Probiotic Properties of Weissella confusa PP29 on Hibiscus sabdariffa L. Media
Abstract
1. Introduction
2. Materials and Methods
2.1. H. sabdariffa L. Extract
2.2. Extract Characterization
2.3. Microorganism
2.4. Fermentation Conditions
2.5. EPS Isolation and Purification
2.6. Gel Permeation Chromatography (GPC) Analysis
2.7. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.8. Nuclear Magnetic Resonance (NMR) Studies
2.9. Thermal Analysis Measurements-Thermogravimetry (TG/DTG)
2.10. Probiotic Activity Determination
2.10.1. Resistance to Low pH
2.10.2. Resistance to Bile Salt
2.10.3. Evaluation of Hydrophobicity and Auto-Aggregation
3. Results and Discussion
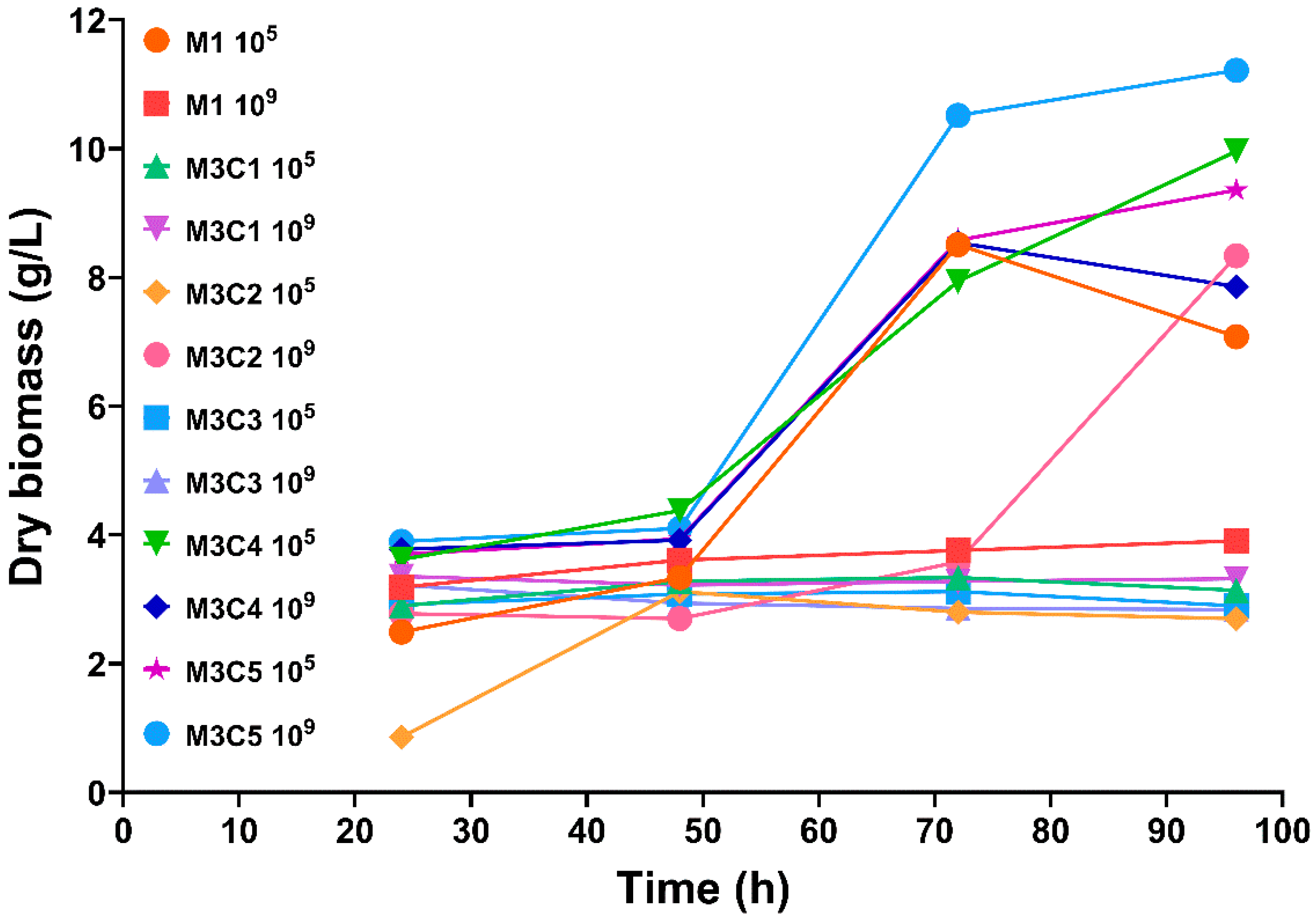
3.1. Dry Biomass Determination
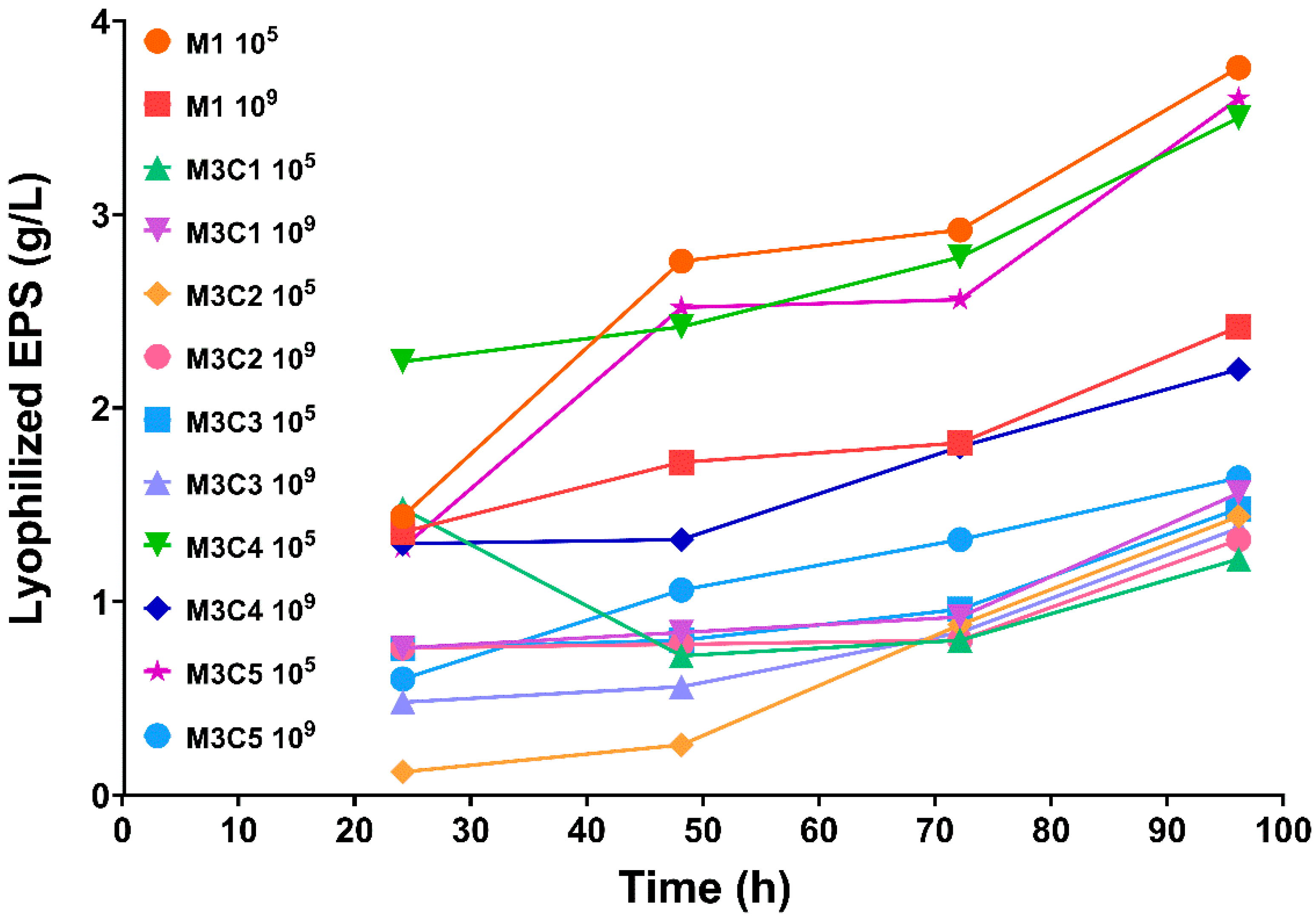
3.2. EPS Quantification from Fermentative Culture Media
3.3. Gel Permeation Chromatography (GPC) Analysis
3.4. Fourier-Transform Infrared Spectroscopy (FTIR)
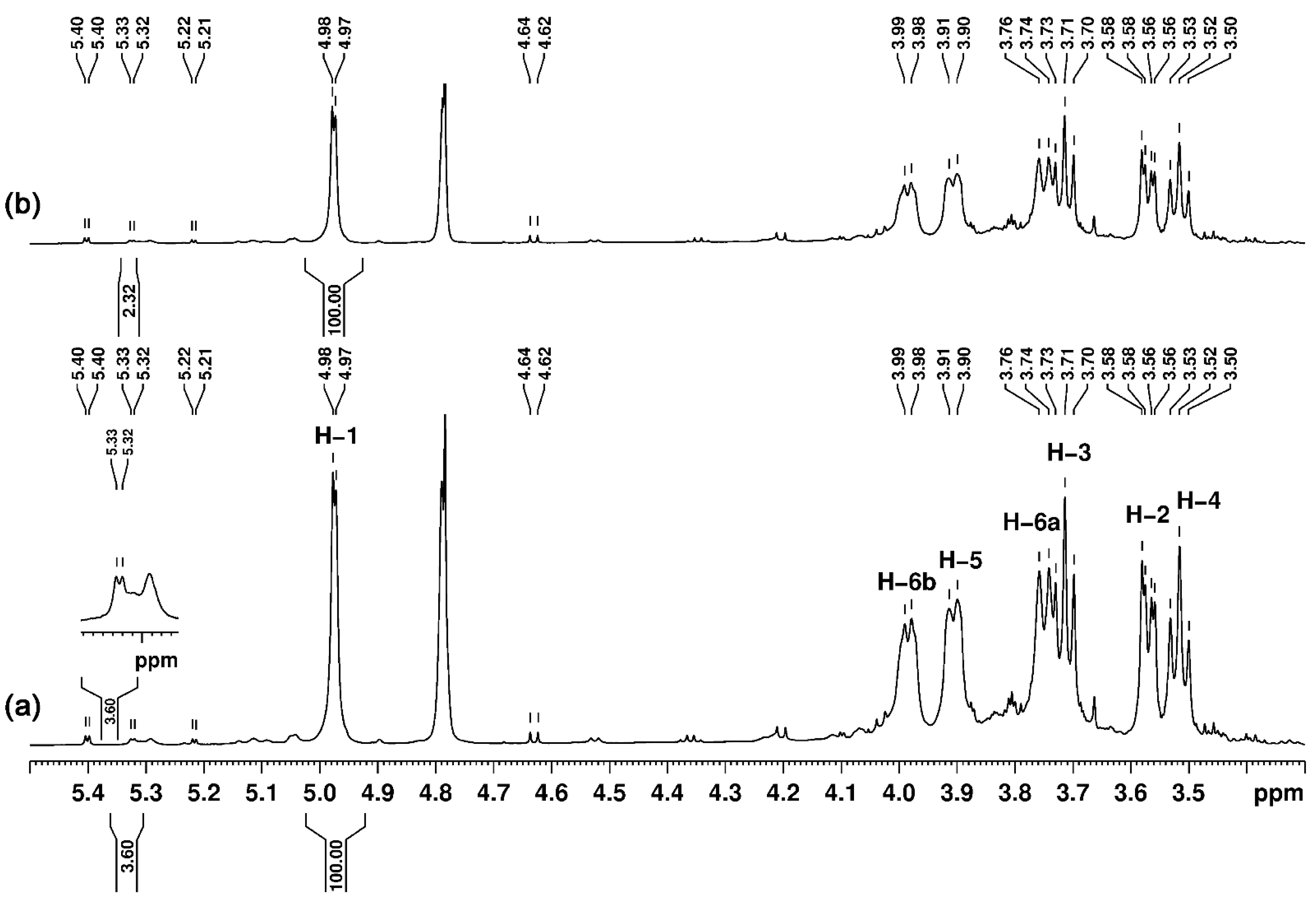
3.5. Nuclear Magnetic Resonance (NMR) Studies
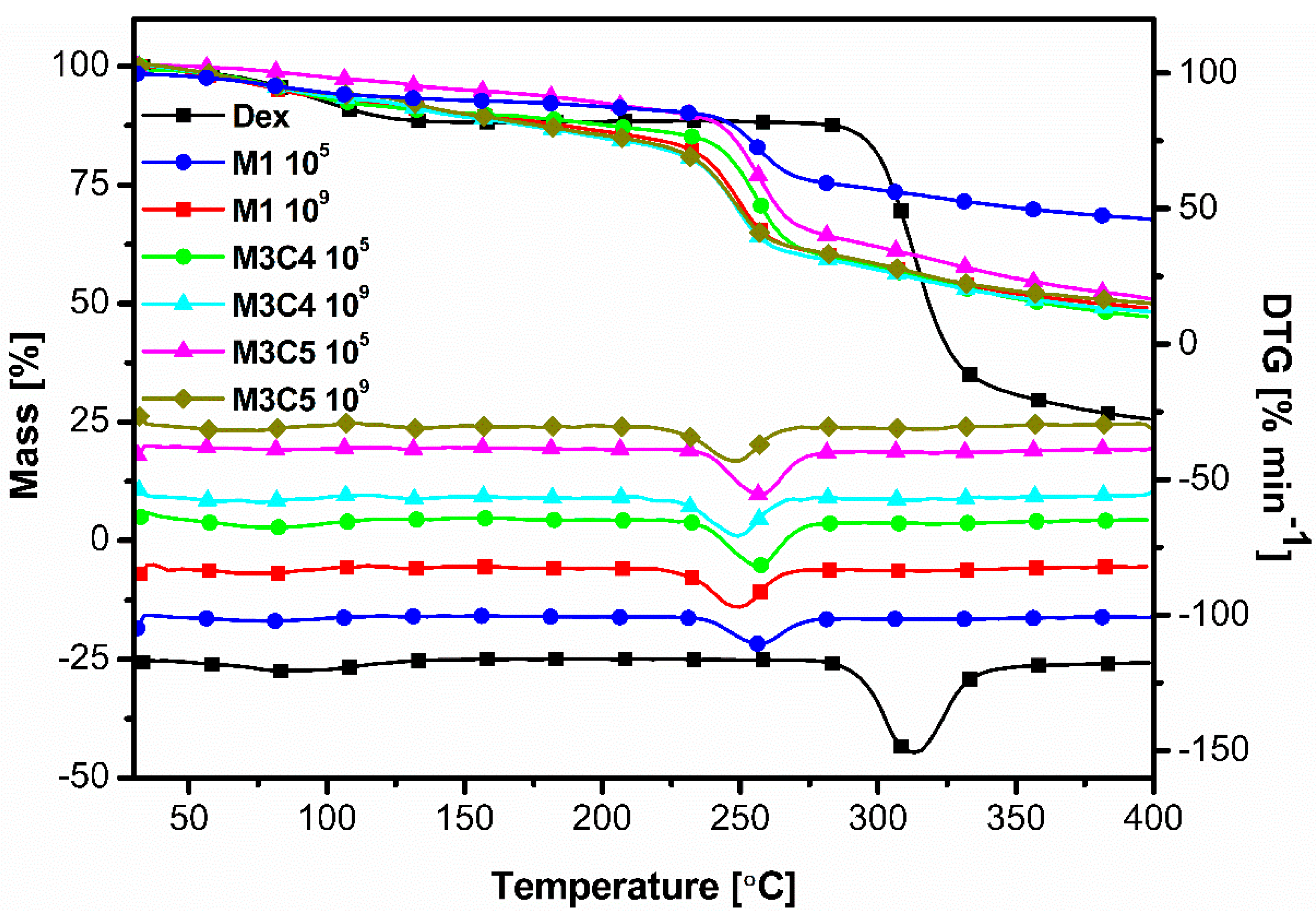
3.6. Thermal Analysis Measurements-Thermogravimetry (TGA)
3.7. Probiotic Activity Determination
3.7.1. Resistance to Low pH
3.7.2. Resistance to Bile Salt
3.7.3. Evaluation of Hydrophobicity and Auto-Aggregation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdallah, E.M. Antibacterial activity of Hibiscus sabdariffa L. calyces against hospital isolates of multidrug resistant Acinetobacter baumannii. J. Acute Dis. 2016, 5, 512–516. [Google Scholar] [CrossRef]
- Jabeur, I.; Pereira, E.; Barros, L.; Calhelha, R.C.; Soković, M.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Hibiscus sabdariffa L. as a source of nutrients, bioactive compounds and colouring agents. Food Res. Int. 2017, 100, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Riaz, G.; Chopra, R. A review on phytochemistry and therapeutic uses of Hibiscus sabdariffa L. Biomed. Pharmacother. 