Formulation of a Functional Probiotic Beverage Using Maesil (Prunus mume) Syrup By-Product Fermented by Lactiplantibacillus plantarum KFOM 0042
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Maesil Syrup By-Product
2.2. Screening of Lactic Acid Bacteria for Fermentation
2.3. Fermentation Optimization and Storage Stability
2.4. Antioxidant Capacity of the MSB Probiotic Beverage
2.4.1. DPPH Radical Scavenging Activity
2.4.2. ABTS Radical Scavenging Activity
2.4.3. Ferric Reducing Antioxidant Power
2.5. Phytochemical Content Analysis of the MSB Probiotic Beverage
2.5.1. Total Polyphenol Content
2.5.2. Total Flavonoids Content
2.6. Organic Acids Analysis of the MSB Probiotic Beverage Using an LC System
3. Results & Discussion
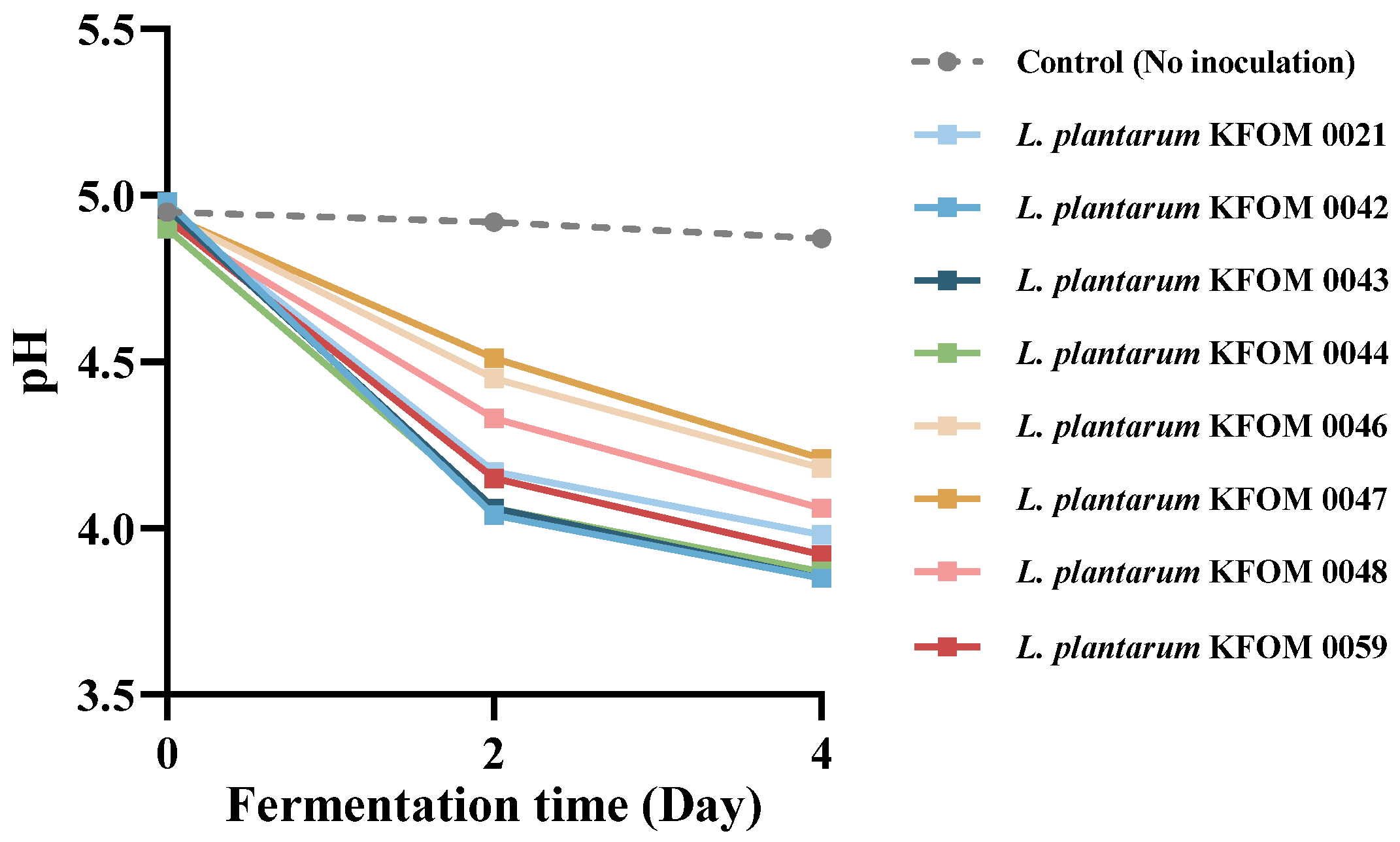
3.1. Selection of LAB for MSB Probiotic Beverage
3.2. Optimization of Fermentation Conditions for MSB Probiotic Beverages
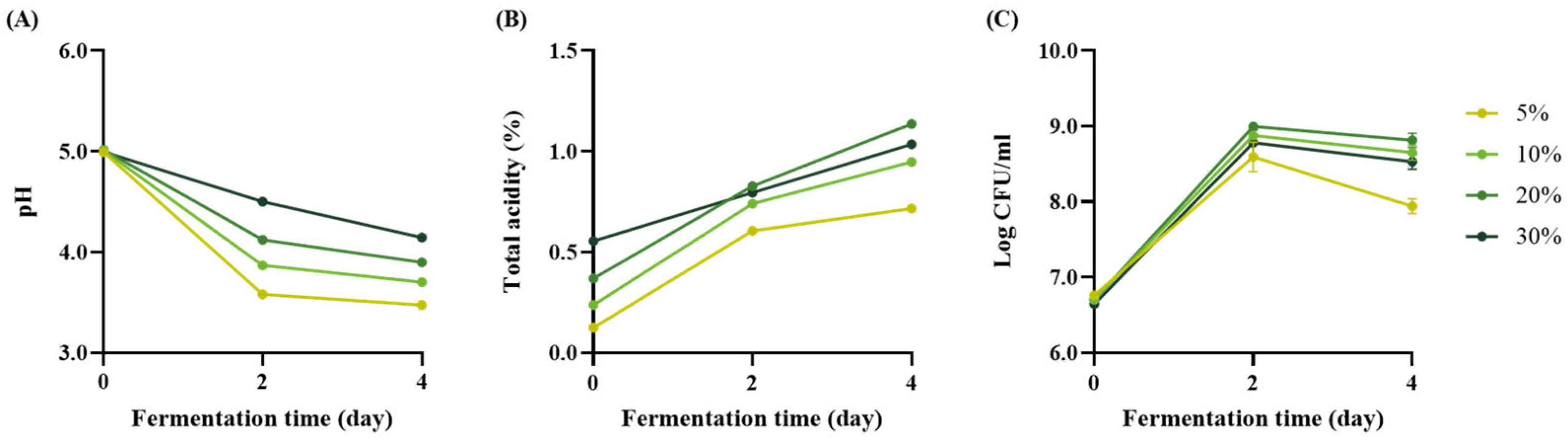
3.2.1. MSB Extract Concentration
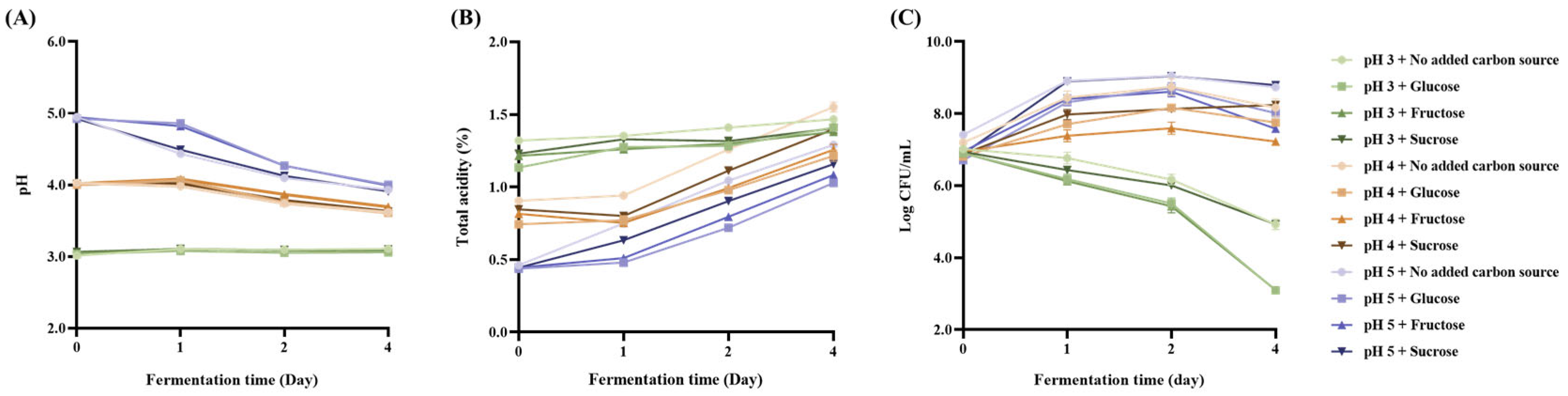
3.2.2. pH and Carbon Source
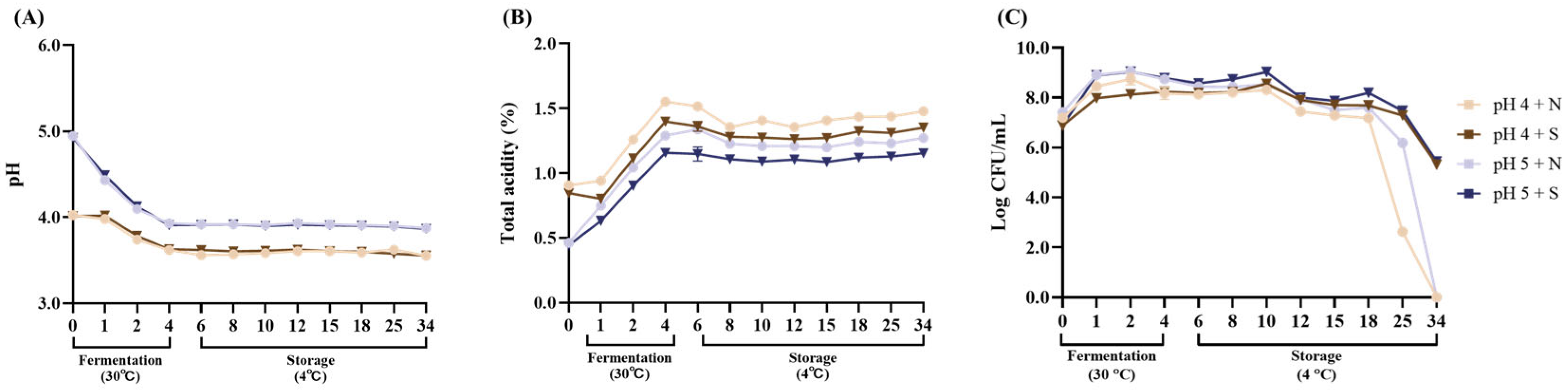
3.3. Evaluation of the Storage Stability of the MSB Probiotic Beverage
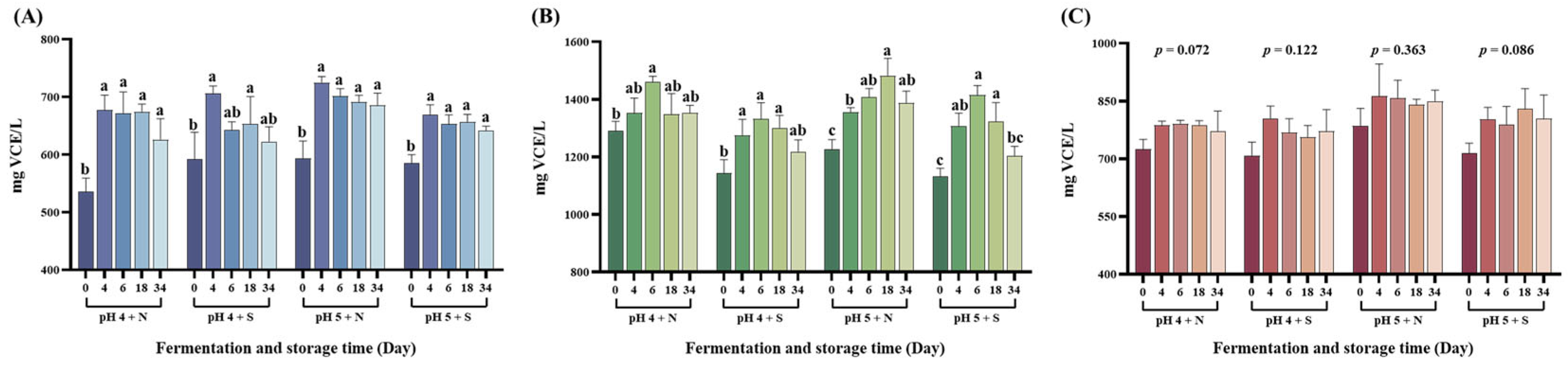
3.4. Measurement of Antioxidant Activity
