A Comparative Study of the Antioxidant and Antidiabetic Properties of Fermented Camel (Camelus dromedarius) and Gir Cow (Bos primigenius indicus) Milk and the Production of Bioactive Peptides via In Vitro and In Silico Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture
2.2. Sample Preparation
2.3. Evaluation of the Antioxidant Activity of CM and GCM via the ABTS Assay
2.4. Assessing Antidiabetic Potentials in Fermented CM and GCM
2.4.1. Evaluation of α-Amylase Inhibition Activity
2.4.2. Evaluation of α-Glucosidase Inhibition Activity
2.5. Assessment of Proteolytic Activity
2.6. Purification and Characterization of Bioactive Peptides with Antioxidant and Antidiabetic Properties
2.6.1. Protein Profiling of Fermented CM and GCM via SDS–PAGE
2.6.2. 2D Gel Electrophoresis
2.6.3. Isoelectric Focusing
2.6.4. Peptide Separation and Fractionation Through RP-HPLC
2.7. Comprehensive Analysis of Isolated Peptides via RPLC/MS
2.7.1. Liquid Chromatography
2.7.2. Comprehensive Data Analysis and Peptide Identification
2.8. Evaluation of FTIR
2.9. Cellular and Inflammatory Response Assessments
2.9.1. Cell Culture
2.9.2. Cell Viability
2.9.3. NO Production
2.9.4. Evaluation of TNF-α, IL-6 and IL-1β Cytokine Levels
2.10. Visualization of Protein Biomarkers via CLSM
2.11. Statistical Analysis
3. Results and Discussion
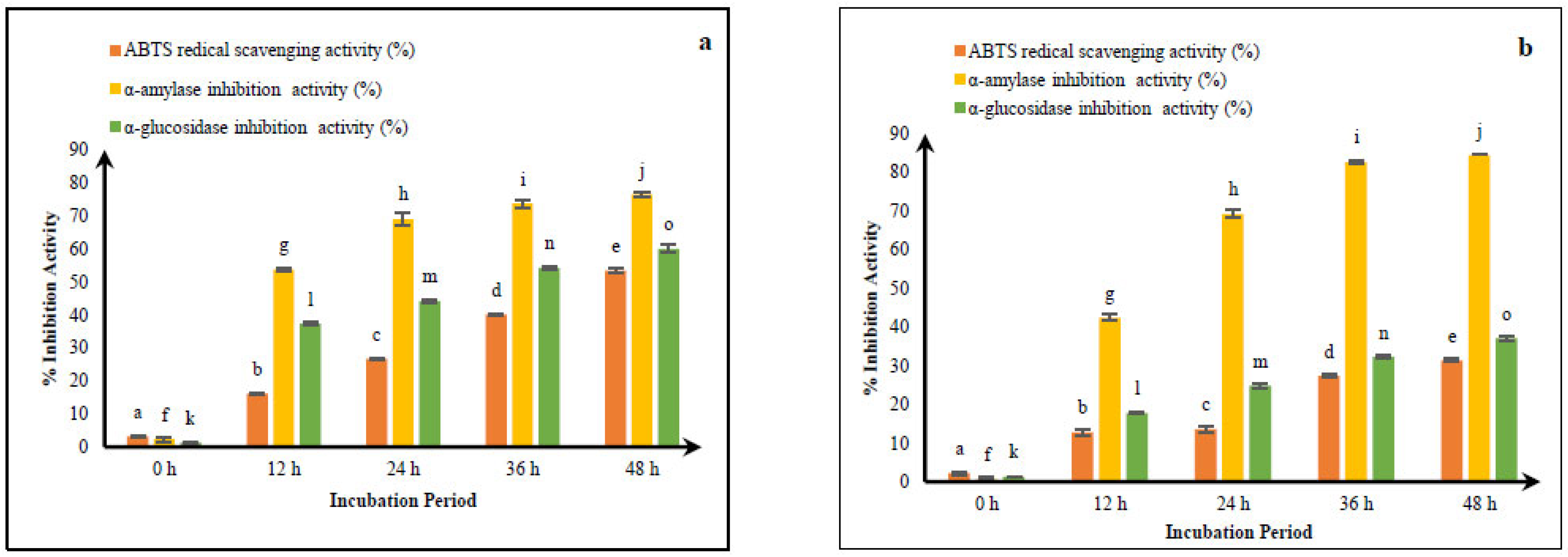
3.1. Comparative ABTS Radical Scavenging of Fermented CM and GCM
3.2. Antidiabetic Activity of Fermented CM and GCM
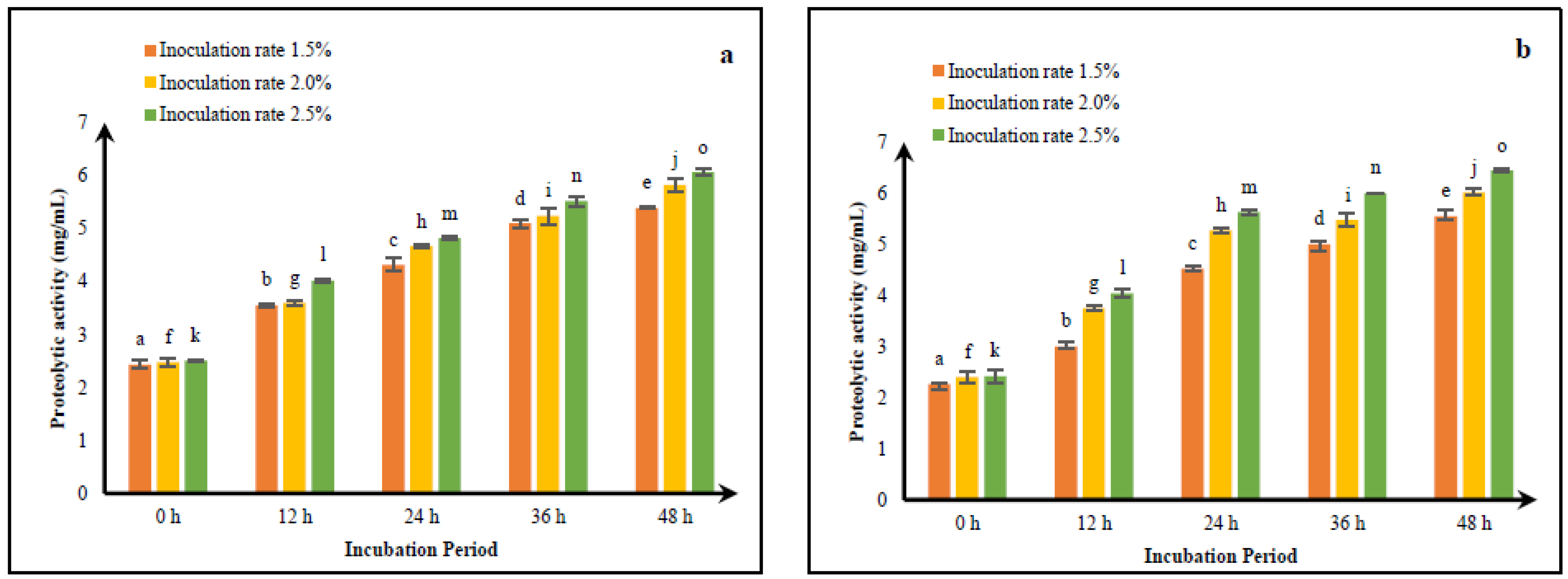
3.3. Optimizing Fermentation Processes of CM and GCM for Improving Peptide Yield
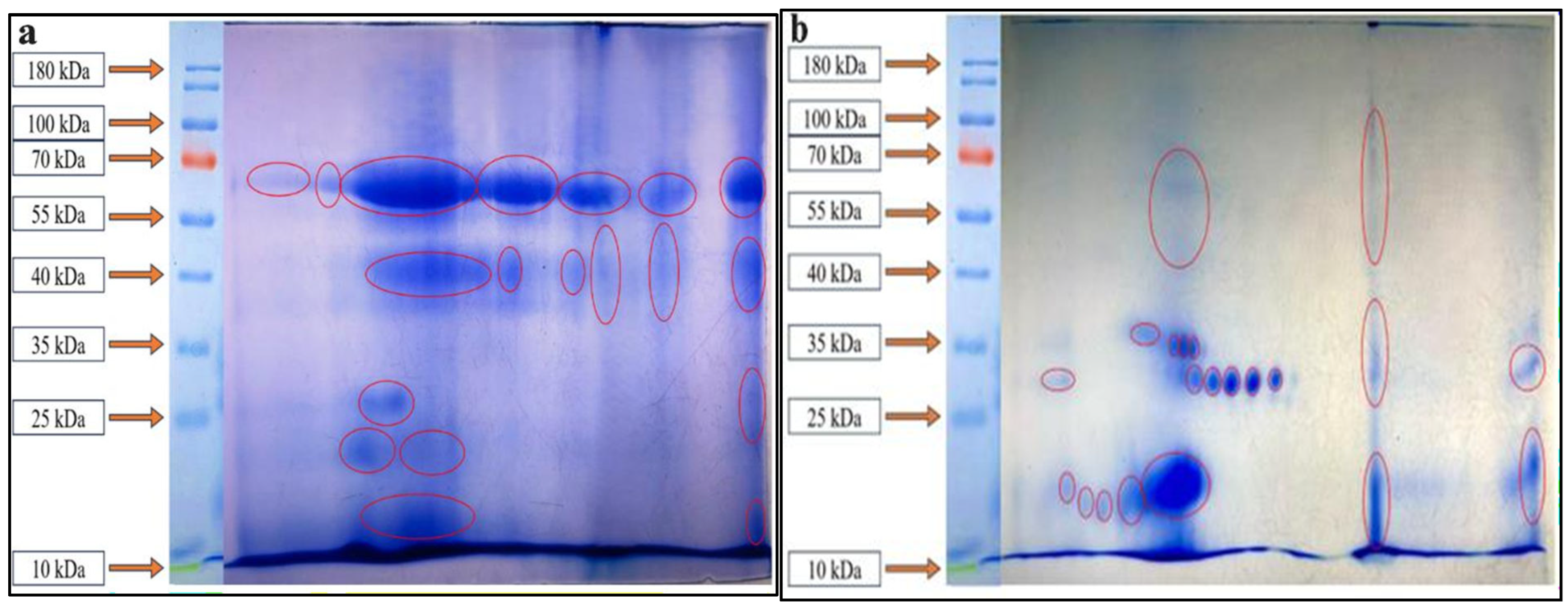
3.4. SDS–PAGE Profiling of Proteins from Fermented CM and GCM
3.5. Characterization of Fermented CM and GCM via 2D Gel Electrophoresis
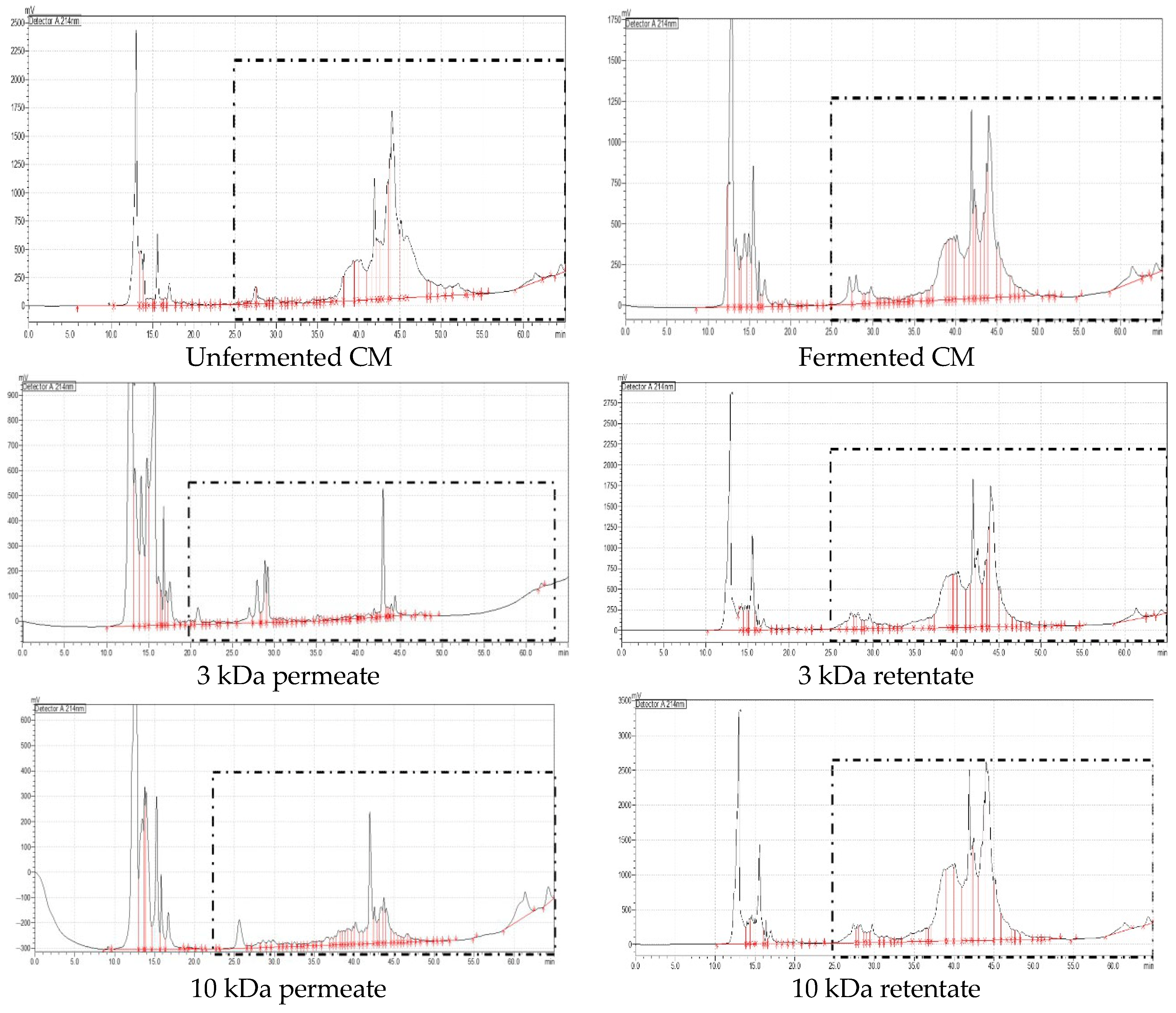
3.6. Evaluation of the Antioxidant and Antidiabetic Activities of UF Fractions of Fermented CM and GCM and RP-HPLC Analysis
3.7. FTIR Analysis of Fermented CM and GCM
3.8. Comprehensive Purification Peptide Analysis Through RP–LC–MS
3.9. Effects of Fermented CM and GCM on the Viability of RAW 264.7 Cells
3.10. Effects of Fermented CM and GCM on LPS-Induced NO Production in RAW 264.7 Cells
3.11. Examination of Cytokine Levels in Raw264.7 Cells
3.12. Visualization of Protein Biomarkers of Fermented CMs and GCMs via CLSM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guyomarc’h, F.; Héquet, F.; Le Féon, S.; Leconte, N.; Garnier-Lambrouin, F.; Auberger, J.; Malnoë, C.; Pénicaud, C.; Gésan-Guiziou, G. Life cycle assessment to quantify the environmental performance of multiproducts food processing systems such as milk fractionation: Importance of subdivision and allocation. J. Food Eng. 2024, 380, 112147. [Google Scholar]
- Seifu, E. Recent advances on camel milk: Nutritional and health benefits and processing implications-A review. AIMS Agric. Food 2022, 7, 777–804. [Google Scholar] [CrossRef]
- Vincenzetti, S.; Cammertoni, N.; Rapaccetti, R.; Santini, G.; Klimanova, Y.; Zhang, J.J.; Polidori, P. Nutraceutical and functional properties of camelids’ milk. Beverages 2022, 8, 12. [Google Scholar] [CrossRef]
- Vitthalrao, B.K. Gir Cow Milk: The Wealth for Human Health. Acta Sci. Vet. Sci. 2021, 3, 9–12. [Google Scholar]
- Gaba, K.; Anand, S. Incorporation of probiotics and other functional ingredients in dairy fat-rich products: Benefits, challenges and opportunities. Dairy 2023, 4, 630–649. [Google Scholar] [CrossRef]
- Riane, K.; Sifour, M.; Ouled-Haddar, H.; Idoui, T.; Bounar, S.; Boussebt, S. Probiotic properties and antioxidant efficiency of Lactobacillus plantarum 15 isolated from milk. J. Microbiol. Biotechnol. Food Sci. 2021, 9, 516–520. [Google Scholar] [CrossRef]
- Singh, N.; Gaur, S. New insights into multifunctional aspects of milk-derived bioactive peptides: A review. Food Chem. Adv. 2024, 4, 100628. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Weiskirchen, R. The role of obesity in type 2 diabetes mellitus—An overview. Int. J. Mol. Sci. 2024, 25, 1882. [Google Scholar] [CrossRef]
- Rais, N.; Ved, A.; Ahmad, R.; Parveen, A. Oxidative Stress and Diabetes Mellitus: Unraveling the Intricate Connection: A Comprehensive Review. J. Pharm. Res. Int. 2024, 36, 13–30. [Google Scholar] [CrossRef]
