Influence of Native Saccharomyces cerevisiae Strains from D.O. “Vinos de Madrid” in the Volatile Profile of White Wines
Abstract
1. Introduction
2. Materials and Methods
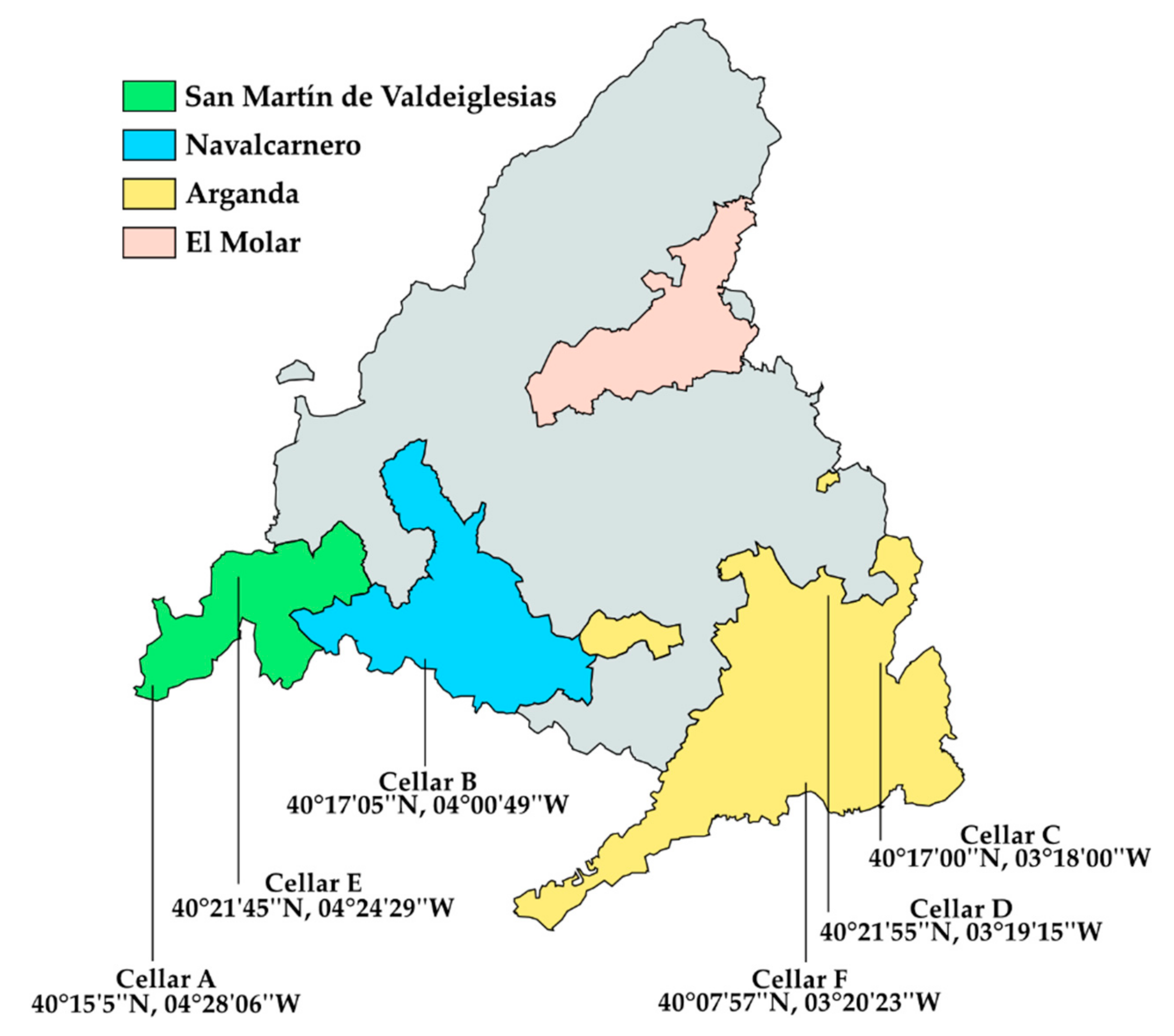
2.1. Yeast Strains, Origin, and Vinification Procedure
2.2. Volatile Fraction Analysis
2.3. Statistical Analysis
3. Results and Discussion
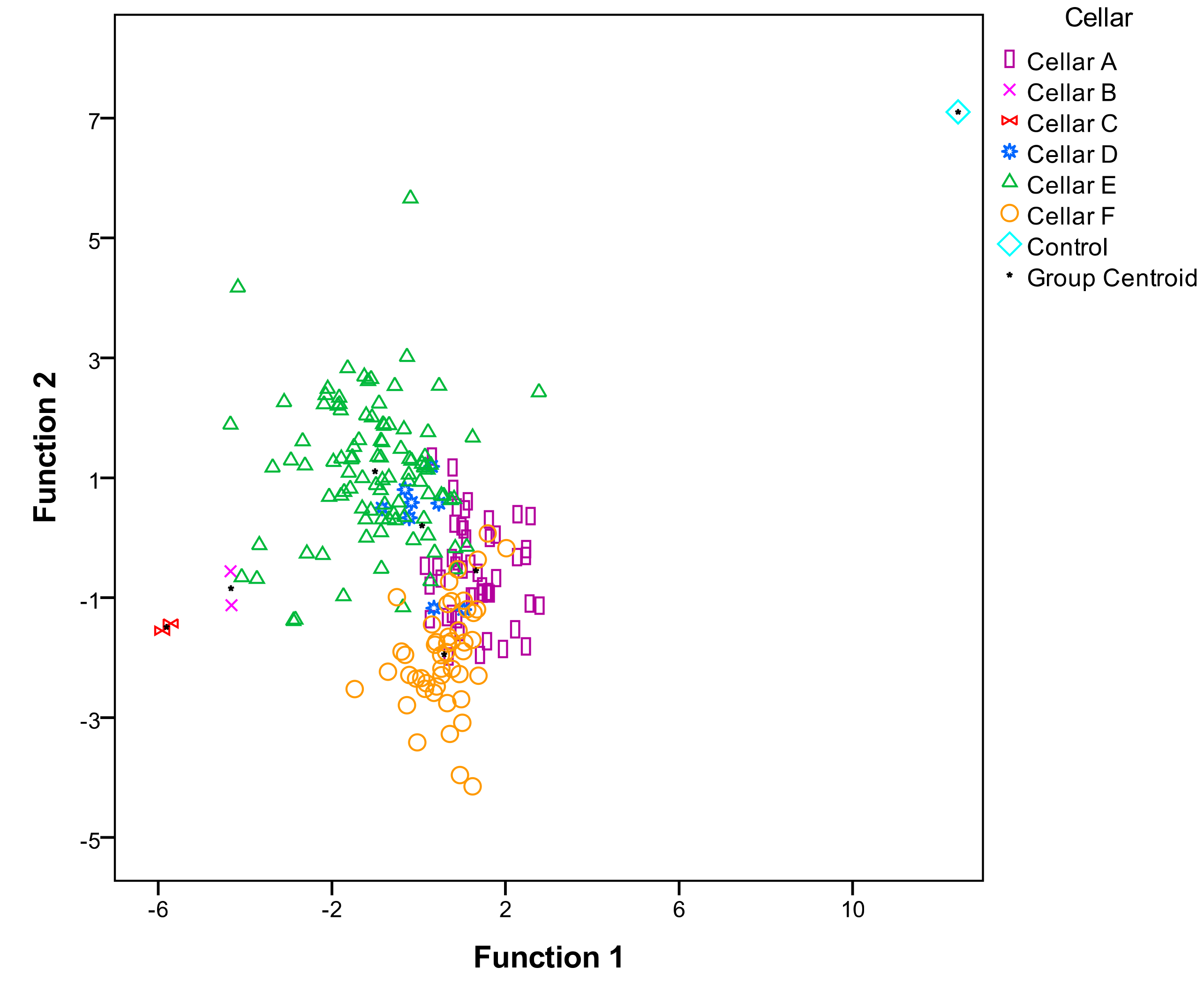
3.1. Aromatic Profile of Wines Elaborated with Different S. cerevisiae Strains
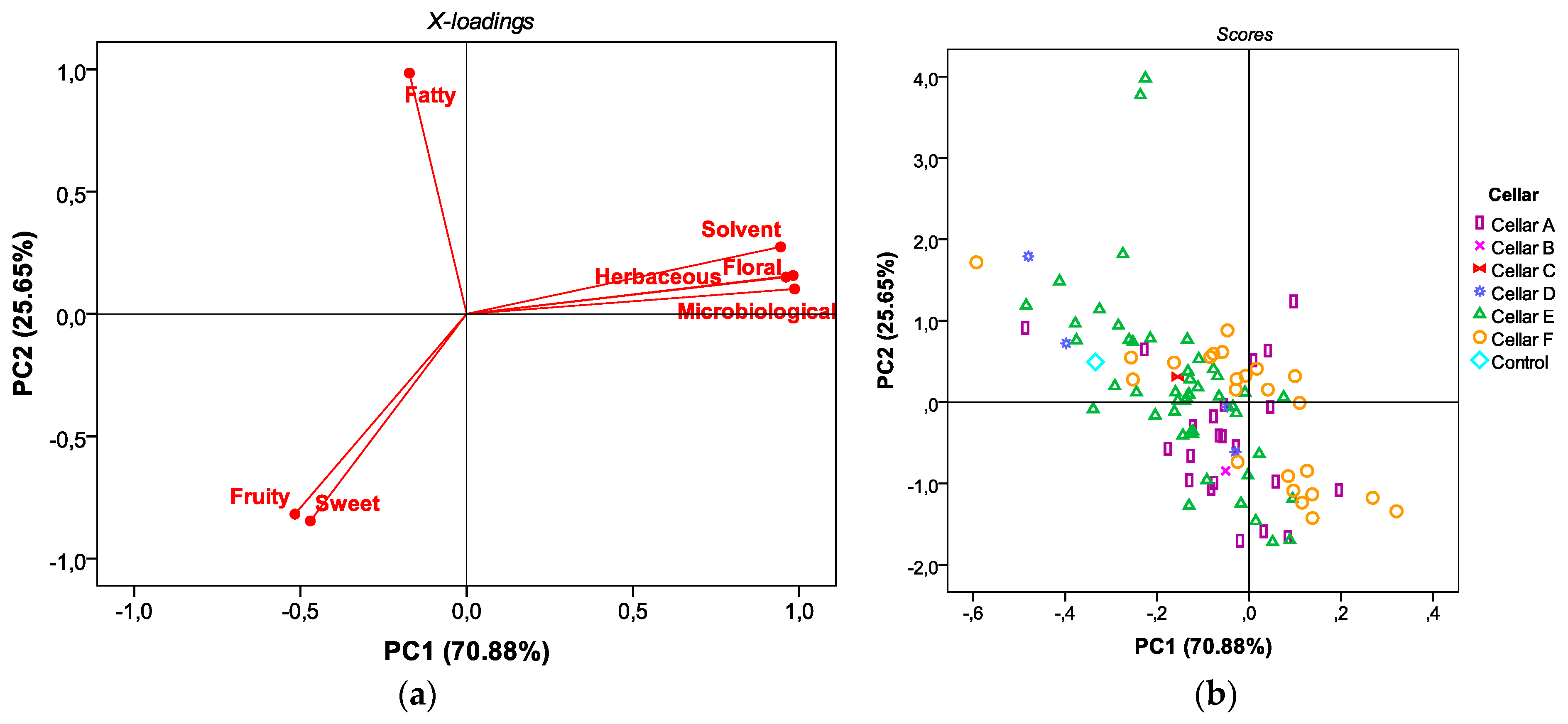
3.2. Principal Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fleet, G.H. Yeast interactions and wine flavour. Int. J. Food Microbiol. 2003, 86, 11–22. [Google Scholar] [CrossRef]
- Styger, G.; Prior, B.; Bauer, F.F. Wine flavor and aroma. J. Ind. Microbiol. Biotechnol. 2011, 38, 1145–1159. [Google Scholar] [CrossRef] [PubMed]
- Lambrechts, M.G.; Pretorius, I.S. Yeast and its importance to wine aroma-A review. S. Afr. J. Enol. Vitic. 2000, 21, 97–129. [Google Scholar] [CrossRef]
- Polaskova, P.; Herszage, J.; Ebeler, S.E. Wine flavor: Chemistry in a glass. Chem. Soc. Rev. 2008, 37, 2478–2489. [Google Scholar] [CrossRef] [PubMed]
- King, E.S.; Kievit, R.L.; Curtin, C.; Swiegers, J.H.; Pretorius, I.S.; Bastian, S.E.P.; Leigh Francis, I. The effect of multiple yeasts co-inoculations on Sauvignon Blanc wine aroma composition, sensory properties and consumer preference. Food Chem. 2010, 122, 618–626. [Google Scholar] [CrossRef]
- Rapp, A. Natural flavours of wine: Correlation between instrumental analysis and sensory perception. Anal. Chem. 1990, 337, 777–785. [Google Scholar] [CrossRef]
- Rocha, S.M.; Rodrigues, F.; Coutinho, P.; Delgadillo, I.; Coimbra, M.A. Volatile composition of Baga red wine: Assessment of the identification of the would-be impact odourants. Anal. Chim. Acta 2004, 513, 257–262. [Google Scholar] [CrossRef]
- Guth, H. Quantitation and sensory studies of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997, 43, 3027–3032. [Google Scholar] [CrossRef]