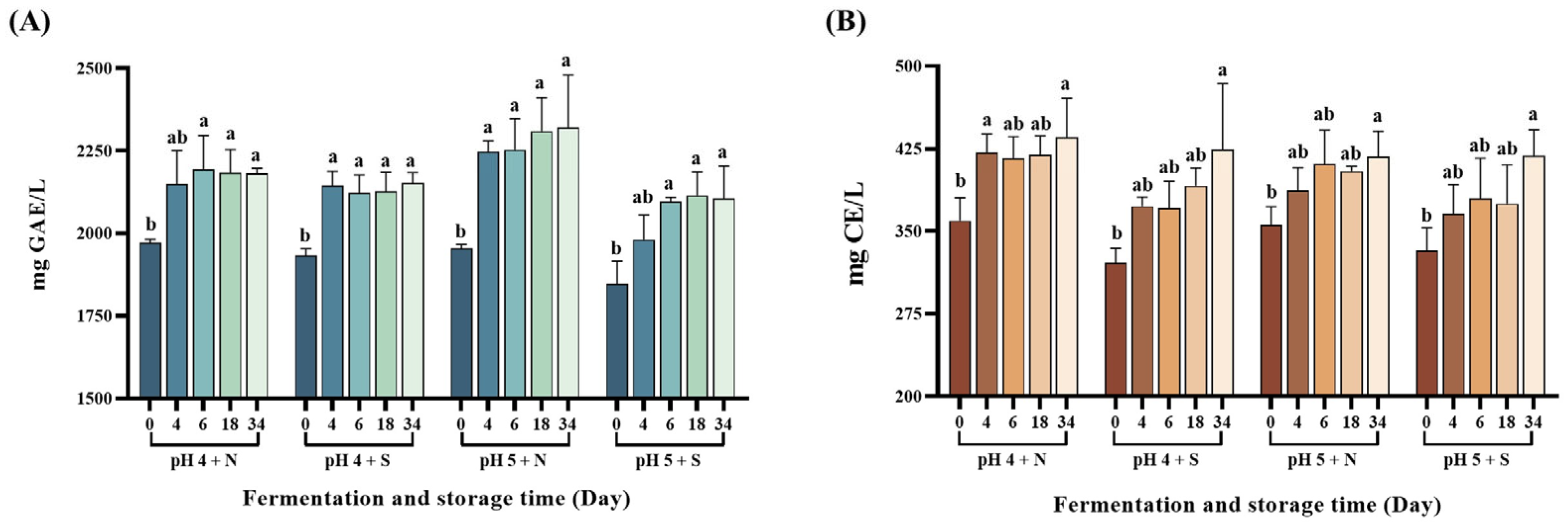
3.5. Measurement of Phytochemical Content
3.6. Quantification of Organic Acid
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, L.; Zhang, H.; Wang, H.; Li, A.C.; Wu, M.; Wang, Q.Z.; Zheng, Z.A. Quality Evaluation and Browning Control in the Multi-Stage Processing of Mume Fructus (Wumei). Foods 2024, 13, 272. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.; Kumar, V.; Bahuguna, A.; Lee, J.S.; Kim, M. The Effect of One-Year Fermentation of Maesil Fruit (Prunus mume) Sugar Syrup on Amygdalin Level: A Natural Toxic Compound. Foods 2024, 13, 2609. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.P.; Tang, Y.; Song, Y.Y.; Du, G.; Li, J. Comprehensive Review of Phytochemical Constituents, Pharmacological Properties, and Clinical Applications of Prunus mume. Front. Pharmacol. 2021, 12, 679378. [Google Scholar] [CrossRef] [PubMed]
- Papun, B.; Wongputtisin, P.; Kanpiengjai, A.; Pisithkul, T.; Manochai, P.; Manowan, K.; Atsaneechantra, A.; Chomsri, N.O. Fermentative Characteristics and Metabolic Profiles of Japanese Apricot Juice Fermented with Lactobacillus acidophilus and Torulaspora delbrueckii. Foods 2024, 13, 3455. