Modelling of the Simultaneous Saccharification and Fermentation for a Pine Sawdust Biorefinery
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization of the Substrate and Liquid Fraction of the Fermentation Process
2.2. Yeast Reactivation
2.3. Pre-Inoculum and Inoculum Preparation
2.4. Simultaneous Saccharification and Fermentation (SSF) Strategy
2.5. Experimental Designs
3. Results and Discussion
3.1. Substrate Characterization
3.2. Simultaneous Saccharification and Fermentation (SSF) Strategy
3.2.1. Saccharomyces cerevisiae Characterization
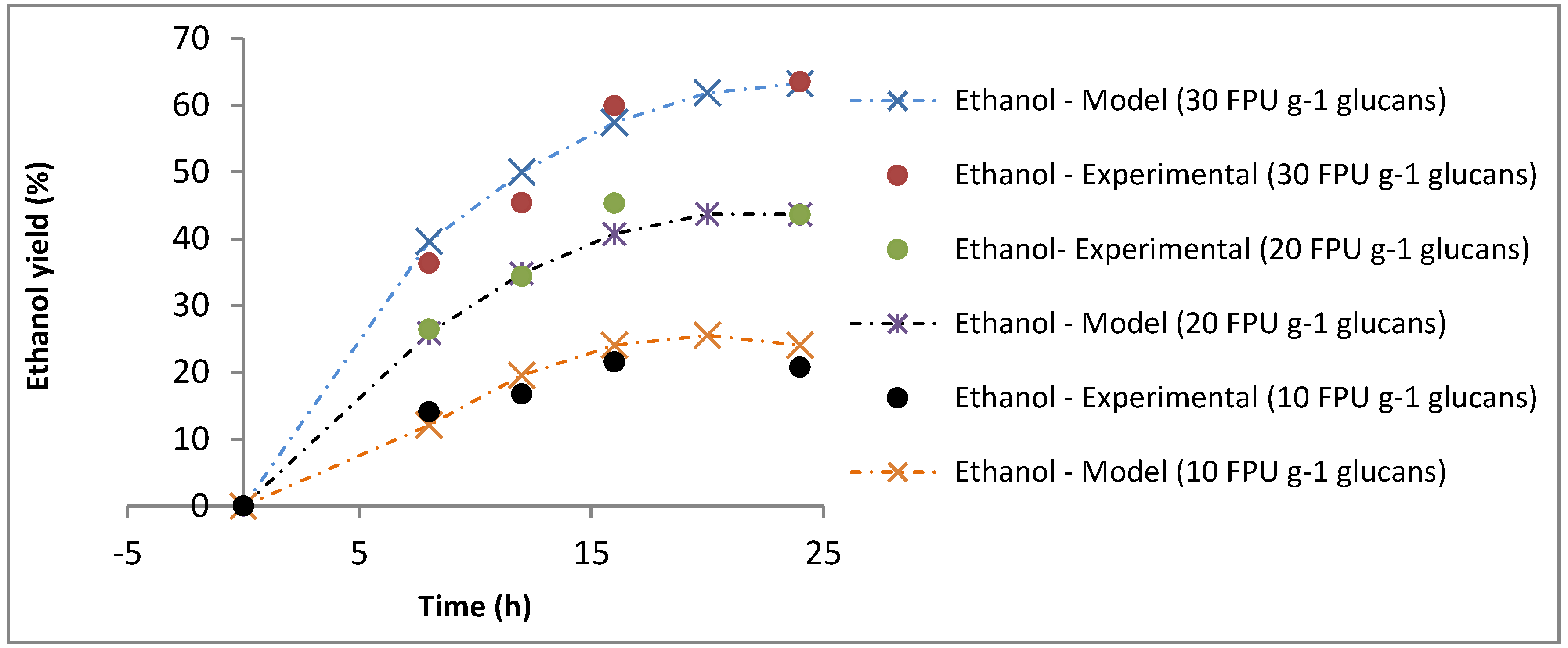
3.2.2. 2G Bioethanol Production
3.3. Experimental Designs
3.3.1. Full Model
3.3.2. Model I
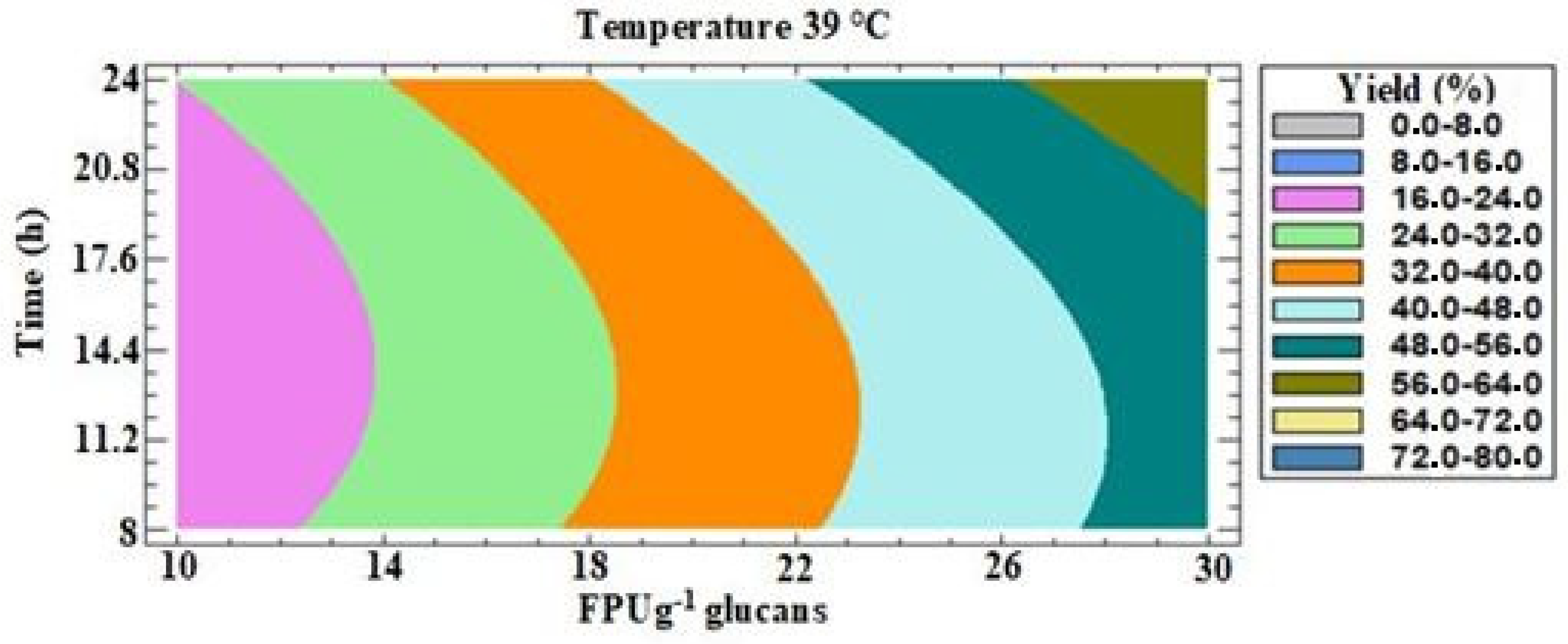
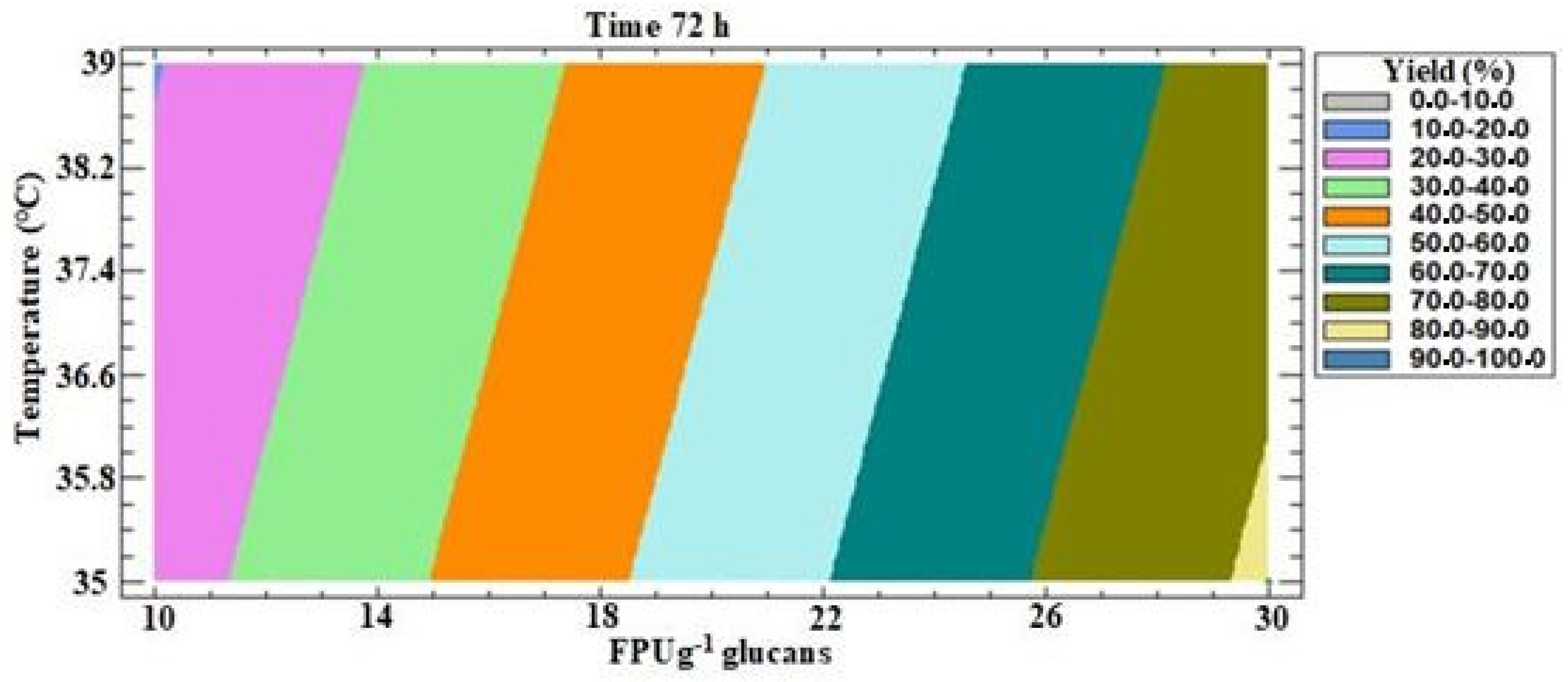
3.3.3. Model II
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vallejos, M.E.; Kruyeniski, J.; Area, M.C. Second-generation bioethanol from industrial wood waste of South American species. Biofuel Res. J. 2017, 15, 654–667. [Google Scholar] [CrossRef][Green Version]
- Area, M.C.; Vallejos, M.E.; Clauser, N.M.; Covinich, L.G.; González, G. Consultoría para el desarrollo de nuevos productos foresto-industriales en la Argentina. La sostenibilidad como un instrumento de desarrollo de sectores productivos estratégicos. 2019; 1–355. [Google Scholar]
- Mendieta, C.M.; Cardozo, R.E.; Felissia, F.E.; Clauser, N.M.; Vallejos, M.E.; Area, M.C. Bioconversion of Wood Waste to Bio-ethylene: A Review. BioResources 2021, 16, 1–27. [Google Scholar] [CrossRef]
- Mendieta, C.M.; Vallejos, M.E.; Felissia, F.E.; Chinga-Carrasco, G.; Area, M.C. Review: Bio-polyethylene from Wood Wastes. J. Polym. Environ. 2019, 28, 1–16. [Google Scholar] [CrossRef]
- Clauser, N.M.; González, G.; Mendieta, C.M.; Kruyeniski, J.; Area, M.C.; Vallejos, M.E. Biomass waste as sustainable raw material for energy and fuels. Sustainability 2021, 13, 794. [Google Scholar] [CrossRef]
