Silage of the By-Products of Mollar de Elche and Wonderful Pomegranate Varieties Preserves Nutritional Value and Antioxidant Activity of Ruminant Feed
Abstract
1. Introduction
2. Materials and Methods
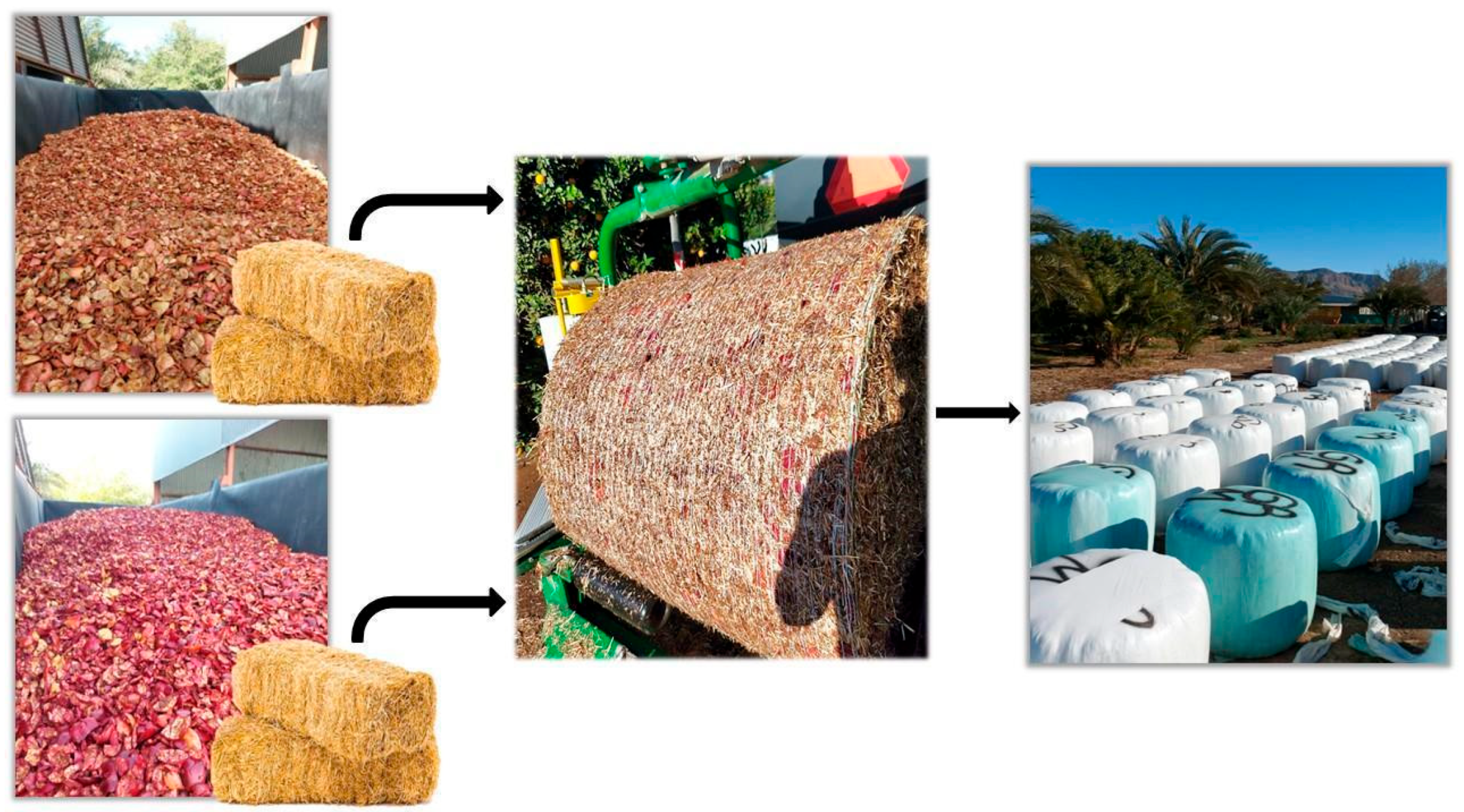
2.1. Facilities, Experimental Design, and Sample Management
2.2. Microbiological Determination
2.3. Quantification of Fermentation Products
2.4. Assessment of Physicochemical Properties
2.5. Antioxidant Capacity
2.6. Statistical Analysis
3. Results
3.1. Microbiology Tracking
3.2. Fermentation Products
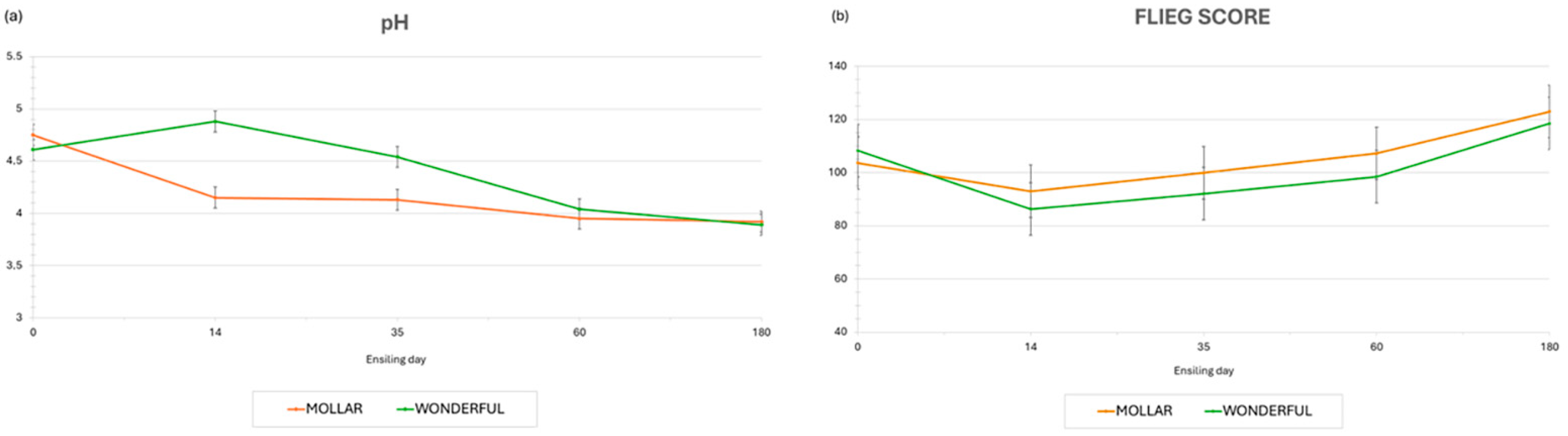
3.3. Physicochemical Parameters and Nutritional Composition
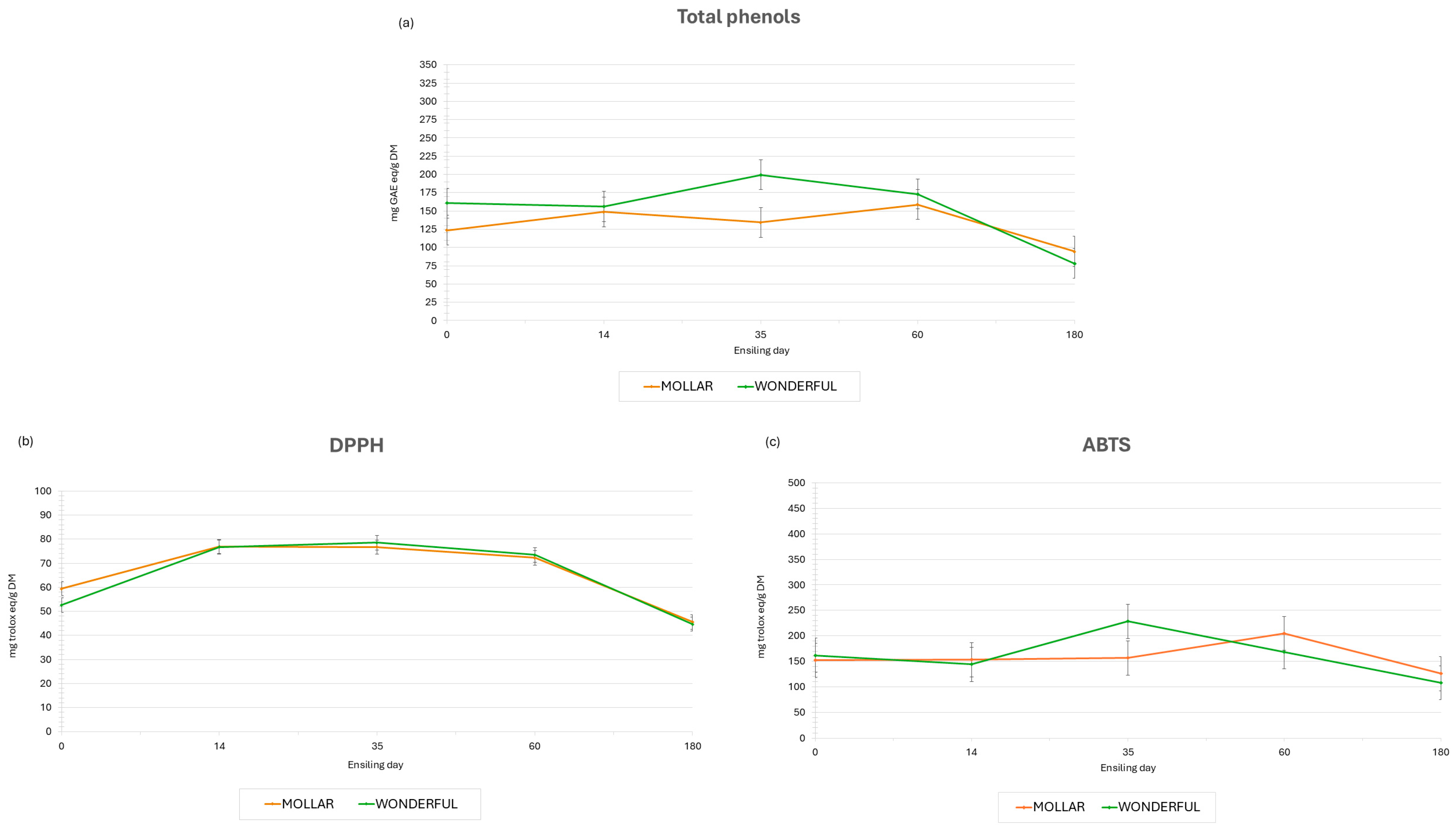
3.4. Antioxidant Capacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis [3-ethylbenzothiazoline-6-sulfonate) |
| ADF | Acid Detergent Fiber |
| ADL | Acid Detergent Lignin |
| AENOR | Spanish Association for Standardization and Certification |
| AOAC | Association of Analytical Communities |
| CP | Crude Protein |
| DM | Dry Matter |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| EE | Ether Extract |
| FM | Fresh Matter |
| GAE | Gallic Acid Equivalents |
| GLM | General Linear Model |
| LAB | Lactic Acid Bacteria |
| PDDO | Protected Designation of Origin |
| NDF | Neutral Detergent Fiber |
| NPN | Non-Protein Nitrogen |
| SDGs | Sustainable Development Goals |
| SEM | Standard Error of the Mean |
| TP | Total Phenolic |
| VFAs | Short-Chain Organic Acids |
References
- Rivas, M.Á.; Casquete, R.; Martín, A.; Córdoba, M.G.; Aranda, E.; Benito, M.J. Strategies to Increase the Biological and Biotechnological Value of Polysaccharides from Agricultural Waste for Application in Healthy Nutrition. Int. J. Environ. Res. Public Health 2021, 18, 5937. [Google Scholar] [CrossRef] [PubMed]
