Abstract
Medicinal mushroom production utilising rural cultivation (solid state fermentation) requires approximately six months compared to culinary mushroom production (7 days). Urban cultivation (submerged liquid fermentation) can be used as a sustainable method of producing medicinal mushroom biomass. In this study, chicken patties were fortified with liquid-fermented Ganoderma lucidum flour (GLF) and Pleurotus djamor mushroom biomass flour (PDF) at concentrations of 3%, 6%, and 9%. These were compared to a negative control (0% mushroom flour chicken patty) and a commercial patty. Chicken patties fortified with 3% PDF and 9% GLF recorded the lowest cooking loss, at 5.55% and 10.3%, respectively. Mushroom chicken patties exhibited lower cooking losses and significant changes in colour and texture compared to control samples. Notably, 3% GLF chicken patty achieved the highest overall acceptability score of 6.55 followed by 9% PDF chicken patty (6.08) (p < 0.05). Biomass flour of liquid-fermented Ganoderma lucidum (ENS-GL) and Pleurotus djamor (ENS-PD) were extracted for their endopolysaccharide and analysed for their functional properties. All elemental, FT-IR, and NMR spectroscopy analyses revealed the existence of a comparable beta-glucan polymer structure, linkages, and absorptions when compared to the Laminarin standard. In addition, ENS-GL also proved to possess higher antimicrobial activities and significant antioxidant levels (DPPH-scavenging activity, ferric reduction potential and total phenolic content) compared to ENS-PD. Overall, this study revealed that sustainable liquid-fermented Ganoderma lucidum, a medicinal mushroom, outperformed Pleurotus djamor, a culinary mushroom, as a potential alternative flour for combating hunger in the future.
1. Introduction
Since ancient times, Greeks, Romans, and Chinese have included mushrooms in their diet, and the popularity of mushrooms has increased over time, notably in many Asian and European countries [1,2]. Edible fungi are a great source of nutrients, promoting health and preventing diseases such as hypercholesterolemia, cancer, and hypertension [3,4]. In addition, they comprise various bioactive compounds: proteins, secondary metabolites, and polysaccharide that are used for the drug development [5,6,7].
Ganoderma lucidum, also known as the ‘Mushroom of Immortality’, is a medicinal mushroom well known as Lingzhi, Reishi in Japan, and Yeongji in South Korea, and has been applied for medicinal purposes since before Common era in China [8,9]. The benefits of Ganoderma lucidum have drawn the attention of researchers and consumers, and they incorporate the mushroom in various food and health-supporting products [10,11]. The primary active ingredients, including polysaccharides, triterpenes, and peptidoglycans, are mainly found in mycelium, fruiting bodies, and spores [12,13]. Medicinal mushroom production utilising rural cultivation or solid state fermentation typically necessitates a longer timeframe of six months [14]. Therefore, there is a sustainable approach that requires a short period of ten days or less to grow medicinal mushroom known as mushroom urban cultivation or submerged liquid fermentation [15].
Pleurotus spp. or oyster mushroom is one of the most cultivated mushroom worldwide and utilised for culinary and commercial purposes and various therapeutic and pharmaceutical activities [16,17]. These species are also well known for their food characteristics, namely nutrition, taste, and physiological function; hence, they are highly regarded for both their sensory characteristics and excellent nutritional profile [17,18]. There are many applications of using black and grey oyster mushroom as a flavour enhancer and fat replacer [19,20,21,22,23]. Pink oyster mushroom or Pleurotus djamor is an edible mushroom and well-known as roseus mushroom, pink oyster or salmon pink oyster because of its pink sporophore, large-sized fruiting bodies, and their tasty flavour [24,25]. It is characterised as one of the rapidly growing Pleurotus species and requires shorter time (ten days) to form fruiting bodies compared to other Pleurotus species [26,27]. In recent research, P. djamor was applied as the fungal dye in the dye sensitised solar cells application [28].
The world’s highest population growth is expected to increase food demand by 70% to 100% by 2050 in order to maintain food security for the projected 9.1 billion people [29]. Mushroom fermentation is one of the approaches that appears promising in the fight for global food security. It provides a means of producing sizable amounts of nutrient-dense food with short harvest intervals, low input needs, and small-scale land requirements [30]. Mushroom production is far less vulnerable to climate-related challenges than many conventional crops because of these qualities as well as the fact that mushrooms require minimal use of soil, water, and energy for growth [30,31]. The recent COVID-19 pandemic and the ensuing global food crisis also highlighted the rising importance of mushrooms as food [31].
According to [32,33], burgers have become increasingly prevalent among processed beef products owing to their simplicity and satisfying sensory attributes. Fast preparation time along with its appealing sensory attributes account for the majority of the high consumption [34,35]. The food industry has become interested in utilising mushrooms in meat products due to their texture and flavour characteristics, as well as their nutritional value, antioxidant activity, and health-promoting qualities [36,37]. In the European Union, there is a constant debate on the usage of protein crops in the manufacturing of protein isolates or concentrates [38]. Numerous nations encourage farmers to explore plant-based substitutes for protein such as soybean, legumes, and mushroom.
Mushrooms are becoming recognised as a prospective food source owing to their nutraceutical and functional attributes. Functional food is defined as food that, beyond offering nutritional advantages, positively affects one or more biological activities [39,40]. Nutraceuticals are defined as food or meal ingredients that offer medical or wellness advantages, including illness prevention and treatment [41,42]. Since the beginning of time, both the mushroom itself and its extracts have been employed in traditional medicine and cuisine due to the fungus’ low calorie content, pleasant flavour, and reputedly invaluable biological properties [43]. In the past, Ganoderma lucidum was consumed greatly as medicine. Recent molecular research has elucidated this historical history by examining the bioactive compounds now present and uncovering the health and nutritional benefits of mushrooms [44]. Liquid fermented Ganoderma lucidum has already been proven to be safe and non-toxic to environment [45,46]. On the other hand, Pleurotus djamor is categorised as a culinary mushroom and is well-known to be edible and safe [47,48].
Drying and grinding preservation techniques turn mushrooms into powder or flour, and are ideal to be utilised in snacks, bakery products, and pasta, as well as meat products [49,50]. Different mushroom powders have been used in place of wheat flour in many studies. For instance, Lentinus edodes powder is used in wheat flour gluten sticks [51]; extruded snacks contain 5–15% of chestnut mushroom (Agrocybe aegerita) [52]; 5–15% Pleurotus sajor-caju is used in biscuits; 10–30% of Lentinula edodes in cookies and steamed buns; and 4–12% Pleurotus ostreatus-based noodles have also proven popular was a wheat flour substitute. In addition, [20] utilised and converted the fruiting bodybase (FBB) of the oyster mushroom into mushroom flour to produce mushroomchicken patty. The flour was incorporated into chicken patties using different formulations and consumer acceptance was measured. In a previous study by [53], Pleurotus djamor powder were supplemented as meat replacer in beef patties’ physicochemical and sensory qualities. Fruiting body biomass of Ganoderma lucidum were also substituted as flour replacer according to different formulation of chicken patties and was found to be low in consumer acceptance [45].
This study aims to utilise liquid-fermented Ganoderma lucidum and Pleurotus djamor as mushroom flour in developing antimicrobial and antioxidative mushroom chicken patties. Furthermore, this study also analyses the presence of beta glucan, antimicrobial and antioxidant activities of endopolysaccharide of both mushrooms which contribute to the functionality of mushroom-chicken patties.
2. Materials and Methods
2.1. Sample Preparations
2.1.1. Ganoderma lucidum Flour (GLF)
The Functional Omics and Bioprocess Development Laboratory, Institute of Biological Sciences, Faculty of Science, Universiti Malaya supplied the sample Malaysian Ganoderma lucidum strain QRS 5120 mushroom mycelium [54]. As described by [55], potato dextrose agar (PDA) powder (39 g/L) was used to grow Ganoderma lucidum on plate and seed culture. The liquid fermentation of Ganoderma lucidum was prepared according to [54] with modification using potato dextrose broth (PDB) (Oxoid no. CM0962, Oxoid Limited, Wade Road, England).
Inoculum preparation involved two seed culture phases, each of which was grown for ten and eleven days in as shaking incubator, respectively, at 30 °C and, at 100 rpm. Mycelial agar from the PDA plate was cut into three squares (1 cm × 1 cm) to initiate the primary seed culture. They were inoculated in a 250 mL Erlenmeyer flask into 100 mL of culture medium. Subsequently, mycelium from the initial seed culture of 20% (v/v) was employed for the second seed culture, having been homogenised for 10 s in a blender to promote the formation of enhanced growing hyphal tips. The culture was inoculated within a 500 mL Erlenmeyer flask containing 100 mL of the total working volume of fresh PDB media and was allowed to incubate at 30 °C with agitation at 100 rpm. The sample was filtered using a Buchner funnel filter paper 0.45 µm (Whatman, Sigma-Aldrich, Dorset, UK), and the mycelial biomass was then washed three times with sterile distilled water. Subsequently, the mycelial biomass was desiccated using a food dehydrator at 35 °C prior to being ground into flour.
2.1.2. Pleurotus djamor Flour (PDF)
The fruiting bodies of Pleurotus djamor were obtained from the Putra Agriculture Centre, Universiti Putra Malaysia. The pretreatment of Pleurotus djamor was performed by cleaning of fruiting bodies and treated by soaking in 0.5 g/100 mL food grade citric acid solution for ten minutes as previously described by [20]. In addition, the treated fruiting bodies were dried in a food dehydrator at 40 °C for a 24 h duration. The dried fungal fruiting bodies were ground into flour.
2.1.3. Endopolysaccharide (ENS)
Firstly, ENS was extracted from biomass flours of the Ganoderma lucidum and Pleurotus djamor. Distilled water was added to the biomass samples (1:10 g/mL). Next, the hot-water extraction method was used by autoclaving distilled water containing biomass flours at 121 °C for 30 min [56]. For characterisation, antimicrobial, and antioxidant analysis, both endopolysaccharide of Ganoderma lucidum (ENS-GL) and Pleurotus djamor (ENS-PD) were utilised because of their solubility in water and solvents.
2.2. Functional Analysis
2.2.1. Elemental Analysis
The content of Carbon (C), Hydrogen (H), Nitrogen (N), and Sulphur (S) of ENS samples was estimated using a Thermo Scientific FlashSmart CHNS/O Elemental Analyzer (Waltham, Massachusetts, MA, USA) device [57].
2.2.2. Fourier Transform Infrared Spectroscopy (FT-IR)
FT-IR spectra of the ENS samples were obtained using an FTIR 3000 spectrophotometer, 134 (Jasco, Japan), following the method of [57].
2.2.3. 1H Nuclear Magnetic Resonance (NMR) Spectroscopy
The NMR spectra of both ENS samples were determined using a DXM 500 FT-NMR spectrometer 138 (Bruker, Karlsruhe, Germany). Every spectrum was performed at a temperature of 80 °C. The chemical shifts (δ) were expressed in parts per million (ppm), and the scan number was 16. The control and reference standard were Laminarin from Laminaria digitata (Sigma-Aldrich, Dorset, UK).
2.2.4. Antimicrobial Activities of the ENS of Ganoderma lucidum and Pleurotus djamor
The target microorganisms were based on WHO list of critical and high priority bacteria [58] and the availability of other bacteria in the Functional Omics and Bioprocess Development laboratory. Antimicrobial activities of mushroom extract samples were performed using the disc diffusion technique described by [57]. Firstly, a Petri dish containing 20 mL of nutrient agar medium was prepared. Subsequently, all test microorganisms were calibrated to 0.5 McFarland standards utilising sterile broth medium. Approximately 200 µL of suspension containing five Gram-positive and five Gram-negative test microorganisms was applied to the prepared solidified agar. Subsequently, the standardised 11 mm sterile discs (blank) (Sigma-Aldrich, Dorset, UK) were immersed in an equivalent absorbed volume containing a precise quantity of extract and delicately placed into the agar overlay. The plates were subsequently incubated overnight at 37 °C, for 48 h at 30 °C, or two days, contingent upon the bacterium’s growth requirements. Gentamicin served as the positive control, whilst ethanol (70%) functioned as the negative control. After incubation, the diameters (mm) of the inhibitory zones were measured. Inhibition zones over 7 mm were considered indicative of good antibacterial activity.