2018, 102, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Van Nguyen Thien, T.; Do, L.T.M.; Dang, P.H.; Huynh, N.V.; Dang, H.P.; Nguyen, T.T.; Tran, K.T.; Nguyen Huu, D.M.; Ton That, Q. A new lignan from the flowers of Hibiscus sabdariffa L. (Malvaceae). Nat. Prod. Res. 2019, 35, 2218–2223. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Thrimawithana, T.; Shukla, R.; Adhikari, B. Extraction and characterization of polyphenolic compounds and potassium hydroxycitrate from Hibiscus sabdariffa. Futur. Foods 2021, 4, 100087. [Google Scholar] [CrossRef]
- Khan, N.H.; Abdulbaqi, I.M.; Darwis, Y.; Aminu, N.; Chan, S.Y. A stability-indicating HPLC-UV method for the quantification of anthocyanin in Roselle (Hibiscus sabdariffa L.) spray-dried extract, oral powder, and lozenges. Heliyon 2022, 8, e09177. [Google Scholar] [CrossRef]
- Maciel, L.G.; do Carmo, M.A.V.; Azevedo, L.; Daguer, H.; Molognoni, L.; de Almeida, M.M.; Granato, D.; Rosso, N.D. Hibiscus sabdariffa anthocyanins-rich extract: Chemical stability, in vitro antioxidant and antiproliferative activities. Food Chem. Toxicol. 2018, 113, 187–197. [Google Scholar] [CrossRef]
- Higginbotham, K.L.; Burris, K.P.; Zivanovic, S.; Davidson, P.M.; Stewart, C.N. Aqueous extracts of Hibiscus sabdariffa calyces as an antimicrobial rinse on hot dogs against Listeria monocytogenes and methicillin-resistant Staphylococcus aureus. Food Control 2014, 40, 274–277. [Google Scholar] [CrossRef]
- Da-Costa-Rocha, I.; Bonnlaender, B.; Sievers, H.; Pischel, I.; Heinrich, M. Hibiscus sabdariffa L.–A phytochemical and pharmacological review. Food Chem. 2014, 165, 424–443. [Google Scholar] [CrossRef] [PubMed]
- Su, C.C.; Wang, C.J.; Huang, K.H.; Lee, Y.J.; Chan, W.M.; Chang, Y.C. Anthocyanins from Hibiscus sabdariffa calyx attenuate in vitro and in vivo melanoma cancer metastasis. J. Funct. Foods 2018, 48, 614–631. [Google Scholar] [CrossRef]
- Goldberg, K.H.; Yin, A.C.; Mupparapu, A.; Retzbach, E.P.; Goldberg, G.S.; Yang, C.F. Components in aqueous Hibiscus rosa-sinensis flower extract inhibit in vitro melanoma cell growth. J. Tradit. Complement. Med. 2017, 7, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Huang, C.C.; Hung, C.H.; Yao, F.Y.; Wang, C.J.; Chang, Y.C. Delphinidin-rich extracts of Hibiscus sabdariffa L. trigger mitochondria-derived autophagy and necrosis through reactive oxygen species in human breast cancer cells. J. Funct. Foods 2016, 25, 279–290. [Google Scholar] [CrossRef]
