfranciscensis during Manufacture of Two Traditional Sourdough Baked Goods. Food Microbiol. 2012, 31, 107–115. [Google Scholar] [CrossRef]
- Coda, R.; Cagno, R.D.; Gobbetti, M.; Rizzello, C.G. Sourdough Lactic Acid Bacteria: Exploration of Non-Wheat Cereal-Based Fermentation. Food Microbiol. 2014, 37, 51–58. [Google Scholar] [CrossRef]
- Brandt, M.J. Industrial Production of Sourdoughs for the Baking Branch—An Overview. Int. J. Food Microbiol. 2019, 302, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Kezer, G. Functional Perspective on Sourdough Bread. Turk. J. Agric. Food Sci. Technol. 2022, 10, 1410–1414. [Google Scholar] [CrossRef]
- Hanson, L.; VandeVusse, L.; Forgie, M.; Malloy, E.; Singh, M.; Scherer, M.; Kleber, D.; Dixon, J.; Hryckowian, A.J.; Safdar, N. A Randomized Controlled Trial of an Oral Probiotic to Reduce Antepartum Group B Streptococcus Colonization and Gastrointestinal Symptoms. Am. J. Obstet. Gynecol. MFM 2023, 5, 100748. [Google Scholar] [CrossRef]
- Lee, Y.; Oh, H.; Jo, M.; Cho, H.; Park, Y. Synergistic Effect of N-3 PUFA and Probiotic Supplementation on Bone Loss Induced by Chronic Mild Stress through the Brain–Gut–Bone Axis. J. Funct. Foods 2023, 100, 105363. [Google Scholar] [CrossRef]
- Dotterud, C.K.; Storrø, O.; Johnsen, R.; Øien, T. Probiotics in Pregnant Women to Prevent Allergic Disease: A Randomized, Double-Blind Trial: Probiotics in Pregnant Women to Prevent Allergic Disease. Br. J. Dermatol. 2010, 163, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Rahim, M.H.A.; Hazrin-Chong, N.H.; Harith, H.H.; Wan-Mohtar, W.A.A.Q.I.; Sukor, R. Roles of Fermented Plant-, Dairy- and Meat-Based Foods in the Modulation of Allergic Responses. Food Sci. Hum. Wellness 2023, 12, 691–701. [Google Scholar] [CrossRef]
- Dekker, J.; Quilter, M.; Qian, H. Comparison of Two Probiotics in Follow-on Formula: Bifidobacterium animalis Subsp. Lactis HN019 Reduced Upper Respiratory Tract Infections in Chinese Infants. Benef. Microbes 2022, 13, 341–353. [Google Scholar] [CrossRef]
- He, F.; Shen, X.; Cheng, R.; Marotta, F. Screening Potential Probiotics Against Obesity and Metabolism Abnormalities in the Elderly. In Gut Microbiota in Aging and Chronic Diseases; Marotta, F., Ed.; Healthy Ageing and Longevity; Springer International Publishing: Cham, Switzerland, 2023; Volume 17, pp. 375–386. ISBN 978-3-031-14022-8. [Google Scholar]
- Shi, S.; Zhang, Q.; Sang, Y.; Ge, S.; Wang, Q.; Wang, R.; He, J. Probiotic Bifidobacterium longum BB68S Improves Cognitive Functions in Healthy Older Adults: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2022, 15, 51. [Google Scholar] [CrossRef]
- Jenkins, G.; Mason, P. The Role of Prebiotics and Probiotics in Human Health: A Systematic Review with a Focus on Gut and Immune Health. Food Nutr. J. 2022, 6, 245. [Google Scholar]
- Staniszewski, A.; Kordowska-Wiater, M. Probiotic and Potentially Probiotic Yeasts—Characteristics and Food Application. Foods 2021, 10, 1306. [Google Scholar] [CrossRef]
- Belicová, A.; Mikulášová, M.; Dušinský, R. Probiotic Potential and Safety Properties of Lactobacillus plantarum from Slovak Bryndza Cheese. BioMed Res. Int. 2013, 2013, e760298. [Google Scholar] [CrossRef] [PubMed]
- Llopis, S.; Hernández-Haro, C.; Monteoliva, L.; Querol, A.; Molina, M.; Fernández-Espinar, M.T. Pathogenic Potential of Saccharomyces Strains Isolated from Dietary Supplements. PLoS ONE 2014, 9, e98094. [Google Scholar] [CrossRef] [PubMed]
- Perricone, M.; Bevilacqua, A.; Corbo, M.R.; Sinigaglia, M. Technological Characterization and Probiotic Traits of Yeasts Isolated from Altamura Sourdough to Select Promising Microorganisms as Functional Starter Cultures for Cereal-Based Products. Food Microbiol. 2014, 38, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liang, Q.; Lu, B.; Shen, H.; Liu, S.; Shi, Y.; Leptihn, S.; Li, H.; Wei, J.; Liu, C.; et al. Whole-Genome Analysis of Probiotic Product Isolates Reveals the Presence of Genes Related to Antimicrobial Resistance, Virulence Factors, and Toxic Metabolites, Posing Potential Health Risks. BMC Genom. 2021, 22, 210. [Google Scholar] [CrossRef]
- Jiang, S.; Cai, L.; Lv, L.; Li, L. Pediococcus Pentosaceus, a Future Additive or Probiotic Candidate. Microb. Cell Factories 2021, 20, 45. [Google Scholar] [CrossRef]
- De Vuyst, L.; Comasio, A.; Kerrebroeck, S.V. Sourdough Production: Fermentation Strategies, Microbial Ecology, and Use of Non-Flour Ingredients. Crit. Rev. Food Sci. Nutr. 2022. [Google Scholar] [CrossRef]
- Aplevicz, K.S.; Ogliari, P.J.; Sant’Anna, E.S. Influence of Fermentation Time on Characteristics of Sourdough Bread. Braz. J. Pharm. Sci. 2013, 49, 233–239. [Google Scholar] [CrossRef]
- Altilia, S.; Foschino, R.; Grassi, S.; Antoniani, D.; Dal Bello, F.; Vigentini, I. Investigating the Growth Kinetics in Sourdough Microbial Associations. Food Microbiol. 2021, 99, 103837. [Google Scholar] [CrossRef]
- Carbonetto, B.; Nidelet, T.; Guezenec, S.; Perez, M.; Segond, D.; Sicard, D. Interactions between Kazachstania Humilis Yeast Species and Lactic Acid Bacteria in Sourdough. Microorganisms 2020, 8, 240. [Google Scholar] [CrossRef]
- Chavan, R.S.; Chavan, S.R. Sourdough Technology-A Traditional Way for Wholesome Foods: A Review. Compr. Rev. Food Sci. Food Saf. 2011, 10, 169–182. [Google Scholar] [CrossRef]
- Warburton, A.; Silcock, P.; Eyres, G.T. Impact of Sourdough Culture on the Volatile Compounds in Wholemeal Sourdough Bread. Food Res. Int. 2022, 161, 111885. [Google Scholar] [CrossRef] [PubMed]
- Gänzle, M.G.; Zheng, J. Lifestyles of Sourdough Lactobacilli–Do They Matter for Microbial Ecology and Bread Quality? Int. J. Food Microbiol. 2019, 302, 15–23. [Google Scholar] [CrossRef]
- Van Kerrebroeck, S.; Maes, D.; De Vuyst, L. Sourdoughs as a Function of Their Species Diversity and Process Conditions, a Meta-Analysis. Trends Food Sci. Technol. 2017, 68, 152–159. [Google Scholar] [CrossRef]
- Siepmann, F.B.; Ripari, V.; Waszczynskyj, N.; Spier, M.R. Overview of Sourdough Technology: From Production to Marketing. Food Bioprocess Technol. 2018, 11, 242–270. [Google Scholar] [CrossRef]
- Limbad, M.; Gutierrez Maddox, N.; Hamid, N.; Kantono, K. Sensory and Physicochemical Characterization of Sourdough Bread Prepared with a Coconut Water Kefir Starter. Foods 2020, 9, 1165. [Google Scholar] [CrossRef] [PubMed]
- Mietton, L.; Samson, M.-F.; Marlin, T.; Godet, T.; Nolleau, V.; Guezenec, S.; Segond, D.; Nidelet, T.; Desclaux, D.; Sicard, D. Impact of Leavening Agent and Wheat Variety on Bread Organoleptic and Nutritional Quality. Microorganisms 2022, 10, 1416. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, L.; Van Kerrebroeck, S.; Leroy, F. Microbial Ecology and Process Technology of Sourdough Fermentation. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2017; Volume 100, pp. 49–160. ISBN 978-0-12-812048-4. [Google Scholar]
- Coelho, M.C.; Malcata, F.X.; Silva, C.C.G. Lactic Acid Bacteria in Raw-Milk Cheeses: From Starter Cultures to Probiotic Functions. Foods 2022, 11, 2276. [Google Scholar] [CrossRef]
- Bintsis, T. Lactic acid bacteria as starter cultures: An update in their metabolism and genetics. AIMS Microbiol. 2018, 4, 665–684. [Google Scholar] [CrossRef]
- Castillo Martinez, F.A.; Balciunas, E.M.; Salgado, J.M.; Domínguez González, J.M.; Converti, A.; de Souza Oliveira, R.P. Lactic Acid Properties, Applications and Production: A Review. Trends Food Sci. Technol. 2013, 30, 70–83. [Google Scholar] [CrossRef]
- Gaglio, R.; Alfonzo, A.; Polizzotto, N.; Corona, O.; Francesca, N.; Russo, G.; Moschetti, G.; Settanni, L. Performances of Different Metabolic Lactobacillus Groups during the Fermentation of Pizza Doughs Processed from Semolina. Fermentation 2018, 4, 61. [Google Scholar] [CrossRef]
