Towards a Complete Exploitation of Brewers’ Spent Grain from a Circular Economy Perspective
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and General Methods
2.2. Microorganisms and Growth Media
2.3. BSG Aqueous Extraction
2.4. Evaluation of Fermentation with BSG Microbial Growth Medium
2.5. Preparation of DESs
2.6. BSG Treatment with DES
2.7. Lignin Solubility in Organic Solvents
2.8. Instruments and Analytic Condition
2.8.1. Thin Layer Chromatography
2.8.2. 1H NMR
2.8.3. Gas-Chromatography/Mass Spectrometry
2.8.4. Gel Permeation Chromatography
2.8.5. Total Sugars Quantification
2.8.6. Phenolic Hydroxyl Group Determination
2.8.7. 31P NMR Analysis
2.8.8. Fourier-Transform Infrared Spectroscopy
2.8.9. Differential Scanning Calorimetry
2.9. Water Reduction in Cement Pastes
3. Results and Discussion
3.1. BSG Waste Pre-Treatment and Sugar Process
3.2. Lignocellulose Process
3.2.1. DES-Mediated Fractionation
3.2.2. BSGT Lignin Characterization
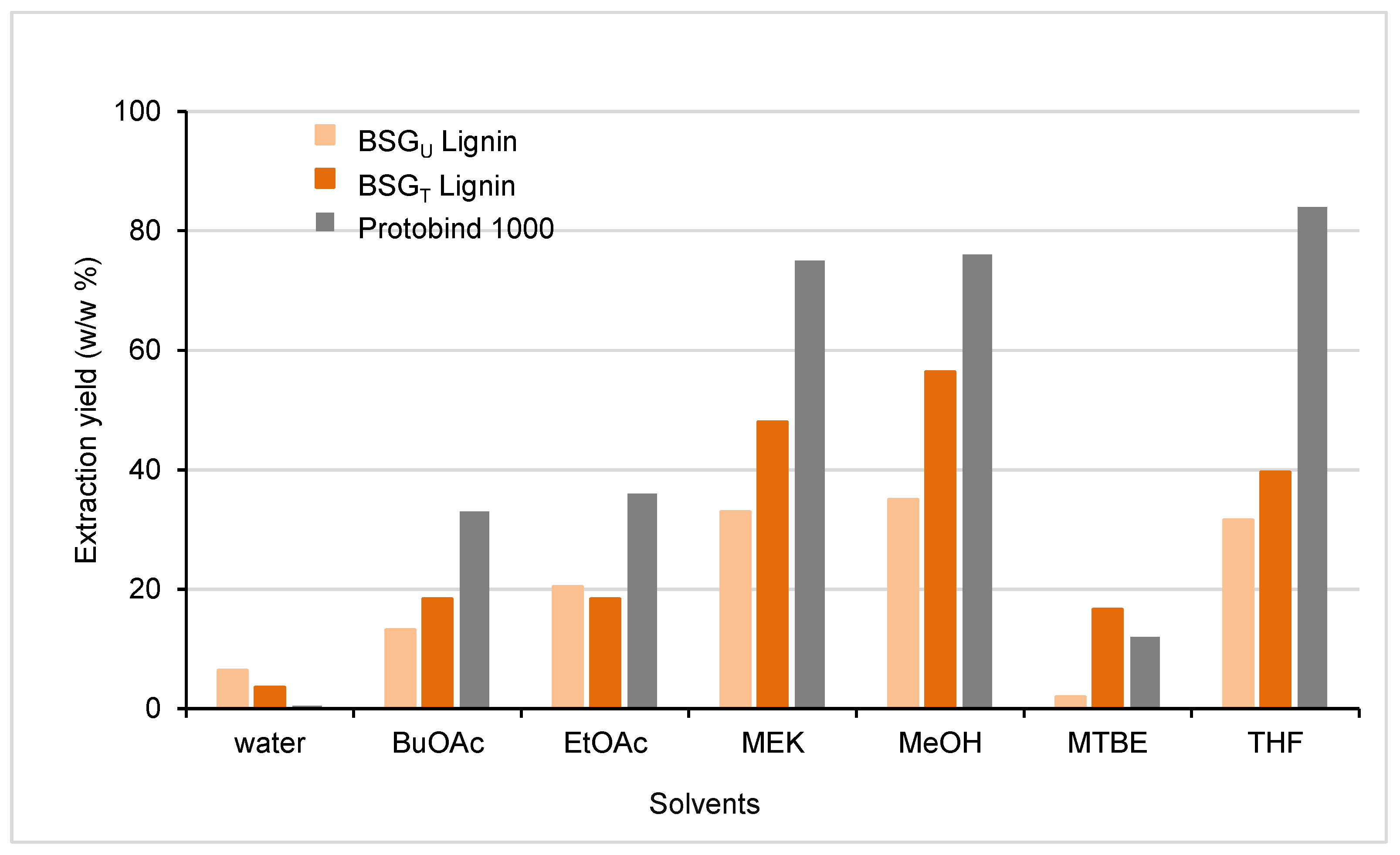
Solvent Solubilization Determination
GPC Results
Sugars Quantification
Phenolic Hydroxyl Group Determination
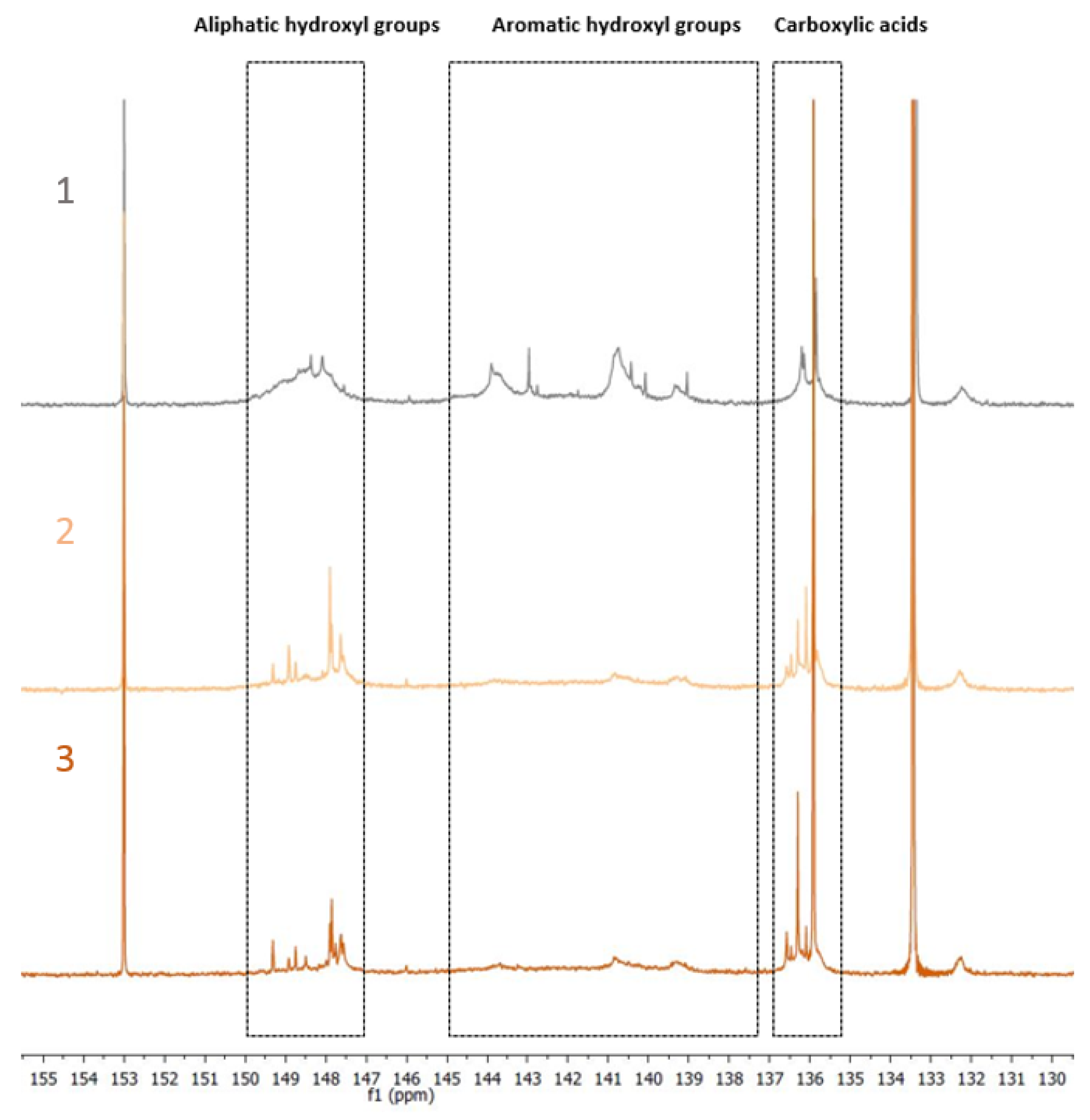
Total Hydroxyl Groups Quantification by 31P NMR
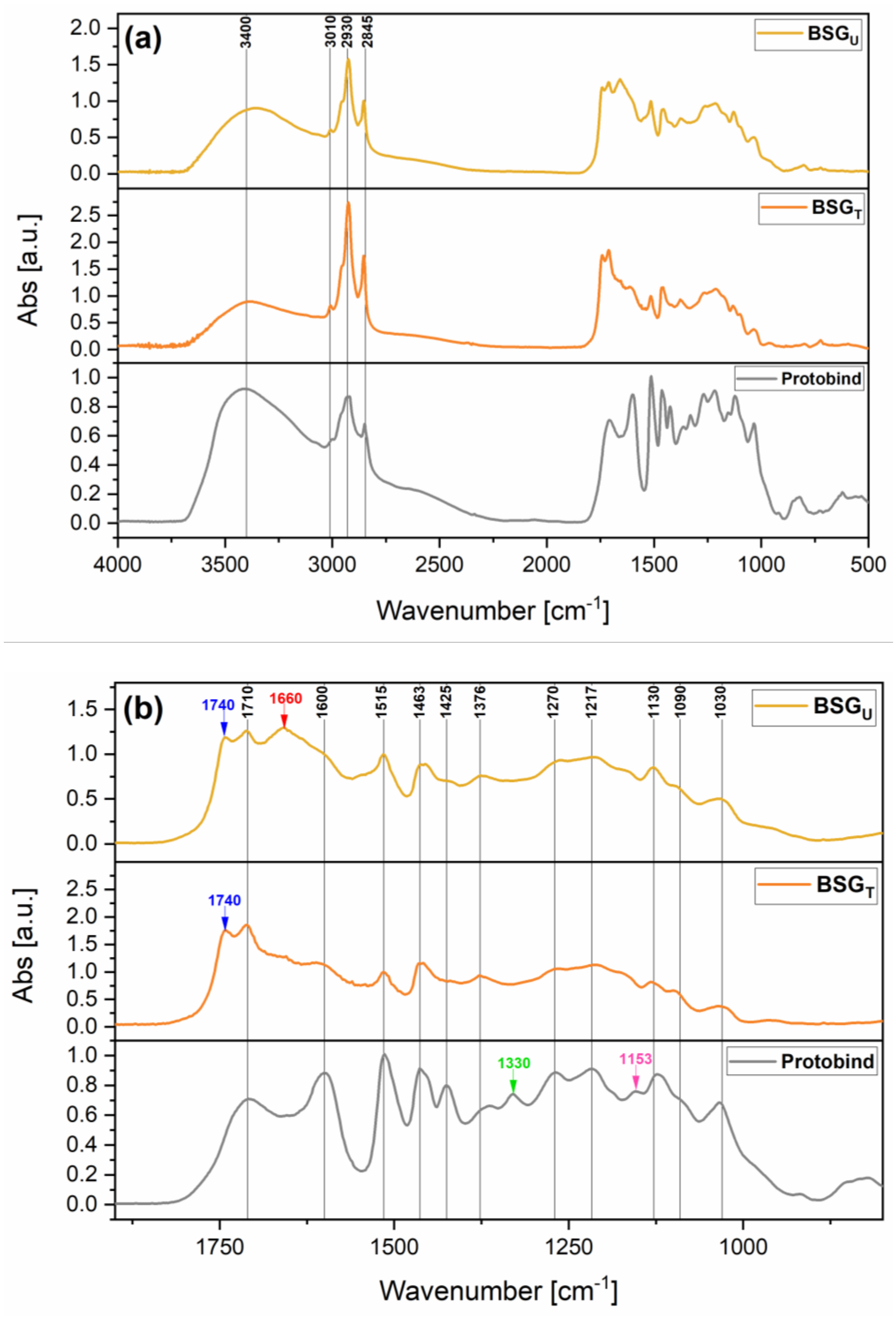
