Beer–The Importance of Colloidal Stability (Non-Biological Haze)
Abstract
:1. Introduction
2. Types and Components of Beer Haze
- Precipitates.
- Bits.
- Haze.
- Invisible haze.
- Visible haze (proteins and pentosanes): can shorten the shelf life of a product [23].
- Native particles, which originate from the beer by coagulation/precipitation,
- Process particles, which originate from materials (e.g., filter aids) added during the process,
- Foreign particles, which enter the beer as accidental contaminants.
- Protein haze–beer contains approx. 500 mgL−1 of different polypeptides. According to Kaersgaard and Hejgaard [36], only 2 mgL−1 are sufficient to cause haze in beer.
- α-glucans
- Calcium oxalate
- Inorganic matter (filter aids, labels, glass particles, etc.)
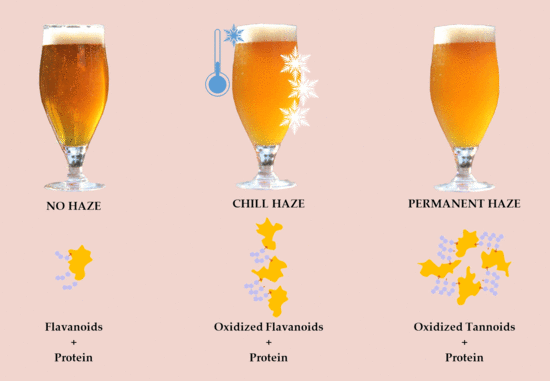
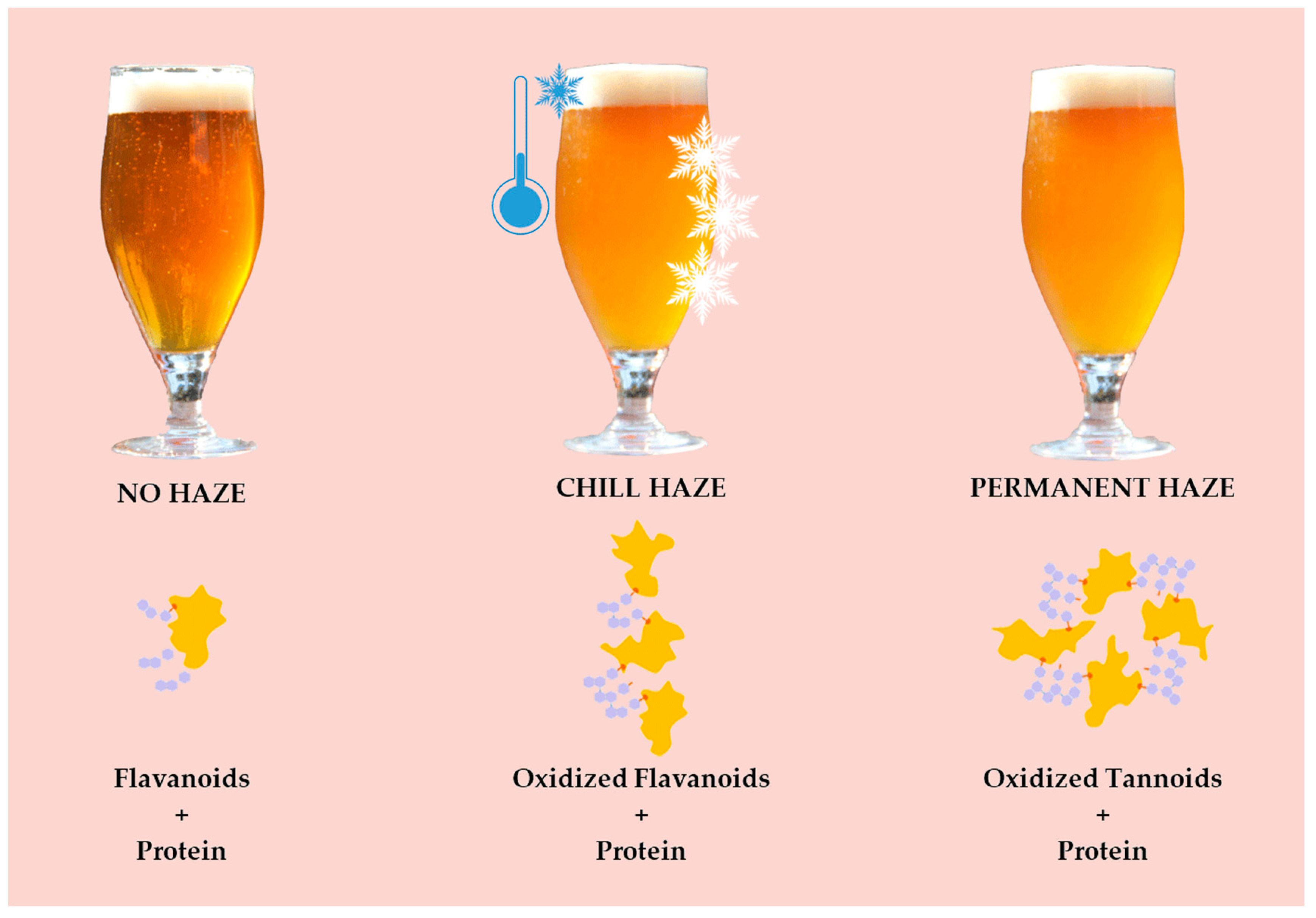
- 2.
- Permanent (irreversible) (Figure 3)–protein + polyphenol; haze active (HA) proteins found in beer originate from barley’s hordein protein fraction. HA–display a high affinity for polyphenols (PPs) and easily form haze [38]. Permanent haze can be caused by Reference [29]:
- oxidation
- aging
- shaking
- metals
- pasteurization
- 3.
- Haze caused by other substances (non-colloidal)
How Beer Properties and Constituents Influence Haze Formation?
- pH—it is well known that pH significantly influences proteins by determining their real charge. Changes in pH result in higher or lower molecular ionization, influence its solubility and space conformation. The optimal pH for haze formation is approx. 4.2–4.4. According to Siebert [10], lower pH significantly lessens haze formation.
- Free amino acids do not influence beer haze because complexes between free amino acids and polyphenols are too small and much more soluble than protein-polyphenol complex [10].
- Alcohol–haze reduction in solutions where alcohol is added can be explained by its polarity (lesser than water) and serves as a solvent in this case. Dioxan finds itself between water and alcohol according to polarity and his ability to reduce haze is greater than alcohol [39].
- Carbohydrates–α- and β-glucans, pentosans/arabinoxylans, glucose, arabinose, polygalacturonic acid, and xylose contribute to haze formation in beer [29].
- Metal ions–iron, copper, zinc, calcium and potassium ions have a tendency to accumulate haze particles. They catalyze oxidation of polyphenols and polymerization of polyphenols with proteins and help in turning reversible haze to permanent [40].
- Oxygen–oxygen in beer encourages the formation of free radicals that deteriorate beer aroma and flavour compounds. This results in old and stale taste [29].