- Wang, C.; Luo, D.; Zheng, L.; Zhao, M. Anti-diabetic mechanism and potential bioactive peptides of casein hydrolysates in STZ/HFD-induced diabetic rats. J. Sci. Food Agric. 2024, 104, 2947–2958. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, K.; Jia, X.; Fu, C.; Yu, H.; Wang, Y. Antioxidant peptides, the guardian of life from oxidative stress. Med. Res. Rev. 2024, 44, 275–364. [Google Scholar] [CrossRef] [PubMed]
- Sah, B.N.P.; Vasiljevic, T.; McKechnie, S.; Donkor, O.N. Effect of probiotics on antioxidant and antimutagenic activities of crude peptide extract from yogurt. Food Chem. 2014, 156, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Telagari, M.; Hullatti, K. In vitro α-amylase and α-glucosidase inhibitory activity of Adiantum caudatum Linn. and Celosia argentea Linn. extracts and fractions. Indian J. Pharmacol. 2015, 47, 425. [Google Scholar]
- Shai, L.J.; Magano, S.R.; Lebelo, S.L.; Mogale, A.M. Inhibitory effects of five medicinal plants on rat alpha-glucosidase: Comparison with their effects on yeast alpha-glucosidase. J. Med. Plants Res. 2011, 5, 2863–2867. [Google Scholar]
- Hati, S.; Sreeja, V.; Solanki, D.; Prajapati, J.B. Influence of proteolytic Lactobacilli on ACE inhibitory activity and release of BAPs. Indian J. Dairy Sci. 2015, 68, 1–8. [Google Scholar]
- Gibbs, B.F.; Zougman, A.; Masse, R.; Mulligan, C. Production and characterization of bioactive peptides from soy hydrolysate and soy fermented food. Food Res. Int. 2004, 37, 123–131. [Google Scholar] [CrossRef]
- Carrasco-Castilla, J.; Hernandez, A.; Alvarez, A.J.; Jimenez-Martinez, C.; Jacinto-Hernandez, C.; Alaiz, M.; Girón-Calle, J.; Vioque, J.; Dávila-Ortiz, G. Antioxidant and metal chelating activities of Phaseolus vulgaris L. var. Jamapa protein isolates, phaseolin and lectin hydrolysates. Food Chem. 2012, 131, 1157–1164. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, N.; Yang, J.; Bu, D.; Wang, J.; Ma, L.; Sun, P. Animal species milk identification by comparison of two-dimensional gel map profile and mass spectrometry approach. Int. Dairy J. 2014, 35, 15–20. [Google Scholar] [CrossRef]
- Panchal, G.; Hati, S.; Sakure, A. Characterization and production of novel antioxidative peptides derived from fermented goat milk by L. fermentum. Food Sci. Technol. 2020, 119, 108887. [Google Scholar] [CrossRef]
- Khakhariya, R.; Sakure, A.A.; Maurya, R.; Bishnoi, M.; Kondepudi, K.K.; Padhi, S.; Rai, A.K.; Liu, Z.; Patil, G.B.; Mankad, M.; et al. A comparative study of fermented buffalo and camel milk with anti-inflammatory, ACE-inhibitory and anti-diabetic properties and release of bioactive peptides with molecular interactions: In vitro, in silico, and molecular study. Food Biosci. 2023, 52, 102373. [Google Scholar] [CrossRef]
- Pipaliya, R.; Basaiawmoit, B.; Sakure, A.A.; Maurya, R.; Bishnoi, M.; Kondepudi, K.K.; Singh, B.P.; Paul, S.; Liu, Z.; Sarkar, P.; et al. Peptidomics-based identification of antihypertensive and antidiabetic peptides from sheep milk fermented using Limosilactobacillus fermentum KGL4 MTCC 25515 with anti-inflammatory activity: In silico, in vitro, and molecular docking studies. Front. Chem. 2024, 12, 1389846. [Google Scholar] [CrossRef] [PubMed]
- Khare, P.; Maurya, R.; Bhatia, R.; Mangal, P.; Singh, J.; Podili, K.; Bishnoi, M.; Kondepudi, K.K. Polyphenol-rich extracts of finger millet and kodo millet ameliorate high-fat diet-induced metabolic alterations. Food Funct. 2020, 11, 9833–9847. [Google Scholar] [CrossRef] [PubMed]
- Steel, R.G.D.; Torrie, J.H. Principles and Procedure of Statistics—A Biometrical Approach; McGraw Hill Kogakusha Ltd.: Tokyo, Japan, 1980; p. 137. [Google Scholar]
- Patel, D.; Sakure, A.; Lodha, D.; Basaiawmoit, B.; Maurya, R.; Das, S.; Bishnoi, M.; Kondepudi, K.K.; Hati, S. Significance of Lactobacillus fermentum on antioxidative and anti-inflammatory activities and ultrafiltration peptide fractions as potential sources of antioxidative peptides from fermented camel milk (Indian breed). J. Am. Nutr. Assoc. 2023, 42, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Bais, B.; Singh, R.; Tak, L.; Gorachiya, P.R.; Kumari, R. Determination of the bioactive potential (antioxidant activity) of camel milk during the fermentation process. J. Camel Pract. Res. 2018, 25, 131–134. [Google Scholar] [CrossRef]
- Al-Nassir, N.S.; Sakr, S.S. In Vitro Digestibility Assessment of Whey from Goat and Camel Milk Fermented with Lactobacillus helveticus for Use as a Base in Formulating Follow-On Formula. Foods 2024, 13, 570. [Google Scholar] [CrossRef]