The minimum inhibitory concentration (MIC) was assessed using microdilution utilising test tubes with minimal modifications. Sterile broth medium was utilised with 0.5 McFarland standards for the modification of bacterial suspension. Both samples were solubilised in sterile water and subjected to serial dilution to concentrations of 200, 100, 20, 10, 8, 5, 3, 2, and 1 mg/mL. The final combination comprised 2.5 mL of chemicals combined with 7.5 mL of a bacterial suspension, resulting in a total working volume of 10 mL. Each test culture was transferred into the tubes and incubated for 24 h at 30 °C. Upon the conclusion of the incubation period, turbidity was regarded as an indicator of bacterial proliferation. The minimum diluted concentration at which the incubated mixture remained clear was therefore designated as the MIC. Sterile L-spreaders were utilised to guarantee consistent dispersion. Thereafter, the quantity representing the MIC, together with at least two higher dilutions, was plated and measured to determine viable colonies specifically for the assessment of minimum bactericidal concentration (MBC). The minimum bactericidal concentration (MBC) is the lowest concentration at which 99% of the bacteria are killed [59].The medium was incubated at 30 °C for 24 h to observe microbial proliferation. The lowest concentration in the medium that produced fewer than five colonies was employed for the MBC.
2.2.5. Antioxidant Assays of the ENS of Ganoderma lucidum and Pleurotus djamor
Preparation of ENS Ethanolic Extracts
The extract was made using dried ENS of Ganoderma lucidum and Pleurotus djamor and ethanol [60]. The ENS samples were stirred for 24 hr at 20 °C at 150 rpm with 1 g per 10 mL of ethanol. The broth samples were centrifuged at 3500× g for 10 min and filtered with Whatman No. 1 filter paper and stored at 4 °C.
DPPH Radical-Scavenging Activity Assay
The DPPH radical-scavenging activity of the ENS extract was assessed using a technique previously documented by [20,61]. The sample was diluted according to concentrations of 5, 10, 20, and 40 mg/mL. The DPPH solution was prepared by dissolving 5.9 mg of DPPH in 100 mL of ethanol. Subsequently, 3.8 mL of the ethanolic DPPH solution was incorporated into 0.2 mL of the extracts. The mixture was agitated rapidly for one minute and thereafter allowed to rest at room temperature in the absence of light for thirty minutes. The absorbance of the combination was measured at 517 nm utilising an ultraviolet–visible (UV-VIS) spectrophotometer (Genesys, Germany). All experiments were performed in triplicate. The radical-scavenging activity of the ENS samples was calculated using Equation (1).
where
AB = Absorbance of blank
AA = Absorbance of sample.
Ferric-Reducing Antioxidant Power (FRAP) Assay
The FRAP analysis was performed using the protocol and procedure provided by the FRAP assay kit (Catalogue Number MAK509, Sigma Aldrich). A 180 μM standard was prepared by mixing 20 μL of 1.8 mM Fe2+ standard with 180 μL of purified water. Next, the standards and samples were diluted and measured using a cuvette. The sample was diluted according to concentrations of 5, 10, 20, and 40 mg/mL. A UV-VIS spectrophotometer (Genesys, Germany) was used to measure the absorbance of the samples at 590 nm, and the Fe3+ reduction potential was obtained by using Equation (2)
where
RS = OD reading of the Sample,
RB = OD reading of Blank,
S = Slope,
DF = Sample dilution factor,
(DF = 1 for undiluted Samples).
Total Phenolic Content (TPC) Assay
TPC assay was conducted based on the protocol and procedure provided by the TPC assay kit (Catalogue Number E-BC-K354-S, Elabscience, Wuhan, China). The standard and samples were diluted according to concentrations of 5, 10, 20, and 40 mg/mL, and absorbance were read at 760 nm using an ultraviolet–visible (UV-VIS) spectrophotometer (Genesys, Germany). Equation (3) was used to obtain the total phenolic content of both samples.
Total phenols content (mg/g tissue) = (ΔA760 − b) ÷ a ×V ÷ W ÷ 1000* × f
ΔA760: Absolute OD (OD Sample − OD Control).
V: the volume of added extraction solution, 2.5 mL of 60% ethanol.
W: Weight of sample, 0.1 g.
*: Unit conversion, 1000 μg = 1 mg.
f: Dilution factor of the sample before the test.
2.3. Preparation of Mushroom Chicken Patties
A total of 2 kg of chicken breast was cut manually using a cleaver and minced in a food processor. The chicken was mixed with ingredients according to Table 1 [20]. The completed chicken mixture was split into 70 g parts, and the patties were hand-formed into a consistent shape measuring 100 mm in diameter and 10 mm in thickness according to [62]. Subsequently, before food analysis, the raw chicken patties were kept in a freezer at −18 °C.

Table 1.
Mushroom chicken patty formulations using both Ganoderma lucidum flour (GLF) and Pleurotus djamor flour (PDF).
2.4. Food Analysis
2.4.1. Cooking Process and Cooking Loss
The patties were weighed before and after cooking for 7 to 8 min at about 176 ± 1 °C, being turned three to four times until an interior temperature of 72 ± 1 °C was attained [20] to calculate the cooking loss of chicken patties. The cooking loss which is also known as weight loss (%) of the chicken patties during the heat treatment [63] were also calculated by using Equation (4) below
where
W1 = raw chicken patty sample weight before cooking, g.
W2 = chicken patty sample weight after cooking, g.
2.4.2. Colour Analysis
The colorimetric analysis of the cooked mushroom chicken patties was conducted using a colorimeter (Minolta model 3500, Minolta Camera Co., Ltd., Osaka, Japan). According to [64], lightness (L*), redness (a*), and yellowness (b*) are colorimetric properties of the developed patties which will be evaluated and contrasted with those of commercially cooked chicken patties (Ramly Chicken Patty 70 g/packet, Ramly Food Processing Sdn. Bhd. Malaysia). The mushroom chicken patties are deemed acceptable if their colouration aligns with that of the commercial chicken patties.
2.4.3. Texture Analysis
The texture profile analysis (TPA) of mushroom chicken patties was performed based on [65]. The studied textural properties were hardness, springiness, gumminess, cohesiveness, and chewiness of the control and mushroom chicken patties, and they were measured using texture analyser (Stable Micro System Model TA.XT 2i/25, Surrey, UK). The measurement was carried out using central cores of each patty sample (2 cm × 2 cm × 2 cm). Each sample was compressed twice (80% of the original height and 2 mm/s crosshead speed) with the help of a compression probe (P 75).
2.4.4. Sensory Analysis
Based on sensory analysis by [20], the mushroom chicken patty samples were assigned with random 3-digit codes and cut into 1 cm × 1 cm square size before being put in labelled small polyethylene plastic containers with a lid. A total of 50 panellists were picked based on the requirements needed (18 years and above and eat chicken meat regularly) and briefed in the evaluation area after sensory evaluation forms and consent forms were distributed. The samples were thereafter allocated to each panellist, accompanied by a cup of drinking water and an unsalted cracker for palate-cleansing during sensory analysis. The mushroom chicken patties were assessed for several sensory attributes including appearance, colour, aroma, texture, taste, aftertaste, and overall acceptability using a 9-point hedonic scale (1 = extremely poor, 2 = very poor, 3 = poor, 4 = bad, 5 = average, 6 = fair, 7 = good, 8 = very good, 9 = excellent) by 50 acceptable panellists who were 18 years old and above and consumed chicken meat regularly (at least twice a week) from the University Putra Malaysia (UPM), Selangor, Malaysia.
2.5. Statistical Analysis
All analyses were conducted in triplicate, and all findings were presented as mean ± standard deviation to ascertain significant differences among means for all tests at α = 0.05 and P-value of 1, using software of GraphPad Prism 5, version 5.0. Furthermore, a radar chart displaying the results of all chicken patties for each sensory feature was prepared and created by using Microsoft Excel (Version 16.45) to tabulate the mean values of each sensory attribute of mushroom chicken patty compositions.
3. Results and Discussions
3.1. Functional Analysis
3.1.1. Elemental Analysis
Elemental analysis was conducted to determine the composition of the ENS-GL and ENS-PD in comparison with laminarin as a control or standard. ENS-GL gives the following composition of (w/w): 36.77% C, 6.06% H, 2.14% N, and 0.14% S (Table 2). ENS-PD, on the other hand, obtained 37.26% C, 5.75% H, 1.75% N, and 0.05% S. ENS-PD has a higher value for C, and lower for other elements compared to ENS-GL. This demonstrates some significant differences between different mushroom species in the elemental intake by mycelia [66].

Table 2.
Elemental composition of endopolysaccharide of Ganoderma lucidum and Pleurotus djamor.
3.1.2. Fourier Transform Infrared Spectroscopy (FT-IR)
The FT-IR spectrum of ENS-GL and ENS-PD presented in Figure 1 and Table 3 summarises the absorption peak of every sample according to vibration mode. The broad and intense absorption peaks of ENS-GL and ENS-PD at 3455 cm−1 and 3432 cm−1 were found to represent the stretching vibration of a hydroxyl group (O–H), which indicated the presence of a polyhydroxy compound. The absorption peak of ENS-PM recorded the highest, which is 2922 cm−1, compared to ENS-GL and laminarin standard indicating a methylene group (CH2) of the stretching vibration of C–H bonds. Other major absorptions by both ENS-GL and ENS-PD recorded the same readings at 1646.08 cm−1, higher than laminarin.
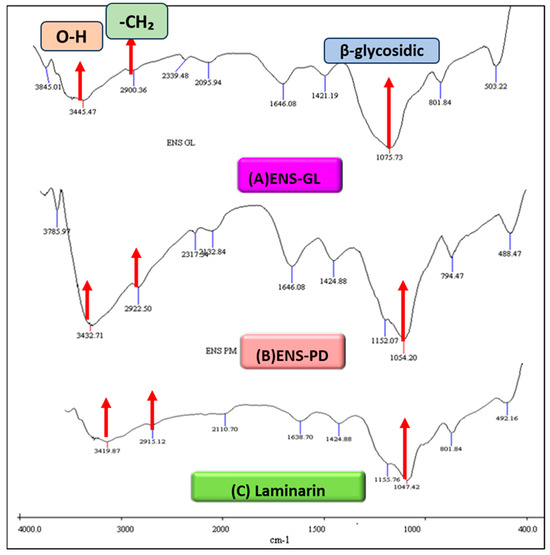
Figure 1.
FT-IR spectra of (A) endopolysaccharide (ENS) from Ganoderma lucidum mycelial biomass, (B) endopolysaccharide (ENS) from Pleurotus djamor biomass, and (C) laminarin is a standard for (1,3)-β-D-glucan from Laminaria digitata (figure by author).
FT-IR spectra in the wave number between 850 and 1200 cm−1 are considered as the “fingerprint” region for carbohydrates, which is unique to a compound in terms of polysaccharide and configuration [67]. Based on the figure, ENS-GL showed the highest absorption at 1075 cm−1, followed by ENS-PM at 1054.20 cm−1 and laminarin at 1047.42 cm−1.

Table 3.
The absorption peak for endopolysaccharide samples in comparison with Laminarin according to vibration mode.
Table 3.