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Zhang, Y.; Guo, Y.L.; Zhu, Y.R.; Xia, W.X.; Dai, Y.; Xia, Y.F. Mume Fructus (Prunus mume Sieb. et Zucc.) extract accelerates colonic mucosal healing of mice with colitis induced by dextran sulfate sodium through potentiation of cPLA2-mediated lysophosphatidylcholine synthesis. Phytomedicine 2023, 119, 154985. [Google Scholar] [CrossRef]
- Go, M.R.; Kim, H.J.; Yu, J.; Choi, S.J. Toxicity and Toxicokinetics of Amygdalin in Maesil (Prunus mume) Syrup: Protective Effect of Maesil against Amygdalin Toxicity. J. Agric. Food Chem. 2018, 66, 11432–11440. [Google Scholar] [CrossRef]
- Ramalingam, S.; Bahuguna, A.; Al-Ansari, M.M.; Shanmugam, G.; Al-Humaid, L.; Lee, J.S.; Kim, M. Whole-genome analysis guided molecular mechanism of cyanogenic glucoside degradation by yeast isolated from Prunus mume fruit syrup. Chemosphere 2022, 307, 136061. [Google Scholar] [CrossRef]
- Yoon, S.H.; Koh, E.; Choi, B.; Moon, B. Effects of Soaking and Fermentation Time on Biogenic Amines Content of Maesil (Prunus mume) Extract. Foods 2019, 8, 592. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Z.; Charalampopoulos, D. Upcycling of melon seed (Cucumis melo L.) oil processing by-product: Evaluation of functional properties and nutritional components as novel ingredient. Chem. Biol. Technol. Agric. 2024, 11, 101. [Google Scholar] [CrossRef]