Fourier-Transform Infrared Spectroscopy
Differential Scanning Calorimetry
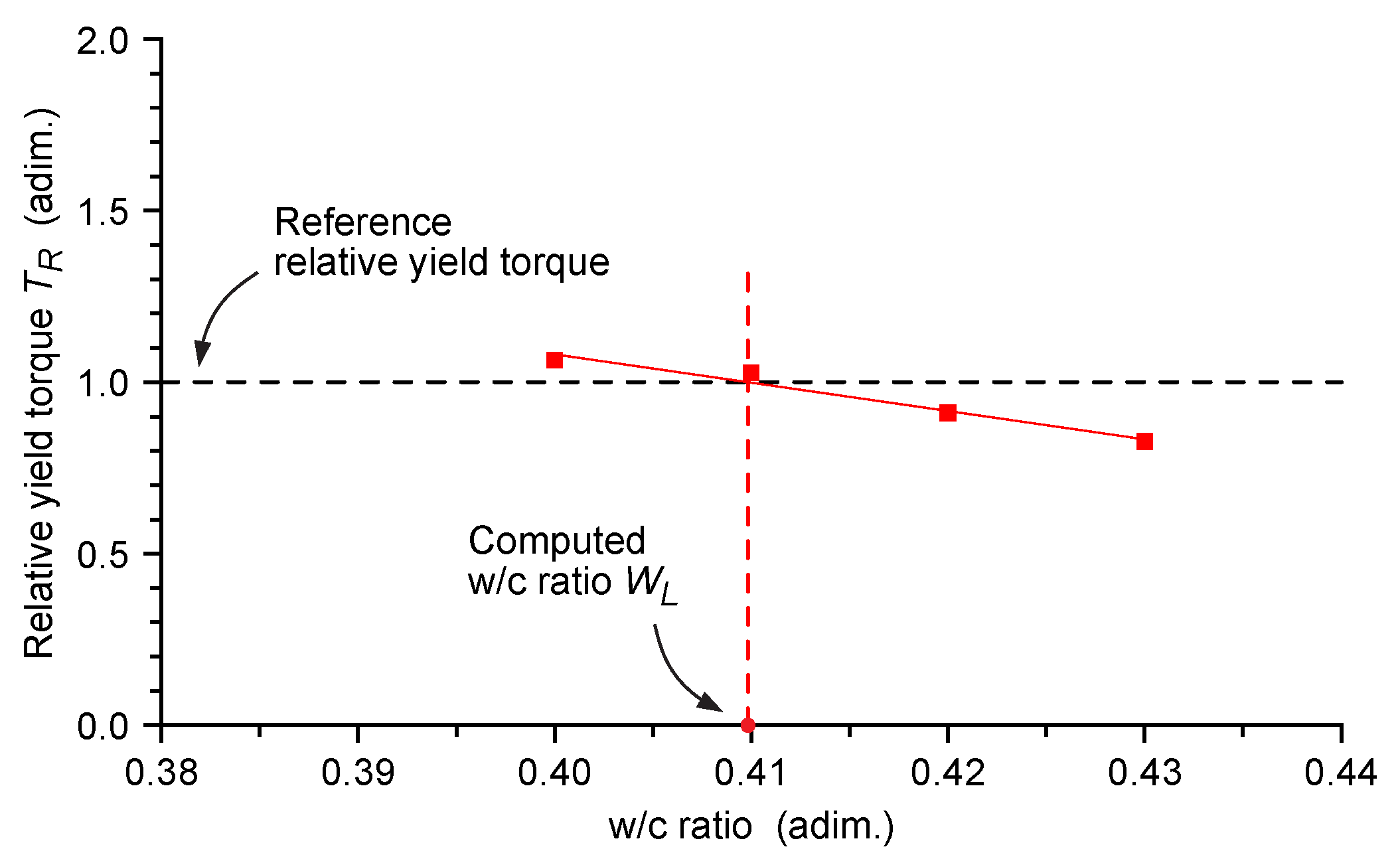
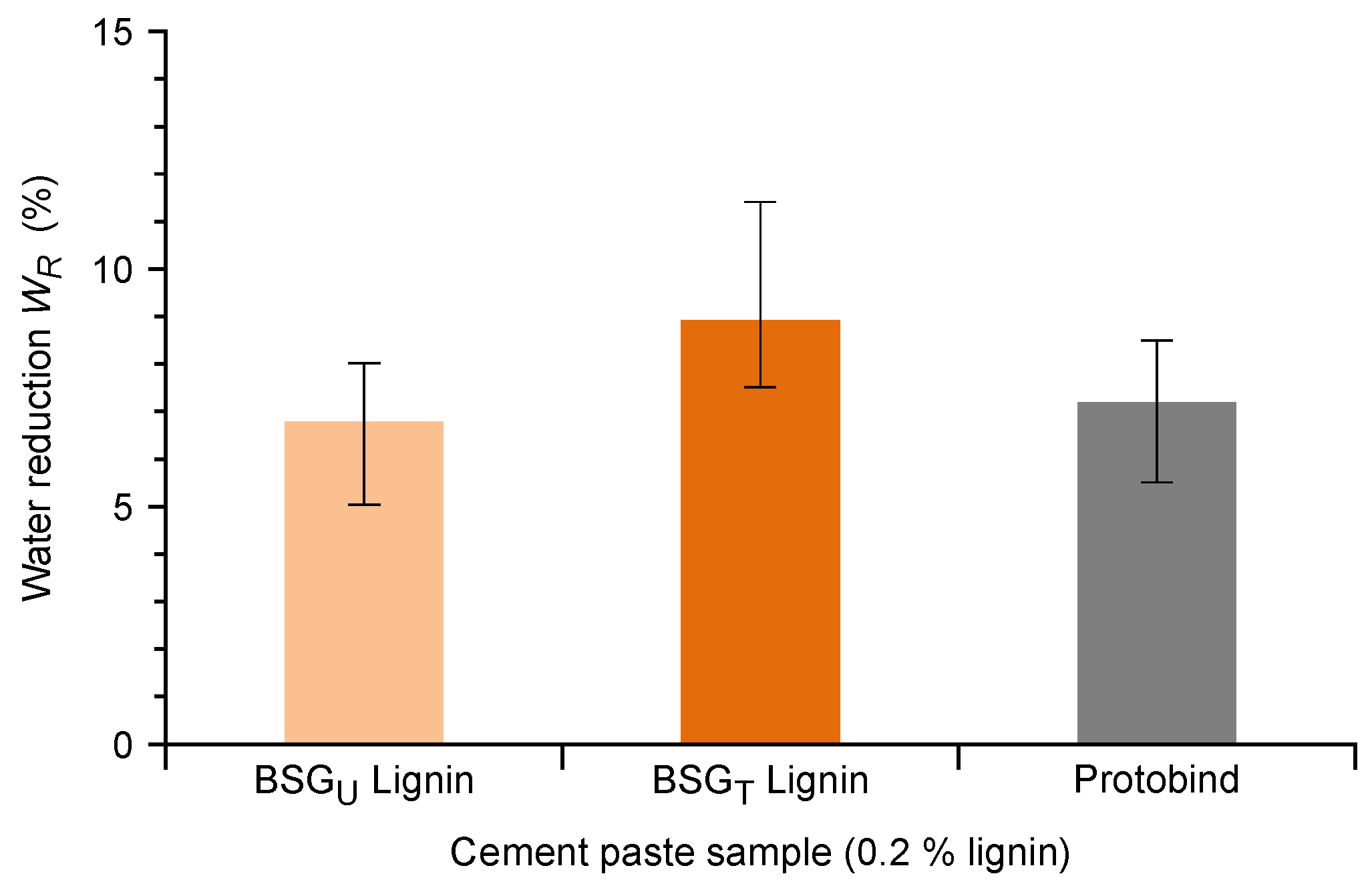
3.2.3. Water Reduction in Cement Pastes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- A New Circular Economy Action Plan for a Cleaner and More Competitive Europe; COM(2020) 98; European Commission, Directorate-General for Environment: Brussels, Belgium, 2020.