The absorption peak for endopolysaccharide samples in comparison with Laminarin according to vibration mode.
| Sample (Absorption Peak, cm−1) | ||||
|---|---|---|---|---|
| Vibration Mode | ENS-GL | ENS-PD | Laminarin | Reference Wavelength |
| Hydroxyl group (O–H) | 3455 | 3432 | 3419 | 3400–3500 |
| Methylene group (-CH2) = water bending | 2900 | 2922 | 2915 | 2900–2922 [68] |
| Symmetric and asymmetric stretching vibration | 1646 | 1646 | 1638 | 1400–1650 [68] |
| Glycosidic linkage (β-configuration) | 1075 | 1054 | 1047 | 850–1200 [67] |
3.1.3. 1H Nuclear Magnetic Resonance (NMR) Spectroscopy
Figure 2 illustrates the 1H NMR spectroscopic analysis of ENS-GL and ENS-PD. By utilising ppm as the standardised unit for NMR investigations, the 1H NMR spectra of ENS-GL and ENS-PD were compared to the standard laminarin (β-1,3-D-glucan) obtained from Laminaria digitata. The ENS’s chemical shifts in δ 2.99 to 4.99 ppm and δ 3.07 to 5.19 ppm in the spectrum show that both molecules are glucans. This can be noticed in Figure 2, respectively. The present study is similar to prior research conducted by [57,68], which examined the properties of laminarin and sulphated laminarin in the 1H-NMR spectrum range of δ 4.49–5.5 ppm. Therefore, the spectra demonstrate that the glycosidic linkages in both ENS-GL and ENS-PM were of the β-type. The β-anomeric protons and carbons can also be observed in the 4–5 ppm range [69]. Based on the analysis of FT-IR and 1H NMR, it can be inferred that the endopolysaccharide consist of (1-3)-β-D-links, resulting in a polymer structure that seems to be a 1,3-β-D-glucan.
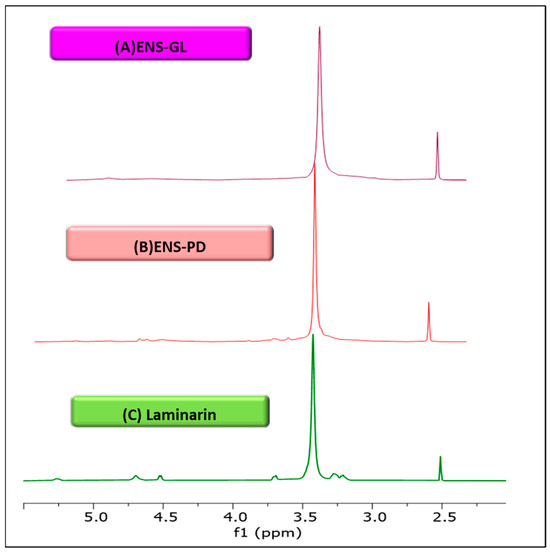
Figure 2.
1H NMR spectra of (1,3)-β-D-glucan. (A) Glucan (G) derived from ENS-Ganoderma lucidum. (B) glucan (G) derived from ENS-Pleurotus djamor. (C) Standard glucan from laminarin (Laminaria digitata) (figure by author).
3.1.4. Antimicrobial Activities
Table 4 depicts the antimicrobial activity of ENS-GL and ENS-PD using the disc diffusion assay. ENS-GL shows the highest diameter of inhibition (mm) against Actinobacteria (15.5 mm at 300 mg/mL) and Staphylococcus epidermis (13 mm at 300 mg/mL), whereas the highest diameter of inhibition (mm) obtained from ENS-PD was against Actinobacteria (9.5 mm at 300 mg/mL). The data shows that the inhibition zone diameters increased with increasing concentration (Table 4). This conforms with the study by [57]. Table 5 records the minimum inhibitory concentrations (MIC) and minimum bactericidal concentrations (MBC) of ENS-GL and ENS-PD. ENS-GL effectively inhibits the growth of bacteria at 1 mg/mL against Actinobacteria. The sample also recorded the lowest MBC which is 5 mg/mL against Actinobacteria. In addition, ENS-PD also inhibits bacterial growth at 5 mg/mL against Actinobacteria, Staphylococcus aureus and Proteus.

Table 4.
Antimicrobial activity of endopolysaccharide of Ganoderma lucidum and Pleurotus djamor using disc diffusion assay.

Table 5.
Minimum inhibitory concentrations and minimum bactericidal concentrations of endopolysaccharide of Ganoderma lucidum and Pleurotus djamor.
The tested concentrations were used to see the trend of MIC were based on [57,70]. The highest tested MIC was 1000 mg/mL, followed by 250, 125, and 62.5 mg/mL. The study is to show the potential and the ability of the endopolysaccharide samples to inhibit the growth of microorganisms. The value of the inhibition diameter is supported based on the study by [70], which produced a similar inhibition diameter of 8.67 mm at higher concentrations (500 mg/mL) compared to this study (at 300 mg/mL). Research reported that polysaccharides of Ganoderma lucidum have the potential to impede the growth of microorganisms such as Staphylococcus aureus, Escherichia coli, and Bacillus subtilis [71]. The antimicrobial activity entails the disruption of bacterial cell walls or the interference with key bacterial enzymes [72]. The varying susceptibility of fungal strains against Gram-positive and Gram-negative bacteria may be attributed to the presence of a membrane around the peptidoglycan of Gram-negative bacteria, which hinders diffusion due to its lipopolysaccharide (LPS) coating [73]. The LPS layer is crucial for maintaining selective permeability [74].
In contrast, Gram-positive bacteria do not possess an outer membrane, but they are distinguished by a dense hydrophilic porous structure that enhances their permeability [75]. As a result, Gram-positive bacteria will exhibit greater sensitivity to mushroom extracts compared to Gram-negative bacteria according to previous studies that utilised Ganoderma lucidum and Pleurotus djamor extracts [73,76], which corresponds to the antimicrobial activity observed in this study.
3.1.5. Antioxidant Activities
DPPH Radical-Scavenging Activity
DPPH radical-scavenging activity is an antioxidant method for assessing the capacity of substances to function as scavengers of free radicals or donors of hydrogen, as well as for evaluating the antioxidant activity of food items [77]. Therefore, the DPPH assay was used to determine the antioxidant activity of ENS-GL and ENS-PD (Figure 3). It was observed that the higher the concentration of ENS samples, the higher the DPPH-scavenging activity (%). Overall, both samples showed substantial values, and ENS-PD showed greater DPPH-scavenging activity (92.6%) in comparison with ENS-GL (91.9%) at a 40 mg/mL concentration.
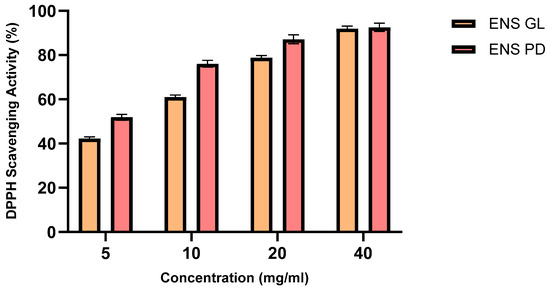
Figure 3.
DPPH-scavenging capacity (%) of endopolysaccharide (ENS) from Ganoderma lucidum mycelial biomass and Pleurotus djamor biomass (Means ± SD, n = 3) (figure by author).
Ganoderma lucidum polysaccharides showed antioxidant activity, which is typically ascribed to their elevated hydrogen levels, which allow them to create stable configurations when interacting with free radicals [78,79]. Unpaired electrons in molecules make free radicals unstable and reactive. By damaging vital macromolecules, free radicals can damage cells and tissues, disrupt homeostasis, and interfere with metabolic processes [80]. This leads to oxidative stress as a result of an overabundance of reactive oxygen species and an imbalance in the metabolism [81].
Multiple studies reported extracted polysaccharides of Ganoderma lucidum to show great reproducibility of DPPH-scavenging activity (%) [82,83]. On the other hand, findings indicated that the polysaccharide extracted from Pleurotus djamor exhibited significant DPPH radical-scavenging activity which conforms with a previous study by Raman et al. [84]. The high reproducibility of DPPH-scavenging activity by both ENS-GL and ENS-PD in mushroom chicken patty can potentially safeguard food quality by preventing oxidative degradation and allowing consumers to mitigate oxidative damage.
Ferric Reducing Antioxidant Power (FRAP)
This antioxidant analysis is conducted to show the ferric reducing ability of samples and also to measure the overall antioxidant capacity [85]. The FRAP test relies on the reduction in the ferric-tripyridyl triazine (Fe(III)-TPTZ) complex to the ferrous-tripyridyl triazine (Fe(II)-TPTZ) by a reductant under acidic conditions [86]. The compound’s reduction capacity can be a valuable predictor of its potential antioxidant action.
According to Figure 4, the result showed that the greater the concentration of ENS-GL and ENS-PD, the greater the ferric reducing capability. In addition, ENS-GL also proved to produce higher reduction potential at only 5 mg/mL (0.007 µM) and increasing gradually until the highest at 40 mg/mL (0.06 µM). On the other hand, ENS-PD only revealed a reduction potential at 40 mg/mL (0.002 µM).
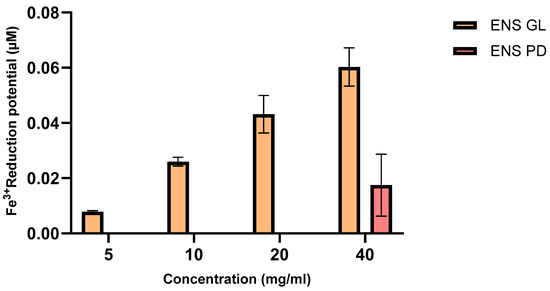
Figure 4.
Fe3+ reduction potential (µM) of endopolysaccharide (ENS) from Ganoderma lucidum mycelial biomass and Pleurotus djamor biomass (Means ± SD, n = 3) (figure by author).
According to a study by [84], the concentration of the mushroom extracts directly correlates with an increase in their reducing power, which validates the present study. In addition, iron is a vital mineral for the correct functioning of the human body, but an excessive amount can lead to harm at the cellular level [87]. The ferrous ion is well recognised as the most potent pro-oxidant element in food systems [88]. Therefore, the chelating activity of mushrooms is highly important for mitigating oxidative stress-induced diseases by effectively removing free ions during consumption.
Total Phenolic Content (TPC)
Phenolic compounds or phenolic acids are found in mushroom extract, typically possessing redox characteristics which are responsible for their antioxidant action [89,90]. In addition, the hydroxyl groups present in most plant and fungi extracts play a crucial role in the process of scavenging free radicals [91,92].
Figure 5 depicts the total phenolic content (mg/g) obtained by both ENS-GL and ENS-PD. The results showed that with increasing concentrations of endopolysaccharide, the total phenolic content (mg/g) also increases. ENS-GL comprised the highest total phenolic content (mg/g) is at 40 mg/mL, 353.51 mg/g, whereas the highest total phenolic content (mg/g) obtained by ENS-PD is at 236.40 mg/g at 40 mg/g.
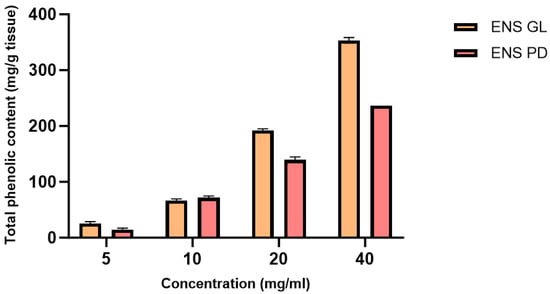
Figure 5.
Total phenolic content (mg/g tissue) of endopolysaccharide (ENS) from Ganoderma lucidum mycelial biomass and Pleurotus djamor biomass (Means ± SD, n = 3) (figure by author).
Based on the study by [93], Ganoderma lucidum extract exhibits the highest total phenol contents (81.34 mg/g) compared to extracts of Ganoderma tropicum and Cannabis indica. In addition, [94] reported that both extracts of Ganoderma lucidum and Pleurotus djamor contain significant phenolic content, where overall, Ganoderma lucidum recorded the highest total phenolic content (402.75 mg/mg) compared to Pleurotus djamor (267.28 mg/g).
3.2. Food Analysis
3.2.1. Cooking Loss
Figure 6 indicates the cooking loss (%) of cooked mushroom chicken patties, with the cooking loss of chicken patties decreasing with the increase in GLF in chicken patties. Chicken patties with 9% GLF showed the lowest value (10.30%) compared to the positive control (26.89%), 3% GLF (23.55%), 6% (13.66%), and the negative control (31.01%). However, the cooking loss of chicken patties increases with the increase in PDF in chicken patties: 3% PDF (5.55), 6% PDF (7.78), and 9% PDF (22.34%).