- Valero, D.; Serrano, M.; Martínez-Romero, D.; Guillén, F.; Castillo, S. Pomegranate Production and Post-Harvest Handling: A Review. Food Rev. Int. 2015, 31, 230–250. [Google Scholar]
- Ain, H.; Ali, A.; Ahmed, S.; Khan, M. Variation in Polyphenol Content and Antioxidant Capacity of Pomegranate Peels from Different Cultivars. Antioxidants 2023, 12, 756. [Google Scholar]
- Tzulker, R.; Glazer, I.; Bar-Ilan, I.; Holland, D.; Aviram, M.; Amir, R. Antioxidant Activity, Polyphenol Content and Related Compounds in Fruit-Peel Extracts. J. Agric. Food Chem. 2007, 55, 1972–1982. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant Activity of Pomegranate Juice and Its Relationship with Phenolic Composition and Processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef] [PubMed]
- Huxley, R.R.; Neil, H.A.W. The Relation between Dietary Flavonol Intake and Coronary Heart Disease Mortality: A Meta-Analysis. Eur. J. Clin. Nutr. 2003, 57, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Whiteley, C.G.; Lee, D.-J. Enzyme Technology and Biological Remediation. Enzym. Microb. Technol. 2003, 33, 897–907. [Google Scholar] [CrossRef]
- de Evan, T.; Marcos, C.N.; Carro, M.D. Chemical Composition and In Vitro Nutritive Evaluation of Pomegranate and Artichoke Fractions as Ruminant Feed. Ruminants 2024, 4, 1–9. [Google Scholar] [CrossRef]
- Jami, E.; Shabtay, A.; Nikbachat, M.; Yosef, E.; Miron, J.; Mizrahi, I. Effects of Adding Concentrated Pomegranate-Residue Extract to Dairy-Cow Rations on Digestibility and Rumen Bacteria. J. Dairy Sci. 2012, 95, 5996–6005. [Google Scholar] [CrossRef]