- Gil, M.; Cabellos, J.M.; Arroyo, T.; Prodanov, M. Characterization of the volatile fraction of young wines from the Denomination of Origin “Vinos de Madrid” (Spain). Anal. Chim. Acta 2006, 563, 145–153. [Google Scholar] [CrossRef]
- Ryan, D.; Prenzler, P.D.; Saliba, A.J.; Scollary, G.R. The significance of low impact odorants in global odour perception. Trends Food Sci. Technol. 2008, 19, 383–389. [Google Scholar] [CrossRef]
- Escudero, A.; Campo, E.; Fariña, L.; Cacho, J.; Ferreira, V. Analytical characterization of the aroma of five premium red wines. Insights into the role of odor families and the concept of fruitiness of wines. J. Agric. Food Chem. 2007, 55, 4501–4510. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.L.; Boss, P.K.; Solomon, P.S.; Trengove, R.D.; Heymann, H.; Ebeler, S.E. Origins of grape and wine aroma. Part 2. Chemical and sensory analysis. Am. J. Enol. Vitic. 2014, 65, 25–42. [Google Scholar] [CrossRef]
- Mina, M.; Tsaltas, D. Contribution of Yeast in Wine Aroma and Flavour. In Yeast—Industrial Applications; Morata, A., Loira, I., Eds.; IntechOpen: London, UK, 2017; pp. 117–134. [Google Scholar] [CrossRef]
- Kutyna, D.R.; Borneman, A.R. Heterologous production of flavour and aroma compounds in Saccharomyces cerevisiae. Genes 2018, 9, 326. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Peinado, R.A.; Moreno, J.; Bueno, J.E.; Moreno, J.A.; Mauricio, J.C. Comparative study of aromatic compounds in two young white wines subjected to pre-fermentative cryomaceration. Food Chem. 2004, 84, 585–590. [Google Scholar] [CrossRef]
- Mestre, M.V.; Maturano, Y.P.; Gallardo, C.; Combina, M.; Mercado, L.; Toro, M.E.; Carrau, F.; Vazquez, F.; Dellacassa, E. Impact on sensory and aromatic profile of low ethanol Malbec wines fermented by sequential culture of Hanseniaspora uvarum and Saccharomyces cerevisiae native yeasts. Fermentation 2019, 5, 65. [Google Scholar] [CrossRef]
- Capone, S.; Tufariello, M.; Siciliano, P. Analytical characterisation of Negroamaro red wines by “Aroma Wheels”. Food Chem. 2013, 141, 2906–2915. [Google Scholar] [CrossRef]