- Nutrizio, M.; Dukić, J.; Sabljak, I.; Samardžija, A.; Fučkar, V.B.; Djekić, I.; Jambrak, A.R. Upcycling of Food By-Products and Waste: Nonthermal Green Extractions and Life Cycle Assessment Approach. Sustainability 2024, 16, 9143. [Google Scholar] [CrossRef]
- Zhao, X.; Korey, M.; Li, K.; Copenhaver, K.; Tekinalp, H.; Celik, S.; Kalaitzidou, K.; Ruan, R.; Ragauskas, A.J.; Ozcan, S. Plastic waste upcycling toward a circular economy. Chem. Eng. J. 2022, 428, 131928. [Google Scholar] [CrossRef]
- Kim, S.O. Review of food upcycling in South Korea: Regulation, limitation, and prospects. Food Sci. Biotechnol. 2023, 32, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, M.; Mirosa, M.; Skeaff, S.; Goodman-Smith, F.; Bremer, P. Not all upcycled food is created equal: What is sustainable? In Public Health Expert Briefing; Public Health Communication Centre Aotearoa: Wellington, New Zealand, 2025. [Google Scholar]
- Ye, H. Emerging Trends in Sustainable Marketing: A Review of Upcycled Food Research and Opportunities for Growth. J. Sustain. Mark. 2023, 4, 63–79. [Google Scholar] [CrossRef]