- Sadhasivam, A.; Sundaram, S.; Rengarajan, K. Pomegranate-Peel Extract as a Natural Antifungal Preservative in Maize Silage. Anim. Feed Sci. Technol. 2022, 286, 115260. [Google Scholar]
- Ahmed, S.; El-Sayed, M.; Mohamed, A.; Youssef, H.; Qureshi, M. Co-Ensiling Pomegranate Peels with Molasses and Berseem Improves Fermentation Quality and Reduces Methane in Buffaloes. Sustainability 2025, in press. [Google Scholar]
- Zhang, X.; Zhang, H.; Wang, D.; Zhang, Y. From Waste to Value: Multi-Omics Reveal Pomegranate Peel Addition Improves Corn Silage Antioxidant Activity and Reduces Methane and Nitrogen Losses. Bioresour. Technol. 2025, 429, 132544. [Google Scholar] [CrossRef]
- Kara, K.; Guclu, B.K.; Baytok, E.; Aktug, E.; Oguz, F.K.; Kamalak, A.; Atalay, A.I. Investigation in Terms of Digestive Values, Silage Quality and Nutrient Content of the Using Pomegranate Pomace in the Ensiling of Apple Pomace with High Moisture Contents. J. Appl. Anim. Res. 2018, 46, 1233–1241. [Google Scholar] [CrossRef]
- Kazemi, S.; Rafiee-Yar, A.; Abdollahzadeh, G.; Ahmadi, F. Pomegranate By-Product Silage Enhances Growth and Meat Quality in Fat-Tailed Lambs. Small Rumin. Res. 2021, 203, 106493. [Google Scholar]
- Eliyahu, K.; Nir, O.; Yosef, E.; Miron, J. Ensiling Pomegranate Pulp with Soy Hulls and Corn Silage Improves Fermentation and Intake in Sheep. Anim. Feed Sci. Technol. 2015, 202, 78–86. [Google Scholar]