- Ali, B.H.; Al Wabel, N.; Blunden, G. Phytochemical, pharmacological and toxicological aspects of Hibiscus sabdariffa L.: A review. Phyther. Res. 2005, 19, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Guardiola, S.; Mach, N. Potencial terapéutico del Hibiscus sabdariffa: Una revisión de las evidencias científicas. Endocrinol. Nutr. 2014, 61, 274–295. [Google Scholar] [CrossRef]
- Amaya-Cruz, D.; Peréz-Ramírez, I.F.; Pérez-Jiménez, J.; Nava, G.M.; Reynoso-Camacho, R. Comparison of the bioactive potential of Roselle (Hibiscus sabdariffa L.) calyx and its by-product: Phenolic characterization by UPLC-QTOF MSE and their anti-obesity effect in vivo. Food Res. Int. 2019, 126, 108589. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Phan-Thi, H.; Pham-Hoang, B.N.; Ho, P.T.; Tran, T.T.T.; Waché, Y. Encapsulation of Hibiscus sabdariffa L. anthocyanins as natural colours in yeast. Food Res. Int. 2018, 107, 275–280. [Google Scholar] [CrossRef]
- Silva, M.; Cueva, C.; Alba, C.; Rodriguez, J.M.; de Pascual-Teresa, S.; Jones, J.; Caturla, N.; Victoria Moreno-Arribas, M.; Bartolomé, B. Gut microbiome-modulating properties of a polyphenol-enriched dietary supplement comprised of hibiscus and lemon verbena extracts. Monitoring of phenolic metabolites. J. Funct. Foods 2022, 91, 105016. [Google Scholar] [CrossRef]
- Laskar, Y.B.; Mazumder, P.B. Insight into the molecular evidence supporting the remarkable chemotherapeutic potential of Hibiscus sabdariffa L. Biomed. Pharmacother. 2020, 127, 110153. [Google Scholar] [CrossRef]
- Nazhand, A.; Souto, E.B.; Lucarini, M.; Souto, S.B.; Durazzo, A.; Santini, A. Ready to Use Therapeutical Beverages: Focus on Functional Beverages Containing Probiotics, Prebiotics and Synbiotics. Beverages 2020, 6, 26. [Google Scholar] [CrossRef]