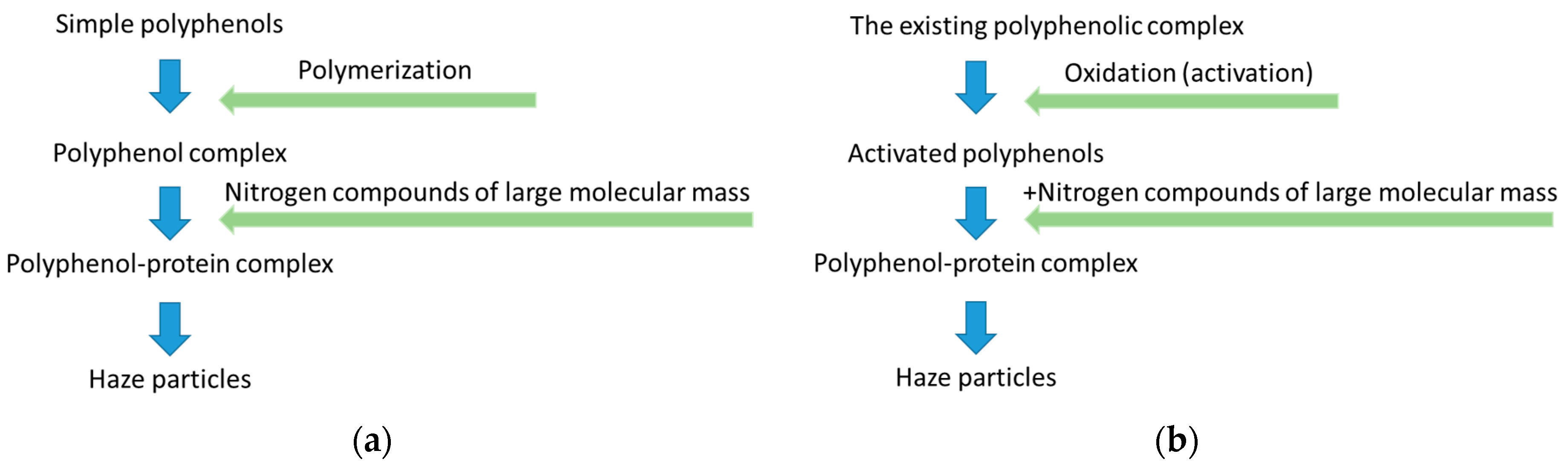
- A simplified schemes of beer colloidal haze formation are shown in Figure 4.
3. Physical-Chemical Properties of Beer
4. Colloidal Stabilization Treatments
Colloidal Stabilization Agents
- Silica–commonly accepted stabilization agents
- Silica gel–industrial preparation of sodium silicate; removes proteins responsible for causing haze. Silica has a highly porous structure to which proteins bind to.
- Silica sol are used as aqueous solutions (15–50% w/w) of silicic acid. In wort or beer, the sol cross-links to form a gel and traps haze-forming material. The gel is then removed during filtration. Sols are mostly used for wort clarification after boiling, but can be used to stabilize beer too [48].
- Polyvinylpolypyrrolidine (PVPP)–PVPP is an adsorbent of great molecular weight, and unit molecular structure of PVPP closely resembles that of the amino acid proline [44]. It is not clear if the PVPP binds to the same part of the polyphenol molecule to which polypeptides bind, or another part, but binding to PVPP makes a strong relationship [49]. PVPP selectively binds to polyphenols responsible for chill and permanent haze. It is insoluble in water and organic solvents, strong acids, and alkali. Before use, it should be mixed with water and left for 15 min to bind water and allow swelling [50]. According to McMurrough [51], PVPP has no significant effect on haze-active polypeptide and Gopal and Rehmanji [50] report that it does not remove polyphenols that enable resistance to staling. PVPP can be used in two forms in brewing [11]:
- In the form of a micronized powder, provided for single use. Particles are very small, but provide a large surface area. This can be added to the beer stream, with a necessary contact time of 5–10 min. This form can be dosed into the cold conditioning tank. Around 10–40 g/hl should be used, and lower dosage are recommended if applied with silica gel. It is removed by filtration.
- Second form has larger particles, but provides smaller surface area and thus can adsorb less polyphenolic compounds. The upside is that this is recyclable and can be regenerated more times [50]. Regenerable PVPP can be added to the brewing process after primary filtration but has to be removed at a separate filter. Regeneration is done by washing with NaOH (1–2% w/vol) at 60–80 °C for 15–30 min, then with dilute acid to restore the pH to four. Regeneration losses are low as 0.5% have been claimed [50].
- Lucilite TR–Lucilite TR has been developed as an alternative to PVPP and even though it is derived from PVP-modified silica gel consisting of amorphous silica coated with, it does not require any time for swelling. For adsorption of polyphenols, it is most effective at lower dosages because at higher dosage, selectivity is poorer as non-haze–active polyphenols are also adsorbed [53].