- Khakhariya, R.; Basaiawmoit, B.; Sakure, A.A.; Maurya, R.; Bishnoi, M.; Kondepudi, K.K.; Padhi, S.; Rai, A.K.; Liu, Z.; Hati, S. Production and characterization of ACE inhibitory and anti-diabetic peptides from buffalo and camel milk fermented with Lactobacillus and yeast: A comparative analysis with in vitro, in silico, and molecular interaction study. Foods 2023, 12, 2006. [Google Scholar] [CrossRef]
- Marcia, J.A.; Aleman, R.S.; Kazemzadeh, S.; Manrique Fernández, V.; Martín Vertedor, D.; Kayanush, A.; Montero Fernández, I. Isolated Fraction of Gastric-Digested Camel Milk Yogurt with Carao (Cassia grandis) Pulp Fortification Enhances the Anti-Inflammatory Properties of HT-29 Human Intestinal Epithelial Cells. Pharmaceuticals 2023, 16, 1032. [Google Scholar] [CrossRef]
- Shukla, P.; Sakure, A.; Pipaliya, R.; Basaiawmoit, B.; Maurya, R.; Bishnoi, M.; Kondepudi, K.K.; Hati, S. Exploring the potential of Lacticaseibacillus paracasei M11 on antidiabetic, anti-inflammatory, and ACE inhibitory effects of fermented dromedary camel milk (Camelus dromedarius) and the release of antidiabetic and antihypertensive peptides. J. Food Biochem. 2022, 46, e14449. [Google Scholar] [CrossRef]
- Jin, Y.; Yan, J.; Yu, Y.; Qi, Y. Screening and identification of DPP-IV inhibitory peptides from deer skin hydrolysates by an integrated approach of LC–MS/MS and in silico analysis. J. Funct. Foods 2015, 18, 344–357. [Google Scholar] [CrossRef]
- Suetsuna, K. Separation and Identification of Antioxidant Peptides from Proteolytic Digest of Dried Bonito. Nippon Suisan Gakkaishi 1999, 65, 92–96. [Google Scholar] [CrossRef]
- Saito, K.; Jin, D.H.; Ogawa, T.; Muramoto, K.; Hatakeyama, E.; Yasuhara, T.; Nokihara, K. Antioxidative properties of tripeptide libraries prepared by combinatorial chemistry. J. Agric. Food Chem. 2003, 51, 3668–3674. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xiong, Y.L.; Zhai, J.; Zhu, H.; Dziubla, T. Fractionation and evaluation of radical scavenging peptides from in vitro digests of buckwheat protein. Food Chem. 2010, 118, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Anna, T.; Alexey, K.; Anna, B.; Vyacheslav, K.; Mikhail, T.; Ulia, M. Effect of in vitro gastrointestinal digestion on bioactivity of poultry protein hydrolysate. Curr. Res. Nutr. Food Sci. J. 2016, 4, 77–86. [Google Scholar] [CrossRef]
- Mine, Y. Egg proteins and peptides in human health-chemistry, bioactivity, and production. Curr. Pharm. Des. 2007, 13, 875–884. [Google Scholar] [CrossRef]
- Chen, H.M.; Muramoto, K.; Yamauchi, F. Structural analysis of antioxidative peptides from Soybean β-Conglycinin. J. Agric. Food Chem. 1995, 43, 574–578. [Google Scholar] [CrossRef]
- Huang, W.Y.; Majumder, K.; Wu, J. Oxygen radical absorbance capacity of peptides from egg white protein ovotransferrin and their interaction with phytochemicals. Food Chem. 2010, 123, 635–641. [Google Scholar] [CrossRef]
- Chen, H.M.; Muramoto, K.; Yamauchi, F.; Nokihara, K. Antioxidant activity of designed peptides based on the antioxidative peptide isolated from digests of a soybean protein. J. Agric. Food Chem. 1996, 44, 2619–2623. [Google Scholar] [CrossRef]
- Suetsuna, K.; Ukeda, H.; Ochi, H. Isolation and characterization of free radical scavenging activities peptides derived from casein. J. Nutr. Biochem. 2000, 11, 128–131. [Google Scholar] [CrossRef]
- Tian, M.; Fang, B.; Jiang, L.; Guo, H.; Cui, J.; Ren, F. Structure-activity relationship of a series of antioxidant tripeptides derived from β-Lactoglobulin using QSAR modeling. Dairy Sci. Technol. 2015, 95, 451–463. [Google Scholar] [CrossRef]
- Liu, W.; Gu, R.; Lin, F.; Lu, J.; Yi, W.; Ma, Y.; Cai, M. Isolation and identification of antioxidative peptides from pilot-scale black-bone silky fowl (Gallus gallus domesticus Brisson) muscle oligopeptides. J. Sci. Food Agric. 2013, 93, 2782–2788. [Google Scholar] [CrossRef]
- Wu, J.; Yi, Z.; Chen, X.; Pan, N.; Su, X.; Shi, H.; Liu, Z. Screening and mechanistic study of antioxidant peptides from Bangia fusco-purpurea targeting the Keap1–Nrf2 pathway. Food Biosci. 2024, 59, 104122. [Google Scholar] [CrossRef]
- Yokomizo, A.; Takenaka, Y.; Takenaka, T. Antioxidative activity of peptides prepared from okara protein. Food Sci. Technol. Res. 2002, 8, 357–359. [Google Scholar] [CrossRef]