- Di Cagno, R.; De Angelis, M.; Gallo, G.; Settanni, L.; Berloco, M.G.; Siragusa, S.; Parente, E.; Corsetti, A.; Gobbetti, M. Genotypic and Phenotypic Diversity of Lactobacillus rossiae Strains Isolated from Sourdough. J. Appl. Microbiol. 2007, 103, 821–835. [Google Scholar] [CrossRef] [PubMed]
- Gänzle, M.; Gobbetti, M. Physiology and Biochemistry of Lactic Acid Bacteria. In Handbook on Sourdough Biotechnology; Springer: New York, NY, USA, 2013; pp. 183–216. ISBN 978-1-4614-5424-3. [Google Scholar]
- Yang, H.; Liu, T.; Zhang, G.; He, G. Intraspecific Diversity and Fermentative Properties of Saccharomyces cerevisiae from Chinese Traditional Sourdough. LWT 2020, 124, 109195. [Google Scholar] [CrossRef]
- Kaseleht, K.; Paalme, T.; Mihhalevski, A.; Sarand, I. Analysis of Volatile Compounds Produced by Different Species of Lactobacilli in Rye Sourdough Using Multiple Headspace Extraction: Volatiles Produced by LAB in Sourdough. Int. J. Food Sci. Technol. 2011, 46, 1940–1946. [Google Scholar] [CrossRef]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-Parabiotics: The New Horizons in Microbial Biotherapy and Functional Foods. Microb. Cell Factories 2020, 19, 168. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. Publisher Correction: The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 671. [Google Scholar] [CrossRef]
- El Sheikha, A.F.; Hu, D.-M.M. Molecular Techniques Reveal More Secrets of Fermented Foods. Crit. Rev. Food Sci. Nutr. 2020, 60, 11–32. [Google Scholar] [CrossRef] [PubMed]
- Weckx, S.; Van Kerrebroeck, S.; De Vuyst, L. Omics Approaches to Understand Sourdough Fermentation Processes. Int. J. Food Microbiol. 2019, 302, 90–102. [Google Scholar] [CrossRef]
- Damián, M.R.; Cortes-Perez, N.G.; Quintana, E.T.; Ortiz-Moreno, A.; Garfias Noguez, C.; Cruceño-Casarrubias, C.E.; Sánchez Pardo, M.E.; Bermúdez-Humarán, L.G. Functional Foods, Nutraceuticals and Probiotics: A Focus on Human Health. Microorganisms 2022, 10, 1065. [Google Scholar] [CrossRef]
- Minervini, F.; Celano, G.; Lattanzi, A.; Tedone, L.; de Mastro, G.; Gobbetti, M.; de Angelis, M. Lactic Acid Bacteria in Durum Wheat Flour Are Endophytic Components of the Plant during Its Entire Life Cycle. Appl. Environ. Microbiol. 2015, 81, 6736–6748. [Google Scholar] [CrossRef]
- Landis, E.A.; Oliverio, A.M.; McKenney, E.A.; Nichols, L.M.; Kfoury, N.; Biango-Daniels, M.; Shell, L.K.; Madden, A.A.; Shapiro, L.; Sakunala, S.; et al. The Diversity and Function of Sourdough Starter Microbiomes. eLife 2021, 10, e61644. [Google Scholar] [CrossRef]
- Menezes, L.A.A.; Sardaro, M.L.S.; Duarte, R.T.D.; Mazzon, R.R.; Neviani, E.; Gatti, M.; De Dea Lindner, J. Sourdough Bacterial Dynamics Revealed by Metagenomic Analysis in Brazil. Food Microbiol. 2020, 85, 103302. [Google Scholar] [CrossRef] [PubMed]
- Groenewald, W.H.; Reenen, C.A.V.; Todorov, S.D.; Toit, M.D.; Witthuhn, R.C.; Holzapfel, W.H.; Dicks, L.M.T. Identification of Lactic Acid Bacteria from Vinegar Flies Based on Phenotypic and Genotypic Characteristics. Am. J. Enol. Vitic. 2006, 57, 519–525. [Google Scholar] [CrossRef]
- Minervini, F.; Dinardo, F.R.; De Angelis, M.; Gobbetti, M. Tap Water Is One of the Drivers That Establish and Assembly the Lactic Acid Bacterium Biota during Sourdough Preparation. Sci. Rep. 2019, 9, 570. [Google Scholar] [CrossRef] [PubMed]
- Dinardo, F.R.; Minervini, F.; De Angelis, M.; Gobbetti, M.; Gänzle, M.G. Dynamics of Enterobacteriaceae and Lactobacilli in Model Sourdoughs Are Driven by PH and Concentrations of Sucrose and Ferulic Acid. LWT 2019, 114, 108394. [Google Scholar] [CrossRef]
- De Angelis, M.; Minervini, F.; Siragusa, S.; Rizzello, C.G.; Gobbetti, M. Wholemeal Wheat Flours Drive the Microbiome and Functional Features of Wheat Sourdoughs. Int. J. Food Microbiol. 2019, 302, 35–46. [Google Scholar] [CrossRef]
- Van Kerrebroeck, S.; Bastos, F.C.C.; Harth, H.; De Vuyst, L. A Low PH Does Not Determine the Community Dynamics of Spontaneously Developed Backslopped Liquid Wheat Sourdoughs but Does Influence Their Metabolite Kinetics. Int. J. Food Microbiol. 2016, 239, 54–64. [Google Scholar] [CrossRef]
- Sakandar, H.A.; Usman, K.; Imran, M. Isolation and Characterization of Gluten-Degrading Enterococcus mundtii and Wickerhamomyces anomalus, Potential Probiotic Strains from Indigenously Fermented Sourdough (Khamir). LWT Food Sci. Technol. 2018, 91, 271–277. [Google Scholar] [CrossRef]
- Fraberger, V.; Unger, C.; Kummer, C.; Domig, K.J. Insights into Microbial Diversity of Traditional Austrian Sourdough. LWT 2020, 127, 109358. [Google Scholar] [CrossRef]
- Fekri, A.; Torbati, M.; Yari Khosrowshahi, A.; Bagherpour Shamloo, H.; Azadmard-Damirchi, S. Functional Effects of Phytate-Degrading, Probiotic Lactic Acid Bacteria and Yeast Strains Isolated from Iranian Traditional Sourdough on the Technological and Nutritional Properties of Whole Wheat Bread. Food Chem. 2020, 306, 125620. [Google Scholar] [CrossRef]
- Arici, M.; Ozulku, G.; Yildirim, R.M.; Sagdic, O.; Durak, M.Z. Biodiversity and Technological Properties of Yeasts from Turkish Sourdough. Food Sci. Biotechnol. 2018, 27, 499–508. [Google Scholar] [CrossRef]
- Vermeulen, N.; Gänzle, M.G.; Vogel, R.F. Glutamine Deamidation by Cereal-Associated Lactic Acid Bacteria. J. Appl. Microbiol. 2007, 103, 1197–1205. [Google Scholar] [CrossRef]
- Zhang, C.; Brandt, M.J.; Schwab, C.; Gänzle, M.G. Propionic Acid Production by Cofermentation of Lactobacillus buchneri and Lactobacillus diolivorans in Sourdough. Food Microbiol. 2010, 27, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Sandra, G.; Schwab, C.; Bello, F.D.; Coffey, A.; Gänzle, M.; Arendt, E. Comparison of the Impact of Dextran and Reuteran on the Quality of Wheat Sourdough Bread. J. Cereal Sci. 2012, 56, 531–537. [Google Scholar] [CrossRef]
- Black, B.A.; Zannini, E.; Curtis, J.M.; Gänzle, M.G. Antifungal Hydroxy Fatty Acids Produced during Sourdough Fermentation: Microbial and Enzymatic Pathways, and Antifungal Activity in Bread. Appl. Environ. Microbiol. 2013, 79, 1866–1873. [Google Scholar] [CrossRef] [PubMed]
- Denkova, R.; Ilieva, S.; Denkova, Z.; Georgieva, L.; Yordanova, M.; Nikolova, D.; Evstatieva, Y. Production of Wheat Bread without Preservatives Using Sourdough Starters. Biotechnol. Biotechnol. Equip. 2014, 28, 889–898. [Google Scholar] [CrossRef]
- Stefańska, I.; Piasecka-Jóźwiak, K.; Kotyrba, D.; Kolenda, M.; Stecka, K.M. Selection of Lactic Acid Bacteria Strains for the Hydrolysis of Allergenic Proteins of Wheat Flour. J. Sci. Food Agric. 2016, 96, 3897–3905. [Google Scholar] [CrossRef]
- Mamhoud, A.; Nionelli, L.; Bouzaine, T.; Hamdi, M.; Gobbetti, M.; Rizzello, C.G. Selection of Lactic Acid Bacteria Isolated from Tunisian Cereals and Exploitation of the Use as Starters for Sourdough Fermentation. Int. J. Food Microbiol. 2016, 225, 9–19. [Google Scholar] [CrossRef]
- Corona, O.; Alfonzo, A.; Ventimiglia, G.; Nasca, A.; Francesca, N.; Martorana, A.; Moschetti, G.; Settanni, L. Industrial Application of Selected Lactic Acid Bacteria Isolated from Local Semolinas for Typical Sourdough Bread Production. Food Microbiol. 2016, 59, 43–56. [Google Scholar] [CrossRef]
- Aslankoohi, E.; Herrera-Malaver, B.; Rezaei, M.N.; Steensels, J.; Courtin, C.M.; Verstrepen, K.J. Non-Conventional Yeast Strains Increase the Aroma Complexity of Bread. PLoS ONE 2016, 11, e0165126. [Google Scholar] [CrossRef]
- Edelmann, M.; Chamlagain, B.; Santin, M.; Kariluoto, S.; Piironen, V. Stability of Added and in Situ-Produced Vitamin B12 in Breadmaking. Food Chem. 2016, 204, 21–28. [Google Scholar] [CrossRef]
- Zhao, C.J.; Gänzle, M.G. Synthesis of Taste-Active γ-Glutamyl Dipeptides during Sourdough Fermentation by Lactobacillus Reuteri. J. Agric. Food Chem. 2016, 64, 7561–7568. [Google Scholar] [CrossRef] [PubMed]