- Isinglass finings–made of natural piscine collagen (98%) from tropical and subtropical fish swim bladders. It is rich in glycine, proline and hydroxyproline. In order to destroy the strong hydrogen bonds and produce soluble collagen, acid hydrolysis has to be applied. By increasing the size and weight of haze particles, finings cause haze particles to settle [11]. Isinglass can be found in four forms–liquid, milled, powdered or freeze dried, and paste. Isinglass molecule is amphoteric, meaning it is both negatively and positively charged. However, the total molecule charge can be described as positive with some negative areas and this is how isinglass can bind and negatively charged yeast cells and positively charged proteins. Ionic bonds are pointed to tannins, yeast cell walls and carrageenan, and hydrogen bonds to tannins, carrageenan and proteins. Since beer holds pH around 4.5, and the optimal pH for isinglass is 4.4, the removal of haze material works well. Isinglass is not allowed in finished beer as it is considered an allergen and some countries demanded it to be declared on food labels, since 2005. In 2015 an Annex II allergens was disclosed to Food allergen labelling and information requirements under the EU Food Information for Consumers Regulation No. 1169/2011: Technical Guidance [54] which included isinglass as an allergen. Walker et al. [55] published an article on the alternatives to isinglass for beer clarification, which included the application of yellow split peas and avian collagen.
- Copper or kettle finings–these are actually carrageenan gels derived from the seaweed Euchema cottonii. Their main ingredients are linear sulphated polysaccharides containing galactose and anhydrogalactose. There are different types but so far κ-type is the most effective in removing proteins, polypeptides, and polyphenols. They can be added 15 min before the end of the boil at pH > 5.2. As described in the chapter authored by Leiper and Miedl [11], copper finings give good wort clarity with a compact, stable, and easy-to-remove sediment. It is important to emphasize that they improve the clarity of cold wort by helping to precipitate trub, but are not so efficient when applied in hot wort. High gravity worts require more finings than normal gravity worts. The application of carrageenan or isinglass in filtered beers results in lower hazes than unfined beers.
- Proteolytic preparations papain has a long history of usage in brewing industry. It was used to remove the albuminoids [56]. It is a mixture of proteinase enzymes obtained from the latex of the Carica papaya fruit. It hydrolyses beer proteins, but not only haze-causing proteins, and can act harmfully to the desirable foam active proteins. It can be applied after transfer to maturation tank or in maturation tank itself. Proteinase preparation–specific to haze-causing polypeptides. It is derived from the fungus Aspergillus niger, as reported by Lopez and Edens [57]. Beer treated with Clarex showed good haze stability following six months of storage at room temperature, with haze levels below 1.5° EBC and little effect on foam stability.
- Tannic acid–has a structure formula resembling polyphenols; it can remove some metals (Fe, Al, Zn and Pb) and polyphenols if they are bound to proteins [58].
- Gelatin is commonly used in the wine and juice industries to remove polyphenols. Gelatin removes haze proteins, but does not react with foam proteins [11].
- Nylon perlon and polyamides are effective for the removal of polyphenols by adsorption, but affect bitterness, foam, and color [47].
- Formaldehyde removal of polyphenols is forbidden in many countries for its cancerous properties [29].
- Antioxidants–ascorbic acid and glucose oxidase are known reduce free radicals [47]. Ascorbic acid is considered to be a stand in for sulphur dioxide (SO2). In presence of heavy metals it can have an adverse effect on beer quality. This is why it is usually added in synergy with some other reductor (K-metabisulphite) [29]. SO2 forms in beer naturally as a result of fermentation, but the original concentration is not sufficient to withhold a staling process. This is why SO2 is introduced to beer in the form of K-sulphite or sulphite acid [29]. Carbonyl-bisulphite adducts are complexes of sulphite. They act as antioxidants and help haze stability [59]. Other antioxidative compounds, hydrogen peroxide, or polyphenols, but are not accepted as EDTA, which is usually employed in heavy metal removal [47].
- Combined treatments is a chamber filled with agarose adsorbent and when beer passes through the chamber haze-active proteins and polyphenols bind to the agarose that can be regenerated using salt and caustic, and is very economic since it can be used several hundreds of times without loss of performance [60].