- De Gobba, C.; Tompa, G.; Otte, J. Bioactive peptides from caseins released by cold active proteolytic enzymes from Arsukibacterium ikkense. Food Chem. 2014, 165, 205–215. [Google Scholar] [CrossRef]
- Tonolo, F.; Fiorese, F.; Moretto, L.; Folda, A.; Scalcon, V.; Grinzato, A.; Rigobello, M.P. Identification of new peptides from fermented milk showing antioxidant properties: Mechanism of action. Antioxidants 2020, 9, 117. [Google Scholar] [CrossRef]
- Ren, Q.; Zhao, H.; Hu, H.; Zhou, Z.; Yang, Z.; Yang, Z. Characterization of a novel metalloprotease produced by Bacillus subtilis JQ-2 and casein-derived bioactive peptides by the protease. Int. Dairy J. 2024, 151, 105866. [Google Scholar] [CrossRef]
- Beermann, C.; Euler, M.; Herzberg, J.; Stahl, B. Antioxidative capacity of enzymatically released peptides from soybean protein isolate. Eur. Food Res. Technol. 2009, 229, 637–646. [Google Scholar] [CrossRef]
- Silva, S.V.; Pihlanto, A.; Malcata, F.X. Bioactive peptides in ovine and caprine cheeselike systems prepared with proteases from Cynara cardunculus. J. Dairy Sci. 2006, 89, 3336–3344. [Google Scholar] [CrossRef]
- Sowmya, K.; Mala, D.; Bhat, M.I.; Kumar, N.; Bajaj, R.K.; Kapila, S.; Kapila, R. Bioaccessible milk casein-derived tripeptide (LLY) mediates overlapping anti-inflammatory and antioxidative effects under cellular (Caco-2) and in vivo milieu. J. Nutr. Biochem. 2018, 62, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Lan, V.T.T.; Ito, K.; Ohno, M.; Motoyama, T.; Ito, S.; Kawarasaki, Y. Analyzing a dipeptide library to identify human dipeptidyl peptidase IV inhibitor. Food Chem. 2015, 175, 66–73. [Google Scholar] [CrossRef]
- Bella, A.M., Jr.; Erickson, R.H.; Kim, Y.S. Rat intestinal brush border membrane dipeptidyl-aminopeptidase IV: Kinetic properties and substrate specificities of the purified enzyme. Arch. Biochem. Biophys. 1982, 218, 156–162. [Google Scholar] [CrossRef]
- Hatanaka, T.; Inoue, Y.; Arima, J.; Kumagai, Y.; Usuki, H.; Kawakami, K.; Mukaihara, T. Production of dipeptidyl peptidase IV inhibitory peptides from defatted rice bran. Food Chem. 2012, 134, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Nongonierma, A.B.; Mooney, C.; Shields, D.C.; FitzGerald, R.J. Inhibition of dipeptidyl peptidase IV and xanthine oxidase by amino acids and dipeptides. Food Chem. 2013, 141, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Gallego, M.; Aristoy, M.C.; Toldrá, F. Dipeptidyl peptidase IV inhibitory peptides generated in Spanish dry-cured ham. Meat Sci. 2014, 96, 757–761. [Google Scholar] [CrossRef]
- Neves, A.C.; Harnedy, P.A.; O’Keeffe, M.B.; FitzGerald, R.J. Bioactive peptides from Atlantic salmon (Salmo salar) with angiotensin converting enzyme and dipeptidyl peptidase IV inhibitory and antioxidant activities. Food Chem. 2017, 218, 396–405. [Google Scholar] [CrossRef]
- Ren, Y.; Liang, K.; Jin, Y.; Zhang, M.; Chen, Y.; Wu, H.; Lai, F. Identification and characterization of two novel α-glucosidase inhibitory oligopeptides from hemp (Cannabis sativa L.) seed protein. J. Funct. Foods 2016, 26, 439–450. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Mooney, C.; Shields, D.C.; FitzGerald, R.J. In silico approaches to predict the potential of milk protein-derived peptides as dipeptidyl peptidase IV (DPP-IV) inhibitors. Peptides 2014, 57, 43–51. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Afsar, S.; Day, L. Differences in the microstructure and rheological properties of low-fat yogurts from goat, sheep, and cow milk. Food Res. Int. 2018, 108, 423–429. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Schwendel, H.; Harland, D.; Day, L. Differences in the yogurt gel microstructure and physicochemical properties of bovine milk containing A1A1 and A2A2 β-casein phenotypes. Food Res. Int. 2018, 112, 217–224. [Google Scholar] [CrossRef]

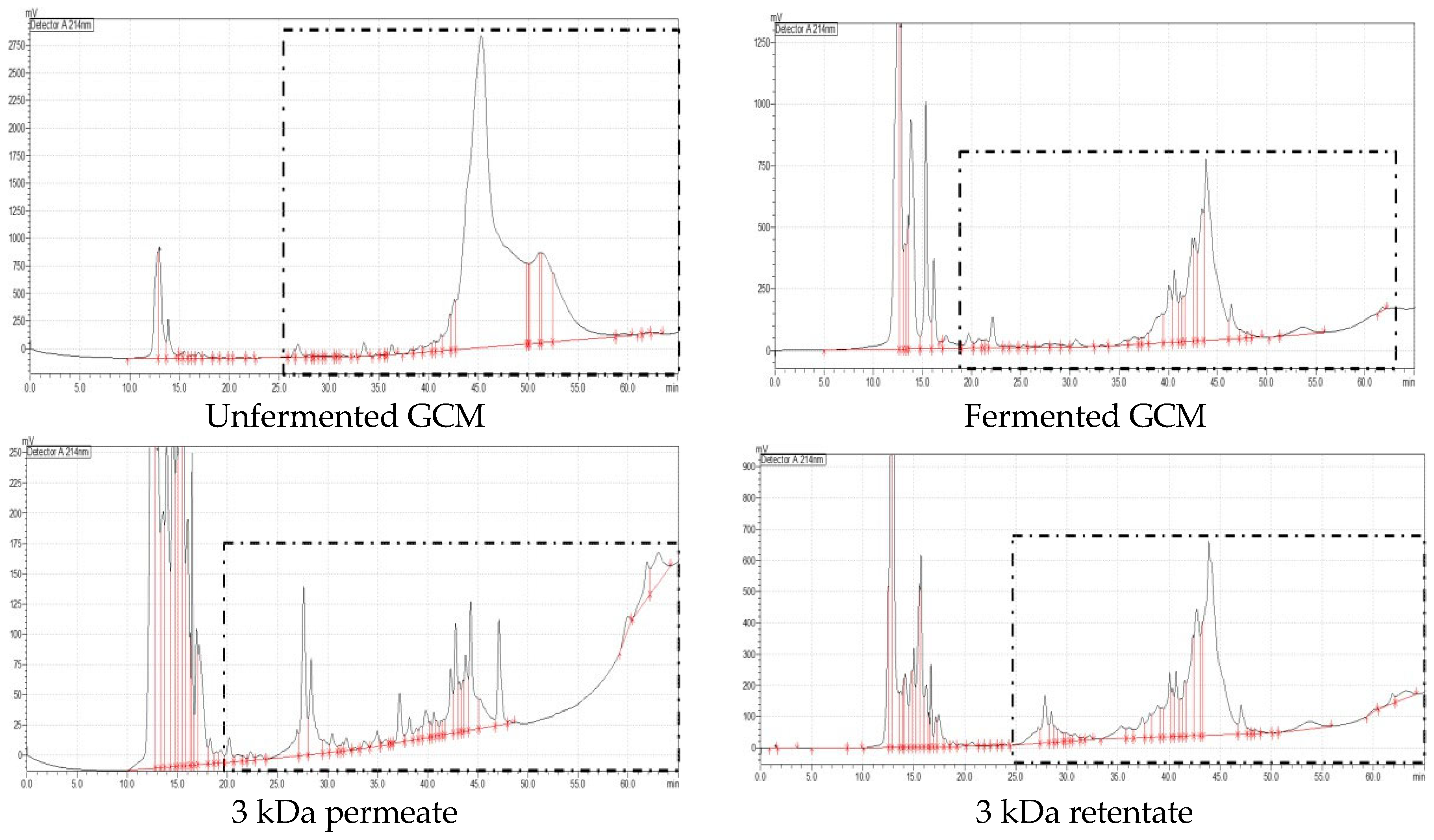


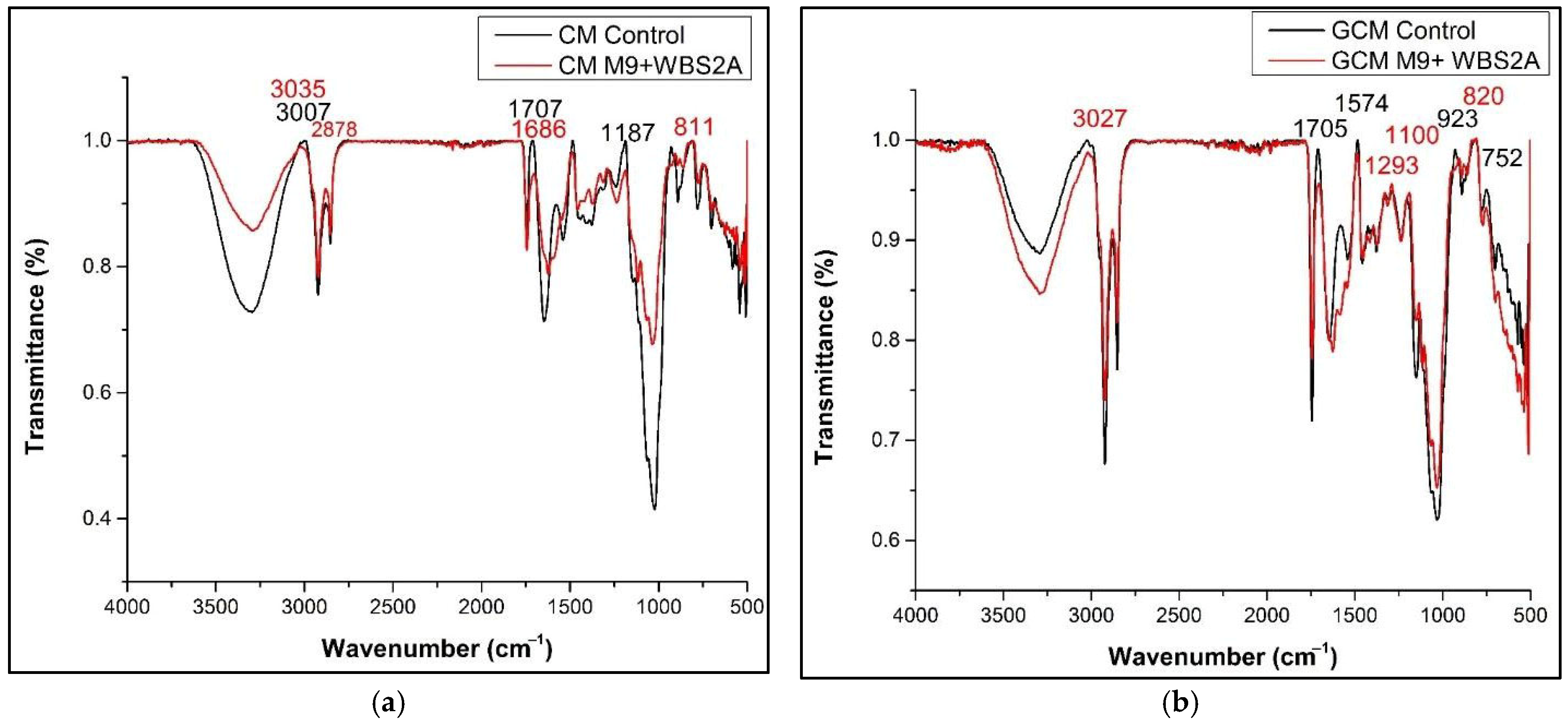

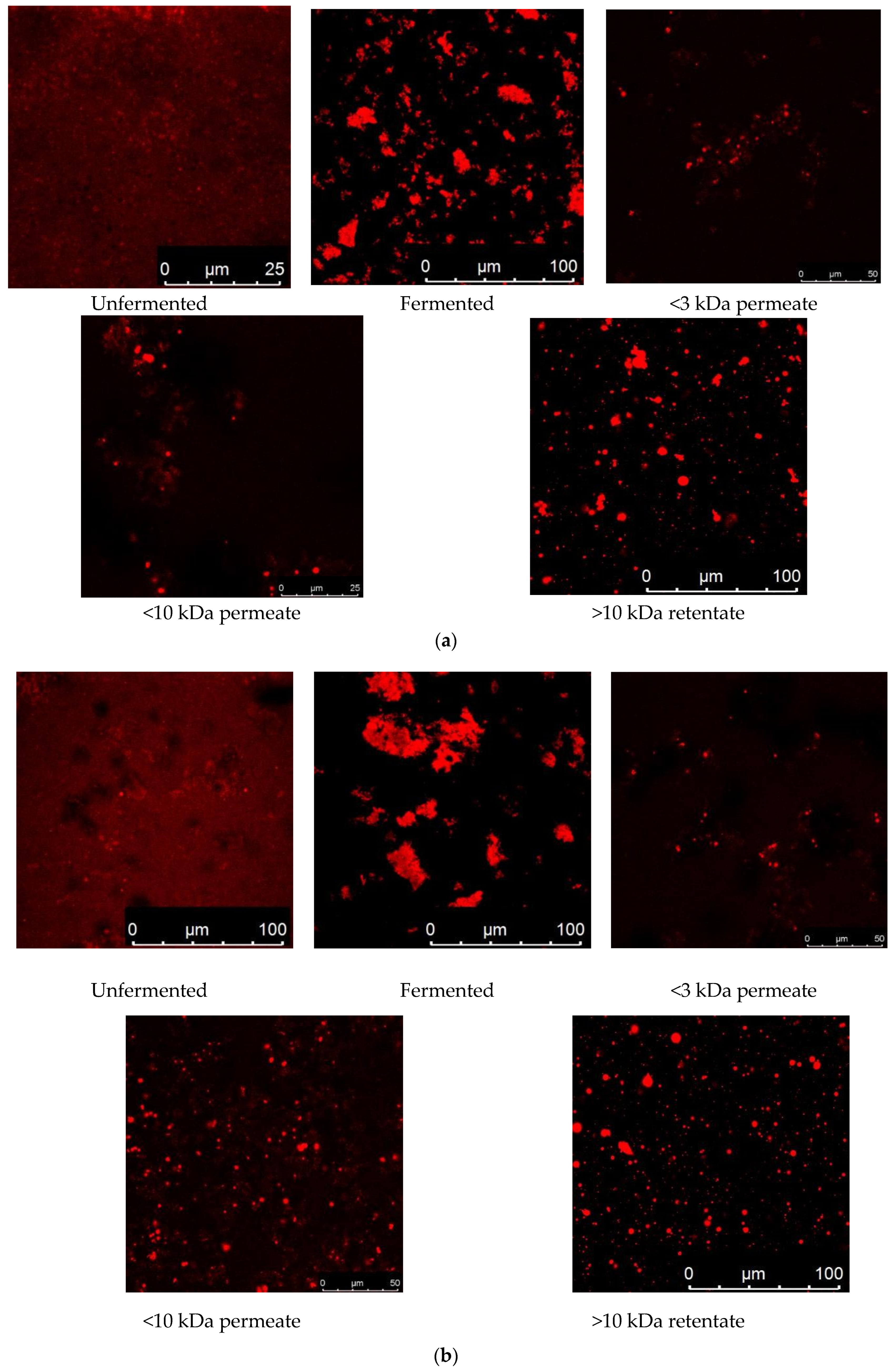
| Milk | Sample | Number of Peaks | Retention Time (min) |
|---|---|---|---|
| Camel milk | <3 kDa | 38 | 24.97 to 48.45 |
| >3 kDa | 48 | 25.89 to 64.34 | |
| <10 kDa | 50 | 25.60 to 64.30 | |
| >10 kDa | 39 | 27.35 to 64.39 | |
| Gir cow milk | <3 kDa | 31 | 26.94 to 47.10 |
| >3 kDa | 35 | 27.16 to 63.20 | |
| <10 kDa | 32 | 27.12 to 49.15 | |