- Scarnato, L.; Montanari, C.; Serrazanetti, D.I.; Aloisi, I.; Balestra, F.; Del Duca, S.; Lanciotti, R. New Bread Formulation with Improved Rheological Properties and Longer Shelf-Life by the Combined Use of Transglutaminase and Sourdough. LWT Food Sci. Technol. 2017, 81, 101–110. [Google Scholar] [CrossRef]
- Elsanhoty, R.M.; Ghonamy, A.G.; El-Adly, N.A.; Fawzy Ramadan, M. Impact of Lactic Acid Bacteria and Bifidobacterium on the Survival of Bacillus subtilus During Fermentation of Wheat Sourdough. J. Food Process. Preserv. 2017, 41, e13086. [Google Scholar] [CrossRef]
- Struyf, N.; Laurent, J.; Verspreet, J.; Verstrepen, K.J.; Courtin, C.M. Saccharomyces cerevisiae and Kluyveromyces marxianus Cocultures Allow Reduction of Fermentable Oligo-, Di-, and Monosaccharides and Polyols Levels in Whole Wheat Bread. J. Agric. Food Chem. 2017, 65, 8704–8713. [Google Scholar] [CrossRef] [PubMed]
- Struyf, N.; Vandewiele, H.; Herrera-Malaver, B.; Verspreet, J.; Verstrepen, K.J.; Courtin, C.M. Kluyveromyces marxianus Yeast Enables the Production of Low FODMAP Whole Wheat Breads. Food Microbiol. 2018, 76, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Karaman, K.; Sagdic, O.; Durak, M.Z. Use of Phytase Active Yeasts and Lactic Acid Bacteria Isolated from Sourdough in the Production of Whole Wheat Bread. LWT Food Sci. Technol. 2018, 91, 557–567. [Google Scholar] [CrossRef]
- Van Kerrebroeck, S.; Comasio, A.; Harth, H.; De Vuyst, L. Impact of Starter Culture, Ingredients, and Flour Type on Sourdough Bread Volatiles as Monitored by Selected Ion Flow Tube-Mass Spectrometry. Food Res. Int. 2018, 106, 254–262. [Google Scholar] [CrossRef]
- Coda, R.; Xu, Y.; Moreno, D.S.; Mojzita, D.; Nionelli, L.; Rizzello, C.G.; Katina, K. Performance of Leuconostoc citreum FDR241 during Wheat Flour Sourdough Type I Propagation and Transcriptional Analysis of Exopolysaccharides Biosynthesis Genes. Food Microbiol. 2018, 76, 164–172. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Coda, R.; Wang, Y.; Verni, M.; Kajala, I.; Katina, K.; Laitila, A. Characterization of Indigenous Pediococcus pentosaceus, Leuconostoc Kimchii, Weissella Cibaria and Weissella Confusa for Faba Bean Bioprocessing. Int. J. Food Microbiol. 2019, 302, 24–34. [Google Scholar] [CrossRef]
- Belz, M.C.E.; Axel, C.; Arendt, E.K.; Lynch, K.M.; Brosnan, B.; Sheehan, E.M.; Coffey, A.; Zannini, E. Improvement of Taste and Shelf Life of Yeasted Low-Salt Bread Containing Functional Sourdoughs Using Lactobacillus amylovorus DSM 19280 and Weisella Cibaria MG1. Int. J. Food Microbiol. 2019, 302, 69–79. [Google Scholar] [CrossRef]
- Siepmann, F.B.; Sousa de Almeida, B.; Waszczynskyj, N.; Spier, M.R. Influence of Temperature and of Starter Culture on Biochemical Characteristics and the Aromatic Compounds Evolution on Type II Sourdough and Wheat Bread. Lwt 2019, 108, 199–206. [Google Scholar] [CrossRef]
- Sadeghi, A.; Ebrahimi, M.; Mortazavi, S.A.; Abedfar, A. Application of the Selected Antifungal LAB Isolate as a Protective Starter Culture in Pan Whole-Wheat Sourdough Bread. Food Control 2019, 95, 297–307. [Google Scholar] [CrossRef]
- Antognoni, F.; Mandrioli, R.; Potente, G.; Taneyo Saa, D.L.; Gianotti, A. Changes in Carotenoids, Phenolic Acids and Antioxidant Capacity in Bread Wheat Doughs Fermented with Different Lactic Acid Bacteria Strains. Food Chem. 2019, 292, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, L.; Qian, H.; Zhang, H.; Li, Y.; Wu, G.; Qi, X.; Xu, M.; Rao, Z. Effect of Selected Strains on Physical and Organoleptic Properties of Breads. Food Chem. 2019, 276, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Kook, S.-Y.; Lee, Y.; Jeong, E.-C.; Kim, S. Immunomodulatory Effects of Exopolysaccharides Produced by Bacillus licheniformis and Leuconostoc mesenteroides Isolated from Korean Kimchi. J. Funct. Foods 2019, 54, 211–219. [Google Scholar] [CrossRef]
- Mantzourani, I.; Plessas, S.; Odatzidou, M.; Alexopoulos, A.; Galanis, A.; Bezirtzoglou, E.; Bekatorou, A. Effect of a Novel Lactobacillus paracasei Starter on Sourdough Bread Quality. Food Chem. 2019, 271, 259–265. [Google Scholar] [CrossRef]
- Sakandar, H.A.; Kubow, S.; Azadi, B.; Faryal, R.; Ali, B.; Ghazanfar, S.; Quraishi, U.M.; Imran, M. Wheat Fermentation With Enterococcus mundtii QAUSD01 and Wickerhamomyces anomalus QAUWA03 Consortia Induces Concurrent Gliadin and Phytic Acid Degradation and Inhibits Gliadin Toxicity in Caco-2 Monolayers. Front. Microbiol. 2019, 9, 3312. [Google Scholar] [CrossRef]
- Ouiddir, M.; Bettache, G.; Leyva Salas, M.; Pawtowski, A.; Donot, C.; Brahimi, S.; Mabrouk, K.; Coton, E.; Mounier, J. Selection of Algerian Lactic Acid Bacteria for Use as Antifungal Bioprotective Cultures and Application in Dairy and Bakery Products. Food Microbiol. 2019, 82, 160–170. [Google Scholar] [CrossRef]
- Li, D.; Zhao, Y.; Fei, T.; Wang, Y.; Lee, B.H.; Shim, J.H.; Xu, B.; Li, Z.; Li, X. Effects of Streptococcus thermophilus GtfB Enzyme on Dough Rheology, Bread Quality and Starch Digestibility. Food Hydrocoll. 2019, 96, 134–139. [Google Scholar] [CrossRef]
- Plessas, S.; Mantzourani, I.; Bekatorou, A. Evaluation of Pediococcus pentosaceus SP2 as Starter Culture on Sourdough Bread Making. Foods 2020, 9, 77. [Google Scholar] [CrossRef]
- Li, Z.; Siepmann, F.B.; Rojas Tovar, L.E.; Chen, X.; Gänzle, M.G. Effect of Copy Number of the SpoVA2mob Operon, Sourdough and Reutericyclin on Ropy Bread Spoilage Caused by Bacillus spp. Food Microbiol. 2020, 91, 103507. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhou, M.; Cui, M.; Wang, Y.; Li, H. Improvement of Whole Wheat Dough Fermentation for Steamed Bread Making Using Selected Phytate-Degrading Wickerhamomyces anomalus P4. J. Cereal Sci. 2021, 100, 103261. [Google Scholar] [CrossRef]
- Bockwoldt, J.A.; Fellermeier, J.; Steffens, E.; Vogel, R.F.; Ehrmann, M.A. β-Glucan Production by Levilactobacillus brevis and Pediococcus claussenii for In Situ Enriched Rye and Wheat Sourdough Breads. Foods 2021, 10, 547. [Google Scholar] [CrossRef]
- Buksa, K.; Kowalczyk, M.; Boreczek, J. Extraction, Purification and Characterisation of Exopolysaccharides Produced by Newly Isolated Lactic Acid Bacteria Strains and the Examination of Their Influence on Resistant Starch Formation. Food Chem. 2021, 362, 130221. [Google Scholar] [CrossRef]
- Blaiotta, G.; Romano, R.; Trifuoggi, M.; Aponte, M.; Miro, A. Development of a Wet-Granulated Sourdough Multiple Starter for Direct Use. Foods 2022, 11, 1278. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, Z.; Ramzan, R.; Abdelazez, A.; Amjad, A.; Afzaal, M.; Zhang, S.; Pan, S. Assessment of the Antimicrobial Potentiality and Functionality of Lactobacillus plantarum Strains Isolated from the Conventional Inner Mongolian Fermented Cheese Against Foodborne Pathogens. Pathogens 2019, 8, 71. [Google Scholar] [CrossRef]
- Koistinen, V.M.; Mattila, O.; Katina, K.; Poutanen, K.; Aura, A.M.; Hanhineva, K. Metabolic Profiling of Sourdough Fermented Wheat and Rye Bread. Sci. Rep. 2018, 8, 5684. [Google Scholar] [CrossRef]
- Angelis, M.D.; Rizzello, C.G.; Fasano, A.; Clemente, M.G.; Simone, C.D.; Silano, M.; Vincenzi, M.D.; Losito, I.; Gobbetti, M. VSL#3 Probiotic Preparation Has the Capacity to Hydrolyze Gliadin Polypeptides Responsible for Celiac Sprue Probiotics and Gluten Intolerance. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2006, 1762, 80–93. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Cassone, A.; Cagno, R.D.; Gobbetti, A.M. Synthesis of Angiotensin I-Converting Enzyme (ACE)-Inhibitory Peptides and γ-Aminobutyric Acid (GABA) during Sourdough Fermentation by Selected Lactic Acid Bacteria. J. Agric. Food Chem. 2008, 56, 6936–6943. [Google Scholar] [CrossRef]
- De Angelis, M.; Damiano, N.; Rizzello, C.G.; Cassone, A.; Di Cagno, R.; Gobbetti, M. Sourdough Fermentation as a Tool for the Manufacture of Low-Glycemic Index White Wheat Bread Enriched in Dietary Fibre. Eur. Food Res. Technol. 2009, 229, 593–601. [Google Scholar] [CrossRef]