- Guérin, M.; Robert-Da Silva, C.; Garcia, C.; Remize, F. Lactic Acid Bacterial Production of Exopolysaccharides from Fruit and Vegetables and Associated Benefits. Fermentation 2020, 6, 115. [Google Scholar] [CrossRef]
- Xiong, L.; Ni, X.; Niu, L.; Zhou, Y.; Wang, Q.; Khalique, A.; Liu, Q.; Zeng, Y.; Shu, G.; Pan, K.; et al. Isolation and Preliminary Screening of a Weissella confusa Strain from Giant Panda (Ailuropoda melanoleuca). Probiotics Antimicrob. Proteins 2019, 11, 535–544. [Google Scholar] [CrossRef]
- de Souza Aquino, J.; Batista, K.S.; Menezes, F.N.D.D.; Lins, P.P.; de Sousa Gomes, J.A.; da Silva, L.A. Models to Evaluate the Prebiotic Potential of Foods. Funct. Food-Improv. Health Adequate Food 2017. [Google Scholar] [CrossRef]
- Devi, P.B.; Kavitake, D.; Jayamanohar, J.; Shetty, P.H. Preferential growth stimulation of probiotic bacteria by galactan exopolysaccharide from Weissella confusa KR780676. Food Res. Int. 2021, 143, 110333. [Google Scholar] [CrossRef] [PubMed]
- Sáyago-Ayerdi, S.G.; Venema, K.; Tabernero, M.; Sarriá, B.; Bravo, L.; Mateos, R. Bioconversion of polyphenols and organic acids by gut microbiota of predigested Hibiscus sabdariffa L. calyces and Agave (A. tequilana Weber) fructans assessed in a dynamic in vitro model (TIM-2) of the human colon. Food Res. Int. 2021, 143, 110301. [Google Scholar] [CrossRef] [PubMed]
- Anghel, N. SPRUCE BARK POLYPHENOLS AS METABOLIC BOOSTERS FOR YEAST DEVELOPMENT. Cellul. Chem. Technol. Cellul. Chem. Technol. 2019, 53, 925–928. [Google Scholar] [CrossRef]
- Rosca, I.; Petrovici, A.R.; Peptanariu, D.; Nicolescu, A.; Dodi, G.; Avadanei, M.; Ivanov, I.C.; Bostanaru, A.C.; Mares, M.; Ciolacu, D. Biosynthesis of dextran by Weissella confusa and its In vitro functional characteristics. Int. J. Biol. Macromol. 2018, 107, 1765–1772. [Google Scholar] [CrossRef]
- Miao, M.; Bai, A.; Jiang, B.; Song, Y.; Cui, S.W.; Zhang, T. Characterisation of a novel water-soluble polysaccharide from Leuconostoc citreum SK24.002. Food Hydrocoll. 2014, 36, 265–272. [Google Scholar] [CrossRef]