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ spent grain: Generation, characteristics and potential applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Thomas, K.; Rahman, P. Brewery wastes. Strategies for sustainability. A review. Asp. Appl. Biol. 2006, 80, 1–11. [Google Scholar]
- Kerby, C.; Vriesekoop, F. An overview of the utilisation of brewery by-products as generated by british craft breweries. Beverages 2017, 3, 24. [Google Scholar] [CrossRef] [Green Version]
- Buffington, J. The economic potential of brewer’s spent grain (bsg) as a biomass feedstock. Adv. Chem. Eng. Sci. 2014, 4, 308–318. [Google Scholar] [CrossRef] [Green Version]
- Salihu, A.; Bala, M. Brewer’s spent grain: A review of its potentials and applications. Afr. J. Biotechnol. 2011, 10, 324–331. [Google Scholar]
- Robertson, J.; I’Anson, K.; Treimo, J.; Faulds, C.; Brocklehurst, T.; Eijsink, V.; Waldron, K. Profiling brewers’ spent grain for composition and microbial ecology at the site of production. LWT Food Sci. Technol. 2010, 43, 890–896. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Duan, Y.; Zhang, H.; Ma, H. A mini-review on brewer’s spent grain protein: Isolation, physicochemical properties, application of protein, and functional properties of hydrolysates. J. Food Sci. 2019, 84, 3330–3340. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Kuhn, D.D.; Ogejo, J.A.; O’Keefe, S.F.; Fraguas, C.F.; Wiersema, B.D.; Jin, Q.; Yu, D.; Huang, H. Wet fractionation process to produce high protein and high fiber products from brewer’s spent grain. Food Bioprod. Process. 2019, 117, 266–274. [Google Scholar] [CrossRef]
- Martínez-Avila, O.; Llenas, L.; Ponsá, S. Sustainable polyhydroxyalkanoates production via solid-state fermentation: Influence of the operational parameters and scaling up of the process. Food Bioprod. Process. 2022, 132, 13–22. [Google Scholar] [CrossRef]
- de Crane d’Heysselaer, S.; Bockstal, L.; Jacquet, N.; Schmetz, Q.; Richel, A. Potential for the valorisation of brewer’s spent grains: A case study for the sequential extraction of saccharides and lignin. Waste Manag. Res. 2021, 734242X211055547. [Google Scholar] [CrossRef]
- Outeiriño, D.; Costa-Trigo, I.; Pinheiro de Souza Oliveira, R.; Pérez Guerra, N.; Domínguez, J.M. A novel approach to the biorefinery of brewery spent grain. Process Biochem. 2019, 85, 135–142. [Google Scholar] [CrossRef]
- Parchami, M.; Ferreira, J.A.; Taherzadeh, M. Brewing process development by integration of edible filamentous fungi to upgrade the quality of brewer’s spent grain (bsg). Bioresources 2021, 16, 1686–1701. [Google Scholar] [CrossRef]
- Dursun, D.; Koulouris, A.; Dalgıç, A.C. Process simulation and techno economic analysis of astaxanthin production from agro-industrial wastes. Waste Biomass Valoriz. 2020, 11, 943–954. [Google Scholar] [CrossRef]
- Caporusso, A.; Capece, A.; De Bari, I. Oleaginous yeasts as cell factories for the sustainable production of microbial lipids by the valorization of agri-food wastes. Fermentation 2021, 7, 50. [Google Scholar] [CrossRef]
- Gillet, S.; Aguedo, M.; Petitjean, L.; Morais, A.R.C.; Lopes, A.M.D.; Lukasik, R.M.; Anastas, P.T. Lignin transformations for high value applications: Towards targeted modifications using green chemistry. Green Chem. 2017, 19, 4200–4233. [Google Scholar] [CrossRef]
- Collins, M.N.; Nechifor, M.; Tanasa, F.; Zanoaga, M.; McLoughlin, A.; Strozyk, M.A.; Culebras, M.; Teaca, C.A. Valorization of lignin in polymer and composite systems for advanced engineering applications—A review. Int. J. Biol. Macromol. 2019, 131, 828–849. [Google Scholar] [CrossRef] [PubMed]
- Tribot, A.; Amer, G.; Abdou Alio, M.; de Baynast, H.; Delattre, C.; Pons, A.; Mathias, J.-D.; Callois, J.-M.; Vial, C.; Michaud, P.; et al. Wood-lignin: Supply, extraction processes and use as bio-based material. Eur. Polym. J. 2019, 112, 228–240. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Greener synthesis of lignin nanoparticles and their applications. Green Chem. 2020, 22, 612–636. [Google Scholar] [CrossRef]
- de Baynast, H.; Tribot, A.; Niez, B.; Audonnet, F.; Badel, E.; Cesar, G.; Dussap, C.-G.; Gastaldi, E.; Massacrier, L.; Michaud, P.; et al. Effects of kraft lignin and corn cob agro-residue on the properties of injected-moulded biocomposites. Ind. Crop. Prod. 2022, 177, 114421. [Google Scholar] [CrossRef]
- Moreno, A.; Sipponen, M.H. Lignin-based smart materials: A roadmap to processing and synthesis for current and future applications. Mater. Horiz. 2020, 7, 2237–2257. [Google Scholar] [CrossRef]