- Díaz, J.R.; Fenoll, J.; Fenoll, A.; Romero, G.; Sendra, E. Procedimiento de Fabricación de Microsilos a Partir de Alcachofas (Cynara scolymus L.) para la Alimentación Animal. U.S. Patent ES2607220B1, 17 January 2018. [Google Scholar]
- Arias, C.; Oliete, B.; Seseña, S.; Jiménez, L.; Palop, L.; Pérez-Guzmán, M.D.; Arias, R. Importance of on-farm management practices on lactate-fermenting Clostridium spp. spore contamination of total mixed ration of Manchega ewe feeding. Small Rumin. Res. 2016, 139, 39–45. [Google Scholar] [CrossRef]
- Liu, F.X.; Fu, S.F.; Bi, X.F.; Chen, F.; Liao, X.J.; Hu, X.S.; Wu, J.H. Physico-chemical and antioxidant properties of four mango (Mangifera indica L.) cultivars in China. Food Chem. 2013, 138, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Cherney, J.H.; Cherney, D.J.R. Assessing Silage Quality. In Silage Science and Technology; Buxton, D.R., Muck, R.E., Harrison, J.H., Eds.; Agronomy Monograph 42; ASA-CSSA-SSSA: Madison, WI, USA, 2003; pp. 141–198. [Google Scholar]
- Association of Official Analytical Chemists. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1999. [Google Scholar]
- Kilic, A. Silo Feed: Instruction, Education and Application Proposals; Bilgehan Press: Izmir, Turkey, 1986. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary neutral detergent fibre and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Licitra, G.; Hernandez, T.M.; Van Soest, P.J. Standardization of Procedures for Nitrogen Fractionation of Ruminant Feeds. Anim. Feed Sci. Technol. 1996, 57, 347–358. [Google Scholar] [CrossRef]
- ISO 10520:1997; Native Starch—Determination of Starch Content—Ewers Polarimetric Method. International Organization for Standardization (ISO): Geneva, Switzerland, 1997.