- Wang, Y.; Gänzle, M.G.; Schwab, C. Exopolysaccharide Synthesized by Lactobacillus Reuteri Decreases the Ability of Enterotoxigenic Escherichia Coli To Bind to Porcine Erythrocytes. Appl. Environ. Microbiol. 2010, 76, 4863–4866. [Google Scholar] [CrossRef] [PubMed]
- Novotni, D.; Curić, D.; Bituh, M.; Colić Barić, I.; Skevin, D.; Cukelj, N. Glycemic Index and Phenolics of Partially-Baked Frozen Bread with Sourdough. Int. J. Food Sci. Nutr. 2011, 62, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, C.G.; Nionelli, L.; Coda, R.; Gobbetti, M. Synthesis of the Cancer Preventive Peptide Lunasin by Lactic Acid Bacteria during Sourdough Fermentation. Nutr. Cancer 2012, 64, 111–120. [Google Scholar] [CrossRef]
- Sanz-Penella, J.M.; Laparra, J.M.; Sanz, Y.; Haros, M. Assessment of Iron Bioavailability in Whole Wheat Bread by Addition of Phytase-Producing Bifidobacteria. J. Agric. Food Chem. 2012, 60, 3190–3195. [Google Scholar] [CrossRef] [PubMed]
- Galle, S.; Schwab, C.; Dal Bello, F.; Coffey, A.; Gänzle, M.G.; Arendt, E.K. Influence of In-Situ Synthesized Exopolysaccharides on the Quality of Gluten-Free Sorghum Sourdough Bread. Int. J. Food Microbiol. 2012, 155, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Sümengen, M.; Dinçer, S.; Kaya, A. Phytase Production from Lactobacillus brevis. Turk. J. Biol. 2012, 36, 533–541. [Google Scholar] [CrossRef]
- Cizeikiene, D.; Juodeikiene, G.; Paskevicius, A.; Bartkiene, E. Antimicrobial Activity of Lactic Acid Bacteria against Pathogenic and Spoilage Microorganism Isolated from Food and Their Control in Wheat Bread. Food Control 2013, 31, 539–545. [Google Scholar] [CrossRef]
- Amari, M.; Arango, L.F.G.; Gabriel, V.; Robert, H.; Morel, S.; Moulis, C.; Gabriel, B.; Remaud-Siméon, M.; Fontagné-Faucher, C. Characterization of a Novel Dextransucrase from Weissella confusa Isolated from Sourdough. Appl. Microbiol. Biotechnol. 2013, 97, 5413–5422. [Google Scholar] [CrossRef]
- De Angelis, M.; Bottacini, F.; Fosso, B.; Kelleher, P.; Calasso, M.; Di Cagno, R.; Ventura, M.; Picardi, E.; van Sinderen, D.; Gobbetti, M. Lactobacillus Rossiae, a Vitamin B12 Producer, Represents a Metabolically Versatile Species within the Genus Lactobacillus. PLoS ONE 2014, 9, e107232. [Google Scholar] [CrossRef]
- Omoba, O.S.; Isah, L.R. Influence of Sourdough Fermentation on Amino Acids Composition, Phenolic Profile, and Antioxidant Properties of Sorghum Biscuits. Prev. Nutr. Food Sci. 2018, 23, 220–227. [Google Scholar] [CrossRef]
- Fraberger, V.; Ammer, C.; Domig, K.J. Functional Properties and Sustainability Improvement of Sourdough Bread by Lactic Acid Bacteria. Microorganisms 2020, 8, 1895. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, Y.; Zhao, M.; Mo, Q.; Feng, F. Weight-Reducing Effect of Lactobacillus plantarum ZJUFT17 Isolated from Sourdough Ecosystem. Nutrients 2020, 12, 977. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Wang, J.; Abdullah; Hafeez, M.A.; Guan, R.; Feng, F. Lactobacillus plantarum ZJUFB2 Prevents High Fat Diet-Induced Insulin Resistance in Association With Modulation of the Gut Microbiota. Front. Nutr. 2021, 8, 754222. [Google Scholar] [CrossRef] [PubMed]
- Schlörmann, W.; Bockwoldt, J.A.; Hübner, S.M.; Wittwer, E.; Reiners, S.; Lorkowski, S.; Dawczynski, C.; Ehrmann, M.A.; Glei, M. Use of the β-Glucan-Producing Lactic Acid Bacteria Strains Levilactobacillus brevis and Pediococcus claussenii for Sourdough Fermentation—Chemical Characterization and Chemopreventive Potential of In Situ-Enriched Wheat and Rye Sourdoughs and Breads. Nutrients 2022, 14, 1510. [Google Scholar] [CrossRef] [PubMed]
- Verni, M.; Vekka, A.; Immonen, M.; Katina, K.; Rizzello, C.G.; Coda, R. Biosynthesis of Γ-aminobutyric Acid by Lactic Acid Bacteria in Surplus Bread and Its Use in Bread Making. J. Appl. Microbiol. 2022, 133, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.T.; Ispirli, H.; Taylan, O.; Alamoudi, M.; Dertli, E. Bioactive and Technological Properties of an α-D-Glucan Synthesized by Weissella cibaria PDER21. Carbohydr. Polym. 2022, 285, 119227. [Google Scholar] [CrossRef]
- Saa, D.T.; Di Silvestro, R.; Nissen, L.; Dinelli, G.; Gianotti, A. Effect of Sourdough Fermentation and Baking Process Severity on Bioactive Fiber Compounds in Immature and Ripe Wheat Flour Bread. Leb. Wiss Technol 2018, 89, 322–328. [Google Scholar] [CrossRef]
- Debonne, E.; Van Bockstaele, F.; Van Driessche, M.; De Leyn, I.; Eeckhout, M.; Devlieghere, F. Impact of Par-Baking and Packaging on the Microbial Quality of Par-Baked Wheat and Sourdough Bread. Food Control 2018, 91, 12–19. [Google Scholar] [CrossRef]
- Zhang, L.; Taal, M.A.; Boom, R.M.; Chen, X.D.; Schutyser, M.A.I. Effect of Baking Conditions and Storage on the Viability of Lactobacillus plantarum Supplemented to Bread. Leb. Wiss Technol 2018, 87, 318–325. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Kazakos, S.; Mantzourani, I.; Plessas, S. Quality Characteristics of Novel Sourdough Breads Made with Functional Lacticaseibacillus paracasei SP5 and Prebiotic Food Matrices. Foods 2022, 11, 3226. [Google Scholar] [CrossRef]
- Koistinen, V.M.; Hedberg, M.; Shi, L.; Johansson, A.; Savolainen, O.; Lehtonen, M.; Aura, A.; Hanhineva, K.; Landberg, R. Metabolite Pattern Derived from Lactiplantibacillus plantarum—Fermented Rye Foods and In Vitro Gut Fermentation Synergistically Inhibits Bacterial Growth. Mol. Nutr. Food Res. 2022, 66, 2101096. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.W.; Chong, A.Q.; Chin, N.L.; Talib, R.A.; Basha, R.K. Sourdough Microbiome Comparison and Benefits. Microorganisms 2021, 9, 1355. [Google Scholar] [CrossRef]
- Reis, J.A.; Paula, A.T.; Casarotti, S.N.; Penna, A.L.B. Lactic Acid Bacteria Antimicrobial Compounds: Characteristics and Applications. Food Eng. Rev. 2012, 4, 124–140. [Google Scholar] [CrossRef]
- Chen, J.; Pang, H.; Wang, L.; Ma, C.; Wu, G.; Liu, Y.; Guan, Y.; Zhang, M.; Qin, G.; Tan, Z. Bacteriocin-Producing Lactic Acid Bacteria Strains with Antimicrobial Activity Screened from Bamei Pig Feces. Foods 2022, 11, 709. [Google Scholar] [CrossRef]
- De Simone, N.; Rocchetti, M.T.; la Gatta, B.; Spano, G.; Drider, D.; Capozzi, V.; Russo, P.; Fiocco, D. Antimicrobial Properties, Functional Characterisation and Application of Fructobacillus fructosus and Lactiplantibacillus plantarum Isolated from Artisanal Honey. Probiotics Antimicrob. Proteins 2022. [Google Scholar] [CrossRef]
- Lavermicocca, P.; Valerio, F.; Evidente, A.; Lazzaroni, S.; Corsetti, A.; Gobbetti, M. Purification and Characterization of Novel Antifungal Compounds from the Sourdough Lactobacillus plantarum Strain 21B. Appl. Environ. Microbiol. 2000, 66, 4084–4090. [Google Scholar] [CrossRef] [PubMed]
- Varsha, K.K.; Priya, S.; Devendra, L.; Nampoothiri, K.M. Control of Spoilage Fungi by Protective Lactic Acid Bacteria Displaying Probiotic Properties. Appl. Biochem. Biotechnol. 2014, 172, 3402–3413. [Google Scholar] [CrossRef]
- Valerio, F.; Conte, A.; Di Biase, M.; Lattanzio, V.M.T.; Lonigro, S.L.; Padalino, L.; Pontonio, E.; Lavermicocca, P. Formulation of Yeast-Leavened Bread with Reduced Salt Content by Using a Lactobacillus plantarum Fermentation Product. Food Chem. 2017, 221, 582–589. [Google Scholar] [CrossRef]
- Hernández-Figueroa, R.H.; Mani-López, E.; López-Malo, A. Antifungal Capacity of Poolish-Type Sourdough Supplemented with Lactiplantibacillus plantarum and Its Aqueous Extracts in Vitro and Bread. Antibiotics 2022, 11, 1813. [Google Scholar] [CrossRef]