| >10 kDa | 37 | 23.09 to 63.10 |
| Sequences | Peptide Ranking Score | Mol. wt. | Prediction | Iso-Electric Point | Net Charge at pH 7 | Hydro-Phobicity | Hydro-Pathicity | Hydro-Philicity |
|---|---|---|---|---|---|---|---|---|
| MKLFFPALLSLGALGLCLAASK | 1.00 | 2265.19 | Non-Toxin | 9.36 | 2 | 0.17 | 1.45 | −0.7 |
| SQPWGLALLLLLLPGTLRAAESHR | 0.83 | 2613.48 | Non-Toxin | 9.95 | 1.5 | −0.02 | 0.39 | −0.43 |
| PSGFQLFGSPAGQKDLLFK | 0.80 | 2037.63 | Non-Toxin | 8.94 | 1 | −0.04 | −0.14 | −0.18 |
| EGIDYWLAHKPLCSEK | 0.68 | 1889.39 | Non-Toxin | 5.46 | −0.5 | −0.14 | −0.63 | 0.14 |
| LLGHLERGRGNLEWK | 0.53 | 1778.29 | Non-Toxin | 9.1 | 1.5 | −0.26 | −0.9 | 0.27 |
| Sequences | Peptide Ranking Score | Mol. wt. | Prediction | Iso- Electric Point | Net Charge at pH 7 | Hydro-Phobicity | Hydro-Pathicity | Hydro-Philicity |
|---|---|---|---|---|---|---|---|---|
| MVMVLSPLLLVFILGLGLTPVAPAQDDYR | 1.00 | 3143.31 | Non-Toxin | 4.21 | −1 | 0.14 | 1.2 | −0.68 |
| GAMMKSFFLVVTILALTLPFLGAQEQNQEQPIR | 0.99 | 3692.92 | Non-Toxin | 6.49 | 0 | 0.01 | 0.45 | −0.45 |
| MMSFVSLLLVGILFHATQAEQLTKCEVFR | 0.98 | 3313.45 | Non-Toxin | 7.07 | 0.5 | 0.04 | 0.88 | −0.54 |
| MMSFVSLLLVGILFHATQAEQLTK | 0.97 | 2678.63 | Non-Toxin | 7.1 | 0.5 | 0.1 | 1 | −0.7 |
| MKCLLLALALTCGAQALIVTQTMK | 0.97 | 2535.6 | Non-Toxin | 8.98 | 2 | 0.09 | 1.26 | −0.65 |
| MKCLLLALALTCGAQALIVTQTMKGLDIQK | 0.94 | 3190.47 | Non-Toxin | 8.94 | 2 | 0.03 | 0.91 | −0.43 |
| MMSFVSLLLVGILFHATQAEQLTKCEVFRELK | 0.94 | 3683.95 | Non-Toxin | 7.07 | 0.5 | 0 | 0.68 | −0.36 |
| MPGPLRLFPQIK | 0.79 | 1396.94 | Non-Toxin | 11.01 | 2 | −0.08 | −0.03 | −0.25 |
| WVTFISLLLLFSSAYSRGVFRR | 0.78 | 2619.43 | Non-Toxin | 11.72 | 3 | 0 | 0.83 | −0.72 |
| NDEYCFNMMKNR | 0.76 | 1564.93 | Non-Toxin | 6.38 | 0 | −0.41 | −1.51 | 0.35 |
| GRNDEYCFNMMKNR | 0.76 | 1778.2 | Non-Toxin | 8.53 | 1 | −0.47 | −1.64 | 0.51 |
| KWVTFISLLLLFSSAYSRGVFR | 0.74 | 2591.42 | Non-Toxin | 11.01 | 3 | 0.03 | 0.86 | −0.72 |
| ELRAMKVLILACLVALALAR | 0.73 | 2168.09 | Non-Toxin | 9.55 | 2 | 0.06 | 1.63 | −0.42 |
| CLLLALALTCGAQALIVTQTMKGLDIQK | 0.72 | 2931.07 | Non-Toxin | 8.36 | 1 | 0.07 | 1.04 | −0.52 |
| KWVTFISLLLLFSSAYSR | 0.69 | 2131.81 | Non-Toxin | 10.01 | 2 | 0.06 | 0.93 | −0.83 |
| PKHPIKHQGLPQEVLNENLLR | 0.66 | 2461.21 | Non-Toxin | 8.94 | 2 | −0.25 | −1 | 0.2 |
| DDPHACYSTVFDKLKHLVDEPQNLIK | 0.65 | 3026.8 | Non-Toxin | 5.31 | −1 | −0.2 | −0.66 | 0.26 |
| MKWVTFISLLLLFSSAYSR | 0.65 | 2263.02 | Non-Toxin | 10.01 | 2 | 0.08 | 0.98 | −0.85 |
| DMPIQAFLLYQEPVLGPVR | 0.55 | 2186.89 | Non-Toxin | 4.38 | −1 | 0 | 0.31 | −0.39 |
| KLLILTCLVAVALARPK | 0.55 | 1822.63 | Non-Toxin | 10.07 | 3 | 0.06 | 1.48 | −0.45 |
| MKLLILTCLVAVALARPK | 0.52 | 1953.84 | Non-Toxin | 10.07 | 3 | 0.07 | 1.51 | −0.5 |
| NYQEAKDAFLGSFLYEYSRR | 0.52 | 2457.96 | Non-Toxin | 6.52 | 0 | −0.27 | −0.97 | 0.13 |
| Sequences | ID | Matched Sequences | Molecular Mass | Source | Scavenging Activity | Reference |
|---|---|---|---|---|---|---|
| SQPWGLALLLLLLPGTLRAAESHR | 8042 | PWG | 358.39 | Soybean protein | ABTS | [32] |
| 8190 | PW | 301.34 | Buckwheat protein | ABTS | [33] | |
| 9082 | WG | 261.28 | Poultry Protein | TRAP | [34] | |
| PSGFQLFGSPAGQKDLLFK | 8134 | KD | 261.27 | Dried bonito | PRAA | [31] |
| EGIDYWLAHKPLCSEK | 7793 | AHK | 354.40 | Egg Proteins | ROS | [35] |
| 7886 | AH | 226.23 | Soybean | Ferric thiocyanate | [36] | |
| 8218 | KP | 243.30 | Egg white protein | ROS | [37] | |
| LLGHLERGRGNLEWK | 3317 | HL | 268.31 | Soybean protein | Ferric thiocyanate | [38] |
| Sequences | ID | Matched Sequences | Molecular Mass | Source | Scavenging Activity | Reference |
|---|---|---|---|---|---|---|
| MVMVLSPLLLVFILGLGLTPVAPAQDDYR | 10749 | LT | 232.27 | Black-bone silky fowl | DPPH | [41] |
| GAMMKSFFLVVTILALTLPFLGAQEQNQEQPIR | 8215 | IR | 287.35 | Egg white protein | ROS | [37] |
| 9086 | MM | 280.4 | Poultry Protein | TRAP | [34] | |
| 10648 | TIL | 345.43 | Bangia fusco-purpurea | ROS | [42] | |
| 10749 | LT | 232.27 | Black-bone silky fowl | DPPH | [41] | |
| MKCLLLALALTCGAQALIVTQTMK | 9162 | KCL | 362.48 | β-lactoglobulin | FRAP | [40] |
| 9163 | LTC | 335.41 | β-lactoglobulin | FRAP | [40] | |
| 9164 | CGA | 249.28 | β-lactoglobulin | FRAP | [40] | |
| 10749 | LT | 232.27 | Black-bone silky fowl | DPPH | [41] | |
| MMSFVSLLLVGILFHATQAEQLTKCEVFRELK | 7888 | EL | 260.28 | Casein | SOSA | [39] |