- Kruyeniski, J.; Ferreira, P.J.; Carvalho, S.; Vallejos, M.E.; Felissia, F.E.; Area, M.C. Physical and chemical characteristics of pretreated pine sawdust and its enzymatic hydrolysis. Ind. Crops Prod. J. 2019, 130, 528–536. [Google Scholar] [CrossRef]
- Yang, C.; Fang, T.J. Kinetics of enzymatic hydrolysis of rice straw by the pretreatment with a bio-based basic ionic liquid under ultrasound. Process Biochem. 2015, 50, 623–629. [Google Scholar] [CrossRef]
- Olsson, L.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates for ethanol production. Enzym. Microb. Technol. 1996, 18, 312–331. [Google Scholar] [CrossRef]
- Kruyeniski, J. Influencia del Pretratamiento de Residuos Forestoindustriales Sobre la Producción de Bioetanol (“In Spanish”). Ph.D. Thesis, Universidad Nacional de Misiones, Misiones, Argentina, 2017. [Google Scholar]
- Mendes, C.V.T.; Vergara, P.; Carbajo, J.M.; Villar, J.C.; Rocha, J.M.; Carvalho, M. Bioconversion of pine stumps to ethanol: Pretreatment and simultaneous saccharification and fermentation. Holzforschung 2019, 74, 212–216. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Kumar, A.; Lukk, T.; Tuohy, M.G.; Gong, L.; Nguyen-tri, P.; Goddard, A.D.; Bill, R.M.; Nayak, S.C.; et al. Lignocellulosic biorefineries: The current state of challenges and strategies for efficient commercialization. Renew. Sustain. Energy Rev. 2021, 148, 111258. [Google Scholar] [CrossRef]
- Sanz-Cervera, S.A. Practicas de Microbiologia, 2nd ed.; Universidad de la Rioja: La Rioja, Spain, 2011. [Google Scholar]
- Mendieta, C.M.; Felissia, F.E.; Arismendy, A.M.; Kruyeniski, J.; Area, M.C. Enzymatic hydrolysis and fermentation strategies for the biorefining of pine sawdust. BioResources 2021, 16, 7474–7491. [Google Scholar] [CrossRef]
- Imlauer, C.M.; Area, M.C.; Raffaeli, N.; Felissia, F.E. Study on soda/ethanol delignification of pine sawdust for a biorefinery. 2022; Unpublished. [Google Scholar]