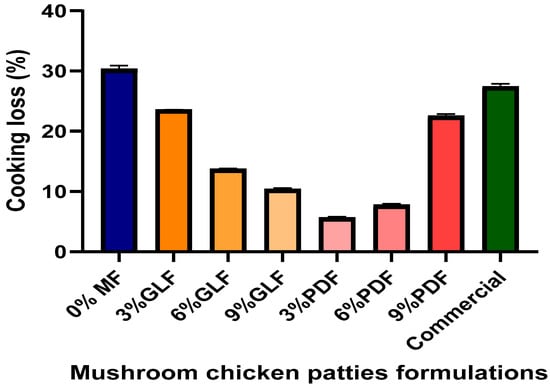
Figure 6.
Cooking loss (%) of control chicken patty with 0% mushroom flour (MF), cooked Ganoderma lucidum flour (GLF), Pleurotus djamor flour (PDF) mushroom chicken patties, and commercial chicken patties. (figure by author).
The cooking loss of chicken patties drastically decreased (p < 0.05) with increasing levels of GLF. The moisture texture of GLF chicken patties might be due to the reduction in potato starch, whereby the binding properties of chicken patties may be compromised, resulting in a softer texture [95]. Heat-induced protein denaturation is the source of cooking loss or shrinkage in meat products. The structural proteins collagen and actomyosin complex are impacted by this denaturation, which results in undesirable alterations in the meat products’ sensory qualities, including hardness and juiciness [96].
Relatively, the cooking loss occurred after cooking due to the contraction of muscle fibres and connective tissues during cooking [97]. The highest cooking loss observed in the control and commercial samples is likely due to fat being readily eliminated during cooking, potentially because of a beef protein matrix with low density and high fat instability [98,99]. In addition, a similar study reported that the high moisture content prevents Pleurotus sajor-caju fibre from forming a three-dimensional matrix within the patties [62,100].
3.2.2. Colour Analysis
The brightness (L*), redness (a*), and yellowness (b*) of the cooked chicken patties were assessed by colour analysis (Table 6). Chicken patties fortified with GLF and PDF exhibited significantly lower L* (p < 0.05) compared to the control (68.56), but all formulations of PDF chicken patty were not significantly different from the commercial (55.98) patties. In addition, 9% PDF chicken patty showed the highest reading for a* (p < 0.05) among formulations and controls, which was expected due to the intense and vibrant colour of the pink mushroom. As for b*, the commercial chicken patty’s reading is significantly different for all formulations and the control, except for the 9% PDF chicken patty.

Table 6.
Colour analysis of seven cooked chicken patties incorporated with Ganoderma lucidum flour (GLF) and Pleurotus djamor flour (PDF).
The significant reading might be related to the increased levels of mushroom flour in the chicken patties led to a more intense and darker look (Figure 7A). The darker look of the GLF chicken patties can be attributed to the browning process that occurs during cooking. The browning of the chicken patties is specifically produced by the polysaccharides caramelisation that occurs in mushrooms during cooking [20,101]. GLF chicken patties recorded the highest L* because of the presence of natural triterpenoid-metabolites, also known as ganoderic acid, a bioactive compound found comprised in Ganoderma species [11]. The disparities in readings among various formulations and controls are primarily attributed to the initial coloration of both the mushrooms and muscle foods, alongside the physical interactions and chemical reactions that may occur between them, which fundamentally influence the impact of mushrooms on meat products [102].
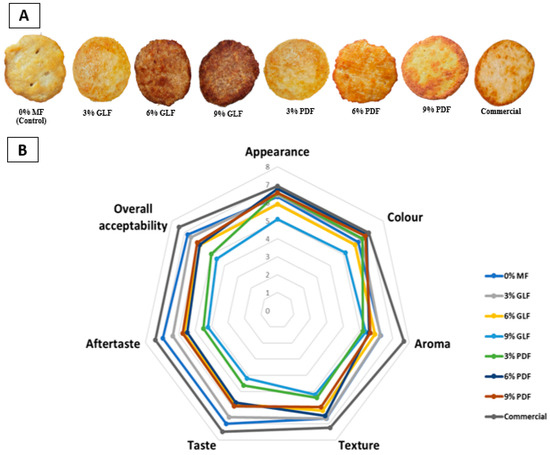
Figure 7.
(A) Cooked control chicken patty, different formulations of mushroom chicken patties and commercial chicken patties. (B) Radar chart of sensory evaluation of chicken patty samples. Fifty panellists examined the sensory test to evaluate colour, odour, taste, texture, and overall acceptability using the 9-point hedonic scale (figure by author).
3.2.3. Texture Analysis
Based on Table 7, all chicken patties fortified with GLF exhibit no significant difference in hardness (1137.63–1653.62) compared to control and commercial patties. However, the chicken patties fortified with 3% and 6% PDF exhibited a considerably greater hardness (p < 0.05) ranging from 2003.49 to 2192.08. The decrease in hardness of mushroom chicken patties might be ascribed to the elevated moisture level in Ganoderma lucidum liquid-fermented and Pleurotus djamor mushrooms [98,102]. It can be seen that the higher the percentage of PDF mushroom flour added, the lower the hardness of PDF chicken patties. It can be seen the texture became crumbly and not well-formed compared to GLF chicken patties, which are denser.

Table 7.
Texture value of cooked chicken patties incorporated with Ganoderma lucidum flour (GLF) and Pleurotus djamor flour (PDF).
The texture of chicken patties ranged from 425.03 (9% PDF) to 1347.23 (9% GLF) and 168.26 (9% PDF) to 648.8 (9% GLF) in terms of gumminess and chewiness, respectively. The chicken patties showed a range of cohesiveness and springiness values, with cohesiveness ranging from 0.47 (6% PDF) to 0.85 (9% GLF) and springiness ranging from 0.376 (9% PDF) to 0.57 (6% PDF). The value range of cohesiveness and springiness is similar to the one reported in a study by [20]. In addition, the 3% GLF showed texture attributes—hardness, gumminess, and chewiness—similar to those of the control. All GLF formulations also exhibited texture values comparable to the commercial sample.
The high errors are due to the different formulations of mushrooms biomass supplemented in chicken patties and compared to commercial chicken patty. Although it is challenging to correlate this engineering parameter with a sensory experience, this approach is highly beneficial for comprehending potential alterations to meat processing procedures to achieve values comparable to those previously approved by customers [103].
The structure of the patties that have been incorporated with both GLF and PDF may undergo certain changes in textural properties throughout the cooking process. The research study by [104] utilising flours derived from mushrooms of the species Pleurotus ostreatus, Agaricus bisporus, and Agaricus brunnescen also stated that the incorporation of non-meat components, the majority of which are derived from plants, into reformed meat products typically results in the production of finished products that are more tender in comparison to the original product.
3.2.4. Sensory Acceptance by Consumers
To elicit, quantify, examine, and comprehend the reactions to the properties of food and substances as they are perceived through the senses of vision, smell, flavour, texture, and sound, sensory evaluation uses scientific methodologies to assess these attributes [105]. Eight samples (Figure 7A), including negative control without mushroom flour (0% MF), GLF chicken patties (3%, 6% and 9%), PDF chicken patties (3%, 6% and 9%), and commercial chicken patties, were tested by 50 panellists for this study. The qualities of chicken patties were evaluated on a nine-point hedonic scale for appearance, colour, scent, texture, taste, aftertaste, and overall acceptance; the results showed above moderate acceptance.
According to Figure 7B, the commercial chicken patty from the supermarket achieved the highest scores in all sensory attributes, indicating its superior sensory profile including the presence of salt and other preservatives. The scores for each attribute include appearance, colour, aroma, texture, taste, aftertaste, and overall acceptability with respective mean scores of 6.92, 6.92, 7.69 7.22, 7.47, 7.41, and 7.45 (Table 8). Additionally, there were no significant differences (p < 0.05) for the majority of sensory attributes between the negative control, commercial, and other formulations of mushroom chicken patties, except for the aftertaste attributes.

Table 8.
Mean scores of the attributes of chicken patties incorporated with Ganoderma lucidum liquid-fermented flour (GLF) and Pleurotus djamor (PDF) flour.
All formulations with GLF (3%, 6%, and 9%) did not differ significantly from the negative control (0% GLF), in terms of appearance. Chicken patty with 9% GLF even obtained the same mean score (6.37) as the negative control chicken patty. However, the positive control (Ramly burger) had the highest score (7.32), indicating a more appealing appearance. According to the visualised representation of the GLF chicken patties in Figure 7A, the addition of GLF led to darker-coloured chicken patties, which correlated with the percentage of GLF used.
In the context of industrial application and production line for a new food product, it is crucial to conduct sensory analysis to evaluate the acceptability and feasibility of manufacturing [106,107]. Therefore, the formulations of samples are chosen in this study (3%, 6%, and 9%). Additionally, the satisfaction of customers with food is determined by its quality, which encompasses sensory or organoleptic attributes [108,109]. Ganoderma lucidum is considered a natural dye or food colourant, and the brownish or blackish appearance observed in the GLF chicken patties was correlated to the total phenolic compounds, especially melanin, found in Ganoderma lucidum [110]. This justification conforms with the results in the study conducted by [10], in which the brown appearance of GL wines becomes less visible as the GL percentages in the wines decrease. Notably, the 6% GLF formulation received a more acceptable colour score (6.74) compared to the 9% GLF formulation (6.30).
The overall acceptability scores using Ganoderma lucidum liquid-fermented biomass as mushroom flour in chicken patty are interestingly higher compared to the preliminary study using fruiting body form and all formulations (3%, 6%, and 9%) of PDF chicken patties. The difference in concentration of ganoderic acids in different forms of mushrooms [111] showed the influence in terms of appearance, flavour, and aftertaste of chicken patties. Hence, this study has achieved the application of Ganoderma lucidum in food products without compromising the attributes’ quality.
3.3. Comparison and Acceptability of Mushroom-Incorporated Savoury Food Products with Previous Studies
The comparison of mean scores of overall acceptability of GLF and PDF mushroom chicken patties and other food products fortified with mushroom flour is shown in Table 9. Ganoderma lucidum liquid-fermented biomass flour and Pleurotus djamor mushroom flour chicken patties indicated the highest acceptance scores compared to all studies. Currently, no study shows the usage of Ganoderma lucidum liquid-fermented biomass flour in food product applications. Therefore, the higher mean score of 6.55 for the 3% GLF (medicinal mushroom) chicken patty proved that the incorporation is valid, compared to a score of 6.08 for the 9% PDF (culinary mushroom) chicken patty. The lowest mean scores were was 10% and 20% for Ganoderma lucidum chicken patty from fruiting bodies form (3.03 and 2.10).

Table 9.
Comparison of Ganoderma lucidum liquid-fermented biomass flour (GLF) and Pleurotus djamor mushroom flour (PDF) chicken patty with food products from previous studies.
3.4. Significance of the Study
The execution of this research to develop functional mushroom biomass flour and incorporate it in food products is relevant to the current settings, which are briefly summarised in (Figure 8).
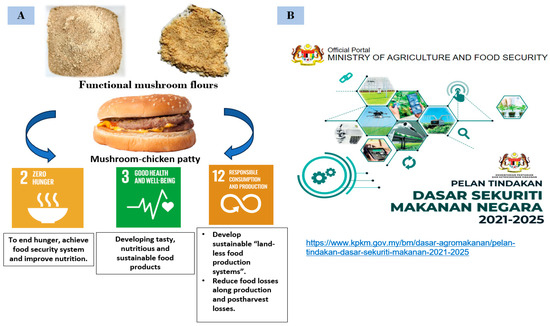
Figure 8.
(A) Outcomes of this study in accordance to Sustainable Development Goals, (B) National Food Security Policy 2021-2025, Retrieved from https://www.kpkm.gov.my/bm/dasar-agromakanan/pelan-tindakan-dasar-sekuriti-makanan-2021-2025 (accessed on 18 May 2025) (figure by author).