- Nikhil Swaraj, A.; Moses, J.A.; Manickam, L. Sustainable food upcycling: Perspectives on manufacturing challenges and certification requirements for large-scale commercialization. Sustain. Food Technol. 2025, 3, 648–664. [Google Scholar] [CrossRef]
- Hwang, J.Y. Optimization of the Lactic Acid Fermentation of Maesil (Prunus mume). Korean J. Food Nutr. 2008, 4, 391–398. [Google Scholar]
- Li, S.; Tao, Y.; Li, D.; Wen, G.; Zhou, J.; Manickam, S.; Han, Y.; Chai, W.S. Fermentation of blueberry juices using autochthonous lactic acid bacteria isolated from fruit environment: Fermentation characteristics and evolution of phenolic profiles. Chemosphere 2021, 276, 130090. [Google Scholar] [CrossRef]
- Ryu, J.Y.; Kang, H.R.; Cho, S.K. Changes Over the Fermentation Period in Phenolic Compounds and Antioxidant and Anticancer Activities of Blueberries Fermented by Lactobacillus plantarum. J. Food Sci. 2019, 84, 2347–2356. [Google Scholar] [CrossRef]
- Li, T.; Jiang, T.; Liu, N.; Wu, C.; Xu, H.; Lei, H. Biotransformation of phenolic profiles and improvement of antioxidant capacities in jujube juice by select lactic acid bacteria. Food Chem. 2021, 339, 127859. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, M.; Zheng, Y.; Miao, K.; Qu, X. The Carbohydrate Metabolism of Lactiplantibacillus plantarum. Int. J. Mol. Sci. 2021, 22, 13452. [Google Scholar] [CrossRef]