- Da Ros, A.; Polo, A.; Rizzello, C.G.; Acin-Albiac, M.; Montemurro, M.; Di Cagno, R.; Gobbetti, M. Feeding with Sustainably Sourdough Bread Has the Potential to Promote the Healthy Microbiota Metabolism at the Colon Level. Microbiol. Spectr. 2021, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Siragusa, S.; De Angelis, M.; Di Cagno, R.; Rizzello, C.G.; Coda, R.; Gobbetti, M. Synthesis of γ-Aminobutyric Acid by Lactic Acid Bacteria Isolated from a Variety of Italian Cheeses. Appl. Environ. Microbiol. 2007, 73, 7283–7290. [Google Scholar] [CrossRef] [PubMed]
- Sarasa, S.B.; Mahendran, R.; Muthusamy, G.; Thankappan, B.; Selta, D.R.F.; Angayarkanni, J. A Brief Review on the Non-Protein Amino Acid, Gamma-Amino Butyric Acid (GABA): Its Production and Role in Microbes. Curr. Microbiol. 2020, 77, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Khanlari, Z.; Moayedi, A.; Ebrahimi, P.; Khomeiri, M.; Sadeghi, A. Enhancement of Γ-aminobutyric Acid (GABA) Content in Fermented Milk by Using Enterococcus faecium and Weissella confusa Isolated from Sourdough. J. Food Process. Preserv. 2021, 45, e15869. [Google Scholar] [CrossRef]
- Arena, M.P.; Capozzi, V.; Russo, P.; Drider, D.; Spano, G.; Fiocco, D. Immunobiosis and Probiosis: Antimicrobial Activity of Lactic Acid Bacteria with a Focus on Their Antiviral and Antifungal Properties. Appl. Microbiol. Biotechnol. 2018, 102, 9949–9958. [Google Scholar] [CrossRef]
- Boakye, P.G.; Kougblenou, I.; Murai, T.; Okyere, A.Y.; Anderson, J.; Bajgain, P.; Philipp, B.; LaPlante, B.; Schlecht, S.; Vogel, C.; et al. Impact of Sourdough Fermentation on FODMAPs and Amylase-Trypsin Inhibitor Levels in Wheat Dough. J. Cereal Sci. 2022, 108, 103574. [Google Scholar] [CrossRef]
- Rashmi, B.S.; Gayathri, D.; Vasudha, M.; Prashantkumar, C.S.; Swamy, C.T.; Sunil, K.S.; Somaraja, P.K.; Prakash, P. Gluten Hydrolyzing Activity of Bacillus spp Isolated from Sourdough. Microb. Cell Factories 2020, 19, 130. [Google Scholar] [CrossRef]
- Gobbetti, M.; Giuseppe Rizzello, C.; Di Cagno, R.; De Angelis, M. Sourdough Lactobacilli and Celiac Disease. Food Microbiol. 2007, 24, 187–196. [Google Scholar] [CrossRef]
- Luti, S.; Mazzoli, L.; Ramazzotti, M.; Galli, V.; Venturi, M.; Marino, G.; Lehmann, M.; Guerrini, S.; Granchi, L.; Paoli, P.; et al. Antioxidant and Anti-Inflammatory Properties of Sourdoughs Containing Selected Lactobacilli Strains Are Retained in Breads. Food Chem. 2020, 322, 126710. [Google Scholar] [CrossRef]
- Colosimo, R.; Gabriele, M.; Cifelli, M.; Longo, V.; Domenici, V.; Pucci, L. The Effect of Sourdough Fermentation on Triticum Dicoccum from Garfagnana: 1H NMR Characterization and Analysis of the Antioxidant Activity. Food Chem. 2020, 305, 125510. [Google Scholar] [CrossRef]
- Nachtigall, C.; Rohm, H.; Jaros, D. Degradation of Exopolysaccharides from Lactic Acid Bacteria by Thermal, Chemical, Enzymatic and Ultrasound Stresses. Foods 2021, 10, 396. [Google Scholar] [CrossRef] [PubMed]
- Gobbetti, M.; De Angelis, M.; Corsetti, A.; Di Cagno, R. Biochemistry and Physiology of Sourdough Lactic Acid Bacteria. Trends Food Sci. Technol. 2005, 16, 57–69. [Google Scholar] [CrossRef]
- Polese, B.; Nicolai, E.; Genovese, D.; Verlezza, V.; La Sala, C.N.; Aiello, M.; Inglese, M.; Incoronato, M.; Sarnelli, G.; De Rosa, T.; et al. Postprandial Gastrointestinal Function Differs after Acute Administration of Sourdough Compared with Brewer’s Yeast Bakery Products in Healthy Adults. J. Nutr. 2018, 148, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, C.; Candal-Uslu, C.; Özhanlı, H.; Arslan-Tontul, S.; Erbas, M. Modulating of Food Glycemic Response by Lactic Acid Bacteria. Food Biosci. 2022, 47, 101685. [Google Scholar] [CrossRef]
- Demirkesen-Bicak, H.; Arici, M.; Yaman, M.; Karasu, S.; Sagdic, O. Effect of Different Fermentation Condition on Estimated Glycemic Index, In Vitro Starch Digestibility, and Textural and Sensory Properties of Sourdough Bread. Foods 2021, 10, 514. [Google Scholar] [CrossRef]
- Rolim, M.E.; Fortes, M.I.; Von Frankenberg, A.; Duarte, C.K. Consumption of Sourdough Bread and Changes in the Glycemic Control and Satiety: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2022. [Google Scholar] [CrossRef]
- Hansen, A.; Schieberle, P. Generation of Aroma Compounds during Sourdough Fermentation: Applied and Fundamental Aspects. Trends Food Sci. Technol. 2005, 16, 85–94. [Google Scholar] [CrossRef]
- Albagli, G.; do Monte Schwartz, I.; Amaral, P.F.F.; Ferreira, T.F.; Finotelli, P.V. How Dried Sourdough Starter Can Enable and Spread the Use of Sourdough Bread. LWT 2021, 149, 111888. [Google Scholar] [CrossRef]
- Mao, H.; Chen, X.D.; Fu, N. Exploring the Integrity of Cellular Membrane and Resistance to Digestive Juices of Dehydrated Lactic Acid Bacteria as Influenced by Drying Kinetics. Food Res. Int. 2022, 157, 111395. [Google Scholar] [CrossRef]
- Gao, X.; Kong, J.; Zhu, H.; Mao, B.; Cui, S.; Zhao, J. Lactobacillus, Bifidobacterium and Lactococcus Response to Environmental Stress: Mechanisms and Application of Cross-Protection to Improve Resistance against Freeze-Drying. J. Appl. Microbiol. 2022, 132, 802–821. [Google Scholar] [CrossRef]
- Rajam, R.; Subramanian, P. Encapsulation of Probiotics: Past, Present and Future. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 46. [Google Scholar] [CrossRef]
- Costa, K.; Silva, L.S.; Kobori, C.N.; da Silva, A.M.; Nicoli, J.R. Microencapsulation of Bifidobacterium longum 51A Cells by Spray Drying and Its Incorporation in Acerola (Malpighia Emarginata) Pulp Powder. Int. J. Food Sci. Technol. 2022, 57, 323–329. [Google Scholar] [CrossRef]
- Katina, K.; Salmenkallio-Marttila, M.; Partanen, R.; Forssell, P.; Autio, K. Effects of Sourdough and Enzymes on Staling of High-Fibre Wheat Bread. LWT Food Sci. Technol. 2006, 39, 479–491. [Google Scholar] [CrossRef]
- Palla, M.; Agnolucci, M.; Calzone, A.; Giovannetti, M.; Di Cagno, R.; Gobbetti, M.; Rizzello, C.G.; Pontonio, E. Exploitation of Autochthonous Tuscan Sourdough Yeasts as Potential Starters. Int. J. Food Microbiol. 2019, 302, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.X.; Zhao, C.J.; Gänzle, M.G. Effect of Glutathione on the Taste and Texture of Type I Sourdough Bread. J. Agric. Food Chem. 2017, 65, 4321–4328. [Google Scholar] [CrossRef] [PubMed]
- Menezes, L.A.A.; Molognoni, L.; de Sá Ploêncio, L.A.; Costa, F.B.M.; Daguer, H.; Dea Lindner, J.D. Use of Sourdough Fermentation to Reducing FODMAPs in Breads. Eur. Food Res. Technol. 2019, 245, 1183–1195. [Google Scholar] [CrossRef]
- van Hijum, S.A.F.T.; Kralj, S.; Ozimek, L.K.; Dijkhuizen, L.; van Geel-Schutten, I.G.H. Structure-Function Relationships of Glucansucrase and Fructansucrase Enzymes from Lactic Acid Bacteria. Microbiol. Mol. Biol. Rev. 2006, 70, 157–176. [Google Scholar] [CrossRef]
- Yu, L.; Qian, Z.; Ge, J.; Du, R. Glucansucrase Produced by Lactic Acid Bacteria: Structure, Properties, and Applications. Fermentation 2022, 8, 629. [Google Scholar] [CrossRef]
- Galle, S.; Arendt, E.K. Exopolysaccharides from Sourdough Lactic Acid Bacteria. Crit. Rev. Food Sci. Nutr. 2014, 54, 891–901. [Google Scholar] [CrossRef]
- Cozzolino, A.; Di Pierro, P.; Mariniello, L.; Sorrentino, A.; Masi, P.; Porta, R. Incorporation of Whey Proteins into Cheese Curd by Using Transglutaminase. Biotechnol. Appl. Biochem. 2003, 38, 289–295. [Google Scholar] [CrossRef]
- Scarnato, L.; Serrazanetti, D.I.; Aloisi, I.; Montanari, C.; Del Duca, S.; Lanciotti, R. Combination of Transglutaminase and Sourdough on Gluten-Free Flours to Improve Dough Structure. Amino Acids 2016, 48, 2453–2465. [Google Scholar] [CrossRef] [PubMed]
- Pourmohammadi, K.; Abedi, E. Enzymatic Modifications of Gluten Protein: Oxidative Enzymes. Food Chem. 2021, 356, 129679. [Google Scholar] [CrossRef]
- BRENDA Information on EC 1.8.1.7—Glutathione-Disulfide Reductase. Available online: https://www.brenda-enzymes.org/enzyme.php?ecno=1.8.1.7#reactschemes (accessed on 20 November 2022).