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Briggs, D.E.; Boulton, C.A.; Brookes, P.A.; Stevens, R. Brewing: Science and Practice; Woodhead Publishing Limited: Cambridge, UK, 2004; p. 881. [Google Scholar]
- Lewis, M.J.; Young, T.W. Brewing, 2nd ed.; Kluwer Academic and Plenum Publishers: New York, NY, USA, 2002; p. 398. [Google Scholar]
- Delvaux, F.; Delvaux, F.R.; Delcour, J.A. Characterisation of the colloidal haze in commercial and pilot scale Belgian white beers. J. Inst. Brew. 2000, 106, 221–227. [Google Scholar] [CrossRef]
- Anderegg, P. Inhibitors of filtration and filterability. Brauerei-Rundschau 1979, 10, 40–43. [Google Scholar]
- Baetsle, G.O. Breweries set specific demands on use of wheat. Voedingsmiddelen-Techuologie 1996, 13, 43–45. [Google Scholar]
- McMurrough, I.; Kelly, K.; Madigan, D. European Brewery Convention Congress. In Proceedings of the European Brewery Convention Congress, Oslo, Norway, 6–10 June 1993; Wijngaarden, M.V., Ed.; IRL Press: Oxford, UK, 1994. [Google Scholar]
- Leipert, K.A.; Stewart, G.G.; McKeown, I.P. Beer polypeptides and silica gel Part, I. Polypeptides involved in haze formation. J. Inst. Brew. 2003, 109, 57–72. [Google Scholar] [CrossRef]
- Leipert, K.A.; Stewart, G.G.; McKeown, I.P.; Nock, T.; Thompson, M.J. Optimising beer stabilisation by the selective removal of tannoids and sensitive proteins. J. Inst. Brew. 2005, 111, 118–127. [Google Scholar] [CrossRef]
- Schur, F. Beer stabilization before filtration. Brauwelt 1980, 120, 1712–1716. [Google Scholar]
- Siebert, K.J.; Carrasco, A.; Lynn, P.Y. Formation of protein polyphenol haze in beverages. J. Agric. Food Chem. 1996, 44, 1997–2005. [Google Scholar] [CrossRef]
- Leiper, K.A.; Miedl, M. Colloidal stability of beer. In Handbook of Alcoholic Beverages Series, Beer: A Quality Perspective; Bamforth, C.W., Russell, I., Stewart, G., Eds.; Elsevier Ltd.: San Diego, CA, USA, 2009; Chapter 4. [Google Scholar]
- Bamforth, C.W. 125th anniversary review: The non-biological instability of beer. J. Inst. Brew. 2011, 117, 488–497. [Google Scholar] [CrossRef]
- Mastanjević, K.; Španić, V.; Horvat, D.; Mastanjević, K.; Šarkanj, B.; Krstanović, V.; Šantek, B. Establishing the impact of Fusarium culmorum infection and fungicide treatment on wheat malt quality. J. Food Process. Preserv. 2018, 17, e13714. [Google Scholar] [CrossRef]
- Lusk, L.T.; Goldstein, H.; Ryder, D. Independent role of beer proteins, melanoidins and polysaccharides in foam formation. J. Am. Soc. Brew. Chem. 1995, 53, 93–103. [Google Scholar] [CrossRef]
- Roza, J.R.; Wallin, C.E.; Bamforth, C.W. A comparison between the instrumental measurement of head retention/lacing and perceived foam quality. Tech. Q. Master Brew. Assoc. Am. 2006, 43, 173–176. [Google Scholar]
- Jackson, G.; Roberts, R.T.; Wainwright, T. Mechanism of beer foam stabilization by propylene glycol alginate. J. Inst. Brew. 1980, 86, 34–37. [Google Scholar] [CrossRef]
- Bamforth, C.W. Bringing matters to a head: The status of research on beer foam. In Proceedings of the European Brewery Convention Congress Foam Symposium, Amsterdam, The Netherlands, 1998; Verlag Hans Carl, Getränke-Fachverlag: Nürnberg, Germany, 1999; pp. 10–23. [Google Scholar]
