3.1. Technological Characterization of S. cerevisiae Strains
In the first phase of the work, 16
S. cerevisiae strains previously isolated from the spontaneous fermentation of grapes were studied for some technological characteristics, such as resistance to antimicrobial compounds (sulfur dioxide, copper, and ethanol) and hydrogen sulfide production (
Table 1). All the strains exhibited a high tolerance to ethanol, growing on plates containing 16%
v/
v of EtOH (data not shown), whereas a certain variability was found for SO
2 and copper resistance.
All the yeasts developing to the highest tested doses of SO
2 were isolated from cellar B and P (except one), while a certain variability was found among the yeasts from cellar M. Furthermore, these last strains showed the highest resistance to copper sulfate, as well as the majority of strains isolated from cellar P. This result is probably related to the application of organic farming methods in cellar M. As it is well known, the use of copper sulfate is allowed in organic viticulture, as copper formulates are effective against a high number of crops pests, and this compound is considered a traditional fungicide, as it has been used against powdery mildew since the 1880s. Yeasts use several mechanisms to respond to environmental stresses, and these mechanisms have been shown to contribute to the adaptation of wine yeast genomes [
30]. It was reported that the acquisition of resistance to copper sulfate could be associated with the ancient use of this fungicide in vineyards, and the acquisition of resistance to copper sulfate is one of the domestication-related traits found among
S. cerevisiae wine strains [
31]. Considering that in the last years, the interest of consumers for organic products, and also for wine, is increasing,
S. cerevisiae strains possessing high copper resistance are very interesting for winemaking. In fact, these starters can tolerate the high level of copper residues present in grape must coming from an organic farming system, assuring a regular evolution of fermentative process [
32].
Regarding hydrogen sulfide production, the 16 strains exhibited a medium and low production level of this compound, and the lowest production level was found among strains isolated from the grapes of cellar B. The killer assay revealed that 81% of the studied strains exhibited killer activity against the reference sensitive strain, which is an interesting result, as the use of starter-possessing killer activity may potentially favor the strain dominance during winemaking.
3.2. Laboratory Fermentations
The fermentative performance of the 16
S. cerevisiae strains was evaluated in inoculated fermentations at laboratory scale. The process lasted 12–15 days; strains isolated from grapes of cellar B exhibited the highest fermentative vigor, since they produced from 1.58 to 2.33 g of CO
2 per 100 mL within 48 h, whereas the maximum level of CO
2 per 100 mL produced after 48 h was 1.67 and 1.96 among strains isolated from samples of cellar P and M, respectively (
Table 2).
The statistical analysis of the main chemical parameters of the experimental wines, produced by 16 different
S. cerevisiae strains, showed main variability among wines obtained with strains from the grapes of cellar M than wines produced with strains from the B and P samples (
Table 2). All the strains completed the fermentations with a residual sugar content, both as glucose and fructose, lower than 1.5 g L
−1 (
Table 2). As regards the other parameters, such as ethanol and volatile acidity, wines obtained with strains from the P samples showed less variability than experimental wines obtained with strains isolated from grapes of the B and M cellars. However, in all the experimental wines, the volatile acidity ranged from 0.3 to 0.67 g L
−1, always being within the acceptable limits. It has been reported that the optimal concentration of acetic acid in wine is 0.2–0.7 g L
−1, and the acceptability level of this parameter is comprised between 0.7–1.1 g L
−1, depending on the style of wine [
33], whereas the OIV [
34] states that the maximum acceptable limit for volatile acidity in most wines is 1.2 g L
−1 of acetic acid.
Quantitative data from the volatile compounds usually present in high quantity in wines, such as acetaldehyde,
n-propanol, isobutanol, amyl alcohols, and ethyl acetate, analyzed in the experimental wines obtained with the selected strains, are reported in
Table 3.
As regards acetaldehyde, only the strain PP2-22 produced too high a level of this compound, whereas the content detected in the other wines was in the usual range (10–75 mg L
−1). Low acetaldehyde level contributes to fruity flavors, while high concentrations (>200 mg L
−1) confer “flatness” in wines [
35].