- King, E.S.; Swiegers, J.H.; Travis, B.; Leigh Francis, I.; Bastian, S.E.P.; Pretorius, I.S. Coinoculated fermentations using Saccharomyces yeasts affect the volatile composition and sensory properties of Vitis vinifera L. cv. Sauvignon Blanc wines. J. Agric. Food Chem. 2008, 56, 10829–10837. [Google Scholar] [CrossRef]
- Romano, P. Metabolic characteristics of wine strains during spontaneous and inoculated fermentation. Food Technol. Biotechnol. 1997, 35, 255–260. [Google Scholar]
- Pretorius, I.S. Tailoring wine yeast for the new millennium: Novel approaches to the ancient art of winemaking. Yeast 2000, 16, 675–729. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I.S. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Martini, A. Biotechnology of natural and winery-associated strains of Saccharomyces cerevisiae. Int. Microbiol. 2003, 6, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Callejon, R.M.; Clavijo, A.; Ortigueira, P.; Troncoso, A.M.; Paneque, P.; Morales, M.L. Volatile and sensory profile of organic red wines produced by different selected autochthonous and commercial Saccharomyces cerevisiae strains. Anal. Chim. Acta 2010, 660, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Tufariello, M.; Chiriatti, M.A.; Grieco, F.; Perrotta, C.; Capone, S.; Rampino, P.; Tristezza, M.; Mita, G.; Grieco, F. Influence of autochthonous Saccharomyces cerevisiae strains on volatile profile of Negroamaro wines. LWT Food Sci. Technol. 2014, 58, 35–48. [Google Scholar] [CrossRef]
- Di Maio, S.; Polizzotto, G.; Di Gangi, E.; Foresta, G.; Genna, G.; Verzera, A.; Scacco, A.; Amore, G.; Oliva, D. Biodiversity of indigenous Saccharomyces populations from old wineries of South-Eastern Sicily (Italy): Preservation and economic potential. PLoS ONE 2012, 7, e30428. [Google Scholar] [CrossRef]
- Tristezza, M.; Vetrano, C.; Bleve, G.; Spano, G.; Capozzi, V.; Logrieco, A.; Mita, G.; Grieco, F. Biodiversity and safety aspects of yeast strains characterized from vineyards and spontaneous fermentations in the Apulia Region, Italy. Food Microbiol. 2013, 36, 335–342. [Google Scholar] [CrossRef]