- Kim, D.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Cheng, Z.; Moore, J.; Yu, L. Relative High-Throughput DPPH Radical Scavenging Capacity Assay. J. Agric. Food Chem. 2006, 54, 7429–7436. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Leite, A.V.; Malta, L.G.; Riccio, M.F.; Eberlin, M.N.; Pastore, G.M.; Maróstica Júnior, M.R. Antioxidant Potential of Rat Plasma by Administration of Freeze-Dried Jaboticaba Peel (Myrciaria jaboticaba Vell Berg). J. Agric. Food Chem. 2011, 59, 2277–2283. [Google Scholar] [CrossRef]
- Campbell, M.; Ortuño, J.; Ford, L.; Davies, D.R.; Koidis, A.; Walsh, P.J.; Theodoridou, K. The Effect of Ensiling on the Nutritional Composition and Fermentation Characteristics of Brown Seaweeds as a Ruminant Feed Ingredient. Animals 2020, 10, 1019. [Google Scholar] [CrossRef]
- Cai, Y.M.; Benno, Y.; Ogawa, M.; Ohamomo, S.; Kumai, S.; Nakase, T. Influence of Lactobacillus spp. from an inoculant and of Weissella and Leuconostoc spp. from forage crops on silage fermentation. Appl. Environ. Microbiol. 1998, 64, 2982–2987. [Google Scholar] [CrossRef]
- Ren, H.; Feng, Y.; Pei, J.; Li, J.; Wang, Z.; Fu, S.; Zheng, Y.; Li, Z.; Peng, Z. Effects of Lactobacillus plantarum additive and temperature on the ensiling quality and microbial community dynamics of cauliflower leaf silages. Bioresour. Technol. 2020, 307, 123238. [Google Scholar] [CrossRef] [PubMed]
- Broberg, A.; Jacobsson, K.; Strom, K.; Schnurer, J. Metabolite profiles of lactic acid bacteria in grass silage. Appl. Environ. Microbiol. 2007, 73, 5547–5552. [Google Scholar] [CrossRef]
- Mohd-Setapar, S.H.; Abd-Talib, N.; Aziz, R. Review on Crucial Parameters of Silage Quality. APCBEE Procedia 2012, 3, 99–103. [Google Scholar] [CrossRef]
- Dolci, P.; Tabacco, E.; Cocolin, L.; Borreani, G. Microbial dynamics during aerobic exposure of corn silage stored under oxygen barrier or polyethylene films. Appl. Environ. Microbiol. 2011, 77, 7499–7507. [Google Scholar] [CrossRef]
- Woolford, M.K. The Silage Fermentation; Marcel Dekker: New York, NY, USA, 1984. [Google Scholar]
- Monllor, P.; Romero, G.; Muelas, R.; Sandoval-Castro, C.A.; Sendra, E.; Díaz, J.R. Ensiling Process in Commercial Bales of Horticultural By-Products from Artichoke and Broccoli. Animals 2020, 10, 831. [Google Scholar] [CrossRef]
- Kung, L.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Sui, H.; Qin, W.; Hu, Z.; Wei, M.; Yong, M.; Wang, C.; Niu, H. Microbial Community and Fermentation Quality of Alfalfa Silage Stored in Farm Bunker Silos in Inner Mongolia, China. Fermentation 2023, 9, 455. [Google Scholar] [CrossRef]
- Vasta, V.; Nudda, A.; Cannas, A.; Lanza, M.; Priolo, A. Alternative feed resources and their effects on the quality of meat and milk from small ruminants. Anim. Feed Sci. Technol. 2008, 146, 223–246. [Google Scholar] [CrossRef]
- McDonald, P.; Henderson, A.R.; Heron, S.J.E. The Biochemistry of Silage; Chalcombe Publications: Marlow Bottom, UK, 1991. [Google Scholar]
- Galvez-Lopez, M.; Navarro, A.; Muelas, R.; Roca, A.; Peris, C.; Romero, G.; Díaz, J.R. Potential of Baled Silage to Preserve White Grape Pomace for Ruminant Feeding. Agriculture 2025, 15, 974. [Google Scholar] [CrossRef]