| 8217 | LK | 259.34 | Egg white protein | ROS | [37] | |
| 9086 | MM | 280.4 | Poultry Protein | TRAP | [34] | |
| 10749 | LT | 232.27 | Black-bone silky fowl | DPPH | [41] | |
| WVTFISLLLLFSSAYSRGVFRR | 7866 | AY | 252.26 | Okara protein | Ferric thiocyanate | [43] |
| NDEYCFNMMKNR | 9086 | MM | 280.4 | Poultry Protein | TRAP | [34] |
| KWVTFISLLLLFSSAYSRGVFR | 7866 | AY | 252.26 | Okara protein | Ferric thiocyanate | [43] |
| ELRAMKVLILACLVALALAR | 7888 | EL | 260.28 | Casein | SOSA | [39] |
| 9355 | LAC | 305.39 | β-lactoglobulin | FRAP | [39] | |
| 9359 | CLV | 333.44 | β-lactoglobulin | FRAP | [40] | |
| CLLLALALTCGAQALIVTQTMKGLDIQK | 9163 | LTC | 335.41 | β-lactoglobulin | FRAP | [40] |
| 9164 | CGA | 249.28 | β-lactoglobulin | FRAP | [40] | |
| 10749 | LT | 232.27 | Black-bone silky fowl | DPPH | [41] | |
| KWVTFISLLLLFSSAYSR | 7866 | AY | 252.26 | Okara protein | Ferric thiocyanate | [43] |
| PKHPIKHQGLPQEVLNENLLR | 8484 | LLR | 400.51 | Casein | ABTS | [44] |
| 9363 | NEN | 375.33 | β-lactoglobulin | FRAP | [40] | |
| 9901 | KHQGLPQEVLNENLL | 1731.94 | Milk protein | ROS | [45] | |
| 10505 | GLPQEVLN | 868.97 | Casein | DPPH | [46] | |
| DDPHACYSTVFDKLKHLVDEPQNLIK | 3317 | HL | 268.31 | Soybean protein | Ferric thiocyanate | [38] |
| 8022 | PHA | 323.34 | Soybean protein | ABTS | [32] | |
| 8217 | LK | 259.34 | Egg white protein | ROS | [37] | |
| MKWVTFISLLLLFSSAYSR | 7866 | AY | 252.26 | Okara protein | Ferric thiocyanate | [43] |
| DMPIQAFLLYQEPVLGPVR | 7872 | LY | 294.34 | Soybean protein isolate | ABTS | [47] |
| 7879 | YQEPVLGP | 901.99 | Ovine cheese, caprine cheese | ABTS | [48] | |
| 10168 | LLY | 407.50 | Milk casein | ABTS | [49] | |
| MKLLILTCLVAVALARPK | 9163 | LTC | 335.41 | β-lactoglobulin | FRAP | [40] |
| 9359 | CLV | 333.44 | β-lactoglobulin | FRAP | [40] | |
| 10749 | LT | 232.27 | Black-bone silky fowl | DPPH | [41] | |
| NYQEAKDAFLGSFLYEYSRR | 7872 | LY | 294.34 | Soybean protein isolate | ABTS | [47] |
| 7928 | YEY | 473.47 | Soybean protein | ABTS | [32] | |
| 8130 | EAK | 346.37 | Dried bonito | PRAA | [31] | |
| 8134 | KD | 261.27 | Dried bonito | PRAA | [31] |
| Sequences | ID | Matched Sequences | Molecular Mass | Source | Inhibition Properties | Reference |
|---|---|---|---|---|---|---|
| MKLFFPALLSLGALGLCLAASK | 3175 | LA | 202.25 | Rat intestinal brush border membrane | Dipeptidyl-aminopeptidase IV | [51] |
| 3179 | PA | 186.20 | Rat intestinal brush border membrane | Dipeptidyl-aminopeptidase IV | [51] | |
| 3182 | LL | 244.32 | Rat intestinal brush border membrane | Dipeptidyl-aminopeptidase IV | [51] | |
| 8506 | FP | 262.30 | Rice bran | Dipeptidyl-aminopeptidase IV | [52] | |
| 8559 | AL | 202.25 | Milk protein | α-glucosidase | [53] | |
| 8560 | SL | 218.24 | Milk protein | α-glucosidase | [53] | |
| 8561 | GL | 188.22 | Milk protein | α-glucosidase | [53] | |
| 8637 | AA | 160.17 | Spanish dry-cured ham | Dipeptidyl-aminopeptidase IV | [54] | |
| 8762 | AS | 176.17 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8831 | MK | 277.38 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8894 | SK | 233.26 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 10028 | FF | 312.36 | Atlantic salmon | Dipeptidyl-aminopeptidase IV | [55] | |
| SQPWGLALLLLLLPGTLRAAESHR | 10360 | LR | 287.35 | Hemp seed protein | α-glucosidase | [56] |
| 3175 | LA | 202.25 | Rat intestinal brush border membrane | Dipeptidyl-aminopeptidase IV | [51] | |
| 3180 | LP | 228.28 | Rice bran | Dipeptidyl-aminopeptidase IV | [52] | |
| 3182 | LL | 244.32 | Rat intestinal brush border membrane | Dipeptidyl-aminopeptidase IV | [51] | |
| 8559 | AL | 202.25 | Milk protein | Dipeptidyl-aminopeptidase IV | [53] | |
| 8561 | GL | 188.22 | Milk protein | Dipeptidyl-aminopeptidase IV | [53] | |
| 8637 | AA | 160.17 | Spanish dry-cured ham | Dipeptidyl-aminopeptidase IV | [54] | |
| 8697 | WG | 261.27 | Milk protein | Dipeptidyl-aminopeptidase IV | [57] | |
| 8758 | AE | 218.20 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8773 | ES | 234.20 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8794 | HR | 311.33 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8855 | PG | 172.18 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8865 | PW | 301.33 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8892 | SH | 242.23 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8905 | TL | 232.27 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| PSGFQLFGSPAGQKDLLFK | 3179 | PA | 186.20 | Rat intestinal brush border membrane | Dipeptidyl-aminopeptidase IV | [51] |