- Capozzi, V.; Garofalo, C.; Chiriatti, M.A.; Grieco, F.; Spano, G. Microbial terroir and food innovation: The case of yeast biodiversity in wine. Microbiol. Res. 2015, 181, 75–83. [Google Scholar] [CrossRef]
- Vaudano, E.; Quinterno, G.; Costantini, A.; Pulcini, L.; Pessione, E.; García-Moruno, E. Yeast distribution in Grignolino grapes growing in a new vineyard in Piedmont and the technological characterization of indigenous Saccharomyces spp. strains. Int. J. Food Microbiol. 2019, 289, 154–161. [Google Scholar] [CrossRef]
- Csoma, H.; Zakany, N.; Capece, A.; Romano, P.; Sipiczki, M. Biological diversity of Saccharomyces yeasts of spontaneously fermenting wines in four wine regions: Comparative genotypic and phenotypic analysis. Int. J. Food Microbiol. 2010, 140, 239–248. [Google Scholar] [CrossRef]
- Cordero-Bueso, G.; Esteve-Zarzoso, B.; Cabellos, J.M.; Gil-Díaz, M.; Arroyo, T. Biotechnological potential of non-Saccharomyces yeasts isolated during spontaneous fermentations of Malvar (Vitis vinifera cv. L.). Eur. Food Res. Technol. 2013, 236, 193–207. [Google Scholar] [CrossRef]
- Arroyo, T. Estudio de la Influencia de Diferentes Tratamientos Enológicos en la Evolución de la Microbiota y en la Calidad de los Vinos Elaborados con la Variedad “Airén”, en la D.O. “Vinos de Madrid”. Ph.D. Thesis, University of Alcalá, Madrid, Spain, 2000. [Google Scholar]
- Cordero-Bueso, G.; Esteve-Zarzoso, B.; Gil-Díaz, M.; García, M.; Cabellos, J.; Arroyo, T. Improvement of Malvar wine quality by use of locally-selected Saccharomyces cerevisiae strains. Fermentation 2016, 2, 7. [Google Scholar] [CrossRef]
- Tello, J.; Cordero-Bueso, G.; Aporta, I.; Cabellos, J.M.; Arroyo, T. Genetic diversity in commercial wineries: Effects of the farming system and vinification management on wine yeasts. J. Appl. Microbiol. 2012, 112, 302–315. [Google Scholar] [CrossRef] [PubMed]