- Gannuscio, R.; Cardamone, C.; Vastolo, A.; Maniaci, G.; Di Grigoli, A.; Todaro, M. Ensiling as a Conservation Technique for Opuntia ficus indica (L.) By-Products: Peel and Pastazzo. Animals 2024, 14, 1148. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.L.M.; Bueno, A.V.I.; Osmari, M.P.; Machado, J.; Nussio, L.G.; Jobim, C.C.; Daniel, J.L.P. Effects of Obligate Heterofermentative Lactic Acid Bacteria Alone or in Combination on the Conservation of Sugarcane Silage. Front. Microbiol. 2021, 12, 643879. [Google Scholar] [CrossRef]
- Filya, I.; Muck, R.E.; Contreras-Govea, F.E. Inoculant effects on alfalfa silage: Fermentation products and nutritive value. J. Dairy Sci. 2007, 90, 5108–5114. [Google Scholar] [CrossRef]
- Peixoto, P.V.; Brust, L.A.C.; França, T.N.; Tokarnia, C.H. Ethanol Poisoning in Cattle by Ingestion of Waste Beer Yeast in Brazil. In Poisoning by Plants, Mycotoxins, and Related Toxins; Riet-Correa, F., Pfister, J.A., Schild, A.L., Wierenga, T.L., Eds.; CAB International: Wallingford, UK, 2011; pp. 494–500. [Google Scholar] [CrossRef]
- Kulyk, M.F.; Zhukov, V.P.; Obertiukh, Y.V.; Vyhovska, I.O.; Honchar, L.O.; Skoromna, O.I.; Tkachenko, T.Y.; Zelinska, I.P. Experimental substantiation of new criteria for silage quality evaluation. Feed. Feed Prod. 2019, 88, 99–106. [Google Scholar] [CrossRef]
- Suksombat, W.; Lounglawan, P. Silage from agricultural by-products in Thailand: Processing and storage. Asian Australas. J. Anim. Sci. 2004, 17, 473–478. [Google Scholar] [CrossRef]
- Ramzan, H.N.; Tanveer, A.; Maqbool, R.; Akram, H.M.; Mirza, M.A. Use of sugarcane molasses as an additive can improve the silage quality of sorghum-sudangrass hybrid. Pak. J. Agric. Sci. 2022, 59, 75–81. [Google Scholar]
- Hatami, A.; Alipour, D.; Hozhabri, F.; Tabatabaei, M. Short Communication: The Effect of Different Inclusion Levels of Polyethylene Glycol as a Silage Additive on Ensilage Characteristics of Pomegranate Peel and In Vitro Rumen Fermentation. Span. J. Agric. Res. 2015, 13, e06SC01. [Google Scholar] [CrossRef]
- Muck, R.E.; Shaver, R.D. Silage Review: Interpretation of Chemical, Microbial and Organoleptic Analyses. J. Dairy Sci. 2018, 101, 4020–4036. [Google Scholar] [CrossRef]
- Kong, Y.; Li, D.; Wan, J.; Wang, S.; Wang, Y.; Liu, W. Carbohydrate Dynamics during Corn-Stover Ensiling with Additives. Bioresour. Technol. 2016, 222, 71–78. [Google Scholar] [CrossRef]
- Van Soest, P.J. Nutritional Ecology of the Ruminant, 2nd ed.; Cornell University Press: Ithaca, NY, USA, 1994. [Google Scholar]
- Tzulker, R.; Glazer, I.; Bar-Ilan, I.; Holland, D.; Aviram, M.; Amir, R. Antioxidant Activity and Related Compounds in Different Accessions of Pomegranate Fruit. J. Agric. Food Chem. 2007, 55, 10038–10045. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Norrapoke, T.; Wanapat, M. Bioactivity of Phytochemicals in Some Lesser-Known Plants and Their Effects on Rumen Fermentation. Anim. Feed Sci. Technol. 2007, 139, 301–322. [Google Scholar] [CrossRef]