- Sluiter, A.; Hames, B.; Hyman, D.; Payne, C.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Wolfe, J. Determination of total solids in biomass and total dissolved solids in liquid process samples. Natl. Renew. Energy Lab. (NREL) 2008, 9, 3–5. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Technical Report-Determination of Structural Carbohydrates and Lignin in Biomass, (NREL/TP-510-42618); Laboratory Analytical Procedure (LAP): Golden, CO, USA, 2004. [Google Scholar]
- De Capriles, C.H.; Mata, S.; Middelveen, M. Preservation of fungi in water (Castellani): 20 years. Mycopathogia 1989, 106, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Medvedeff, M.G.; Vedoya, M.C.; Mereles-Rodriguez, B.E.; Chade, M.E. Cátedra de Micología: Guía de Trabajos Prácticos; Editorial Universitaria de Misiones: Posadas, Misiones, 2005. [Google Scholar]
- Dowe, N.; McMillan, J. Technical Report-SSF Experimental Protocols -Lignocellulosic Biomass Hydrolysis and Fermentation, (NREL/TP-510-42630); Laboratory Analytical Procedure (LAP): Golden, CO, USA, 2008. [Google Scholar]
- Xue, Y.; Jameel, H.; Park, S. Strategies to recycle enzymes and their impact on enzymatic hydrolysis for bioethanol production. BioResources 2012, 7, 602–615. [Google Scholar]
- Von Schenck, A.; Berglin, N.; Uusitalo, J. Ethanol from Nordic wood raw material by simplified alkaline soda cooking. Appl. Energy 2013, 102, 229–240. [Google Scholar] [CrossRef]
- Reis, V.R.; Paula, A.; Bassi, G.; Carolina, J.; Ceccato-antonini, S.R. Characteristics of Saccharomyces cerevisiae yeasts exhibiting rough colonies and pseudohyphal morphology with respect to alcoholic fermentation. Braz. J. Microbiol. 2013, 44, 1121–1131. [Google Scholar] [CrossRef]