- García, M.; Greetham, D.; Wimalasena, T.T.; Phister, T.G.; Cabellos, J.M.; Arroyo, T. The phenotypic characterization of yeast strains to stresses inherent to wine fermentation in warm climates. J. Appl. Microbiol. 2016, 121, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Vaudano, E.; García-Moruno, E. Discrimination of Saccharomyces cerevisiae wine strains using microsatellite multiplex PCR and band pattern analysis. Food Microbiol. 2008, 25, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Ortega, C.; López, R.; Cacho, J.; Ferreira, V. Fast analysis of important wine volatile compounds-Development and validation of a new method based on gas chromatographic-flame ionisation detection analysis of dichloromethane microextracts. J. Chromatogr. A 2001, 923, 205–214. [Google Scholar] [CrossRef]
- Office Internationale de la Vigne et du Vin. International Code of Oenological Practices; OIV: Paris, France, 2009. [Google Scholar]
- Bely, M.; Rinaldi, A.; Dubourdieu, D. Influence of assimilable nitrogen on volatile acidity production by Saccharomyces cerevisiae during high sugar fermentation. J. Biosci. Bioeng. 2003, 96, 507–512. [Google Scholar] [CrossRef]
- Moyano, L.; Zea, L.; Moreno, J.; Medina, M. Analytical study of aromatic series in sherry wines subjected to biological aging. J. Agric. Food Chem. 2002, 50, 7356–7361. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Duan, S.; Zhao, L.; Gao, Z.; Luo, M.; Song, S.; Xu, W.; Zhang, C.; Ma, C.; Wang, S. Aroma characterization based on aromatic series analysis in table grapes. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Belda, I.; Ruiz, J.; Esteban-Fernández, A.; Navascués, E.; Marquina, D.; Santos, A.; Moreno-Arribas, M.V. Microbial contribution to wine aroma and its intended use for wine quality improvement. Molecules 2017, 22, 189. [Google Scholar] [CrossRef]
- Etiévant, X.P. Wine. In Volatile Compounds in Foods and Beverages; Maarse, H., Ed.; Marcel Dekker: New York, NY, USA, 1991; pp. 483–546. [Google Scholar]
- Aznar, M.; López, R.; Cacho, J.; Ferreira, V. Prediction of aged red wine aroma properties from aroma chemical composition. Partial least squares regression models. J. Agric. Food Chem. 2003, 51, 2700–2707. [Google Scholar] [CrossRef]