- Petrovici, A.R.; Nicolescu, A.; Silion, M.; Roșca, I.; Ciolacu, D. Biopolymer biosynthesis by lactic acid bacteria strain in four different culture media. Rev. Roum. Chim. 2018, 63, 637–642. [Google Scholar]
- Krausova, G.; Hyrslova, I.; Hynstova, I. In Vitro Evaluation of Adhesion Capacity, Hydrophobicity, and Auto-Aggregation of Newly Isolated Potential Probiotic Strains. Ferment. 2019, 5, 100. [Google Scholar] [CrossRef]
- Mushtaq, M.; Gani, A.; Noor, N.; Masoodi, F.A. Phenotypic and probiotic characterization of isolated LAB from Himalayan cheese (Kradi/Kalari) and effect of simulated gastrointestinal digestion on its bioactivity. LWT 2021, 149, 111669. [Google Scholar] [CrossRef]
- Collins, M.D.; Samelis, J.; Metaxopoulos, J.; Wallbanks, S. Taxonomic studies on some leuconostoc-like organisms from fermented sausages: Description of a new genus Weissella for the Leuconostoc paramesenteroides group of species. J. Appl. Bacteriol. 1993, 75, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Hainal, A.-R.; Ignat, I.; Volf, I.; Popa, V.I. TRANSFORMATION OF POLYPHENOLS FROM BIOMASS BY SOME YEAST SPECIES. Cellul. Chem. Technol. 2011, 45, 211–219. [Google Scholar]
- Petrovici, A.R.; Roşca, I.; Dodi, G.; Nicolescu, A.; Avǎdanei, M.; Varganici, C.D.; Ciolacu, D. Effects of culture medium composition on biosynthesis of exopolysaccharides. Cellul. Chem. Technol. 2017, 51, 821–830. [Google Scholar]
- Wu, S.; Lu, H.Y.; Chen, Q.H.; Xie, H.C.; Jiao, Y.C. Anthocyanin extract from Lycium ruthenicum enhanced production of biomass and polysaccharides during submerged fermentation of Agaricus bitorquis (Quél.) Sacc. Chaidam. Bioprocess Biosyst. Eng. 2021, 44, 2303–2313. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Deng, L.; Li, S.; Tan, T. Structural characterization of a water-insoluble (1 → 3)-α-d-glucan isolated from the Penicillium chrysogenum. Carbohydr. Polym. 2007, 67, 133–137. [Google Scholar] [CrossRef]
- Maina, N.H.; Tenkanen, M.; Maaheimo, H.; Juvonen, R.; Virkki, L. NMR spectroscopic analysis of exopolysaccharides produced by Leuconostoc citreum and Weissella confusa. Carbohydr. Res. 2008, 343, 1446–1455. [Google Scholar] [CrossRef]