Firstly, the study is in line with the Malaysia’s announced National Food Security Policy 2021–2025 (DSMN Action Plan) which encompasses the availability and sustainability of food supply in Malaysia following unexpected situations like the COVID-19 pandemic. The production of high-protein mushroom biomass flour, which can be fermented in laboratories promotes sustainability and enhances food security.
To eradicate hunger, achieve zero hunger, and attain better nutrition and sustainable agriculture by 2030 are among the Sustainable Development Goals. The global COVID-19 pandemic worsened the hunger crisis, resulting in nutritional deficiency and malnutrition of infants. The number of undernourished people has risen from 690 million in 2019 to 720–811 million [115]. The most undernourished people are found in Asia (381 million). Africa ranks second (250 million) after the Caribbean and Latin America (48 million) [116]. Hence, mushrooms are utilised to balance mainly protein deficiency and for populations that do not eat animal proteins due to unavailability or religious belief [117]. Aligned with sustainable development goals (SDGs) [118], this food product development will help to achieve both the second and third goals which are zero hunger and good health and well-being, respectively, by 2030.
World Food Program (WFP) and Food and Agriculture Organization (FAO) and, World Health Organization (WHO) interpret hunger from many perspectives. These encompass food insecurity, food supply shortages, chronic undernourishment, reduced food intake accompanied by bodily manifestations of hunger, and persistent concern over the timing and availability of their next meal [119]. Around 702 to 828 million individuals faced hunger in 2021 [120], while over 1 billion people globally endure hunger, malnutrition, and food insecurity, with a significant proportion residing in Sub-Saharan Africa and South Asia. The index of hunger globally has been increasing due to undernutrition and child mortality [121].
The FAO [120] reported that global hunger escalated further in 2021, contrary to anticipations that the COVID-19 pandemic would have subsided and food security would begin to improve. By 2021, almost 30% of the global population, equating to around 2.3 billion individuals, experienced moderate to severe food insecurity. The global population was anticipated to reach 8 billion on 15 November 2022, is now projected to approach 9 billion by around 2037 and forecasted to attain 10 billion around 2058 [122], which would provide increases of 50%, 60%, and 55% in the demands for water, energy, and food, correspondingly [123,124]. This forecast leads to further ramifications and possibilities. Significant manufacturing waste is one of the issues. The World Bank forecasts that global garbage, projected at 2.01 billion tons annually in 2016, would rise by more than 70% by 2050 [125]. The global output of mushrooms has risen, resulting in around 53 million tons of mushroom waste, since 1 kg of mushrooms necessitates 5 kg of mushroom media substrate [126]. Currently, over 12.74 million tonnes of mushrooms are consumed globally, and projections indicate that by 2026, the global mushroom market value is expected to attain 20.84 million tonnes [4,127]. Furthermore, increasing waste mushroom substrate (WMS) arises from augmented mushroom cultivation [128].
In addition, mushrooms rapidly degrade due to browning, wilting, and liquefaction, resulting in texture, fragrance, and flavour loss, making them unmarketable [129]. The high moisture content and delicate texture result in a short post-harvest life and can be maintained only for 24 h under tropical conditions [130]. Therefore, the production of Ganoderma lucidum liquid-fermented biomass is in line with SDG 12, which is responsible for consumption and production using the landless food concept.
The core principle of the circular economy is to proactively enhance and acknowledge every phase of production to reduce the waste produced by industrial processes [131]. Agriculture is seen as a relevant domain for the implementation of the circular economy due to its focus on substantial waste generation, constraints in nutrient circulation, and environmental sustainability [132,133]. The growing human need for protein-rich food and the limitations of current technology have prompted the pursuit of cost-effective alternatives for generating protein-rich meals [134]. In Nigeria, where individuals turn to mushroom gardening to address malnutrition, poverty, and hunger, mushrooms exemplify a viable means of achieving food security due to their high fibre and protein content [117].
3.5. Potential Future Studies
Mushrooms have been extensively utilised as an ingredient and substitute in several food products [20,22,135,136]. This research serves as a foundation for further investigations into the uses and optimisation of different medicinal mushrooms as liquid-fermented biomass in other savoury food items. Additionally, biomass flour and mushroom chicken patties may be developed for industrial-scale manufacturing, establishing connections with primary ingredient suppliers and facilitating worldwide marketability. Further analysis for this study should include the flavouring of the patties and more research on the nutritional profile of each mushroom–chicken burger to enhance commercialisation. The utilisation of mushroom-based flour can also be commercialised as a gluten-free filler material with nutritional advantages in the production of functional food.
4. Conclusions
The study focused on the production of biomass flour from medicinal mushroom (Ganoderma lucidum-GL) through liquid fermentation, and the culinary mushroom (Pleurotus djamor-PD), to analyse their functional properties and develop mushroom chicken patties.
The ENS were extracted from both mushroom biomasses and assessed for characterisation, antimicrobial, and antioxidant properties. The structure of extracted ENS-GL and ENS-PD was successfully characterised using elemental analysis, Fourier transform infrared spectroscopy (FT-IR), and NMR spectroscopy. In addition, ENS-GL and ENS-PD also prove that they can act as antibacterial agents, by hindering the growth of bacteria and exhibiting a significant level of antioxidant activities.
Consequently, 3% GLF chicken patty was accepted overall, achieving the maximum score of 6.55, appealing to consumers’ preferences due to its attractive appearance (6.43), colour (6.37), aroma (6.24), texture (6.65), taste (6.59), and aftertaste (6.35) compared to other GLF and PDF formulations. In addition, the lowest cooking loss observed in the 9% GLF and 3% PDF chicken patties suggests a more optimal eating quality. However, for commercialisation purposes, it is recommended to incorporate a minimum of 3% GLF, as this formulation obtained the highest overall acceptability score of 6.55.
Given the present increase in demand for health food, Ganoderma lucidum and Pleurotus djamor have proven to be alternative flours containing functional properties (antimicrobial and antioxidant) in a formulated chicken patties product. The medicinal mushroom Ganoderma lucidum and Pleurotus djamor biomass, with a high protein content and a good nutritional profile, were successfully used as an alternative flour to replace potato starch in chicken patty formulations.
This study provides a blueprint for food companies to develop chicken patties from laboratory-grown biomass powder. It also highlights its relevance and importance, both domestically and globally in terms of achieving three of the Sustainable Development Goals (Goal 2: Zero Hunger, Goal 3: Good Health and Well-Being, and Goal 12: Responsible Consumption and Production) and is following Malaysia’s National Food Security Policy (DSMN Action Plan) 2021–2025.
Author Contributions
Conceptualization, W.A.A.Q.I.W.-M. and N.A.Z.-A.; writing—original draft preparation, N.A.Z.-A., Z.I. and A.A.J.; writing—review and editing, A.A.J., N.A.Z.-A., C.S.Y., M.A.U.-R., Z.I., M.H.A.R., S.R. and N.H.; visualisation, W.A.A.Q.I.W.-M., N.A.Z.-A. and C.S.Y.; supervision, W.A.A.Q.I.W.-M., Z.I. and A.A.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been funded by Universiti Malaya Partnership Grant. Thank you to the Universiti Malaya Partnership Grant ‘’Transforming Soy Sauce Processing Liquid Waste into Essential Polyunsaturated Fatty Acids Utilising Thraustochytrids from Malaysian Mangroves (MG004-2025)’’ and ‘’Formulation of Lampam Sungai Sustainable Fish Feed Using Petronas Waste (MG002-2025)’’.
Institutional Review Board Statement
The study was conducted in accordance with the Universiti Malaya Research Ethics Guidelines, and approved by the Universiti Malaya Research Ethics Committee (UMREC) of Universiti Malaya (UM.TNC2/UMREC_4045) and December 2024 till December 2027).
Informed Consent Statement
Written informed consent has been obtained from the consumers to publish this paper.
Data Availability Statement
All data sets are available upon request.
Acknowledgments
Thank you to the Universiti Malaya Partnership Grant ‘’Transforming Soy Sauce Processing Liquid Waste Into Essential Polyunsaturated Fatty Acids Utilising Thraustochytrids From Malaysian Mangroves (MG004-2025)’’ and ‘’Formulation of Lampam Sungai Sustainable Fish Feed Using Petronas Waste (MG002-2025)’’.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| GLF | Ganoderma lucidum flour |
| Pleurotus djamor flour | |
| ENS-GL | Endopolysaccharide of Ganoderma lucidum |
| ENS-PD | Endopolysaccharide of Pleurotus djamor |
| FT-IR | Fourier Transform Infrared Spectroscopy |
| 1H NMR | Nuclear Magnetic Resonance with respect to hydrogen-1 nuclei |
References
- Liu, S.; Liu, H.; Li, J.; Wang, Y. Research Progress on Elements of Wild Edible Mushrooms. J. Fungi 2022, 8, 964. [Google Scholar]
- Letcher, A. Shroom: A Cultural History of the Magic Mushroom; Faber & Faber: London, UK, 2024. [Google Scholar]
- Kumar, A.; Devi, R.; Dhalaria, R.; Tapwal, A.; Verma, R.; Rashid, S.; Elossaily, G.M.; Khan, K.A.; Chen, K.T.; Verma, T. Nutritional, Nutraceutical, and Medicinal Potential of Cantharellus cibarius Fr.: A Comprehensive Review. Food Sci. Nutr. 2025, 13, 4641. [Google Scholar]