The wines produced by strains from M grapes exhibited the highest variability for this compound, whereas similar values of acetaldehyde content were found among samples obtained by strains isolated from B grapes. Generally, the strains from M grapes were characterized by the highest variability for the production level of these secondary compounds. In fact, significant differences were detected among the different fermentations performed by M strains for almost all the compounds, except isobutanol. Among the analyzed compounds, the most variable was the isoamyl alcohol, which was produced at significant different levels in all three groups of experimental wines.
As already reported by other studies [
36,
37,
38], these results confirmed that from fermentation of the same grape must, different strains of
S. cerevisiae can produce significantly different amounts of aromatic compounds, as a consequence of both the differential ability of wine yeast strains to release varietal volatile compounds from grape precursors and to synthesize de novo volatile compounds.
A multivariate analysis of variance (MANOVA), based on strain origin as an independent factor, was performed on the data obtained from an analysis of experimental wines, both chemical parameters and aromatic compounds. This analysis showed significant differences between fermentation obtained by strains of different origin (Wilk’s lambda 0.0001089, p < 0.00009776).
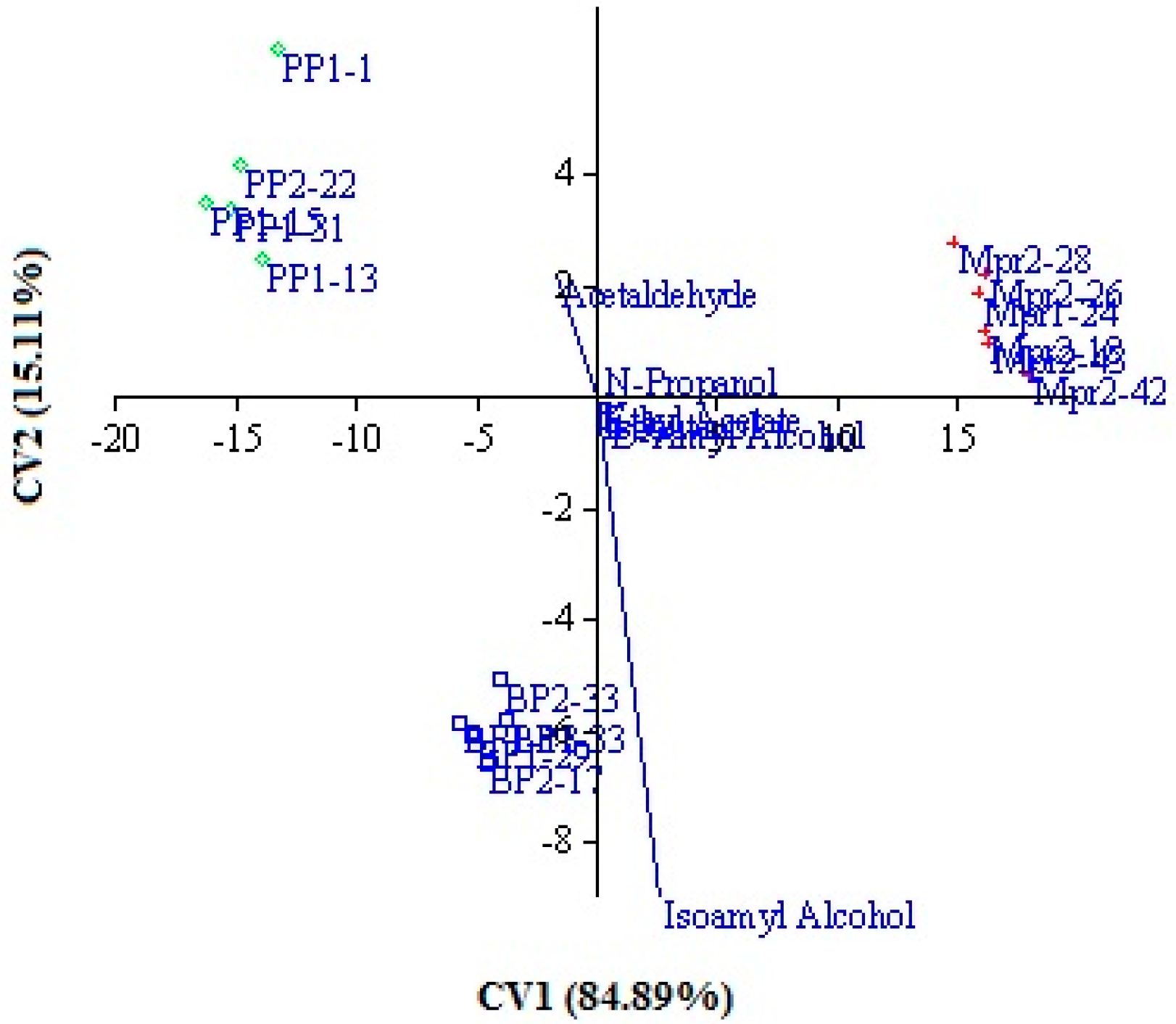
Furthermore, the same data were submitted to canonical variate analysis (CVA), considering the strain origin as an independent factor. This approach allows to visualize the relative position of the different production levels of secondary compounds and chemical parameters of experimental wines in the multivariate statistical space, by maximizing the variation related to the strain origin (