- Xu, D.; Tang, K.; Hu, Y.; Xu, X.; Gänzle, M.G. Effect of Glutathione Dehydrogenase of Lactobacillus sanfranciscensis on Gluten Properties and Bread Volume in Type i Wheat Sourdough Bread. J. Agric. Food Chem. 2018, 66, 9770–9776. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Khurshid, S.; Sarwar, A.; Aziz, T.; Naveed, M.; Ali, U.; Makhdoom, S.I.; Nadeem, A.A.; Khan, A.A.; Sameeh, M.Y.; et al. Enhancing Bread Quality and Shelf Life via Glucose Oxidase Immobilized on Zinc Oxide Nanoparticles—A Sustainable Approach towards Food Safety. Sustainability 2022, 14, 14255. [Google Scholar] [CrossRef]
- Xu, X.; Yang, Q.; Luo, Z.; Xiao, Z. Effects of Sourdough Fermentation and an Innovative Compound Improver on the Baking Performance, Nutritional Quality, and Antistaling Property of Whole Wheat Bread. ACS Food Sci. Technol. 2022, 2, 825–835. [Google Scholar] [CrossRef]
- Visvanathan, R.; Jayathilake, C.; Liyanage, R.; Sivakanesan, R. Applicability and Reliability of the Glucose Oxidase Method in Assessing α-Amylase Activity. Food Chem. 2019, 275, 265–272. [Google Scholar] [CrossRef]
- Decamps, K.; Joye, I.J.; Courtin, C.M.; Delcour, J.A. Glucose and Pyranose Oxidase Improve Bread Dough Stability. J. Cereal Sci. 2012, 55, 380–384. [Google Scholar] [CrossRef]
- Decamps, K.; Joye, I.J.; Rakotozafy, L.; Nicolas, J.; Courtin, C.M.; Delcour, J.A. The Bread Dough Stability Improving Effect of Pyranose Oxidase from Trametes multicolor and Glucose Oxidase from Aspergillus niger: Unraveling the Molecular Mechanism. J. Agric. Food Chem. 2013, 61, 7848–7854. [Google Scholar] [CrossRef]
- Manhivi, V.E.; Amonsou, E.O.; Kudanga, T. Laccase-Mediated Crosslinking of Gluten-Free Amadumbe Flour Improves Rheological Properties. Food Chem. 2018, 264, 157–163. [Google Scholar] [CrossRef]
- Mayolo-Deloisa, K.; González-González, M.; Rito-Palomares, M. Laccases in Food Industry: Bioprocessing, Potential Industrial and Biotechnological Applications. Front. Bioeng. Biotechnol. 2020, 8, 222. [Google Scholar] [CrossRef]
- Niño-Medina, G.; Gutiérrez-Soto, G.; Urías-Orona, V.; Hernández-Luna, C.E. Effect of Laccase from Trametes maxima CU1 on Physicochemical Quality of Bread. Cogent Food Agric. 2017, 3, 1328762. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Zoumpopoulou, G.; Georgalaki, M.; Alexandraki, V.; Kazou, M.; Anastasiou, R.; Tsakalidou, E. Sourdough Bread. In Innovations in Traditional Foods; Elsevier: Amsterdam, The Netherlands, 2019; pp. 127–158. ISBN 978-0-12-814887-7. [Google Scholar]
- IUBMB International Union of Biochemistry and Molecular Biology. Available online: https://iubmb.qmul.ac.uk/ (accessed on 20 November 2022).
- Diana, M.; Rafecas, M.; Quílez, J. Free Amino Acids, Acrylamide and Biogenic Amines in Gamma-Aminobutyric Acid Enriched Sourdough and Commercial Breads. J. Cereal Sci. 2014, 60, 639–644. [Google Scholar] [CrossRef]
- Polak, T.; Mejaš, R.; Jamnik, P.; Kralj Cigić, I.; Poklar Ulrih, N.; Cigić, B. Accumulation and Transformation of Biogenic Amines and Gamma-Aminobutyric Acid (GABA) in Chickpea Sourdough. Foods 2021, 10, 2840. [Google Scholar] [CrossRef] [PubMed]
- Venturi, M.; Galli, V.; Pini, N.; Guerrini, S.; Granchi, L. Use of Selected Lactobacilli to Increase γ-Aminobutyric Acid (GABA) Content in Sourdough Bread Enriched with Amaranth Flour. Foods 2019, 8, 218. [Google Scholar] [CrossRef]
- Calabrese, F.M.; Ameur, H.; Nikoloudaki, O.; Celano, G.; Vacca, M.; JFLemos, W., Jr.; Manzari, C.; Vertè, F.; Di Cagno, R.; Pesole, G.; et al. Metabolic Framework of Spontaneous and Synthetic Sourdough Metacommunities to Reveal Microbial Players Responsible for Resilience and Performance. Microbiome 2022, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Pourmohammadi, K.; Abedi, E. Hydrolytic Enzymes and Their Directly and Indirectly Effects on Gluten and Dough Properties: An Extensive Review. Food Sci. Nutr. 2021, 9, 3988–4006. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, S.; Bajaj, B.K.; Kumar, A.; Tiwari, S.K.; Singh, B. A Review on Biotechnological Potential of Multifarious Enzymes in Bread Making. Process Biochem. 2020, 99, 290–306. [Google Scholar] [CrossRef]
- Bala, A.; Singh, B. Concomitant Production of Cellulase and Xylanase by Thermophilic Mould Sporotrichum thermophile in Solid State Fermentation and Their Applicability in Bread Making. World J. Microbiol. Biotechnol. 2017, 33, 109. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.; Martarello, R.; de Souza, P.M.; de Freitas, M.M.; Barros, K.V.G.; Ferreira Filho, E.X.; Homem-de-Mello, M.; Magalhães, P.O. Optimization of Xylanase Production from Aspergillus foetidus in Soybean Residue. Enzyme Res. 2018, 2018, 6597017. [Google Scholar] [CrossRef]
- Guo, Y.; Gao, Z.; Xu, J.; Chang, S.; Wu, B.; He, B. A Family 30 Glucurono-Xylanase from Bacillus subtilis LC9: Expression, Characterization and Its Application in Chinese Bread Making. Int. J. Biol. Macromol. 2018, 117, 377–384. [Google Scholar] [CrossRef]
- Adigüzel, A.O.; Tunçer, M. Production, Characterization and Application of a Xylanase from Streptomyces sp. AOA40 in Fruit Juice and Bakery Industries. Food Biotechnol. 2016, 30, 189–218. [Google Scholar] [CrossRef]
- García-Mantrana, I.; Monedero, V.; Haros, M. Myo-Inositol Hexakisphosphate Degradation by Bifidobacterium pseudocatenulatum ATCC 27919 Improves Mineral Availability of High Fibre Rye-Wheat Sour Bread. Food Chem. 2015, 178, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Chanderman, A.; Puri, A.K.; Permaul, K.; Singh, S. Production, Characteristics and Applications of Phytase from a Rhizosphere Isolated Enterobacter sp. ACSS. Bioprocess Biosyst. Eng. 2016, 39, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, B.; Satyanarayana, T. Recombinant HAP Phytase of the Thermophilic Mold Sporotrichum Thermophile: Expression of the Codon-Optimized Phytase Gene in Pichia pastoris and Applications. Mol. Biotechnol. 2016, 58, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Buddhiwant, P.; Bhavsar, K.; Kumar, V.R.; Khire, J.M. Phytase Production by Solid-State Fermentation of Groundnut Oil Cake by Aspergillus niger: A Bioprocess Optimization Study for Animal Feedstock Applications. Prep. Biochem. Biotechnol. 2016, 46, 531–538. [Google Scholar] [CrossRef]
- Benabda, O.; M’hir, S.; Kasmi, M.; Mnif, W.; Hamdi, M. Optimization of Protease and Amylase Production by Rhizopus oryzae Cultivated on Bread Waste Using Solid-State Fermentation. J. Chem. 2019, 2019, e3738181. [Google Scholar] [CrossRef]
- Trabelsi, S.; Ben Mabrouk, S.; Kriaa, M.; Ameri, R.; Sahnoun, M.; Mezghani, M.; Bejar, S. The Optimized Production, Purification, Characterization, and Application in the Bread Making Industry of Three Acid-Stable Alpha-Amylases Isoforms from a New Isolated Bacillus subtilis Strain US586. J. Food Biochem. 2019, 43, e12826. [Google Scholar] [CrossRef]
- Rana, N.; Verma, N.; Vaidya, D.; Dipta, B. Application of Bacterial Amylase in Clarification of Juices and Bun Making. J. Pharm. Phytochem. 2017, 6, 859–864. [Google Scholar]
- Shivlata, L.; Satyanarayana, T. Characteristics of Raw Starch-Digesting α-Amylase of Streptomyces badius DB-1 with Transglycosylation Activity and Its Applications. Appl. Biochem. Biotechnol. 2017, 181, 1283–1303. [Google Scholar] [CrossRef]
- Kiesenhofer, D.P.; Mach, R.L.; Mach-Aigner, A.R. Glucose Oxidase Production from Sustainable Substrates. Curr. Biotechnol. 2017, 6, 238–244. [Google Scholar] [CrossRef]
- Hassan, B.A.; Jebor, M.A.; Ali, Z.M. Purification and Characterization of the Glucose Oxidase from Penicillium notatum. Int. J. Pharm. Qual. Assur. 2018, 9, 55–63. [Google Scholar] [CrossRef]