- Goldstein, H.; Ting, P. Post kettle bittering compounds: Analysis, taste, foam and light stability. In Proceedings of the European Brewery Convention, Monograph XXII—Symposium on Hops, Zoeterwoude, The Netherlands, May/June 1994; Fachverlag Hans Carl: Nürnberg, Germany, 1994; pp. 154–162. [Google Scholar]
- Lusk, L.T.; Murakami, A.; Nielsen, L.; Kay, S.; Ryder, D. Beer photooxidation creates two compounds with aromas indistinguishable from 3-methyl-2-butene-1-thiol. J. Am. Soc. Brew. Chem. 2009, 67, 189–192. [Google Scholar] [CrossRef]
- Templar, J.; Arrigan, K.; Simpson, W.J. Formation, measurement and significance of lightstruck flavour in beer: A review. Brew. Dig. 1995, 70, 18–25. [Google Scholar]
- Kristina, M.; Vinko, K.; Krešimir, M.; Bojan, Š. Malting and Brewing Industries Encounter Fusarium spp. Related Problems. Fermentation. 2018, 4, 3. [Google Scholar]
- Bamforth, C.W. Beer haze. J. Am. Soc. Brew. Chem. 1999, 57, 81–90. [Google Scholar] [CrossRef]
- Siebert, K.J.; Troukhanova, N.V.; Lynn, P.Y. Nature of polyphenol protein interactions. J. Agric. Food Chem. 1996, 44, 80–85. [Google Scholar] [CrossRef]
- Aron, P.M.; Shellhammer, T.H. A discussion of polyphenols in beer physical and flavour stability. J. Inst. Brew. 2010, 116, 369–380. [Google Scholar] [CrossRef]
- Letters, R. Origin of carbohydrates in beer sediments. J. Inst. Brew. 1969, 75, 54–80. [Google Scholar] [CrossRef]
- Coote, N.; Kirsop, B.H. A haze consisting largely of pentosan. J. Inst. Brew. 1976, 82, 34. [Google Scholar] [CrossRef]
- Gjertsen, P. Beta-glucans in malting and brewing. I Influence of beta-glucans on the filtration of strong beers. Proc. Am. Soc. Brew. Chem. 1966, 24, 113–120. [Google Scholar] [CrossRef]
- Lewis, M.J.; Poerwantaro, W.M. Release of haze material from the cell walls of agitated yeast. J. Am. Soc. Brew. Chem. 1991, 49, 43–46. [Google Scholar] [CrossRef]
- Krstanović, V. The Influence of Partial Substitution of Malt with Maize Grits and Wheat on Quality Parameters and Colloidal Stability of Beer. Master’s Thesis, University of Zagreb, Zagreb, Croatia, July 2000. [Google Scholar]
- Sharpe, F.R.; Channon, P.J. Beer haze caused by can lid lubricant. In Proceedings of the 21th Congress of European Brewery Convention, Madrid, Spain, 10–14 May 1987; pp. 599–606. [Google Scholar]
- Bamforth, C.W. Processing and packaging and their effects on beer stability. Ferment 1988, 1, 49–53. [Google Scholar]
- Walker, M.D.; Boume, D.T.; Wenn, R.V. The influence of malt-derived bacteria on the haze and filterability of wort and beer. In Proceedings of the 26th Congress of the European Brewery Convention, Maastricht, The Netherlands, 1997; pp. 191–198. [Google Scholar]
- Bengough, W.I.; Harris, G. General composition of nonbiological hazes of beer and some factors in their formation. Part, I. J. Inst. Brew. 1955, 61, 134–135. [Google Scholar] [CrossRef]
- Glenister, P.R. Beer Deposits: A Laboratory Guide and Pictorial Atlas for the Study of the Various Particles Found in the Deposits of Beer and Ale; Miles Laboratories: Chicago, IL, USA, 1975. [Google Scholar]
- Steiner, E.; Becker, T.; Gastl, M. Turbidity and haze formation in beer—Insights and overview. J. Inst. Brew. 2010, 116, 360–368. [Google Scholar] [CrossRef]