- Chaves, M.; Zea, L.; Moyano, L.; Medina, M. Changes in color and odorant compounds during oxidative aging of Pedro Ximenez sweet wines. J. Agric. Food Chem. 2007, 55, 3592–3598. [Google Scholar] [CrossRef] [PubMed]
- Van Gemert, L.J.; Nettenbreijer, A.H. Compilation of Odour Threshold Values in Air and Water; National Institute for Water Supply/Central Institute for Nutrition and Food Research TNO: Zeist and Voorburg, The Netherlands, 1977. [Google Scholar]
- Culleré, L.; Cacho, J.; Ferreira, V. An assessment of the role played by some oxidation-related aldehydes in wine aroma. J. Agric. Food Chem. 2007, 55, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Yunoki, K.; Tanji, M.; Murakami, Y.; Yasui, Y.; Hirose, S.; Ohnishi, M. Fatty acid compositions of commercial red wines. Biosci. Biotechnol. Biochem. 2004, 68, 2623–2626. [Google Scholar] [CrossRef] [PubMed]
- Rankine, B.C.; Fornachon, J.C.M.; Annette Bridson, D. Diacetyl in Australian dry red table wines and its significance in wine quality. Vitis 1969, 8, 129–134. [Google Scholar]
- Knight, S.; Klaere, S.; Fedrizzi, B.; Goddard, M.R. Regional microbial signatures positively correlate with differential wine phenotypes: Evidence for a microbial aspect to terroir. Sci. Rep. 2015, 5, 14233. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P. New developments in wine microbiology. Am. J. Enol. Vitic. 1985, 36, 1–10. [Google Scholar]
- Saberi, S.; Cliff, M.A.; van Vuuren, H.J.J. Impact of mixed S. cerevisiae strains on the production of volatiles and estimated sensory profiles of Chardonnay wines. Food Res. Int. 2012, 48, 725–735. [Google Scholar] [CrossRef]
- Capece, A.; Romaniello, R.; Pietrafesa, R.; Romano, P. Indigenous Saccharomyces cerevisiae yeasts as a source of biodiversity for the selection of starters for specific fermentations. BIO Web Conf. 2014, 3, 02003. [Google Scholar] [CrossRef]