- de Haro, J.C.; Allegretti, C.; Smit, A.T.; Turri, S.; D’Arrigo, P.; Griffini, G. Biobased polyurethane coatings with high biomass content: Tailored properties by lignin selection. ACS Sustain. Chem. Eng. 2019, 7, 11700–11711. [Google Scholar] [CrossRef]
- Garcia Gonzalez, M.N.; Levi, M.; Turri, S.; Griffini, G. Lignin nanoparticles by ultrasonication and their incorporation in waterborne polymer nanocomposites. J. Appl. Polym. Sci. 2017, 134, 45318. [Google Scholar] [CrossRef]
- de Carlos Haro, J.; Magagnin, L.; Turri, S.; Griffini, G. Lignin-based anticorrosion coatings for the protection of aluminum surfaces. ACS Sustain. Chem. Eng. 2019, 7, 6213–6222. [Google Scholar] [CrossRef]
- Lu, J.J.; Cheng, M.Y.; Zhao, C.; Li, B.; Peng, H.H.; Zhang, Y.J.; Shao, Q.J.; Hassan, M. Application of lignin in preparation of slow-release fertilizer: Current status and future perspectives. Ind. Crop. Prod. 2022, 176, 114267. [Google Scholar] [CrossRef]
- Chen, W.J.; Zhao, C.X.; Li, B.Q.; Yuan, T.Q.; Zhang, Q. Lignin-derived materials and their applications in rechargeable batteries. Green Chem. 2022, 24, 565–584. [Google Scholar] [CrossRef]
- Budnyak, T.M.; Slabon, A.; Sipponen, M.H. Lignin–inorganic interfaces: Chemistry and applications from adsorbents to catalysts and energy storage materials. ChemSusChem 2020, 13, 4344–4355. [Google Scholar] [CrossRef]
- de Haro, J.C.; Tatsi, E.; Fagiolari, L.; Bonomo, M.; Barolo, C.; Turri, S.; Bella, F.; Griffini, G. Lignin-based polymer electrolyte membranes for sustainable aqueous dye-sensitized solar cells. ACS Sustain. Chem. Eng. 2021, 9, 8550–8560. [Google Scholar] [CrossRef]
- Edmeades, R.M.; Hewlett, P.C. Cement admixtures. In Lea’s Chemistry of Cement and Concrete, 4th ed.; Hewlett, P.C., Ed.; Butterworth-Heinemann: Oxford, UK, 1998; pp. 841–905. [Google Scholar]
- Lou, H.M.; Lai, H.R.; Wang, M.X.; Pang, Y.X.; Yang, D.J.; Qiu, X.Q.; Wang, B.; Zhang, H.B. Preparation of lignin-based superplasticizer by graft sulfonation and investigation of the dispersive performance and mechanism in a cementitious system. Ind. Eng. Chem. Res. 2013, 52, 16101–16109. [Google Scholar] [CrossRef]
- Zheng, T.; Zheng, D.F.; Qiu, X.Q.; Yang, D.J.; Fan, L.; Zheng, J.M. A novel branched claw-shape lignin-based polycarboxylate superplasticizer: Preparation, performance and mechanism. Cem. Concr. Res. 2019, 119, 89–101. [Google Scholar] [CrossRef]
- Gupta, C.; Nadelman, E.; Washburn, N.R.; Kurtis, K.E. Lignopolymer superplasticizers for low-co2 cements. ACS Sustain. Chem. Eng. 2017, 5, 4041–4049. [Google Scholar] [CrossRef]
- Kalliola, A.; Vehmas, T.; Liitia, T.; Tamminen, T. Alkali-O2 oxidized lignin—A bio-based concrete plasticizer. Ind. Crop. Prod. 2015, 74, 150–157. [Google Scholar] [CrossRef]
- Li, S.Y.; Li, Z.Q.; Zhang, Y.D.; Liu, C.; Yu, G.; Li, B.; Mu, X.D.; Peng, H. Preparation of concrete water reducer via fractionation and modification of lignin extracted from pine wood by formic acid. ACS Sustain. Chem. Eng. 2017, 5, 4214–4222. [Google Scholar] [CrossRef]
- Allegretti, C.; Boumezgane, O.; Rossato, L.; Strini, A.; Troquet, J.; Turri, S.; Griffini, G.; D’Arrigo, P. Tuning lignin characteristics by fractionation: A versatile approach based on solvent extraction and membrane-assisted ultrafiltration. Molecules 2020, 25, 2893. [Google Scholar] [CrossRef] [PubMed]
- D’Arrigo, P.; Allegretti, C.; Tamborini, S.; Formantici, C.; Galante, Y.; Pollegioni, L.; Mele, A. Single-batch, homogeneous phase depolymerization of cellulose catalyzed by a monocomponent endocellulase in ionic liquid [bmim][cl]. J. Mol. Catal. B Enzym. 2014, 106, 76–80. [Google Scholar] [CrossRef]
- Allegretti, C.; Fontanay, S.; Rischka, K.; Strini, A.; Troquet, J.; Turri, S.; Griffini, G.; D’Arrigo, P. Two-step fractionation of a model technical lignin by combined organic solvent extraction and membrane ultrafiltration. ACS Omega 2019, 4, 4615–4626. [Google Scholar] [CrossRef] [Green Version]
- Haist, M.; Link, J.; Nicia, D.; Leinitz, S.; Baumert, C.; von Bronk, T.; Cotardo, D.; Pirharati, M.E.; Fataei, S.; Garrecht, H.; et al. Interlaboratory study on rheological properties of cement pastes and reference substances: Comparability of measurements performed with different rheometers and measurement geometries. Mater. Struct. 2020, 53, 92. [Google Scholar] [CrossRef]
- Johnson, E.A. Phaffia rhodozyma: Colorful odyssey. Int. Microbiol. 2003, 6, 169–174. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, X.; Too, H.-P. Microbial astaxanthin biosynthesis: Recent achievements, challenges, and commercialization outlook. Appl. Microbiol. Biotechnol. 2020, 104, 5725–5737. [Google Scholar] [CrossRef]