- El-Ramady, H.; Abdalla, N.; Badgar, K.; Llanaj, X.; Törős, G.; Hajdú, P.; Eid, Y.; Prokisch, J. Edible mushrooms for sustainable and healthy human food: Nutritional and medicinal attributes. Sustainability 2022, 14, 4941. [Google Scholar] [CrossRef]
- Bhambri, A.; Srivastava, M.; Mahale, V.G.; Mahale, S.; Karn, S.K. Mushrooms as Potential Sources of Active Metabolites and Medicines. Front. Microbiol. 2022, 13, 2022. [Google Scholar]
- Li, H.; Gao, J.A.; Zhao, F.; Liu, X.; Ma, B. Bioactive peptides from edible mushrooms—The preparation, mechanisms, structure—Activity relationships and prospects. Foods 2023, 12, 2935. [Google Scholar] [PubMed]
- Kumar, K.; Mehra, R.; Guiné, R.P.F.; Lima, M.J.; Kumar, N.; Kaushik, R.; Ahmed, N.; Yadav, A.N.; Kumar, H. Edible Mushrooms: A Comprehensive Review on Bioactive Compounds with Health Benefits and Processing Aspects. Foods 2021, 10, 2996. [Google Scholar] [CrossRef]
- Klaus, A.; Wan-Mohtar, W.A.A.Q.I. Ganoderma in Traditional Culture. In Ganoderma; CRC Press: Boca Raton, FL, USA, 2024; pp. 35–60. [Google Scholar]
- Yangzom, R.; Wangchuk, P. Ganoderma lucidum (Lingzhi Mushroom): Its Medicinal Uses, Biomolecules and Therapeutic Applications; Royal Society of Chemistry: London, UK, 2023; Volume 10, pp. 221–241. [Google Scholar]
- Nguyen, A.N.; Johnson, T.E.; Jeffery, D.W.; Capone, D.L.; Danner, L.; Bastian, S.E. Sensory and chemical drivers of wine consumers’ preference for a new Shiraz wine product containing Ganoderma lucidum extract as a novel ingredient. Foods 2020, 9, 224. [Google Scholar] [CrossRef]
- El Sheikha, A.F. Nutritional profile and health benefits of Ganoderma lucidum “Lingzhi, Reishi, or Mannentake” as functional foods: Current scenario and future perspectives. Foods 2022, 11, 1030. [Google Scholar] [CrossRef]
- Wang, J.; Cao, B.; Zhao, H.; Feng, J. Emerging roles of Ganoderma lucidum in anti-aging. Aging Dis. 2017, 8, 691. [Google Scholar]
- Sułkowska-Ziaja, K.; Trepa, M.; Olechowska-Jarząb, A.; Nowak, P.; Ziaja, M.; Kała, K.; Muszyńska, B. Natural compounds of fungal origin with antimicrobial activity—Potential cosmetics applications. Pharmaceuticals 2023, 16, 1200. [Google Scholar] [CrossRef]
- Miller, S.L.; Sayner, A. Cultivation of Medicinal Mushrooms for a World in Need. In Wild Mushrooms and Health: Diversity, Phytochemistry, Medicinal Benefits, and Cultivation; CRC Press: Boca Raton, FL, USA, 2023; Volume 95. [Google Scholar]
- Klaus, A.; Wan-Mohtar, W.A.A.Q.I. Cultivation strategies of edible and medicinal mushrooms. In Wild Mushrooms; CRC Press: Boca Raton, FL, USA, 2022; pp. 23–65. [Google Scholar]
- Mahari, W.A.W.; Peng, W.; Nam, W.L.; Yang, H.; Lee, X.Y.; Lee, Y.K.; Liew, R.K.; Ma, N.L.; Mohammad, A.; Sonne, C. A review on valorization of oyster mushroom and waste generated in the mushroom cultivation industry. J. Hazard. Mater. 2020, 400, 123156. [Google Scholar]
- Törős, G.H.; El-Ramady, H.; Prokisch, J. Edible mushroom of Pleurotus spp.: A case study of oyster mushroom (Pleurotus ostreatus L.). Environ. Biodivers. Soil Secur. 2022, 6, 51–59. [Google Scholar]
- Corrêa, R.C.G.; Brugnari, T.; Bracht, A.; Peralta, R.M.; Ferreira, I.C. Biotechnological, nutritional and therapeutic uses of Pleurotus spp. (Oyster mushroom) related with its chemical composition: A review on the past decade findings. Trends Food Sci. Technol. 2016, 50, 103–117. [Google Scholar]
- Kotasthane, T. Oyster Mushroom and its value added products. Res. Sq. 2021, preprint. [Google Scholar]
- Wan-Mohtar, W.A.A.Q.I.; Halim-Lim, S.A.; Kamarudin, N.Z.; Rukayadi, Y.; Abd Rahim, M.H.; Jamaludin, A.A.; Ilham, Z. Fruiting-body-base flour from an Oyster mushroom waste in the development of antioxidative chicken patty. J. Food Sci. 2020, 85, 3124–3133. [Google Scholar]
- Shams, E.A.; Yousef, N.S.; El-Shazly, H.A. Chemical, Sensory and Rheological Evaluation of Karish Cheese Made by Oyster Mushroom Mycelium. J. Food Dairy Sci. 2020, 11, 187–193. [Google Scholar]
- Wan-Mohtar, W.; Mahmud, N.; Supramani, S.; Ahmad, R.; Zain, N.A.M.; Hassan, N.A.; Peryasamy, J.; Halim-Lim, S.A. Fruiting-body-base flour from an oyster mushroom-a waste source of antioxidative flour for developing potential functional cookies and steamed-bun. AIMS Agric. Food 2018, 3, 481–492. [Google Scholar]
- Wan Rosli, W.; Maihiza, N.; Raushan, M. The ability of oyster mushroom in improving nutritional composition, β-glucan and textural properties of chicken frankfurter. Int. Food Res. J. 2015, 22, 311–317. [Google Scholar]
- Jegadeesh, R.; Lakshmanan, H.; Kab-Yeul, J.; Sabaratnam, V.; Raaman, N. Cultivation of Pink Oyster mushroom Pleurotus djamor var. roseus on various agro-residues by low cost technique. J. Mycopathol. Res. 2018, 56, 213–220. [Google Scholar]
- Phonemany, M.; Sysouphanthong, P.; Rujanapun, N.; Thongklang, N.; Charoensup, R. Preliminary trial of the cultivation of Pleurotus djamor var. fuscopruinosus from southern Thailand using sawdust substrate and applications. Res. Sq. 2024, preprint. [Google Scholar]
- Hasan, M.; Khatun, M.; Sajib, M.; Rahman, M.; Rahman, M.; Roy, M.; Miah, M.; Ahmed, K. Effect of wheat bran supplement with sugarcane bagasse on growth, yield and proximate composition of pink oyster mushroom (Pleurotus djamor). Am. J. Food Sci. Technol. 2015, 3, 150–157. [Google Scholar]
- Whitaker, R., III. The Effect of Temperature and Substrate Composition on the Cultivation of Pleurotus djamor. Master’s Thesis, North Carolina Agricultural and Technical State University, Greensboro, NC, USA, 2024. [Google Scholar]
- Pirdaus, N.A.; Ahmad, N.; Dahlan, N.Y.; Redzuan, A.N.; Zalizan, A.H.; Muhammad-Sukki, F.; Bani, N.A.; Abdul Patah, M.F.; Wan-Mohtar, W.A.A.Q.I. Performance of yellow and pink oyster mushroom dyes in dye sensitized solar cell. Sci. Rep. 2024, 14, 23757. [Google Scholar]
- Rai, P.K. Role of water-energy-food nexus in environmental management and climate action. Energy Nexus 2023, 11, 100230. [Google Scholar]
- Yaeger, W.; Jawed, M.; Tauman, D.; Ho, P.; Ali, A.; Gomanie, N.N.; Mehta, K. Modular methods for oyster mushroom cultivation in low-resource settings. In Proceedings of the 2022 IEEE Global Humanitarian Technology Conference (GHTC), Santa Clara, CA, USA, 8–11 September 2022; pp. 30–37. [Google Scholar]
- Llanaj, X.; Törős, G.; Hajdú, P.; Abdalla, N.; El-Ramady, H.; Kiss, A.; Solberg, S.Ø.; Prokisch, J. Biotechnological Applications of Mushrooms under the Water-Energy-Food Nexus: Crucial Aspects and Prospects from Farm to Pharmacy. Foods 2023, 12, 2671. [Google Scholar] [PubMed]
- Heck, R.T.; Saldaña, E.; Lorenzo, J.M.; Correa, L.P.; Fagundes, M.B.; Cichoski, A.J.; de Menezes, C.R.; Wagner, R.; Campagnol, P.C.B. Hydrogelled emulsion from chia and linseed oils: A promising strategy to produce low-fat burgers with a healthier lipid profile. Meat Sci. 2019, 156, 174–182. [Google Scholar]
- Mercês, Z.d.C.d.; Salvadori, N.M.; Evangelista, S.M.; Cochlar, T.B.; Rios, A.d.O.; Oliveira, V.R.d. Hybrid and plant-based burgers: Trends, challenges, and physicochemical and sensory qualities. Foods 2024, 13, 3855. [Google Scholar] [CrossRef]
- Mizi, L.; Cofrades, S.; Bou, R.; Pintado, T.; López-Caballero, M.; Zaidi, F.; Jiménez-Colmenero, F. Antimicrobial and antioxidant effects of combined high pressure processing and sage in beef burgers during prolonged chilled storage. Innov. Food Sci. Emerg. Technol. 2019, 51, 32–40. [Google Scholar]
- Ilić, J.; Djekic, I.; Tomasevic, I.; Oosterlinck, F.; van den Berg, M.A. Materials properties, oral processing, and sensory analysis of eating meat and meat analogs. Annu. Rev. Food Sci. Technol. 2022, 13, 193–215. [Google Scholar]
- Reis, F.S.; Martins, A.; Vasconcelos, M.H.; Morales, P.; Ferreira, I.C. Functional foods based on extracts or compounds derived from mushrooms. Trends Food Sci. Technol. 2017, 66, 48–62. [Google Scholar]
- Losoya-Sifuentes, C.; Cruz, M.; del Refugio Rocha-Pizaña, M.; Loredo-Treviño, A.; Belmares, R. Edible Mushrooms: A Nutrient-Rich Ingredient for Healthier Food Products–A Review. Curr. Nutr. Rep. 2025, 14, 9. [Google Scholar]
- Banach, J.; van der Berg, J.; Kleter, G.; van Bokhorst-van de Veen, H.; Bastiaan-Net, S.; Pouvreau, L.; van Asselt, E. Alternative proteins for meat and dairy replacers: Food safety and future trends. Crit. Rev. Food Sci. Nutr. 2023, 63, 11063–11080. [Google Scholar] [PubMed]
- Vetter, J. Biological values of cultivated mushrooms—A review. Acta Aliment. 2019, 48, 229–240. [Google Scholar]
- Vlaicu, P.A.; Untea, A.E.; Varzaru, I.; Saracila, M.; Oancea, A.G. Designing nutrition for health—Incorporating dietary by-products into poultry feeds to create functional foods with insights into health benefits, risks, bioactive compounds, food component functionality and safety regulations. Foods 2023, 12, 4001. [Google Scholar]
- Pandey, A.K.; Rajan, S.; Sarsaiya, S.; Jain, S.K. Mushroom for the national circular economy. Int. J. Sci. Res. Biol. Sci. 2020, 7, 61–69. [Google Scholar]
- Pandey, P.; Pal, R.; Koli, M.; Malakar, R.K.; Verma, S.; Kumar, N.; Kumar, P. A traditional review: The utilization of nutraceutical as a traditional cure for the modern world at current prospectus for multiple health conditions. J. Drug Deliv. Ther. 2024, 14, 154–163. [Google Scholar]
- Mingyi, Y.; Belwal, T.; Devkota, H.P.; Li, L.; Luo, Z. Trends of utilizing mushroom polysaccharides (MPs) as potent nutraceutical components in food and medicine: A comprehensive review. Trends Food Sci. Technol. 2019, 92, 94–110. [Google Scholar]
- Bell, V.; Silva, C.; Guina, J.; Fernandes, T. Mushrooms as future generation healthy foods. Front. Nutr. 2022, 9, 1050099. [Google Scholar]
- Wan-Mohtar, W.A.A.Q.I.; Zahia-Azizan, N.A.; Rui Yeong, T.; Ilham, Z.; Jamaludin, A.A. Mushroom-bioreactor biomass as bioactive protein source: Synergy of mushroom rural and urban cultivation. Org. Agric. 2024, 14, 459–466. [Google Scholar]
- Taufek, N.M.; Harith, H.H.; Abd Rahim, M.H.; Ilham, Z.; Rowan, N.; Wan-Mohtar, W.A.A.Q.I. Performance of mycelial biomass and exopolysaccharide from Malaysian Ganoderma lucidum for the fungivore red hybrid Tilapia (Oreochromis sp.) in Zebrafish embryo. Aquac. Rep. 2020, 17, 100322. [Google Scholar]
- Aa, B.; Ab, O.; M, P.; Ns, Y. Mushroom cultivation in tropical Africa: Successes, challenges, and opportunities. J. Agric. Food Res. 2024, 18, 101264. [Google Scholar]