- Cheng, N.; Koda, K.; Tamai, Y.; Yamamoto, Y.; Takasuka, T.E.; Uraki, Y. Optimization of simultaneous saccharification and fermentation conditions with amphipathic lignin derivatives for concentrated bioethanol production. Bioresour. Technol. 2017, 232, 126–132. [Google Scholar] [CrossRef]
- Olofsson, K.; Bertilsson, M.; Lidén, G. A short review on SSF-An interesting process option for ethanol production from lignocellulosic feedstocks. Biotechnol. Biofuels 2008, 1, 1754–6834. [Google Scholar] [CrossRef] [PubMed]
- Linde, M.; Galbe, M.; Zacchi, G. Simultaneous saccharification and fermentation of steam-pretreated barley straw at low enzyme loadings and low yeast concentration. Enzym. Microb. Technol. 2007, 40, 1100–1107. [Google Scholar] [CrossRef]
- Barboza- Devos, R.J.; Colla, L.M. Bioresource Technology Reports Simultaneous saccharification and fermentation to obtain bioethanol: A bibliometric and systematic study. Bioresour. Techol. Rep. 2022, 17, 100924. [Google Scholar] [CrossRef]
- Stephen, J.D.; Mabee, W.E.; Saddler, J.N. Will second-generation ethanol be able to compete with first-generation ethanol? Opportunities for cost reduction. Biofuels Bioprod. Biorefin. 2012, 6, 159–176. [Google Scholar] [CrossRef]

| Factor | +1 | 0 | −1 |
|---|---|---|---|
| A = Enzyme load (FPU g−1 glucans) | 30 | 20 | 10 |
| B = Temperature (°C) | 39 | 37 | 35 |
| Full Model = Time (h) | 72 | 4 | |
| Model I C = Time (h) | 24 | 16 | 8 |
| Model II C = Time (h) | 72 | 48 | 24 |
| Chemical Composition | Glucans (% odm *) | Xylans (% odm) | Galactans (% odm) | Mannans (% odm) | Arabinans (% odm) | Lignin (% odm) |
|---|---|---|---|---|---|---|
| Pine sawdust | 40.9 | 7.5 | 2.6 | 14.8 | 0.8 | 29.2 |
| Soda-ethanol pulp | 80.2 | 7.2 | 0.3 | 8.4 | - | 3.7 |
| Temperature (°C) | Time | 4 | 8 | 12 | 16 | 24 | 48 | 72 |
|---|---|---|---|---|---|---|---|---|
| Enzymatic load = 30 FPU g−1 Glucans | ||||||||
| 35 °C | Produced ethanol (gL−1) | 1.27 | 3.35 | 4.02 | 5.37 | 5.69 | n.d. | 9.21 |