Figure 1). The canonical variate analysis of the data demonstrated a clear discrimination between wines in the function of isolation origin of inoculated strains. In fact, wines obtained by inoculating strains from grapes of the same isolation origin were grouped together (i.e., all the wines produced by strains isolated from the P samples were grouped in the left-high panel). The first and second canonical axes, which accounted for 84.89% and 15.11% respectively of the total variance, discriminate the three groups. This distribution confirms that the production level of secondary compounds and chemical parameters are significantly different in the three groups. The main differences are due to amyl alcohols, acetaldehyde, and volatile acidity. These results confirm that strain origin affects strain metabolic activity, probably as a consequence of the selective pressure exerted by the environment on natural microflora [
39]. It was reported that yeast strains develop physiological and metabolic adaptations in response to specific environmental conditions [
40], and different authors [
41,
42] demonstrated the existence of a correlation between strain origin and the characteristics of wines obtained inoculating yeasts strains isolated from different wine regions. In this way, it might be possible to associate specific indigenous strains with a specific region, or with a “terroir”, a term that was traditionally associated only with grape variety, climate, and soil. Recent studies [
41,
43,
44] put evidence in on the existence of a microbiological aspect of “terroir”, highlighting that wine microbioma (microorganisms influencing both vine growth and wine characteristics) exhibits regional differentiation.
3.3. Pilot-Scale Vinification: Monitoring of Starter Dominance Ability
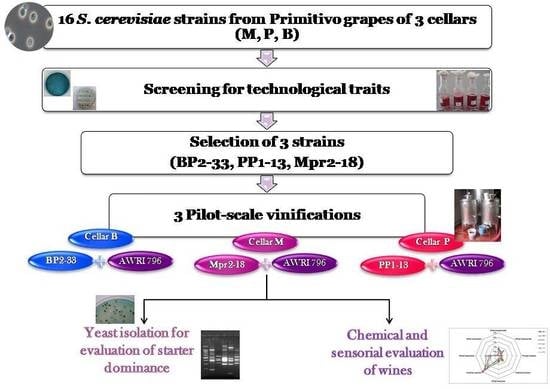
On the basis of these results, three indigenous strains, one from each winery, BP2-33, PP1-13 and Mpr2-18, possessing suitable oenological characteristics, such as high resistance to antimicrobial compounds, medium/low H
2S production (
Table 1), good fermentative performance (
Table 2), and the balanced production of secondary compounds (
Table 3), were selected as starters to perform pilot-scale vinification in the three cellars (B, P, and M). These strains were tested in comparison to the commercial strain AWRI796, which is commonly used in the three wineries.