- Phonemany, M.; Sysouphanthong, P.; Rujanapun, N.; Sarker, S.D.; Nahar, L.; Puttarak, P.; Hiransai, P.; Thongklang, N.; Charoensup, R. Identification and therapeutic efficacy of Pleurotus djamor var fuscopruinosus. Sci. Rep. 2025, 15, 18929. [Google Scholar]
- Moutia, I.; Lakatos, E.; Kovács, A.J. Impact of Dehydration Techniques on the Nutritional and Microbial Profiles of Dried Mushrooms. Foods 2024, 13, 3245. [Google Scholar] [CrossRef]
- Sangeeta, S.; Sharma, D.; Ramniwas, S.; Mugabi, R.; Uddin, J.; Nayik, G.A. Revolutionizing Mushroom processing: Innovative techniques and technologies. Food Chem. X 2024, 23, 101774. [Google Scholar] [PubMed]
- Xie, S.; Li, H.; Li, N.; Liu, Z.; Xu, D.; Hu, L.; Mo, H. Lentinus edodes Powder Improves the Quality of Wheat Flour Gluten Sticks. Foods 2023, 12, 1755. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Vargas, E.; Rodriguez, J.A.; Domínguez, R.; Lorenzo, J.M.; Sosa, M.E.; Andrés, S.C.; Rosmini, M.; Pérez-Alvarez, J.A.; Teixeira, A.; Santos, E.M. Edible Mushrooms as a Natural Source of Food Ingredient/Additive Replacer. Foods 2021, 10, 2687. [Google Scholar] [CrossRef]
- Bermúdez, R.; Rangel-Vargas, E.; Lorenzo, J.M.; Rodríguez, J.A.; Munekata, P.E.S.; Teixeira, A.; Pateiro, M.; Romero, L.; Santos, E.M. Effect of Partial Meat Replacement by Hibiscus sabdariffa By-Product and Pleurotus djamor Powder on the Quality of Beef Patties. Foods 2023, 12, 391. [Google Scholar] [CrossRef]
- Supramani, S.; Rahayu Ahmad, Z.I.; Annuar, M.S.M.; Klaus, A.; Wan-Mohtar, W.A.A.Q.I. Optimisation of biomass, exopolysaccharide and intracellular polysaccharide production from the mycelium of an identified Ganoderma lucidum strain QRS 5120 using response surface methodology. AIMS Microbiol. 2019, 5, 19. [Google Scholar]
- Abdullah, N.R.; Sharif, F.; Azizan, N.H.; Hafidz, I.F.M.; Supramani, S.; Usuldin, S.R.A.; Ahmad, R.; Wan-Mohtar, W.A.A.Q.I. Pellet diameter of Ganoderma lucidum in a repeated-batch fermentation for the trio total production of biomass-exopolysaccharide-endopolysaccharide and its anti-oral cancer beta-glucan response. AIMS Microbiol. 2020, 6, 379. [Google Scholar]
- Nik Ubaidillah, N.H.; Abdullah, N.; Sabaratnam, V. Isolation of the intracellular and extracellular polysaccharides of Ganoderma neojaponicum (Imazeki) and characterization of their immunomodulatory properties. Electron. J. Biotechnol. 2015, 18, 188–195. [Google Scholar]
- Wan-Mohtar, W.A.A.Q.I.; Young, L.; Abbott, G.M.; Clements, C.; Harvey, L.M.; McNeil, B. Antimicrobial properties and cytotoxicity of sulfated (1, 3)-β-D-glucan from the mycelium of the mushroom Ganoderma lucidum. J. Microbiol. Biotechnol. 2016, 26, 999–1010. [Google Scholar]
- Roque-Borda, C.A.; da Silva, P.B.; Rodrigues, M.C.; Azevedo, R.B.; Di Filippo, L.; Duarte, J.L.; Chorilli, M.; Festozo Vicente, E.; Pavan, F.R. Challenge in the Discovery of New Drugs: Antimicrobial Peptides against WHO-List of Critical and High-Priority Bacteria. Pharmaceutics 2021, 13, 773. [Google Scholar] [PubMed]
- Ben Akacha, B.; Švarc-Gajić, J.; Elhadef, K.; Ben Saad, R.; Brini, F.; Mnif, W.; Smaoui, S.; Ben Hsouna, A. The Essential Oil of Tunisian Halophyte Lobularia maritima: A Natural Food Preservative Agent of Ground Beef Meat. Life 2022, 12, 1571. [Google Scholar] [CrossRef]
- Vamanu, E. In vitro antimicrobial and antioxidant activities of ethanolic extract of lyophilized mycelium of Pleurotus ostreatus PQMZ91109. Molecules 2012, 17, 3653–3671. [Google Scholar] [CrossRef] [PubMed]
- Elhadef, K.; Chaari, M.; Akermi, S.; Ennouri, K.; Ben Hlima, H.; Fourati, M.; Chakchouk Mtibaa, A.; Ennouri, M.; Sarkar, T.; Shariati, M.A.; et al. Gelatin-sodium alginate packaging film with date pits extract: An eco-friendly packaging for extending raw minced beef shelf life. Meat Sci. 2024, 207, 109371. [Google Scholar]
- Wan Rosli, W.; Solihah, M. Effect on the addition of Pleurotus sajor-caju (PSC) on physical and sensorial properties of beef patty. Int. Food Res. J. 2012, 19, 993–999. [Google Scholar]
- Pang, B.; Bowker, B.; Zhuang, H.; Yang, Y.; Zhang, J. Research Note: Comparison of 3 methods used for estimating cook loss in broiler breast meat. Poult. Sci. 2020, 99, 6287–6290. [Google Scholar]
- Tomasevic, I.; Tomovic, V.; Ikonic, P.; Rodriguez, J.M.L.; Barba, F.J.; Djekic, I.; Nastasijevic, I.; Stajic, S.; Zivkovic, D. Evaluation of poultry meat colour using computer vision system and colourimeter: Is there a difference? Br. Food J. 2019, 121, 1078–1087. [Google Scholar]
- Banerjee, D.K.; Das, A.K.; Banerjee, R.; Pateiro, M.; Nanda, P.K.; Gadekar, Y.P.; Biswas, S.; McClements, D.J.; Lorenzo, J.M. Application of enoki mushroom (Flammulina Velutipes) stem wastes as functional ingredients in goat meat nuggets. Foods 2020, 9, 432. [Google Scholar] [CrossRef]
- Marek, S.; Piotr, R.; Przemysław, N.; Anna, B.; Monika, G.; Kalač, P.; Agnieszka, J.; Sylwia, B.; Lidia, K.; Mirosław, M. Comparison of multielemental composition of Polish and Chinese mushrooms (Ganoderma spp.). Eur. Food Res. Technol. 2017, 243, 1555–1566. [Google Scholar]
- Chen, C.; Shao, Y.; Tao, Y.; Wen, H. Optimization of dynamic microwave-assisted extraction of Armillaria polysaccharides using RSM, and their biological activity. LWT-Food Sci. Technol. 2015, 64, 1263–1269. [Google Scholar]
- Usuldin, S.R.A.; Mahmud, N.; Ilham, Z.; Ikram, N.K.K.; Ahmad, R.; Wan-Mohtar, W.A.A.Q.I. In-depth spectral characterization of antioxidative (1, 3)-β-D-glucan from the mycelium of an identified tiger milk mushroom Lignosus rhinocerus strain ABI in a stirred-tank bioreactor. Biocatal. Agric. Biotechnol. 2020, 23, 101455. [Google Scholar]
- Zhang, J.; Liu, Y.; Tang, Q.; Zhou, S.; Feng, J.; Chen, H. Polysaccharide of Ganoderma and its bioactivities. Ganoderma Health Biol. Chem. Ind. 2019, 1181, 107–134. [Google Scholar]
- Idu, M.; Okojie, S.O.; Gabriel, B.O. In-vitro microbicidal activity of Ganoderma lucidum aqueous extract against selected pathogenic bacteria and yeast. J. Herb. Med. 2025, 50, 100990. [Google Scholar]
- Swallah, M.S.; Bondzie-Quaye, P.; Wu, Y.; Acheampong, A.; Sossah, F.L.; Elsherbiny, S.M.; Huang, Q. Therapeutic potential and nutritional significance of Ganoderma lucidum–a comprehensive review from 2010 to 2022. Food Funct. 2023, 14, 1812–1838. [Google Scholar] [PubMed]
- Zhou, J.; Cai, Y.; Liu, Y.; An, H.; Deng, K.; Ashraf, M.A.; Zou, L.; Wang, J. Breaking down the cell wall: Still an attractive antibacterial strategy. Front. Microbiol. 2022, 13, 952633. [Google Scholar]
- Mustafin, K.; Bisko, N.; Blieva, R.; Al-Maali, G.; Krupodorova, T.; Narmuratova, Z.; Saduyeva, Z.; Zhakipbekova, A. Antioxidant and antimicrobial potential of Ganoderma lucidum and Trametes versicolor. Turk. J. Biochem. 2022, 47, 483–489. [Google Scholar]
- Stephens, M.; von der Weid, P.-Y. Lipopolysaccharides modulate intestinal epithelial permeability and inflammation in a species-specific manner. Gut Microbes 2020, 11, 421–432. [Google Scholar]
- Jubeh, B.; Breijyeh, Z.; Karaman, R. Resistance of gram-positive bacteria to current antibacterial agents and overcoming approaches. Molecules 2020, 25, 2888. [Google Scholar] [CrossRef]
- Illuri, R.; Eyini, M.; Kumar, M.; Prema, P.; Nguyen, V.-H.; Bukhari, N.A.; Hatamleh, A.A.; Balaji, P. Bio-prospective potential of Pleurotus djamor and Pleurotus florida mycelial extracts towards Gram positive and Gram negative microbial pathogens causing infectious disease. J. Infect. Public Health 2022, 15, 297–306. [Google Scholar]
- Kedare, S.B.; Singh, R. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar]
- Esmaelifar, M.; Hatamian-Zarmi, A.; Alvandi, H.; Azizi, M.; Mokhtari-Hosseini, Z.B.; Ebrahimi-Hoseinzadeh, B. Optimization of antioxidant activities and intracellular polysaccharide contents using Agaricus bisporus extract as elicitor in submerged fermenting Ganoderma lucidum: Optimization of IPS production from Ganoderma lucidum. Appl. Food Biotechnol. 2021, 8, 297–306. [Google Scholar]
- Ekiz, E.; Oz, E.; Abd El-Aty, A.; Proestos, C.; Brennan, C.; Zeng, M.; Tomasevic, I.; Elobeid, T.; Çadırcı, K.; Bayrak, M. Exploring the potential medicinal benefits of Ganoderma lucidum: From metabolic disorders to coronavirus infections. Foods 2023, 12, 1512. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.N.A.D. The role of free radicals and reactive oxygen species in biological systems-a comprehensive review. Int. J. Drug Res. Dent. Sci. 2022, 4, 28–41. [Google Scholar]
- Clare, K.; Dillon, J.F.; Brennan, P.N. Reactive oxygen species and oxidative stress in the pathogenesis of MAFLD. J. Clin. Transl. Hepatol. 2022, 10, 939. [Google Scholar]
- Modi, H.; Shah, P.; Shukla, M.; Lahiri, S.K. Determination of total phenolic content and antioxidant activity of Ganoderma lucidum collected from Dang district of Gujarat, India. Nat. Prod. Indian J. 2014, 10, 75–83. [Google Scholar]
- Shah, P.; Modi, H. Comparative study of DPPH, ABTS and FRAP assays for determination of antioxidant activity. Int. J. Res. Appl. Sci. Eng. Technol. 2015, 3, 636–641. [Google Scholar]
- Raman, J.; Sivakumar, A.; Lakshmanan, H.; Raaman, N.; Shin, H.-J. Antioxidant activity of partially characterized polysaccharides from the edible mushroom Pleurotus djamor var. roseus. J. Mushroom 2021, 19, 140–149. [Google Scholar]
- Swargiary, A.; Roy, M.K.; Verma, A.K. In vitro study of the antioxidant, antiproliferative, and anthelmintic properties of some medicinal plants of Kokrajhar district, India. J. Parasit. Dis. 2021, 45, 1123–1134. [Google Scholar]
- Daniel, A.; Temikotan, T. Antioxidant and Radical Scavenging of Piliostigma reticulatum using FRAP and DPPH. J. Pharm. Res. Dev. 2021, 2, 1–6. [Google Scholar]
- Mohammadifar, S.; Fallahi Gharaghoz, S.; Asef Shayan, M.R.; Vaziri, A. Comparison between antioxidant activity and bioactive compounds of Ganoderma applanatum (Pers.) Pat. and Ganoderma lucidum (Curt.) P. Karst from Iran. Iran. J. Plant Physiol. 2020, 11, 3417–3424. [Google Scholar]
- Sen, S.; Chakraborty, R. Food in health preservation and promotion: A special focus on the interplay between oxidative stress and pro-oxidant/antioxidant. In Exploring the Nutrition and Health Benefits of Functional Foods; IGI Global: Hershey, PA, USA, 2017; pp. 265–300. [Google Scholar]