| 3182 | LL | 244.32 | Rat intestinal brush border membrane | Dipeptidyl-aminopeptidase IV | [51] | |
| 8505 | SP | 202.20 | Rice bran | Dipeptidyl-aminopeptidase IV | [52] | |
| 8760 | AG | 146.14 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8779 | FQ | 293.31 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8782 | GF | 222.23 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8862 | PS | 202.20 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8874 | QL | 259.30 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| EGIDYWLAHKPLCSEK | 3175 | LA | 202.25 | Rat intestinal brush border membrane | Dipeptidyl-aminopeptidase IV | [51] |
| 8519 | KP | 243.30 | Rice bran | Dipeptidyl-aminopeptidase IV | [52] | |
| 8558 | EK | 275.30 | Milk protein | α-glucosidase | [53] | |
| 8638 | PL | 228.28 | Spanish dry-cured ham | Dipeptidyl-aminopeptidase IV | [54] | |
| 8677 | WL | 317.38 | Milk protein | α-glucosidase | [57] | |
| 8761 | AH | 226.23 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8770 | EG | 204.18 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8785 | GI | 188.22 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8947 | YW | 367.39 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| LLGHLERGRGNLEWK | 3182 | LL | 244.32 | Rat intestinal brush border membrane | Dipeptidyl-aminopeptidase IV | [51] |
| 8557 | HL | 268.31 | Milk protein | α-glucosidase | [53] | |
| 8676 | WK | 332.39 | Milk protein | α-glucosidase | [57] | |
| 8776 | EW | 333.33 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8784 | GH | 212.20 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8845 | NL | 245.27 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8882 | RG | 231.25 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] |
| Sequences | ID | Matched Sequences | Molecular Mass | Source | Inhibition Properties | Reference |
|---|---|---|---|---|---|---|
| DDPHACYSTVFDKLKHLVDEPQNLIK | 3184 | HA | 226.23 | Rat intestinal brush border membrane | Dipeptidyl-aminopeptidase IV | [51] |
| 8557 | HL | 268.31 | Milk protein | α-amylase | [53] | |
| 8767 | DP | 230.21 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8811 | KH | 283.32 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8821 | LI | 244.32 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8825 | LV | 230.30 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8845 | NL | 245.27 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8856 | PH | 252.26 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8861 | PQ | 243.25 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8875 | QN | 260.24 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8912 | TV | 218.24 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8915 | VD | 232.23 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8917 | VF | 264.31 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8945 | YS | 268.26 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| DMPIQAFLLYQEPVLGPVR | 3169 | GP | 172.18 | Spanish dry-cured ham | α-amylase | [54] |
| 3171 | MP | 246.32 | Rice bran | α-amylase | [52] | |
| 3182 | LL | 244.32 | Rat intestinal brush border membrane | Dipeptidyl-aminopeptidase IV | [51] | |
| 8555 | FL | 278.34 | Milk protein | Dipeptidyl-aminopeptidase IV | [53] | |
| 8593 | VLGP | 384.46 | Milk protein | Dipeptidyl-aminopeptidase IV | [53] | |
| 8594 | VR | 273.33 | Milk protein | Dipeptidyl-aminopeptidase IV | [53] | |
| 8759 | AF | 236.26 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8805 | IQ | 259.30 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8857 | PI | 228.28 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8864 | PV | 214.26 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8867 | QA | 217.22 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8869 | QE | 275.25 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8922 | VL | 230.30 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8943 | YQ | 309.31 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 9116 | GPV | 271.31 | Deer skin hydrolysates | Dipeptidyl-aminopeptidase IV | [30] | |
| ELRAMKVLILACLVALALAR | 3172 | VA | 188.22 | Milk protein | Dipeptidyl-aminopeptidase IV | [53] |