In order to evaluate the capacity to dominate the fermentative process of these three indigenous strains in comparison to the commercial starter, yeast isolation was performed at different fermentation times (beginning, middle, and end) in each vinification trial. All the yeasts isolated were identified by restriction analysis of the ITS region, and all the colonies identified as
S. cerevisiae were characterized by amplification of the interdelta region (with primer pair δ2/δ12). The strain dominance at the different sampling times, expressed as a percentage of colonies showing the same molecular profiles of the inoculated starter in the six fermentations, was reported in
Table 4. All the indigenous starters displayed a dominance capacity higher than that of the commercial strain in all the cellars. In fact, all the colonies isolated from the vessel inoculated with the indigenous starter BP2-33 showed one unique profile throughout the entire fermentation process and corresponding to the inoculated yeast, whereas the indigenous starters tested in the cellars P and M showed a slightly lower dominance level, corresponding to 90.5 (PP1-13) and 96.3% (Mpr2-18) at the end of the process. The commercial starter dominated the fermentation in cellar M, with a dominance of 100% in the final step of the process, and in cellar B, where the dominance ability was about 80% at the end of fermentation, and only two molecular profiles different from the profile of the inoculated starter were found (
Table 4). On the contrary, this starter showed a very low dominance level in cellar P, which was equal to 44% and 63.6% in the middle and final phases of fermentation, respectively. Isolates from a vessel inoculated with the commercial strain in cellar P showed nine different interdelta profiles during the middle stage of fermentation, and three of these profiles (c, e, l,
Table 4) were found also in the final phases of the fermentation.
In this case, other
S. cerevisiae strains, different from the inoculated starter, participated significantly in the fermentative process. Other authors reported low dominance percentages of commercial yeasts in inoculated winery fermentation [
41]. In a study reporting the distribution of wine yeasts in different commercial wineries [
45], the authors found a general displacement of the autochthonous strains of
S. cerevisiae by the commercial strain inoculated, although not in all the wineries analyzed. In fact, in one cellar, the commercial strain, although predominant in the process, allowed the growth of other two strains, whereas in another winery, no imposition of commercial strain was observed. Different reasons were reported to justify the non-implantation of commercial yeast starters, such as the use of lower doses of dry yeast than those recommended, osmotic and oxidative stress, or the use of inappropriate procedures of rehydration [
44]. In our study, commercial and indigenous starters were prepared in the laboratory by following the same procedure for all of them; as a consequence, the different dominance level cannot be related to the employed modality. In order to find the characteristics explaining the low dominance level of some starters,
S. cerevisiae strains different from inoculated starters were studied. In particular, among the yeasts isolated from all the fermentations and showing interdelta profiles different from those of the inoculated starters, a strain representative of each interdelta profile was selected. These strains were tested for resistance to antimicrobial compounds (EtOH, SO
2, and copper) and killer activity. All these natural isolates showed variable resistance levels to SO
2 and copper, whereas almost all of them showed higher ethanol tolerance than inoculated starters (data not shown). In fact, the maximum dose of ethanol tolerated was 20%
v/
v for all the isolates, whereas the starters were grown until 16%
v/
v of EtOH. As regards the killer activity, variable results were found: some isolates exhibited killer activity against reference strains, whereas others were sensitive to killer protein (data not shown). These results might suggest that the competitiveness of the
S. cerevisiae population of grape must toward inoculated starters was correlated to high ethanol tolerance. Therefore, indigenous strains were characterized by higher ethanol tolerance than that the inoculated starters developed during the process. However, other traits not investigated in this study might be involved in the high competiveness of indigenous