- Kozarski, M.; Klaus, A.; Jakovljevic, D.; Todorovic, N.; Vunduk, J.; Petrović, P.; Niksic, M.; Vrvic, M.M.; Van Griensven, L. Antioxidants of edible mushrooms. Molecules 2015, 20, 19489–19525. [Google Scholar] [CrossRef] [PubMed]
- Mwangi, R.W.; Macharia, J.M.; Wagara, I.N.; Bence, R.L. The antioxidant potential of different edible and medicinal mushrooms. Biomed. Pharmacother. 2022, 147, 112621. [Google Scholar]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef]
- Wang, W.; Tan, J.; Nima, L.; Sang, Y.; Cai, X.; Xue, H. Polysaccharides from fungi: A review on their extraction, purification, structural features, and biological activities. Food Chem. X 2022, 15, 100414. [Google Scholar]
- Bristy, A.T.; Islam, T.; Ahmed, R.; Hossain, J.; Reza, H.M.; Jain, P. Evaluation of total phenolic content, HPLC analysis, and antioxidant potential of three local varieties of mushroom: A comparative study. Int. J. Food Sci. 2022, 2022, 3834936. [Google Scholar]
- Bustillos, R.; Francisco, C.; Dulay, R. Liquid culture and antioxidant properties of Ganoderma lucidum and Pleurotus djamor. Int. J. Biol. Pharm. Allied Sci. 2018, 7, 576–583. [Google Scholar]
- Hong, S.; Shen, Y.; Li, Y. Physicochemical and functional properties of texturized vegetable proteins and cooked patty textures: Comprehensive characterization and correlation analysis. Foods 2022, 11, 2619. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.; Marais, J.; Strydom, P.E.; Hoffman, L.C. Effects of increasing internal end-point temperatures on physicochemical and sensory properties of meat: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2843–2872. [Google Scholar]
- Wang, J.; Yang, P.; Han, D.; Huang, F.; Li, X.; Song, Y.; Wang, H.; Liu, J.; Zheng, J.; Zhang, C. Role of intramuscular connective tissue in water holding capacity of porcine muscles. Foods 2022, 11, 3835. [Google Scholar] [CrossRef]
- Wan Rosli, W.; Solihah, M.; Aishah, M.; Nik Fakurudin, N.; Mohsin, S. Colour, textural properties, cooking characteristics and fibre content of chicken patty added with oyster mushroom (Pleurotus sajor-caju). Int. Food Res. J. 2011, 18, 621–627. [Google Scholar]
- Lee, S.; Jo, K.; Jeon, H.; Choi, Y.-S.; Jung, S. Recent strategies for improving the quality of meat products. J. Anim. Sci. Technol. 2023, 65, 895. [Google Scholar]
- Mazumder, M.A.R.; Sujintonniti, N.; Chaum, P.; Ketnawa, S.; Rawdkuen, S. Developments of plant-based emulsion-type sausage by using grey oyster mushrooms and chickpeas. Foods 2023, 12, 1564. [Google Scholar]
- Abdul-Malek, A.; Nazimah, H.; Razifah, M.; Bellere, A.; Raseetha, S. Beneficial properties of edible mushrooms and their potential utilisation of mushroom waste in food products. Food Res 2023, 7, 21–36. [Google Scholar]
- Ibrahim, H.S.S.; Huda-Faujan, N. Physicochemical Properties and Nutritional Composition of Chicken Patties with Grey Oyster Mushroom Stems. Malays. J. Sci. Health Technol. 2024, 10, 50–57. [Google Scholar]
- Paredes, J.; Cortizo-Lacalle, D.; Imaz, A.M.; Aldazabal, J.; Vila, M. Application of texture analysis methods for the characterization of cultured meat. Sci. Rep. 2022, 12, 3898. [Google Scholar]
- Botella-Martínez, C.; Muñoz-Tebar, N.; Lucas-González, R.; Pérez-Álvarez, J.A.; Fernández-López, J.; Viuda-Martos, M. Assessment of chemical, physico-chemical and sensory properties of low-sodium beef burgers formulated with flours from different mushroom types. Foods 2023, 12, 3591. [Google Scholar]
- Stone, H.; Bleibaum, R.; Thomas, H.A. Sensory Evaluation Practices; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Marsanasco, M.; Márquez, A.L.; Wagner, J.R.; Chiaramoni, N.S.; Alonso, S.d.V. Bioactive compounds as functional food ingredients: Characterization in model system and sensory evaluation in chocolate milk. J. Food Eng. 2015, 166, 55–63. [Google Scholar]
- Ruiz-Capillas, C.; Herrero, A.M.; Pintado, T.; Delgado-Pando, G. Sensory analysis and consumer research in new meat products development. Foods 2021, 10, 429. [Google Scholar] [CrossRef]
- Miran, B.; Thomas, T.W. Consumer priorities in food quality characteristics:: Empirical findings from Turkiye. South-East. Eur. J. Econ. 2023, 21, 7–40. [Google Scholar]
- Liu, J.; Ellies-Oury, M.; Stoyanchev, T.; Hocquette, J. Consumer perception of beef quality and how to control, improve and predict it? Focus on eating quality. Foods 2022, 11, 1732. [Google Scholar] [CrossRef]
- Ahmad, N.; Vunduk, J.; Klaus, A.; Dahlan, N.Y.; Ghosh, S.; Muhammad-Sukki, F.; Dufossé, L.; Bani, N.A.; Wan-Mohtar, W.A.A.Q.I. Roles of medicinal mushrooms as natural food dyes and dye-sensitised solar cells (DSSC): Synergy of zero hunger and affordable energy for sustainable development. Sustainability 2022, 14, 13894. [Google Scholar] [CrossRef]
- Liang, C.; Tian, D.; Liu, Y.; Li, H.; Zhu, J.; Li, M.; Xin, M.; Xia, J. Review of the molecular mechanisms of Ganoderma lucidum triterpenoids: Ganoderic acids A, C2, D, F, DM, X and Y. Eur. J. Med. Chem. 2019, 174, 130–141. [Google Scholar]
- Wannasupchue, W.; Siriamornpun, S.; Huaisan, K.; Huaisan, J.; Meeso, N. Effect of adding Ling-zhi (Ganoderma lucidum) on oxidative stability, textural and sensory properties of smoked fish sausage. Thai J. Agric. Sci. 2011, 5, 505–512. [Google Scholar]
- Ghobadi, R.; Mohammadi, R.; Chabavizade, J.; Sami, M. Effect of Ganoderma lucidum powder on oxidative stability, microbial and sensory properties of emulsion type sausage. Adv. Biomed. Res. 2018, 7, 24. [Google Scholar]
- Süfer, Ö.; Bozok, F.; Demir, H. Usage of edible mushrooms in various food products. Turk. J. Agric.-Food Sci. Technol. 2016, 4, 144–149. [Google Scholar]
- United Nations, End Hunger, Achieve Food Security and Improved Nutrition and Promote Sustainable Agriculture. 2021. Available online: https://unstats.un.org/sdgs/report/2020/goal-02/ (accessed on 16 March 2025).
- World Health Organisation. In Brief, The State of Food Security and Nutrition in the World. 2020. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/9a0fca06-5c5b-4bd5-89eb-5dbec0f27274/content (accessed on 9 April 2025).
- Niazi, A.R.; Ghafoor, A. Different ways to exploit mushrooms: A review. All Life 2021, 14, 450–460. [Google Scholar]
- United Nations. Zero Hunger. 2022. Available online: https://www.un.org/sustainabledevelopment/hunger/2022 (accessed on 1 March 2025).
- Nigam, A. Improving global hunger index. Agric. Res. 2019, 8, 132–139. [Google Scholar]
- FAO, 2022. The World is at a Critical Juncture. 2022. Available online: https://www.fao.org/interactive/state-of-food-security-nutrition/2021/en/ (accessed on 24 February 2025).
- Mansoor, S.; Khan, T.; Farooq, I.; Shah, L.R.; Sharma, V.; Sonne, C.; Rinklebe, J.; Ahmad, P. Drought and global hunger: Biotechnological interventions in sustainability and management. Planta 2022, 256, 97. [Google Scholar]
- Zeifman, L.; Hertog, S.; Kantorova, V.; Wilmoth, J. A World of 8 Billion; United Nations Department of Economic and Social Affairs: New York, NY, USA, 2022. [Google Scholar]
- Kumar, H.; Bhardwaj, K.; Sharma, R.; Nepovimova, E.; Cruz-Martins, N.; Dhanjal, D.S.; Singh, R.; Chopra, C.; Verma, R.; Abd-Elsalam, K.A. Potential usage of edible mushrooms and their residues to retrieve valuable supplies for industrial applications. J. Fungi 2021, 7, 427. [Google Scholar]
- Islam, S.M.F.; Karim, Z. World’s demand for food and water: The consequences of climate change. In Desalination-Challenges and Opportunities; Abadi Farahani, M.H.D., Vatanpour, V., et al., Eds.; IntechOpen: London, UK, 2019; pp. 1–27. [Google Scholar]
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank Publications: Washington, DC, USA, 2018. [Google Scholar]
- Islam, M.S.; Kasim, S.; Alam, K.M.; Amin, A.M.; Geok Hun, T.; Haque, M.A. Changes in chemical properties of banana pseudostem, mushroom media waste, and chicken manure through the co-composting process. Sustainability 2021, 13, 8458. [Google Scholar] [CrossRef]
- Atallah, E.; Zeaiter, J.; Ahmad, M.N.; Leahy, J.J.; Kwapinski, W. Hydrothermal carbonization of spent mushroom compost waste compared against torrefaction and pyrolysis. Fuel Process. Technol. 2021, 216, 106795. [Google Scholar]
- Zaini, N.A.M.; Azizan, N.A.Z.; Abd Rahim, M.H.; Jamaludin, A.A.; Raposo, A.; Raseetha, S.; Zandonadi, R.P.; BinMowyna, M.N.; Raheem, D.; Lho, L.H. A narrative action on the battle against hunger using mushroom, peanut, and soybean-based wastes. Front. Public Health 2023, 11, 1175509. [Google Scholar]
- Akhil, G. Comparative Evaluation of Different Species of Oyster Mushroom Suitable to Kerala. Ph.D. Thesis, Department of Plant Pathology, College of Agriculture, Padannakkad, Padanakkad, India, 2022. [Google Scholar]
- Zheng, C.; Li, J.; Liu, H.; Wang, Y. Review of postharvest processing of edible wild-grown mushrooms. Food Res. Int. 2023, 173, 113223. [Google Scholar]
- Al-Thani, N.A.; Al-Ansari, T. Comparing the convergence and divergence within industrial ecology, circular economy, and the energy-water-food nexus based on resource management objectives. Sustain. Prod. Consum. 2021, 27, 1743–1761. [Google Scholar]
- Fassio, F.; Tecco, N. Circular economy for food: A systemic interpretation of 40 case histories in the food system in their relationships with SDGs. Systems 2019, 7, 43. [Google Scholar] [CrossRef]
- Velasco-Muñoz, J.F.; Aznar-Sánchez, J.A.; López-Felices, B.; Román-Sánchez, I.M. Circular economy in agriculture. An analysis of the state of research based on the life cycle. Sustain. Prod. Consum. 2022, 34, 257–270. [Google Scholar]
- Singh, M.; Trivedi, N.; Enamala, M.K.; Kuppam, C.; Parikh, P.; Nikolova, M.P.; Chavali, M. Plant-based meat analogue (PBMA) as a sustainable food: A concise review. Eur. Food Res. Technol. 2021, 247, 2499–2526. [Google Scholar]
- Sogari, G.; Li, J.; Wang, Q.; Lefebvre, M.; Huang, S.; Mora, C.; Gómez, M.I. Toward a reduced meat diet: University North American students’ acceptance of a blended meat-mushroom burger. Meat Sci. 2022, 187, 108745. [Google Scholar]
- Yahya, F.; Ting, H.T. Effect of Different Ratios of Chicken Meat to Fresh Osyter Mushroom (Pleurotus sajor-caju) on the Physicochemical Properties and Sensory Acceptability of Sausages. Int. J. Food Agric. Nat. Resour. 2020, 1, 7–14. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).