- Shabtay, A.; Eitam, H.; Tadmor, Y.; Orlov, A.; Meir, A.; Weinberg, P.; Chen, Y.; Brosh, A.; Izhaki, I.; Kerem, Z.; et al. Nutritive and Antioxidative Potential of Pomegranate Industrial By-Products as Beef Cattle Feed. J. Agric. Food Chem. 2008, 56, 10063–10070. [Google Scholar] [CrossRef]
| Variety | Days of Ensiling | SEM | F p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 14 | 35 | 60 | 180 | v | d | x | ||
| Aerobic bacteria (log cfu/g FM) | |||||||||
| Mollar | 7.63 | 5.84 | 5.33 | 6.94 | 4.60 | 0.29 | F = 2.2 p = 0.171 | F = 18.7 p = < 0.0001 | F = 3.1 p = 0.068 |
| Wonderful | 7.05 | 5.81 | 6.31 | 6.84 | 5.67 | ||||
| Lactic bacteria (log cfu/g FM) | |||||||||
| Mollar | 5.87 | 8.74 | 7.97 | 7.73 | 7.06 | 0.53 | F = 0.3 p = ns | F = 8.5 p = 0.003 | F = 0.04 p = ns |
| Wonderful | 5.55 | 8.43 | 7.96 | 7.70 | 6.85 | ||||
| Enterobacteria (log cfu/g FM) | |||||||||
| Mollar | 6.40 | 1.09 | 0.00 | 0.00 | 0.00 | 0.25 | F = 4.6 p = ns | F = 217.4 p = 0.0001 | F = 16.38 p = 0.0002 |
| Wonderful | 5.00 | 3.82 | 0.00 | 0.00 | 0.00 | ||||
| Molds (log cfu/g FM) | |||||||||
| Mollar | 3.60 | 3.03 | 2.34 | 3.48 | 1.15 | 0.55 | F = 16.1 p = 0.002 | F = 7.6 p = 0.0045 | F = 0.33 p = ns |
| Wonderful | 5.72 | 4.26 | 3.72 | 4.39 | 2.50 | ||||
| Yeast (log cfu/g FM) | |||||||||
| Mollar | 6.05 | 7.31 | 6.81 | 4.58 | 5.26 | 0.54 | F = 0.20 p = ns | F = 8.9 p = 0.0024 | F = 2.83 p = ns |
| Wonderful | 6.70 | 7.24 | 6.0 | 6.0 | 3.33 | ||||
| Butyric spores (log cfu/g FM) | |||||||||
| Mollar | 1.79 | 3.08 | 2.55 | 2.72 | 0.15 | 0.32 | F = 0.07 p = ns | F = 8.18 p = 0.005 | F = 5.81 p = 0.017 |
| Wonderful | 1.82 | 2.31 | 1.95 | 2.55 | 1.90 | ||||
| Variety | Days of Ensiling | SEM | F p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 14 | 35 | 60 | 180 | v | d | x | ||
| Sucrose (g/kg DM) | |||||||||
| Mollar | 19.24 | 8.72 | 8.55 | 9.79 | 0.28 | 1.27 | F = 2.60 p = ns | F = 44.7 p = <0.001 | F = 0.98 p = ns |
| Wonderful | 15.10 | 8.62 | 8.55 | 7.83 | 0.00 | ||||
| Glucose (g/kg DM) | |||||||||
| Mollar | 59.86 | 12.20 | 7.27 | 5.63 | 4.53 | 6.38 | F = 0.09 p = ns | F = 23.66 p = <0.001 | F = 0.18 p = ns |
| Wonderful | 55.23 | 11.46 | 11.26 | 10.48 | 6.80 | ||||
| Fructose (g/kg DM) | |||||||||
| Mollar | 95.67 | 29.26 | 19.08 | 18.97 | 17.78 | 9.45 | F = 0.11 p = ns | F = 22.49 p = <0.001 | F = 0.16 p = ns |
| Wonderful | 86.14 | 32.87 | 21.67 | 16.90 | 13.43 | ||||
| Lactic acid (g/kg DM) | |||||||||
| Mollar | 1.47 | 28.00 | 33.81 | 27.53 | 38.70 | 3.07 | F = 2.07 p = ns | F = 50.92 p = <0.001 | F = 4.96 p = 0.018 |
| Wonderful | 1.26 | 14.12 | 23.89 | 26.5 | 49.74 | ||||
| Acetic acid (g/kg DM) | |||||||||
| Mollar | 1.06 | 5.13 | 10.6 | 18.5 | 11.92 | 2.67 | F = 2.68 p = ns | F = 10.23 p = 0.0015 | F = 2.64 p = ns |
| Wonderful | 0.85 | 1.63 | 5.03 | 8.00 | 17.86 | ||||
| Ethanol (g/kg DM) | |||||||||
| Mollar | 1.78 | 54.59 | 46.43 | 38.84 | 27.95 | 5.19 | F = 8.35 p = 0.016 | F = 36.12 p = <0.001 | F = 1.39 p = ns |
| Wonderful | 1.53 | 59.47 | 52.60 | 60.01 | 43.44 | ||||