S. cerevisiae strains present in grape must.
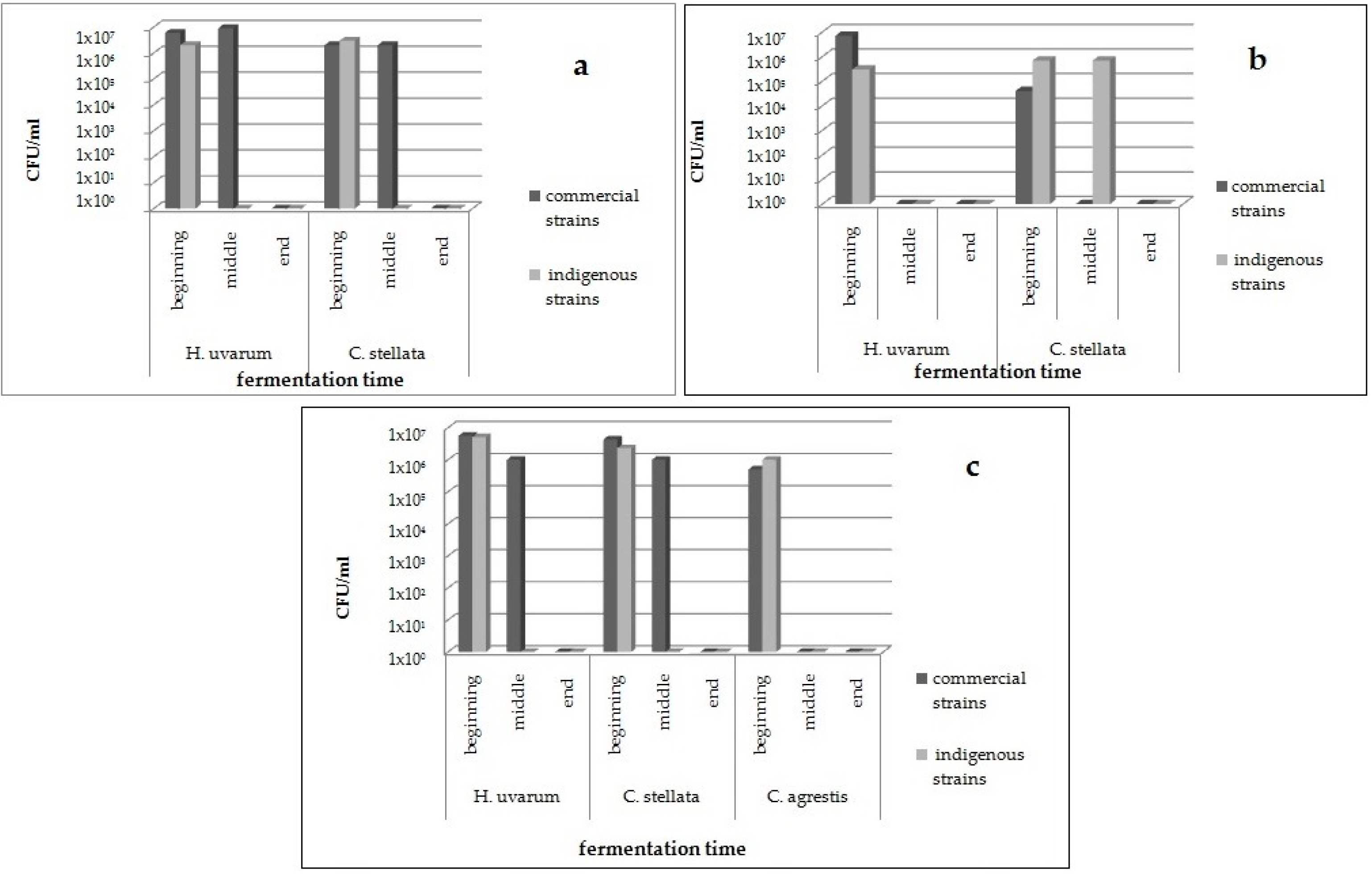
As regards the colonies identified as non-
Saccharomyces, the population distribution of the different species over all the fermentations is reported in
Figure 2. All the colonies isolated from fermentations performed in cellars B and P belonged to
Hanseniaspora uvarum and
Candida stellata, whereas in the cellar M besides these two species,
C. agrestis was also found. In cellar B,
H. uvarum and
C. stellata were found both at the beginning and middle phases of fermentations inoculated with commercial strain, and only in the first stage of the process, when the indigenous selected BP2-33 strain was inoculated (
Figure 2a). In cellar P, the same non-
Saccharomyces species was detected only in the first stage of fermentation conducted by both the commercial strain and the indigenous starter PP1-13, whereas
C. stellata was found also in the middle time of fermentation guided by PP1-13 (
Figure 2b). As shown in
Figure 2c, in cellar M, at the beginning of the process,
H. uvarum,
C. stellata, and
C. agrestis were isolated from both the inoculated fermentations, whereas in the middle phase of the process,
H. uvarum and
C. stellata were present only among colonies isolated from a vessel inoculated with commercial starter, as already reported for the trials performed in cellar B (
Figure 2a). This result might indicate that the indigenous starters selected for cellars B and M are highly competitive against non-
Saccharomyces yeasts compared with the indigenous strain selected for cellar P. Other authors found that the presence of non-
Saccharomyces species throughout the fermentation process is a consequence of the type of strain used as starter culture. Tofalo et al. [
46] reported the presence of non-
Saccharomyces species throughout all the processes; in this study, an indigenous
S. cerevisiae strain starter with low alcoholic performance was used as a starter culture, and this could have allowed the growth of non-
Saccharomyces strains. These findings underline the fundamental role of the screening program followed in the laboratory to select the most promising indigenous