- Kaersgaard, P.; Hejgaard, J. Antigenic beer macromolecules: An experimental survey of purification methods. J. Inst. Brew. 1979, 85, 103–111. [Google Scholar] [CrossRef]
- Rehmanji, M.; Mola, A.; Narayanan, K.; Gopal, C. Superior colloidal stabilization of beer by combined treatment with silica (Xerogel) and PVPP, polyclar plus 730. In Proceedings of the MBAA 112th Anniversary Conwention, Keystone, Colorado 1999 International Specialty Products, Wayne, NJ, USA, 2000; pp. 113–118. [Google Scholar]
- Gorinstein, S.; Moshe, R.; Wolfe, F.H.; Berliner, M.; Rotenstreich, A.; Tilis, K. Characterization of stabilized and unstabilized beers. J. Food Biochem. 1990, 14, 161–172. [Google Scholar] [CrossRef]
- Asano, K.; Shinagawa, K.; Hashimoto, N. Characterization of haze-forming proteins of beer and their roles in chill haze formation. J. Am. Soc. Brew. Chem. 1982, 40, 147–154. [Google Scholar] [CrossRef]
- Anger, H.M. Assuring nonbiological stability of beer as an important factor for guaranteeing minimum self-life. Brauwelt Int. 1996, 2, 142–150. [Google Scholar]
- Leonel, M.; Moll, M.; Dodds, J.A.; Leitzelement, M. Beer colloidal haze and the filtration process. MBAA Tech. Q. 1986, 23, 44–48. [Google Scholar]
- Titze, J.; Christian, M.; Jacob, F.; Parlar, H.; Ilberg, V. The possibilities of particle analysis demonstrated by the measurement of the colloidal stability of filtered beer. J. Inst. Brew. 2010, 116, 405–412. [Google Scholar] [CrossRef]
- Siebert, K.J. Effects of protein polyphenol interactions on beverage haze, stabilization, and analysis. J. Agric. Food Chem. 1999, 47, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Siebert, K.J.; Lynn, P.Y. Mechanism of beer colloidal stabilization. J. Am. Soc. Brew. Chem. 1997, 55, 73–78. [Google Scholar] [CrossRef]
- Schur, F. Beer stabilization. Schweiz. Brau. Rundsch. 1979, 90, 4–12. [Google Scholar]
- Siebert, K.J.; Stenroos, L.E.; Reid, D.S. Characterization of amorphous-particle haze. J. Am. Soc. Brew. Chem. 1981, 39, 1–11. [Google Scholar] [CrossRef]
- Basarova, G. The structure-function relationship of polymeric sorbents for colloidal stabilization of beer. Food Struct. 1990, 9, 175–194. [Google Scholar]
- Niemsche, K.; Oppermann, A. Clarification of wort and beer with silica sol. Brew. Int. 2004, 4, 30–32. [Google Scholar]
- McMurrough, I.; O’Rourke, T. New insight into the mechanism of achieving colloidal stability. MBAA Tech. Q. 1997, 34, 271–277. [Google Scholar]
- Gopal, C.; Rehmanji, M. PVPP—The route to effective beer stabilisation. Brew Guard 2000, 129, 1–4. [Google Scholar]
- McMurrough, I. Colloidal stabilization of beer. Ferment 1995, 8, 39–45. [Google Scholar]
- McMurrough, I.; Madigan, D.; Kelly, R.J.; Smyth, M.R. The role of flavanoid polyphenols in beer stability. J. Am. Soc. Brew. Chem. 1996, 54, 141–148. [Google Scholar] [CrossRef]
- Rehmanji, M.; Mola, A.; Narayanan, K.; Gopal, C. Superior colloidal stabilization of beer by combined treatment with silica (xerogel) and PVPP, polyclar plus 730. MBAA Tech. Q 2000, 37, 113–118. [Google Scholar]
- Food Allergen Labelling and Information Requirements under the EU Food Information for Consumers Regulation No. 1169/2011: Technical Guidance. Available online: https://www.food.gov.uk/sites/default/files/media/document/food-allergen-labelling-technical-guidance.pdf (accessed on 12 October 2018).