- Yogananth, N.; Chanthru, A.; Sivanesan, D. Optimization of Various Parameters for the Production of Glucose Oxidase Using Aspergillus niger. Int. J. Curr. Microbiol. App. Sci. 2012, 1, 23–28. [Google Scholar]
- Hamid, H.M.; Khalil-ur-Rehman; Zia, M.A.; Asgher, M. Optimization of Various Parameters for the Production of Glucose Oxidase from Rice Polishing Using Aspergillus niger. Biotechnol. Pak. 2003, 2, 1–7. [Google Scholar]
- Bhange, K.; Chaturvedi, V.; Bhatt, R. Simultaneous Production of Detergent Stable Keratinolytic Protease, Amylase and Biosurfactant by Bacillus subtilis PF1 Using Agro Industrial Waste. Biotechnol. Rep. 2016, 10, 94–104. [Google Scholar] [CrossRef]
- Ozdenefe, M.S.; Dincer, S.; Unal, M.U.; Kayis, F.B.; Takci, H.A.M.; Arkut, A. Optimization of Culture Conditions for Alkaline Protease Production from Waste Breads Using Bacillus subtilis. Romanian Biotechnol. Lett. 2017, 22, 12597–12610. [Google Scholar]
- Sangeetha, R.; Geetha, A.; Arulpandi, I. Optimization of Protease and Lipase Production by Bacillus pumilus SG 2 Isolated from an Industrial Effluent. Internet J Microbiol 2008, 5, 2. [Google Scholar]
- Nema, A.; Patnala, S.H.; Mandari, V.; Kota, S.; Devarai, S.K. Production and Optimization of Lipase Using Aspergillus niger MTCC 872 by Solid-State Fermentation. Bull. Natl. Res. Cent. 2019, 43, 82. [Google Scholar] [CrossRef]
- Tanyol, M.; Uslu, G.; Yönten, V. Optimization of Lipase Production on Agro-Industrial Residue Medium by Pseudomonas fluorescens (NRLL B-2641) Using Response Surface Methodology. Biotechnol. Biotechnol. Equip. 2015, 29, 64–71. [Google Scholar] [CrossRef]
- Iqbal, S.A.; Rehman, A. Characterization of Lipase from Bacillus subtilis I-4 and Its Potential Use in Oil Contaminated Wastewater. Braz. Arch. Biol. Technol. 2015, 58, 789–797. [Google Scholar] [CrossRef]
- Kumar, D.J.M.; Rejitha, R.; Devika, S.; Balakumaran, M.D.; Rebecca, A.I.N.; Kalaichelvan, P.T. Production, Optimization and Purification of Lipase from Bacillus sp. MPTK 912 Isolated from Oil Mill Effluent. Adv. Appl. Sci. Res. 2012, 3, 930–938. [Google Scholar]
- Mahmoud, A.-M.Y.; Asif, A.M.J.-F.; Hani, Z.A. Production, Purification and Characterization of Cellulase from Streptomyces sp. Afr. J. Microbiol. Res. 2014, 8, 348–354. [Google Scholar] [CrossRef]
- Swathy, R.; Rambabu, K.; Banat, F.; Ho, S.-H.; Chu, D.-T.; Show, P.L. Production and Optimization of High Grade Cellulase from Waste Date Seeds by Cellulomonas Uda NCIM 2353 for Biohydrogen Production. Int. J. Hydrog. Energy 2020, 45, 22260–22270. [Google Scholar] [CrossRef]
- Verma, N.; Kumar, V.; Bansal, M.C. Utility of Luffa cylindrica and Litchi chinensis Peel, an Agricultural Waste Biomass in Cellulase Production by Trichoderma reesei under Solid State Cultivation. Biocatal. Agric. Biotechnol. 2018, 16, 483–492. [Google Scholar] [CrossRef]
- Oort, M.V.; Whitehurst, R.J. Enzymes in Food Technology; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 978-1-4443-0994-2. [Google Scholar]
- Dalmaso, G.Z.L.; Ferreira, D.; Vermelho, A.B. Marine Extremophiles: A Source of Hydrolases for Biotechnological Applications. Mar. Drugs 2015, 13, 1925–1965. [Google Scholar] [CrossRef]
- Kanazawa, S.; Sanabria, M.; Monteiro, M. Influence of the Fermentation Methods on the Resistant Starch Formation by X-Ray Diffraction. SN Appl. Sci. 2021, 3, 191. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch Retrogradation: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef]
- Corsetti, A.; Gobbetti, M.; De Marco, B.; Balestrieri, F.; Paoletti, F.; Russi, L.; Rossi, J. Combined Effect of Sourdough Lactic Acid Bacteria and Additives on Bread Firmness and Staling. J Agric Food Chem 2000, 48, 3044–3051. [Google Scholar] [CrossRef]
- Muir, J.G.G.; Varney, J.E.E.; Ajamian, M.; Gibson, P.R.R. Gluten-Free and Low-FODMAP Sourdoughs for Patients with Coeliac Disease and Irritable Bowel Syndrome: A Clinical Perspective. Int. J. Food Microbiol. 2019, 290, 237–246. [Google Scholar] [CrossRef]
- Vermelho, A.B.; Couri, S. Methods to Determine Enzymatic Activity; Bentham Science Publishers: Saif Zone, Sharjah, United Arab Emirates, 2013. [Google Scholar]
- Melim Miguel, A.S.; Souza, T.; da Costa Figueiredo, E.V.; Paulo Lobo, B.W.; Maria, G. Enzymes in Bakery: Current and Future Trends. In Food Industry; Muzzalupo, I., Ed.; InTech: London, UK, 2013; ISBN 978-953-51-0911-2. [Google Scholar]
- Li, Z.; Dong, Y.; Zhou, X.; Xiao, X.; Zhao, Y.; Yu, L. Dough Properties and Bread Quality of Wheat–Barley Composite Flour as Affected by β-Glucanase. Cereal Chem. J. 2014, 91, 631–638. [Google Scholar] [CrossRef]
- Lei, X.G.; Porres, J.M. Phytase Enzymology, Applications, and Biotechnology. Biotechnol. Lett. 2003, 25, 1787–1794. [Google Scholar] [CrossRef]
- Ries, E.F. Estudo Da Produção, Caracterização e Aplicação de Nova Fitase de Saccharomyces cerevisiae. Ph.D. Thesis, Universidade Estadual de Campinas, Campinas, Brazil, 2010; 130p. [Google Scholar]
- Aslam, M.F.; Ellis, P.R.; Berry, S.E.; Latunde-Dada, G.O.; Sharp, P.A. Enhancing Mineral Bioavailability from Cereals: Current Strategies and Future Perspectives. Nutr. Bull. 2018, 43, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Baye, K.; Guyot, J.-P.; Icard-Vernière, C.; Rochette, I.; Mouquet-Rivier, C. Enzymatic Degradation of Phytate, Polyphenols and Dietary Fibers in Ethiopian Injera Flours: Effect on Iron Bioaccessibility. Food Chem. 2015, 174, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Brouns, F. Phytic Acid and Whole Grains for Health Controversy. Nutrients 2022, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Puig, E.; Monedero, V.; Haros, M. Bread with Whole Quinoa Flour and Bifidobacterial Phytases Increases Dietary Mineral Intake and Bioavailability. LWT Food Sci. Technol. 2015, 60, 71–77. [Google Scholar] [CrossRef]
- Nuobariene, L.; Hansen, Å.S.; Arneborg, N. Isolation and Identification of Phytase-Active Yeasts from Sourdoughs. LWT Food Sci. Technol. 2012, 48, 190–196. [Google Scholar] [CrossRef]
- Nuobariene, L.; Arneborg, N.; Hansen, Å.S. Phytase Active Yeasts Isolated From Bakery Sourdoughs. In Proceedings of the 9th Baltic Conference on Food Science and Technology “Food for Consumer Well-Being”, Jelgava, Latvia, 8–9 May 2014; pp. 223–227. [Google Scholar]
- Mohammadi, M.; Zoghi, A.; Azizi, M.H. Effect of Xylanase and Pentosanase Enzymes on Dough Rheological Properties and Quality of Baguette Bread. J. Food Qual. 2022, 2022, 2910821. [Google Scholar] [CrossRef]
- Martínez-Anaya, M.A.; Devesa, A. Influence of Enzymes in Sourdough Wheat Breadmaking. Changes in Pentosans / Influencia de Las Enzimas y Los Iniciadores Microbianos En Panificación. Cambios En Las Pentosanas. Food Sci. Technol. Int. 2000, 6, 109–116. [Google Scholar] [CrossRef]
- Leahu, A.; Codină, G.G.; Mironeasa, S.; Roşu, A.-I. Effects of A2 Phospholipase on Dough Rheological Properties and Bread Characteristics. Food Environ. Saf. J. 2011, 10, 1. [Google Scholar]
- Gandra, K.M.; Del Bianchi, M.; Godoy, V.P.; Queiroz, F.P.C.; Steel, C.J. Aplicação de Lipase e Monoglicerídeo Em Pão de Forma Enriquecido Com Fibras. Cienc. E Tecnol. Aliment. 2008, 28, 182–192. [Google Scholar] [CrossRef]
- Gobbetti, M.; De Angelis, M.; Di Cagno, R.; Calasso, M.; Archetti, G.; Rizzello, C.G. Novel Insights on the Functional/Nutritional Features of the Sourdough Fermentation. Int. J. Food Microbiol. 2018, 302, 103–113. [Google Scholar] [CrossRef]