- Kothari, D.; Tingirikari, J.M.R.; Goyal, A. In vitro analysis of dextran from Leuconostoc mesenteroides NRRL B-1426 for functional food application. Bioact. Carbohydrates Diet. Fibre 2015, 6, 55–61. [Google Scholar] [CrossRef]
- Zhou, Q.; Feng, F.; Yang, Y.; Zhao, F.; Du, R.; Zhou, Z.; Han, Y. Characterization of a dextran produced by Leuconostoc pseudomesenteroides XG5 from homemade wine. Int. J. Biol. Macromol. 2018, 107, 2234–2241. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Liu, P.; Ahmed, Z.; Xiao, P.; Bai, X. Physical characterization of exopolysaccharide produced by Lactobacillus plantarum KF5 isolated from Tibet Kefir. Carbohydr. Polym. 2010, 82, 895–903. [Google Scholar] [CrossRef]
- Grispoldi, L.; Giglietti, R.; Traina, G.; Cenci-Goga, B. How to Assess in vitro Probiotic Viability and the Correct Use of Neutralizing Agents. Front. Microbiol. 2020, 11, 204. [Google Scholar] [CrossRef]
- Fessard, A.; Remize, F. Why Are Weissella spp. Not Used as Commercial Starter Cultures for Food Fermentation? Ferment. 2017, 3, 38. [Google Scholar] [CrossRef]
- Monteagudo-Mera, A.; Rodríguez-Aparicio, L.; Rúa, J.; Martínez-Blanco, H.; Navasa, N.; García-Armesto, M.R.; Ferrero, M.Á. In vitro evaluation of physiological probiotic properties of different lactic acid bacteria strains of dairy and human origin. J. Funct. Foods 2012, 4, 531–541. [Google Scholar] [CrossRef]
- Korcz, E.; Varga, L. Exopolysaccharides from lactic acid bacteria: Techno-functional application in the food industry. Trends Food Sci. Technol. 2021, 110, 375–384. [Google Scholar] [CrossRef]
- Huang, H.C.; Chang, W.T.; Wu, Y.H.; Yang, B.C.; Xu, M.R.; Lin, M.K.; Chen, H.J.; Cheng, J.H.; Lee, M.S. Phytochemicals levels and biological activities in Hibiscus sabdariffa L. were enhanced using microbial fermentation. Ind. Crops Prod. 2022, 176, 114408. [Google Scholar] [CrossRef]
- Sáyago-Ayerdi, S.G.; Zamora-Gasga, V.M.; Venema, K. Changes in gut microbiota in predigested Hibiscus sabdariffa L. calyces and Agave (Agave tequilana weber) fructans assessed in a dynamic in vitro model (TIM-2) of the human colon. Food Res. Int. 2020, 132, 109036. [Google Scholar] [CrossRef]