S. cerevisiae strains able to efficiently ferment the musts.
As expected, these non-Saccharomyces yeasts were not found at the end of all the pilot-scale fermentations performed in the three cellars.
3.4. Pilot-Scale Vinification: Analysis of Wines
The chemical parameters detected in the wines obtained by the six pilot-scale vinifications, performed in the three different cellars, are shown in the
Table 5. The ethanol content ranged between 11.74–15.12
v/
v, with the highest level in wines obtained in cellar M, both by inoculating indigenous and commercial starters. As a consequence, the wines obtained in this cellar contained the lowest amounts of sugar residuals. All six wines contained a similar level of volatile acidity, which was comprised in the desirable range.
The aromatic compounds detected in the six wines were reported in
Table 6, in which the volatile compounds were subdivided into four chemical classes—namely, esters, alcohols, terpenes, and aldehydes. Higher alcohols represented the most abundant group in the analyzed samples, followed by esters and aldehydes. Fusel alcohols can contribute a positive flavor in the wine when present in concentrations below 300 mg L
−1, whereas concentrations above 400 mg L
−1 have a detrimental effect on wine aroma [
9]. In all the analyzed wines, the concentration of total fusel alcohols was below 400 mg L
−1, except for wines produced in cellar M, both by the indigenous and commercial starters (
Table 6), and the most abundant alcohol in all the wines was isoamyl alcohol. As regards wines produced in cellar B, the content of higher alcohols in wine produced by the indigenous starter was significantly higher than the level detected in wine produced by AWRI796, except for
n-propanol content. In addition, for wines produced in cellars M and P, statistically significant differences between wines from indigenous and commercial starters were found for almost all the higher alcohols detected in this study.
The esters are an important group that can significantly affect wine aroma. The fermentation esters associated with wine fruitiness are divided in two groups: acetate esters (mainly: ethyl acetate, 2-phenyl ethyl acetate, 3-methyl-1-butanol acetate or isoamyl acetate, hexyl acetate) and ethyl fatty acid esters. Among the esters, the main wine ester is ethyl acetate, which can contribute a desirable fruit fragrance at concentrations lower than 150 mg L
−1, whereas it contributes an unpleasant odor at concentrations higher than this value. The highest amounts of this compound were detected in wines produced in cellar B, both by indigenous and commercial starters. However, any pilot-scale wine showed an ethyl acetate content less than 50 mg L
−1. Other esters present in high concentrations were isoamyl acetate (banana aroma) and ethyl hexanoate (fruity, floral aroma), both produced by all the starters in concentrations higher than their threshold values of 0.03 mg L
−1 and 0.05 mg L
−1, respectively [
47]. As already reported for higher alcohols, statistically significant differences for the content of all the esters detected in this study were found between wines obtained with commercial and indigenous strains, in particular in cellar M. It was reported [
48,
49,
50] that ester concentration is affected by different fermentation conditions, such as temperature, aeration, and sugar content, other than yeast strain used. By considering that the same fermentative conditions were used in each cellar, the yeast starters used by the winemakers plays a fundamental role on the ester content of the wines.
As regards aldehydes, it’s well known that acetaldehyde represents more than 90% of the total aldehyde content in wine. Its aroma threshold value is 100 mg L
−1; low levels of this compound give a desirable fruity aroma to the wines, whereas an excessive content produces an apple-like off-flavor in the wine, and levels more than 200 mg L
−1 cause wine flatness [
51]. The highest amount of acetaldehyde was found in the wine produced in cellar P by the commercial strain (57.74 mg L
−1), although in all the wines, this compound did not exceed its threshold value.