- Walker, S.L.; Donet Camarena, M.C.; Freeman, G. Alternatives to isinglass for beer clarification. J. Inst. Brew. 2007, 113, 347–354. [Google Scholar] [CrossRef]
- Esnault, E. Beer stabilization with papain. Brew Guard 1995, 124, 47–49. [Google Scholar]
- Lopez, M.; Edens, L. Effective prevention of chill-haze in beer using an acid proline-specifi c endoprotease from Aspergillus niger. J. Agric. Food Chem. 2005, 53, 7944–7949. [Google Scholar] [CrossRef] [PubMed]
- Mussche, R.A. Beer stabilization with gallotannin. Brew Guard 1994, 123, 44–49. [Google Scholar]
- Kaneda, H.; Osawa, T.; Kawakishi, S.; Munekata, M.; Koshino, S. Contribution of carbonyl-bisulfi te adducts to beer stability. J. Agric. Food Chem. 1994, 42, 2428–2432. [Google Scholar] [CrossRef]
- Jany, A.; Katzke, M. CSS—A new beer stabilization process. MBAA Tech. Q. 2002, 39, 96–98. [Google Scholar]


| Instability Factor | Stabilization Agent | Technological Process of Application | Course of Action | Advantage | Shortcomings |
|---|---|---|---|---|---|
| Polyphenolic compounds | PVPP | Before or after filtration | adsorption | Lighter colour | Removal of low-molecular polyphenols (decreased antioxidative potential) |
| Gelatin | Precipitation | Removes proteins Leaves foam proteins intact | |||
| Nylon | adsorption | damage bitterness, foam and colour | |||
| Formaldehyde | fermentation | Polymerization and precipitation | Lighter colour | Cancerous; forbidden for usage | |
| Lucilite TR | adsorption | Effective with PVPP | |||
| Proteins | Bentonite | Lagering tank, 8 days before decantation | Non-selective adsorption | Increased filterability Lighter colour | Affects bitterness, colour, flavour and foam Takes 24 h to react Beer loss 1–3% |
| Polyamide resin | Lagering tank, 1 day before decantation | adsorption | |||
| Tend to dissolve in beer | |||||
| Enzymes (papain, proteinases) | Lagering tank or pressure tank | Hydrolysis | |||
| Silica gels and sols | Gels are removed by filtration Sols are used after boiling | Commonly used | |||
| Carbohydrates | Enzymes (amylase, amyloglucosidase, glucanase) | Fermentation or lagering tank | Foam instability Tend to dissolve in beer | ||
| Heavy metals | EDTA | Fermentation | Forming complexes and precipitation | Influences the potability | |
| Tannic acid (Gallotannin) | wort following boiling and during maturation filtration | effective against flavor staling and light instability | It can damage foam proteins | ||
| Oxygen | Ascorbic acid | Filtration | reduction | Lighter colour | Influences the potability |
| Enzymes (glucose-oxidase, glucose-catalase) | |||||
| SO2K-, Na-sulphite Carbonyl-bisulphite adducts | Reduced oxidation and retards staling Retards flavour deterioration | ||||
| Cystein, Lysin-HCl | Influences the potability | ||||
| CO2 | Physical removal | ||||
| Proteins and polyphenols | agar | adsorption | Does not influence foam stability | ||
| Isinglass finings | Declared as allergen | ||||
| Copper finings | give good wort clarity with a compact, stable, easy-to-remove sediment |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastanjević, K.; Krstanović, V.; Lukinac, J.; Jukić, M.; Vulin, Z.; Mastanjević, K. Beer–The Importance of Colloidal Stability (Non-Biological Haze). Fermentation 2018, 4, 91. https://doi.org/10.3390/fermentation4040091
Mastanjević K, Krstanović V, Lukinac J, Jukić M, Vulin Z, Mastanjević K. Beer–The Importance of Colloidal Stability (Non-Biological Haze). Fermentation. 2018; 4(4):91. https://doi.org/10.3390/fermentation4040091
Chicago/Turabian StyleMastanjević, Kristina, Vinko Krstanović, Jasmina Lukinac, Marko Jukić, Zdravko Vulin, and Krešimir Mastanjević. 2018. "Beer–The Importance of Colloidal Stability (Non-Biological Haze)" Fermentation 4, no. 4: 91. https://doi.org/10.3390/fermentation4040091
APA StyleMastanjević, K., Krstanović, V., Lukinac, J., Jukić, M., Vulin, Z., & Mastanjević, K. (2018). Beer–The Importance of Colloidal Stability (Non-Biological Haze). Fermentation, 4(4), 91. https://doi.org/10.3390/fermentation4040091