| Experiment Code | Anthocyanins’ Concentration in Culture Media, μg/mL | Description |
|---|---|---|
| M1 105 | - | The PP29 strain fermented in M1 inoculated with 105 CFU/mL |
| M1 109 | - | The PP29 strain fermented in M1 inoculated with 109 CFU/mL |
| M3C1 105 | 1 | The PP29 strain fermented in M3C1 inoculated with 105 CFU/mL |
| M3C1 109 | 1 | The PP29 strain fermented in M3C1 inoculated with 109 CFU/mL |
| M3C2 105 | 2 | The PP29 strain fermented in M3C2 inoculated with 105 CFU/mL |
| M3C2 109 | 2 | The PP29 strain fermented in M3C2 inoculated with 109 CFU/mL |
| M3C3 105 | 3 | The PP29 strain fermented in M3C3 inoculated with 105 CFU/mL |
| M3C3 109 | 3 | The PP29 strain fermented in M3C3 inoculated with 109 CFU/mL |
| M3C4 105 | 1000 | The PP29 strain fermented in M3C4 inoculated with 105 CFU/mL |
| M3C4 109 | 1000 | The PP29 strain fermented in M3C4 inoculated with 109 CFU/mL |
| M3C5 105 | 2000 | The PP29 strain fermented in M3C5 inoculated with 105 CFU/mL |
| M3C5 109 | 2000 | The PP29 strain fermented in M3C5 inoculated with 109 CFU/mL |
| Sample, 109 Initial CPU | Fraction | Mn, Da | Mw, Da | PDI |
|---|---|---|---|---|
| M1 | 1 | 2.0 × 106 | 2.2 × 106 | 1.1 |
| 2 | 6.1 × 104 | 6.3 × 104 | 1.03 | |
| M3C4 | 1 | 8.6 × 105 | 1.2 × 106 | 1.39 |
| 2 | 4.9 × 105 | 5.6 × 105 | 1.14 | |
| 3 | 1.2 × 105 | 2.7 × 105 | 2.25 | |
| M3C5 | 1 | 9.1 × 105 | 1.3 × 106 | 1.42 |
| 2 | 6.8 × 105 | 8.1 × 105 | 1.19 | |
| 3 | 6.7 × 104 | 8.8 × 104 | 1.31 |
| Degradation Temperature, °C | 249 | 255 | 312 |
|---|---|---|---|
| Samples | M1 109 | M1 105 | Dex |
| M3C4 109 | M3C4 105 | ||
| M3C5 109 | M3C5 105 |
| Experiments | pH 7 | pH 3 | pH 2 |
|---|---|---|---|
| M1 105 | 100 | 3.00 ± 0.12 | 0.00 ± 0.00 |
| M1 109 | 100 | 25.00 ± 1.08 | 4.89 ± 0.98 |
| M3C4 105 | 100 | 39.56 ± 1.57 | 5.02 ± 0.22 |
| M3C4 109 | 100 | 40.59 ± 1.69 | 10.28 ± 0.50 |
| M3C5 105 | 100 | 42.05 ± 1.77 | 21.05 ± 0.98 |
| M3C5 109 | 100 | 72.74 ± 3.19 | 53.51 ± 2.42 |