| YP/T (%) | 11.11 | 29.51 | 35.36 | 47.28 | 50.06 | n.d. | 81.00 | |
| PP/T (gL−1 h−1) | 0.32 | 0.42 | 0.34 | 0.34 | 0.24 | n.d. | 0.13 | |
| Yeast concentration (gL−1) | n.d. | 1.85 | 1.93 | 2.94 | 3.73 | 3.66 | 3.80 | |
| 37 °C | Produced ethanol (gL−1) | 1.34 | 1.74 | 1.85 | 5.70 | 4.92 | 8.28 | 9.07 |
| YP/T (%) | 11.82 | 15.35 | 16.32 | 43.27 | 50.20 | 72.87 | 79.82 | |
| PP/T (gL−1 h−1) | 0.34 | 0.22 | 0.15 | 0.36 | 0.21 | 0.17 | 0.13 | |
| Yeast concentration (gL−1) | 1.54 | 1.78 | 1.88 | 2.75 | 3.24 | 3.63 | 3.73 | |
| 39 °C | Produced ethanol (gL−1) | 2.62 | 4.13 | 5.15 | 6.81 | 7.22 | 7.93 | 8.08 |
| YP/T (%) | 23.07 | 36.36 | 45.38 | 59.92 | 63.51 | 69.83 | 71.1 | |
| PP/T (gL−1 h−1) | 0.66 | 0.52 | 0.43 | 0.43 | 0.30 | 0.17 | 0.11 | |
| Yeast concentration (gL−1) | 1.21 | 2.16 | 2.32 | 2.93 | 3.34 | 3.48 | 3.48 | |
| Enzymatic load = 20 FPU g−1 glucans | ||||||||
| 35 °C | Produced ethanol (gL−1) | 1.75 | 2.37 | 3.05 | 3.66 | 4.12 | n.d. | 5.87 |
| YP/T (%) | 12.7 | 20.86 | 26.82 | 32.20 | 36.26 | n.d. | 51.63 | |
| PP/T (gL−1 h−1) | 0.44 | 0.30 | 0.25 | 0.23 | 0.17 | n.d. | 0.08 | |
| Yeast concentration (gL−1) | n.d. | 1.77 | 2.03 | 2.09 | 3.26 | 3.94 | 3.99 | |
| 37 °C | Produced ethanol (gL−1) | 0.61 | 1.72 | 3.20 | 3.98 | 4.56 | 4.83 | 5.98 |
| YP/T (%) | 5.37 | 15.13 | 28.19 | 35.06 | 40.13 | 42.53 | 52.61 | |
| PP/T (gL−1 h−1) | 0.15 | 0.22 | 0.27 | 0.25 | 0.19 | 0.10 | 0.08 | |
| Yeast concentration (gL−1) | 1.45 | 1.63 | 1.88 | 2.07 | 3.10 | 3.69 | 3.95 | |
| 39 °C | Produced ethanol (gL−1) | 1.75 | 3.01 | 3.91 | 5.15 | 4.96 | 5.57 | 5.55 |
| YP/T (%) | 15.41 | 26.46 | 34.38 | 45.34 | 43.63 | 48.99 | 48.84 | |
| PP/T (gL−1 h−1) | 0.44 | 0.38 | 0.33 | 0.32 | 0.21 | 0.12 | 0.08 | |
| Yeast concentration (gL−1) | 1.15 | 2.11 | 2.13 | 2.78 | 3.03 | 3.67 | 3.81 | |
| Enzymatic load = 10 FPU g−1 glucans | ||||||||
| 35 °C | Produced ethanol (gL−1) | 0.57 | 1.19 | 1.25 | 1.87 | 2.41 | n.d. | 2.62 |