| 3175 | LA | 202.25 | Rat intestinal brush border membrane | Dipeptidyl-aminopeptidase IV | [51] | |
| 8559 | AL | 202.25 | Milk protein | Dipeptidyl-aminopeptidase IV | [53] | |
| 8802 | IL | 244.32 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8821 | LI | 244.32 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8825 | LV | 230.30 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8831 | MK | 277.38 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8922 | VL | 230.30 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| KWVTFISLLLLFSSAYSRGVFR | 3182 | LL | 244.32 | Rat intestinal brush border membrane | Dipeptidyl-aminopeptidase IV | [51] |
| 8556 | WV | 303.35 | Milk protein | Dipeptidyl-aminopeptidase IV | [53] | |
| 8560 | SL | 218.24 | Milk protein | Dipeptidyl-aminopeptidase IV | [53] | |
| 8765 | AY | 252.26 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8780 | FR | 321.37 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8786 | GV | 174.19 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8818 | KW | 332.39 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8882 | RG | 231.25 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8917 | VF | 264.31 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8927 | VT | 218.24 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8945 | YS | 268.26 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| NDEYCFNMMKNR | 8777 | EY | 310.30 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] |
| 8778 | FN | 279.30 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8831 | MK | 277.38 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8833 | MM | 280.40 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8840 | ND | 247.20 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8846 | NM | 263.31 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8849 | NR | 288.30 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| NYQEAKDAFLGSFLYEYSRR | 8555 | FL | 278.34 | Milk protein | Dipeptidyl-aminopeptidase IV | [53] |
| 8759 | AF | 236.26 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8777 | EY | 310.30 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8869 | QE | 275.25 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8889 | RR | 330.38 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8943 | YQ | 309.31 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] | |
| 8945 | YS | 268.26 | Soy protein hydrolysates | Dipeptidyl-aminopeptidase IV | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhuva, B.; Basaiawmoit, B.; Sakure, A.A.; Mankad, P.M.; Rawat, A.; Bishnoi, M.; Kondepudi, K.K.; Patel, A.; Sarkar, P.; Hati, S. A Comparative Study of the Antioxidant and Antidiabetic Properties of Fermented Camel (Camelus dromedarius) and Gir Cow (Bos primigenius indicus) Milk and the Production of Bioactive Peptides via In Vitro and In Silico Studies. Fermentation 2025, 11, 391. https://doi.org/10.3390/fermentation11070391
Bhuva B, Basaiawmoit B, Sakure AA, Mankad PM, Rawat A, Bishnoi M, Kondepudi KK, Patel A, Sarkar P, Hati S. A Comparative Study of the Antioxidant and Antidiabetic Properties of Fermented Camel (Camelus dromedarius) and Gir Cow (Bos primigenius indicus) Milk and the Production of Bioactive Peptides via In Vitro and In Silico Studies. Fermentation. 2025; 11(7):391. https://doi.org/10.3390/fermentation11070391
Chicago/Turabian StyleBhuva, Brijesh, Bethsheba Basaiawmoit, Amar A. Sakure, Pooja M. Mankad, Anita Rawat, Mahendra Bishnoi, Kanthi Kiran Kondepudi, Ashish Patel, Preetam Sarkar, and Subrota Hati. 2025. "A Comparative Study of the Antioxidant and Antidiabetic Properties of Fermented Camel (Camelus dromedarius) and Gir Cow (Bos primigenius indicus) Milk and the Production of Bioactive Peptides via In Vitro and In Silico Studies" Fermentation 11, no. 7: 391. https://doi.org/10.3390/fermentation11070391
APA StyleBhuva, B., Basaiawmoit, B., Sakure, A. A., Mankad, P. M., Rawat, A., Bishnoi, M., Kondepudi, K. K., Patel, A., Sarkar, P., & Hati, S. (2025). A Comparative Study of the Antioxidant and Antidiabetic Properties of Fermented Camel (Camelus dromedarius) and Gir Cow (Bos primigenius indicus) Milk and the Production of Bioactive Peptides via In Vitro and In Silico Studies. Fermentation, 11(7), 391. https://doi.org/10.3390/fermentation11070391