Other compounds detected in this study were terpenes, which are responsible for wine varietal flavor, although they are not present at high levels in wine. It has been reported that besides grapes, yeasts are also involved in the production of terpenes. As shown in
Table 6, four terpenes were identified in these wines. The most represented was linalool, which has a rose-like floral aroma and contributes positively to wine aroma, although in these wines, it was detected at a value below its threshold value of 25 μg L
−1, and β-damascenone, which has been reported to possess sweet and exotic flavor notes. In addition, for almost all these compounds, wines produced by AWRI796 contained levels significantly different from the content detected in wines obtained with indigenous strains, particularly for wines produced in cellars B and M.
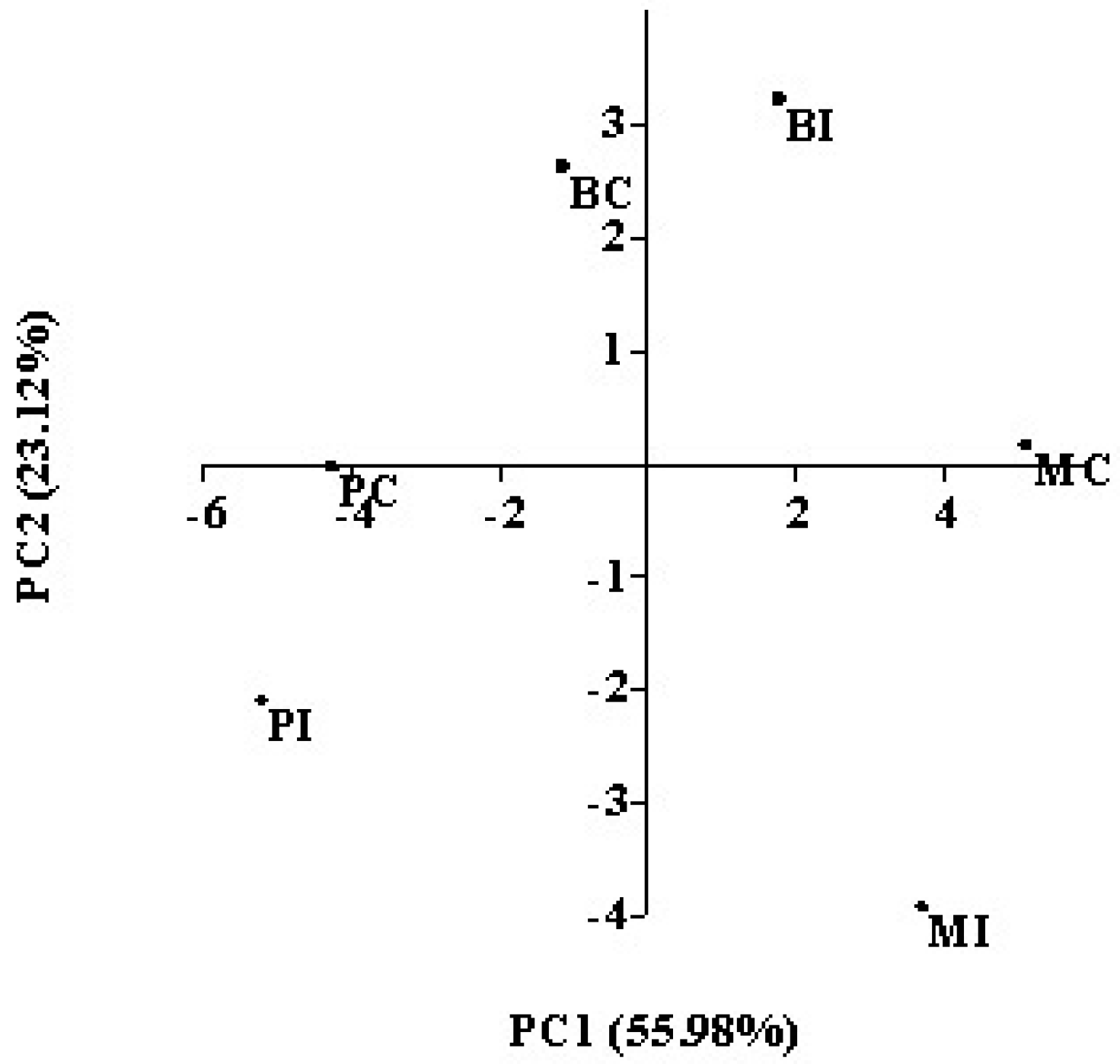
All the parameters determined in wines obtained by indigenous and commercial strains in the three cellars were submitted to principal component analysis (PCA). The first two components account for about 79% of the total variance. The first principal component (PC1) explained 55.98% of data variability, and was correlated with ethyl propanoate, isobutanol, 2-propanol, 1-butanol, 2-phenylethyl acetate, and benzaldehyde, while propyl acetate, ethyl hexanoate, ethyl acetate, and
n-propanol contribute more strongly to the second principal component (PC2). The plot of the six wines on the plane defined by these first two components is shown in
Figure 3. The PCA of the wines revealed that the wines obtained in the same cellar by inoculating the two strains (indigenous and commercial) differed in the aromatic profile, as they were located in different quadrants. Only wines obtained in cellar P with both starters were located in the same quadrant. In both the fermentation trials performed in cellar P, the starter dominance was the lowest one (
Table 4), mainly for fermentation performed by the commercial starter. In this trial, a high participation of indigenous
S. cerevisiae strains to fermentative process occurred, which might affect the composition, making the wine obtained by commercial starter similar to wine produced by inoculating the indigenous strain previously isolated and selected from the same grape must. This result confirms that dominance or competitiveness of a yeast starter strain could make a significant impact on the aromatic characteristic of wine by dominating its sensorial quality or eliminating the influence of the
S. cerevisiae population naturally present in fermenting grape must [