| YP/T (%) | 5.04 | 9.50 | 11.89 | 16.41 | 21.18 | n.d. | 23.07 | |
| PP/T (gL−1 h−1) | 0.14 | 0.15 | 0.10 | 0.12 | 0.10 | n.d. | 0.04 | |
| Yeast concentration (gL−1) | n.d. | 0.88 | 1.06 | 1.40 | 2.89 | 3.43 | 3.77 | |
| 37 °C | Produced ethanol (gL−1) | 0.00 | 0.46 | 1.48 | 1.74 | 2.09 | 3.03 | 3.82 |
| YP/T (%) | 0.00 | 4.06 | 13.03 | 15.31 | 18.41 | 26.69 | 33.59 | |
| PP/T (gL−1 h−1) | 0.00 | 0.06 | 0.12 | 0.11 | 0.09 | 0.06 | 0.05 | |
| Yeast concentration (gL−1) | 0.65 | 1.61 | 0.99 | 1.39 | 2.45 | 3.21 | 3.85 | |
| 39 °C | Produced ethanol (gL−1) | 1.02 | 1.61 | 1.91 | 2.45 | 2.36 | 2.22 | 2.00 |
| YP/T (%) | 9.02 | 14.11 | 16.77 | 21.56 | 20.79 | 19.52 | 17.62 | |
| PP/T (gL−1 h−1) | 0.26 | 0.20 | 0.16 | 0.15 | 0.10 | 0.05 | 0.03 | |
| Yeast concentration (gL−1) | 0.62 | 1.88 | 1.37 | 1.14 | 2.71 | 3.31 | 3.74 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendieta, C.M.; Kruyeniski, J.; Felissia, F.E.; Area, M.C. Modelling of the Simultaneous Saccharification and Fermentation for a Pine Sawdust Biorefinery. Fermentation 2022, 8, 130. https://doi.org/10.3390/fermentation8030130
Mendieta CM, Kruyeniski J, Felissia FE, Area MC. Modelling of the Simultaneous Saccharification and Fermentation for a Pine Sawdust Biorefinery. Fermentation. 2022; 8(3):130. https://doi.org/10.3390/fermentation8030130
Chicago/Turabian StyleMendieta, Carolina Mónica, Julia Kruyeniski, Fernando Esteban Felissia, and María Cristina Area. 2022. "Modelling of the Simultaneous Saccharification and Fermentation for a Pine Sawdust Biorefinery" Fermentation 8, no. 3: 130. https://doi.org/10.3390/fermentation8030130
APA StyleMendieta, C. M., Kruyeniski, J., Felissia, F. E., & Area, M. C. (2022). Modelling of the Simultaneous Saccharification and Fermentation for a Pine Sawdust Biorefinery. Fermentation, 8(3), 130. https://doi.org/10.3390/fermentation8030130