- Stachowiak, B.; Szulc, P. Astaxanthin for the food industry. Molecules 2021, 26, 2666. [Google Scholar] [CrossRef]
- Jacobson, G.K.; Jolly, S.O.; Sedmak, J.J.; Skatrud, T.J.; Wasileski, J.M. Astaxanthin Over-Producing Strains of Phaffia Rhodozyma. U.S. Patent 5,466,599, 14 November 1995. [Google Scholar]
- Huang, C.; Chen, X.F.; Xiong, L.; Chen, X.D.; Ma, L.L.; Chen, Y. Single cell oil production from low-cost substrates: The possibility and potential of its industrialization. Biotechnol. Adv. 2013, 31, 129–139. [Google Scholar] [CrossRef]
- Qadeer, S.; Khalid, A.; Mahmood, S.; Anjum, M.; Ahmad, Z. Utilizing oleaginous bacteria and fungi for cleaner energy production. J. Clean. Prod. 2017, 168, 917–928. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Allergens, F.; Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.-I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; et al. Safety of Yarrowia lipolytica yeast biomass as a novel food pursuant to regulation (eu) 2015/2283. EFSA J. 2019, 17, e05594. [Google Scholar]
- Wei, Y.; Siewers, V.; Nielsen, J. Cocoa butter-like lipid production ability of non-oleaginous and oleaginous yeasts under nitrogen-limited culture conditions. Appl. Microbiol. Biotechnol. 2017, 101, 3577–3585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, H.U.; Park, J.M. Biodiesel production by various oleaginous microorganisms from organic wastes. Bioresour. Technol. 2018, 256, 502–508. [Google Scholar] [CrossRef]
- Yi, J.S.; Yoo, H.W.; Kim, E.J.; Yang, Y.H.; Kim, B.G. Engineering streptomyces coelicolor for production of monomethyl branched chain fatty acids. J. Biotechnol. 2020, 307, 69–76. [Google Scholar] [CrossRef]
- Ran-Ressler, R.R.; Khailova, L.; Arganbright, K.M.; Adkins-Rieck, C.K.; Jouni, Z.E.; Koren, O.; Ley, R.E.; Brenna, J.T.; Dvorak, B. Branched chain fatty acids reduce the incidence of necrotizing enterocolitis and alter gastrointestinal microbial ecology in a neonatal rat model. PLoS ONE 2011, 6, e29032. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Xiaohan, W.; Chen, Y.; Jin, W.; Jin, Q.; Wang, X. Enrichment of branched chain fatty acids from lanolin via urea complexation for infant formula use. LWT Food Sci. Technol. 2020, 117, 108627. [Google Scholar] [CrossRef]
- Wang, W.; Lee, D.-J. Lignocellulosic biomass pretreatment by deep eutectic solvents on lignin extraction and saccharification enhancement: A review. Bioresour. Technol. 2021, 339, 125587. [Google Scholar] [CrossRef]
- Procentese, A.; Raganati, F.; Olivieri, G.; Russo, M.E.; Rehmann, L.; Marzocchella, A. Deep eutectic solvents pretreatment of agro-industrial food waste. Biotechnol. Biofuels 2018, 11, 37. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef] [Green Version]
- Sar, T.; Arifa, V.H.; Hilmy, M.R.; Ferreira, J.A.; Wikandari, R.; Millati, R.; Taherzadeh, M.J. Organosolv pretreatment of oat husk using oxalic acid as an alternative organic acid and its potential applications in biorefinery. Biomass Conv. Bioref. 2022. [Google Scholar] [CrossRef]
- Allegretti, C.; Fontanay, S.; Krauke, Y.; Luebbert, M.; Strini, A.; Troquet, J.; Turri, S.; Griffini, G.; D’Arrigo, P. Fractionation of soda pulp lignin in aqueous solvent through membrane-assisted ultrafiltration. ACS Sustain. Chem. Eng. 2018, 6, 9056–9064. [Google Scholar] [CrossRef]
- Faix, O. Fourier transform infrared spectroscopy. In Methods in Lignin Chemistry; Lin, S.Y., Dence, C.W., Eds.; Springer: Berlin/Heidelberg, Germany, 1992; pp. 83–109. [Google Scholar]
- Boeriu, C.; Bravo, D.; Gosselink, R.; Dam, J. Characterisation of structure-dependent functional properties of lignin with infrared spectroscopy. Ind. Crop. Prod. 2004, 20, 205–218. [Google Scholar] [CrossRef]
- Rencoret, J.; Prinsen, P.; Gutiérrez, A.; Martínez, Á.T.; del Río, J.C. Isolation and structural characterization of the milled wood lignin, dioxane lignin, and cellulolytic lignin preparations from brewer’s spent grain. J. Agric. Food Chem. 2015, 63, 603–613. [Google Scholar] [CrossRef] [PubMed]
- He, W.M.; Fatehi, P. Preparation of sulfomethylated softwood kraft lignin as a dispersant for cement admixture. RSC Adv. 2015, 5, 47031–47039. [Google Scholar] [CrossRef]
- Takahashi, S.; Hosoya, S.; Hattori, M.; Morimoto, M.; Uraki, Y.; Yamada, T. Performance of softwood soda-anthraquinone lignin-polyethylene glycol derivatives as water-reducing admixture for concrete. J. Wood Chem. Technol. 2015, 35, 348–354. [Google Scholar] [CrossRef]