52]. Furthermore, the wines obtained inoculating the same starter, the commercial strain AWRI796, were located in three different quadrants, indicating differences in the chemical composition of wines obtained by using the same yeast strain, but in different wineries. Although the same grape variety was used in all the cellars, each of them were located in different geographical areas, which can be affected by the composition of grape must, i.e., the precursor content. In fact, factors that are also related to the vineyard growing area, such as seasonal weather differences, soil composition, and vineyard management were reported to affect the development and retention of grape aroma compounds, and consequently the aroma of the wines produced [
53]. This result emphasizes that the effective impact of yeast strains on the aroma properties is dependent on a network of effects; other strain metabolisms and other factors, such as raw material composition and winemaking procedure, amongst others, have to be considered.
The wines obtained during pilot-scale vinifications were submitted to sensory evaluation in order to evaluate if there were significant differences on the overall organoleptic quality of wines obtained in the three cellars. As reported in
Table 7, the results of a one-way ANOVA model performed on the sensory data showed a significant effect of samples on liking scores (F = 11.79;
p < 0.00; L.S.D. = 0.49). The sample associated with the highest liking score was the wine obtained in cellar P by inoculating the commercial starter (sample PC), followed by wines obtained in the three different cellars by inoculating the indigenous starters (samples BI, MI, PI), whereas the lowest liking scores were associated with wines produced by inoculating the commercial starter in cellar B and M (samples BC and MC). By considering that a score of 5 was taken as the lower limit of acceptability, all the wines fermented with indigenous starters reached the threshold of acceptability, whereas among wines produced by the commercial strain, only the wine from cellar P attained a liking score much higher than the threshold of acceptability. It has to be underlined that during the fermentation of this wine, the inoculated starter showed a low dominance ability and a high participation of indigenous strains was found (
Table 4), which might affect both the aromatic and sensorial qualities of wine. However, these results indicate a predilection of the tasters toward the wines produced with selected indigenous starters or with a high contribution of indigenous
S. cerevisiae strains, suggesting the need to support the use of indigenous starters for safeguarding the biodiversity of vineyard yeasts. It was found [
54] that the winery environment and commercial strain use significantly alter the composition of
S. cerevisiae population present in the first phase of spontaneous fermentation, indicating that a population of yeast descended from commercial strains might be resident in the winery facilities. As a consequence, the promotion of indigenous starter cultures represents a promising tool to limit the erosion of natural biodiversity induced by the wide use of commercial starters in winemaking.