| Variety | Days of Ensiling | SEM | F p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 14 | 35 | 60 | 180 | v | d | x | ||
| Dry Matter (g/kg FM) | |||||||||
| Mollar | 412 | 325 | 326 | 350 | 355 | 15.7 | F = 0.91 p = ns | F = 14.07 p = <0.001 | F = 1.51 p = ns |
| Wonderful | 469 | 325 | 318 | 312 | 363 | ||||
| Neutral Detergent Fiber (g/kg DM) | |||||||||
| Mollar | 491 | 479 | 482 | 485 | 490 | 23.9 | F = 0.00 p = ns | F = 1.75 p = ns | F = 1.57 p = ns |
| Wonderful | 448 | 435 | 512 | 493 | 539 | ||||
| Acid Detergent Fiber (g/kg DM) | |||||||||
| Mollar | 332 | 333 | 361 | 337 | 308 | 16.3 | F = 0.18 p = ns | F = 2.50 p = ns | F = 1.00 p = ns |
| Wonderful | 314 | 317 | 374 | 340 | 348 | ||||
| Ether Extract (g/kg DM) | |||||||||
| Mollar | 0.75 | 0.70 | 0.90 | 0.90 | 0.90 | 0.72 | F = 4.74 p = 0.059 | F = 0.91 p = ns | F = 0.95 p = ns |
| Wonderful | 0.75 | 0.85 | 0.85 | 0.90 | 1.05 | ||||
| Crude Protein (g/kg DM) | |||||||||
| Mollar | 34.5 | 40.5 | 41.0 | 42.0 | 43.5 | 1.67 | F = 0.04 p = ns | F = 8.59 p = 0.0014 | F = 0.27 p = ns |
| Wonderful | 33.0 | 42.0 | 41.0 | 41.0 | 45.0 | ||||
| Non-Protein Nitrogen (g/kg DM) | |||||||||
| Mollar | 6.00 | 6.50 | 5.50 | 6.00 | 6.00 | 1.03 | F = 0.03 p = ns | F = 0.32 p = ns | F = 0.05 p = ns |
| Wonderful | 6.00 | 7.00 | 6.00 | 6.00 | 5.50 | ||||
| Starch (g/kg DM) | |||||||||
| Mollar | 27.0 | 52.0 | 53.5 | 39.5 | 32.5 | 9.47 | F = 3.89 p = ns | F = 0.67 p = ns | F = 1.01 p = ns |
| Wonderful | 33.0 | 32.5 | 23.5 | 32.0 | 27.0 | ||||
| Total Sugar (g/kg DM) | |||||||||
| Mollar | 123 | 30.0 | 19.0 | 13.0 | 22.0 | 5.21 | F = 0.77 p = ns | F = 90.49 p = <0.001 | F = 1.45 p = ns |
| Wonderful | 115 | 46.0 | 20.5 | 20.0 | 19.5 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galvez-Lopez, M.; Zemzmi, J.; Ilea, M.I.M.; Hernández, F.; Rodríguez, M.; Díaz, J.R.; Romero, G. Silage of the By-Products of Mollar de Elche and Wonderful Pomegranate Varieties Preserves Nutritional Value and Antioxidant Activity of Ruminant Feed. Fermentation 2025, 11, 392. https://doi.org/10.3390/fermentation11070392
Galvez-Lopez M, Zemzmi J, Ilea MIM, Hernández F, Rodríguez M, Díaz JR, Romero G. Silage of the By-Products of Mollar de Elche and Wonderful Pomegranate Varieties Preserves Nutritional Value and Antioxidant Activity of Ruminant Feed. Fermentation. 2025; 11(7):392. https://doi.org/10.3390/fermentation11070392
Chicago/Turabian StyleGalvez-Lopez, Marina, Jihed Zemzmi, Mihaela Iasmina Madalina Ilea, Francisca Hernández, Martín Rodríguez, José Ramón Díaz, and Gema Romero. 2025. "Silage of the By-Products of Mollar de Elche and Wonderful Pomegranate Varieties Preserves Nutritional Value and Antioxidant Activity of Ruminant Feed" Fermentation 11, no. 7: 392. https://doi.org/10.3390/fermentation11070392
APA StyleGalvez-Lopez, M., Zemzmi, J., Ilea, M. I. M., Hernández, F., Rodríguez, M., Díaz, J. R., & Romero, G. (2025). Silage of the By-Products of Mollar de Elche and Wonderful Pomegranate Varieties Preserves Nutritional Value and Antioxidant Activity of Ruminant Feed. Fermentation, 11(7), 392. https://doi.org/10.3390/fermentation11070392