- Noguerol-Pato, R.; González-Barreiro, C.; Cancho-Grande, B.; Martínez, M.C.; Santiago, J.L.; Simal-Gándara, J. Floral, spicy and herbaceous active odorants in Gran Negro grapes from shoulders and tips into the cluster, and comparison with Brancellao and Mouratón varieties. Food Chem. 2012, 135, 2771–2782. [Google Scholar] [CrossRef]
- Lorenzo, C.; Garde-Cerdan, T.; Lara, J.F.; Martínez-Gil, A.M.; Pardo, F.; Salinas, M.R. Volatile composition of wines elaborated from organic and non-organic grapes. Ferment. Technol. 2015, 4. [Google Scholar] [CrossRef]
- Francis, I.L.; Newton, J.L. Determining wine aroma from compositional data. Aust. J. Grape Wine Res. 2005, 11, 114–126. [Google Scholar] [CrossRef]
- Dalton, P.; Doolittle, N.; Nagata, H.; Breslin, P.A.S. The merging of the senses: Integration of subthreshold taste and smell. Nat. Neurosci. 2000, 3, 431–432. [Google Scholar] [CrossRef] [PubMed]
- Ishii, A.; Roudnitzky, N.; Béno, N.; Bensafi, M.; Hummel, T.; Rouby, C.; Thomas-Danguin, T. Synergy and masking in odor mixtures: An electrophysiological study of orthonasal vs. retronasal perception. Chem. Senses 2008, 33, 553–561. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Atanasova, B.; Thomas-Danguin, T.; Langlois, D.; Nicklaus, S.; Chabanet, C.; Etiévant, P. Perception of wine fruity and woody notes: Influence of peri-threshold odorants. Food Qual. Prefer. 2005, 16, 504–510. [Google Scholar] [CrossRef]
| Compound | ODE | OTV 1 | Aromatic Serie 2 |
|---|---|---|---|
| 1-Propanol | Alcohol, ripe fruit | 9 a | 1, 5 |
| 1-Butanol | Soap, fatty, diesel | 150 a | 1 |
| Isobutanol | Bitter, fusel, alcohol | 40 b | 1 |
| Isoamyl alcohol | Harsh, bitter | 30 b | 1 |
| (Z)-3-Hexen-1-ol | Lemon, fresh | 0.4 b | 3, 5 |
| 1-Hexanol | Green grass, fresh | 8 b | 3 |
| Metionol | Garlic | 1 b | 3 |
| Benzyl alcohol | Pleasant, soft | 200 c | 2, 4 |
| β-Phenylethyl alcohol | Flowery, roses | 14 b | 2, 4 |
| Ethyl butyrate | Fruity, sweet, apple | 0.02 b | 2, 5 |
| Ethyl isovalerate | Fruity, sweet, banana | 0.003 b | 2, 5 |
| Isoamyl acetate | Banana, sweet, fruity | 0.03 b | 2, 5 |