- Childs, C.M.; Perkins, K.M.; Menon, A.; Washburn, N.R. Interplay of anionic functionality in polymer-grafted lignin superplasticizers for portland cement. Ind. Eng. Chem. Res. 2019, 58, 19760–19766. [Google Scholar] [CrossRef]
- Ji, D.; Luo, Z.Y.; He, M.; Shi, Y.J.; Gu, X.L. Effect of both grafting and blending modifications on the performance of lignosulphonate-modified sulphanilic acid-phenol-formaldehyde condensates. Cem. Concr. Res. 2012, 42, 1199–1206. [Google Scholar] [CrossRef]



| Entry | Microbial Strain | Growth Conditions 1 | Biomass Productivity 2 | Fatty Acid Productivity 3 |
|---|---|---|---|---|
| 1 | Phaffia rhodozyma (DMS 5626) | 6 days at 22 °C, pH 6.5 | 3.6 g/L (135 g/Kg BSG) | - |
| 2 | Yarrowia lipolytica (DSM 8218) | 4 days at 25 °C, pH 6.5 | 1.5 g/L (55 g/Kg BSG) | 200 mg/L (7.5 g/Kg BSG) |
| 3 | Yarrowia lipolytica (DSM 70562) | 4 days at 25 °C, pH 6.5 | 1.6 g/L (61 g/Kg BSG) | 220 mg/L (8.2 g/Kg BSG) |
| 4 | Rhodococcus opacus (DSM 43205) | 4 days at 28 °C, pH 7.0 | 2.4 g/L (89 g/Kg BSG) | 547 mg/L (20.5 g/Kg BSG) |
| 5 | Streptomyces cavourensis (DSM 112466) | 4 days at 28 °C, pH 7.0 | 2.1 g/L (80 g/Kg BSG) | 220 mg/L (8.2 g/Kg BSG) |
| 6 | Streptomyces albidoflavus (DSM 112467) | 4 days at 28 °C, pH 7.0 | 1.7 g/L (64 g/Kg BSG) | 180 mg/L (6.7 g/Kg BSG) |
| Microbial Strain | Branched Chain FAs (%) 1 | Linear FAs (%) 1 | Undetermined FAs (%) 1,2 | ||||
|---|---|---|---|---|---|---|---|
| iso | anteiso | Others | SFAs 3 | MUFAs 4 | PUFAs 5 | ||
| Yarrowia lipolytica (DSM 70562) | - | - | - | 16.5 | 9.0 | 72.0 | 2.5 |
| Rhodococcus opacus (DSM 43205) | - | - | 4.0 6; 15.1 7 | 44.6 | 21.7 | 9.1 | 5.5 |
| Streptomyces cavourensis (DSM 112466) | 28.4 | 37.7 | 6.8 6 | 15.0 | 3.7 | 7.5 | 0.9 |
| Streptomyces albidoflavus (DSM 112467) | 18.6 | 39.1 | 8.1 6 | 15.2 | 7.0 | 5.8 | 6.2 |
| Composition DES HBA/HBD | Molar Ratio (HBA/HBD) | Density of Pure DES (g/cm3) | BSGU Cellulose Recovery (% w/w Biomass) | BSGU Lignin Recovery (% w/w Biomass) |
|---|---|---|---|---|
| Choline chloride/Formic acid | 1/2 | 1.147 | ns | ns |
| Choline chloride/Acetic acid | 1/2 | 1.103 | 39 | 7 |
| Choline chloride/L-Lactic acid | 1/5 | 1.184 | 25 | 10 |
| Betaine Glycine/Formic acid | 1/2 | 1.161 | 55 | 9 |
| Betaine Glycine/Acetic acid | 1/2 | 1.107 | 32 | 8 |
| Betaine Glycine/L-Lactic acid | 1/5 | 1.203 | 53 | 7 |
| Sample | Mn (g/mol) | Mw (g/mol) | Ð |
|---|---|---|---|
| BSGU Lignin | 820 | 1580 | 1.93 |
| BSGT Lignin | 670 | 1630 | 2.43 |
| Protobind 1000 | 830 | 2800 | 3.37 |
| Sample | Reducing Sugars/Biomass (w/w) | Reducing Sugars/Biomass after Hydrolysis (w/w) |
|---|---|---|
| BSGU Lignin | 0.25% | 2.9% |
| BSGT Lignin | 0.18% | 3.3% |
| Protobind 1000 | 0.34% | 13% |
| Sample | Vanillin Equivalent Content (mmol/g) |
|---|---|
| BSGU Lignin | 1.1 |
| BSGT Lignin | 1.2 |
| Protobind 1000 | 3.1 |
| Sample | -OH Aliphatic (mmol/g) | -OH Aromatic (mmol/g) | -COOH (mmol/g) |
|---|---|---|---|
| BSGU Lignin | 2.27 | 2.77 | 2.30 |
| BSGT Lignin | 1.78 | 3.21 | 3.99 |
| Protobind 1000 | 3 | 4.67 | 1.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allegretti, C.; Bellinetto, E.; D’Arrigo, P.; Griffini, G.; Marzorati, S.; Rossato, L.A.M.; Ruffini, E.; Schiavi, L.; Serra, S.; Strini, A.; et al. Towards a Complete Exploitation of Brewers’ Spent Grain from a Circular Economy Perspective. Fermentation 2022, 8, 151. https://doi.org/10.3390/fermentation8040151
Allegretti C, Bellinetto E, D’Arrigo P, Griffini G, Marzorati S, Rossato LAM, Ruffini E, Schiavi L, Serra S, Strini A, et al. Towards a Complete Exploitation of Brewers’ Spent Grain from a Circular Economy Perspective. Fermentation. 2022; 8(4):151. https://doi.org/10.3390/fermentation8040151
Chicago/Turabian StyleAllegretti, Chiara, Emanuela Bellinetto, Paola D’Arrigo, Gianmarco Griffini, Stefano Marzorati, Letizia Anna Maria Rossato, Eleonora Ruffini, Luca Schiavi, Stefano Serra, Alberto Strini, and et al. 2022. "Towards a Complete Exploitation of Brewers’ Spent Grain from a Circular Economy Perspective" Fermentation 8, no. 4: 151. https://doi.org/10.3390/fermentation8040151
APA StyleAllegretti, C., Bellinetto, E., D’Arrigo, P., Griffini, G., Marzorati, S., Rossato, L. A. M., Ruffini, E., Schiavi, L., Serra, S., Strini, A., Tessaro, D., & Turri, S. (2022). Towards a Complete Exploitation of Brewers’ Spent Grain from a Circular Economy Perspective. Fermentation, 8(4), 151. https://doi.org/10.3390/fermentation8040151