| Experiments | 0.0 % Bile Salt in QSRS | 0.1 % Bile Salt in QSRS | 0.2 % Bile Salt in QSRS | 0.3 % Bile Salt in QSRS |
|---|---|---|---|---|
| M1 105 | 100 | 21.00 ± 0.91 | 18.59 ± 0.79 | 2.85 ± 0.12 |
| M1 109 | 100 | 42.85 ± 1.77 | 29.05 ± 1.22 | 5.02 ± 0.23 |
| M3C4 105 | 100 | 59.61 ± 2.84 | 31.05 ± 1.32 | 8.05 ± 0.37 |
| M3C4 109 | 100 | 72.74 ± 3.28 | 39.05 ± 1.68 | 15.08 ± 0.75 |
| M3C5 105 | 100 | 78.02 ± 3.25 | 57.02 ± 2.37 | 23.58 ± 1.15 |
| M3C5 109 | 100 | 86.25 ± 4.07 | 69.05 ± 2.75 | 42.05 ± 2.02 |
| Experiments | A%, 1 h | A%, 6 h | A%, 24 h | H%, Hexane | H%, Chloroform |
|---|---|---|---|---|---|
| M1 105 | 3.10 ± 0.14 | 8.35 ± 0.36 | 9.28 ± 0.41 | 42.5 ± 2.02 | 38.52 ± 1.83 |
| M1 109 | 5.90 ± 0.25 | 11.02 ± 0.50 | 13.25 ± 0.62 | 49.2 ± 2.29 | 39.58 ± 1.92 |
| M3C4 105 | 12.80 ± 0.56 | 28.25 ± 1.10 | 31.02 ± 1.49 | 59.8 ± 2.78 | 42.52 ± 1.77 |
| M3C4 109 | 19.25 ± 0.81 | 31.05 ± 1.36 | 32.25 ± 1.59 | 67.58 ± 2.93 | 49.85 ± 2.08 |
| M3C5 105 | 25.29 ± 1.14 | 49.85 ± 2.33 | 52.35 ± 2.40 | 72.85 ± 3.05 | 70.21 ± 3.18 |
| M3C5 109 | 41.02 ± 1.86 | 59.80 ± 2.86 | 61.25 ± 2.92 | 79.58 ± 3.73 | 78.58 ± 3.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimofte, A.; Simionescu, N.; Petrovici, A.-R.; Spiridon, I. Probiotic Properties of Weissella confusa PP29 on Hibiscus sabdariffa L. Media. Fermentation 2022, 8, 553. https://doi.org/10.3390/fermentation8100553
Dimofte A, Simionescu N, Petrovici A-R, Spiridon I. Probiotic Properties of Weissella confusa PP29 on Hibiscus sabdariffa L. Media. Fermentation. 2022; 8(10):553. https://doi.org/10.3390/fermentation8100553
Chicago/Turabian StyleDimofte, Alexandra, Natalia Simionescu, Anca-Roxana Petrovici, and Iuliana Spiridon. 2022. "Probiotic Properties of Weissella confusa PP29 on Hibiscus sabdariffa L. Media" Fermentation 8, no. 10: 553. https://doi.org/10.3390/fermentation8100553
APA StyleDimofte, A., Simionescu, N., Petrovici, A.-R., & Spiridon, I. (2022). Probiotic Properties of Weissella confusa PP29 on Hibiscus sabdariffa L. Media. Fermentation, 8(10), 553. https://doi.org/10.3390/fermentation8100553