| Ethyl hexanoate | Pineapple, apple | 0.014 b | 5 |
| Ethyl-3-hydroxybutyrate | Fruity | 20 c | 5 |
| Hexyl acetate | Fruity, green, pear | 1 d | 5 |
| 2-Phenylethyl acetate | Flowery, lilac | 0.25 b | 4 |
| Diethyl succinate | Camphor | 100 d | 5, 6 |
| Ethyl octanoate | Fresh, flowery, pineapple | 0.58 a | 2, 4, 5 |
| Ethyl lactate | Lactic | 154 a | 6 |
| Isobutyric acid | Rancid, butter, cheese | 0.05 e | 7 |
| Butyric acid | Butter, cheese, stinky | 0.173 b | 7 |
| Isovaleric acid | Cheese | 0.033 b | 7 |
| Hexanoic acid | Cheese | 0.42 b | 7 |
| Octanoic acid | Sweet, cheesy | 0.5 b | 7 |
| Decanoic acid | Rancid, fatty | 1 b | 7 |
| Diacetyl | Butter | 0.1 b | 7 |
| Furfural | Bread, toasty, candy | 15 d | 6 |
| Benzaldehyde | Sweet, candy, wood | 5 b | 2, 4 |
| Phenylacetaldehyde | Roses | 1 f | 4 |
| Acetoin | Butter | 150 a | 7 |
| γ-Butyrolactone | Coconut | 35 c | 2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García, M.; Esteve-Zarzoso, B.; Crespo, J.; Cabellos, J.M.; Arroyo, T. Influence of Native Saccharomyces cerevisiae Strains from D.O. “Vinos de Madrid” in the Volatile Profile of White Wines. Fermentation 2019, 5, 94. https://doi.org/10.3390/fermentation5040094
García M, Esteve-Zarzoso B, Crespo J, Cabellos JM, Arroyo T. Influence of Native Saccharomyces cerevisiae Strains from D.O. “Vinos de Madrid” in the Volatile Profile of White Wines. Fermentation. 2019; 5(4):94. https://doi.org/10.3390/fermentation5040094
Chicago/Turabian StyleGarcía, Margarita, Braulio Esteve-Zarzoso, Julia Crespo, Juan Mariano Cabellos, and Teresa Arroyo. 2019. "Influence of Native Saccharomyces cerevisiae Strains from D.O. “Vinos de Madrid” in the Volatile Profile of White Wines" Fermentation 5, no. 4: 94. https://doi.org/10.3390/fermentation5040094
APA StyleGarcía, M., Esteve-Zarzoso, B., Crespo, J., Cabellos, J. M., & Arroyo, T. (2019). Influence of Native Saccharomyces cerevisiae Strains from D.O. “Vinos de Madrid” in the Volatile Profile of White Wines. Fermentation, 5(4), 94. https://doi.org/10.3390/fermentation5040094