- Zhu, K.-X.; Zhou, H.-M.; Qian, H.-F. Proteins Extracted from Defatted Wheat Germ: Nutritional and Structural Properties. Cereal Chem. 2006, 83, 69–75. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Nionelli, L.; Coda, R.; De Angelis, M.; Gobbetti, M. Effect of Sourdough Fermentation on Stabilisation, and Chemical and Nutritional Characteristics of Wheat Germ. Food Chem. 2010, 119, 1079–1089. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Morton, F.R.; Kok, C.Y.; Kong, J.; Barrett, A.J. The MEROPS Database of Proteolytic Enzymes, Their Substrates and Inhibitors in 2017 and a Comparison with Peptidases in the PANTHER Database. Nucleic Acids Res. 2018, 36, D320–D325. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, N.; Kretzer, J.; Machalitza, H.; Vogel, R.F.; Gänzle, M.G. Influence of Redox-Reactions Catalysed by Homo- and Hetero-Fermentative Lactobacilli on Gluten in Wheat Sourdoughs. J. Cereal Sci. 2006, 43, 137–143. [Google Scholar] [CrossRef]
- Wieser, H. Chemistry of Gluten Proteins. Food Microbiol. 2007, 24, 115–119. [Google Scholar] [CrossRef]
- Bietz, J.; Wall, J. Identity of High Molecular Weight Gliadin and Ethanol-Soluble Glutenin Subunits of Wheat: Relation to Gluten Structure. Cereal Chem. 1980, 57, 415–421. [Google Scholar]
- Gänzle, M.G.; Loponen, J.; Gobbetti, M. Proteolysis in Sourdough Fermentations: Mechanisms and Potential for Improved Bread Quality. Trends Food Sci. Technol. 2008, 19, 513–521. [Google Scholar] [CrossRef]
- De Souza, T.S.P.; de Andrade, C.J.; Koblitz, M.G.B.; Fai, A.E.C. Microbial Peptidase in Food Processing: Current State of the Art and Future Trends. Catal. Lett. 2023, 153, 114–137. [Google Scholar] [CrossRef]
- Di Cagno, R.; Rizzello, C.G.; De Angelis, M.; Cassone, A.; Giuliani, G.; Benedusi, A.; Limitone, A.; Surico, R.F.; Gobbetti, M. Use of Selected Sourdough Strains of Lactobacillus for Removing Gluten and Enhancing the Nutritional Properties of Gluten-Free Bread. J. Food Prot. 2008, 71, 1491–1495. [Google Scholar] [CrossRef]
- Thiele, C.; Grassl, S.; Gänzle, M. Gluten Hydrolysis and Depolymerization during Sourdough Fermentation. J. Agric. Food Chem. 2004, 52, 1307–1314. [Google Scholar] [CrossRef]
- Shan, L.; Qiao, S.-W.; Arentz-Hansen, H.; Molberg, Ø.; Gray, G.M.; Sollid, L.M.; Khosla, C. Identification and Analysis of Multivalent Proteolytically Resistant Peptides from Gluten: Implications for Celiac Sprue. J. Proteome Res. 2005, 4, 1732–1741. [Google Scholar] [CrossRef] [PubMed]
- Engström, N.; Sandberg, A.S.; Scheers, N. Sourdough Fermentation of Wheat Flour Does Not Prevent the Interaction of Transglutaminase 2 with A2-Gliadin or Gluten. Nutrients 2015, 7, 2134. [Google Scholar] [CrossRef] [PubMed]
- Dunaevsky, Y.E.; Tereshchenkova, V.F.; Belozersky, M.A.; Filippova, I.Y.; Oppert, B.; Elpidina, E.N. Effective Degradation of Gluten and Its Fragments by Gluten-Specific Peptidases: A Review on Application for the Treatment of Patients with Gluten Sensitivity. Pharmaceutics 2021, 13, 1603. [Google Scholar] [CrossRef] [PubMed]
- Reale, A.; Di Stasio, L.; Di Renzo, T.; De Caro, S.; Ferranti, P.; Picariello, G.; Addeo, F.; Mamone, G. Bacteria Do It Better! Proteomics Suggests the Molecular Basis for Improved Digestibility of Sourdough Products. Food Chem. 2021, 359, 129955. [Google Scholar] [CrossRef]
- Alvarez-Sieiro, P.; Redruello, B.; Ladero, V.; Martín, M.C.; Fernández, M.; Alvarez, M.A. Screening Sourdough Samples for Gliadin-Degrading Activity Revealed Lactobacillus casei Strains Able to Individually Metabolize the Coeliac-Disease-Related 33-Mer Peptide. Can. J. Microbiol. 2016, 62, 422–430. [Google Scholar] [CrossRef]
- Lancetti, R.; Sciarini, L.; Pérez, G.T.; Salvucci, E. Technological Performance and Selection of Lactic Acid Bacteria Isolated from Argentinian Grains as Starters for Wheat Sourdough. Curr. Microbiol. 2021, 78, 255–264. [Google Scholar] [CrossRef]
- Zhao, C.J.; Schieber, A.; Gänzle, M.G. Formation of Taste-Active Amino Acids, Amino Acid Derivatives and Peptides in Food Fermentations—A Review. Food Res. Int. 2016, 89, 39–47. [Google Scholar] [CrossRef]
- Feng, W.; Ma, S.; Wang, X. Quality Deterioration and Improvement of Wheat Gluten Protein in Frozen Dough. Grain Oil Sci. Technol. 2020, 3, 29–37. [Google Scholar] [CrossRef]
- Schopf, M.; Scherf, K.A. Water Absorption Capacity Determines the Functionality of Vital Gluten Related to Specific Bread Volume. Foods 2021, 10, 228. [Google Scholar] [CrossRef]
- Codex Alimentarius. Agenda Item 11 Cx/Nfsdu 19/41/11. Joint FAO/WHO Food Standards Programme. In Proceedings of the Codex Committee on Nutrition and Foods for Special Dietary Uses Forty-First Session, Dusseldorf, Germany, 24–29 November 2019. [Google Scholar]
- FDA. Generally Recognized as Safe (GRAS). Available online: https://www.fda.gov/food/food-ingredients-packaging/generally-recognized-safe-gras (accessed on 10 October 2022).
- Anvisa Resolução Da Diretoria Colegiada—RDC No 241, DE 26 DE JULHO DE 2018; Imprensa Nacional: Brasília, Brazil, 2018.
- Planalto. LEI Nº 13.123. 2015. Available online: https://www.planalto.gov.br/ccivil_03/_ato2015-2018/2015/lei/l13123.htm (accessed on 10 October 2022).
- De Melo Pereira, G.V.; de Oliveira Coelho, B.; Magalhães Júnior, A.I.; Thomaz-Soccol, V.; Soccol, C.R. How to Select a Probiotic? A Review and Update of Methods and Criteria. Biotechnol. Adv. 2018, 36, 2060–2076. [Google Scholar] [CrossRef]
- FAO; WHO. Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation; FAO Food and Nutrition Paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006; ISBN 978-92-5-105513-7. [Google Scholar]
- Legifrance. France Décret N°93-1074. 1993. Available online: https://www.legifrance.gouv.fr/loda/id/JORFTEXT000000727617/ (accessed on 10 October 2022).
- Eur Regulamento (UE) 2015/2283 do Parlamento Europeu e do Conselho. Available online: https://eur-lex.europa.eu/legal-content/PT/TXT/PDF/?uri=CELEX:32015R2283&from=EN (accessed on 6 October 2022).
- Europa Safety Assessment and Regulatory Aspects of Micro-Organisms in Feed and Food Applications. Available online: https://food.ec.europa.eu/system/files/2020-12/sci-com_scan-old_report_out85.pdf (accessed on 4 October 2022).
- Eur-Lex Establishing the Union List of Novel Foods in Accordance with Regulation (EU) 2015/2283 of the European Parliament and of the Council on Novel Foods. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R2470&from=EN (accessed on 2 October 2022).
- Balachandra Nair, R.; Ramachandranna, P.C. Patenting of Microorganisms: Systems and Concerns. J. Commer. Biotechnol. 2010, 16, 337–347. [Google Scholar] [CrossRef]
- Planalto. LEI Nº 9.279. 1996. Available online: https://www.planalto.gov.br/ccivil_03/leis/l9279.htm (accessed on 10 October 2022).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akamine, I.T.; Mansoldo, F.R.P.; Vermelho, A.B. Probiotics in the Sourdough Bread Fermentation: Current Status. Fermentation 2023, 9, 90. https://doi.org/10.3390/fermentation9020090
Akamine IT, Mansoldo FRP, Vermelho AB. Probiotics in the Sourdough Bread Fermentation: Current Status. Fermentation. 2023; 9(2):90. https://doi.org/10.3390/fermentation9020090
Chicago/Turabian StyleAkamine, Ingrid Teixeira, Felipe R. P. Mansoldo, and Alane Beatriz Vermelho. 2023. "Probiotics in the Sourdough Bread Fermentation: Current Status" Fermentation 9, no. 2: 90. https://doi.org/10.3390/fermentation9020090
APA StyleAkamine, I. T., Mansoldo, F. R. P., & Vermelho, A. B. (2023). Probiotics in the Sourdough Bread Fermentation: Current Status. Fermentation, 9(2), 90. https://doi.org/10.3390/fermentation9020090