- Yilmaz, B.; Bangar, S.P.; Echegaray, N.; Suri, S.; Tomasevic, I.; Manuel Lorenzo, J.; Melekoglu, E.; Rocha, J.M.; Ozogul, F. The Impacts of Lactiplantibacillus plantarum on the Functional Properties of Fermented Foods: A Review of Current Knowledge. Microorganisms 2022, 10, 826. [Google Scholar] [CrossRef]
- Ferreira, I.; de Sousa Melo, D.; Menezes, A.G.T.; Fonseca, H.C.; de Assis, B.B.T.; Ramos, C.L.; Magnani, M.; Dias, D.R.; Schwan, R.F. Evaluation of potentially probiotic yeasts and Lactiplantibacillus plantarum in co-culture for the elaboration of a functional plant-based fermented beverage. Food Res. Int. 2022, 160, 111697. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ni, Y.; Yu, Q.; Fan, L. Evaluation of co-fermentation of L. plantarum and P. kluyveri of a plant-based fermented beverage: Physicochemical, functional, and sensory properties. Food Res. Int. 2023, 172, 113060. [Google Scholar] [CrossRef] [PubMed]
- Meenu, M.; Kaur, S.; Kaur, M.; Mradula, M.; Khandare, K.; Xu, B.; Pati, P.K. The golden era of fruit juices-based probiotic beverages: Recent advancements and future possibilities. Process Biochem. 2024, 142, 113–135. [Google Scholar] [CrossRef]
- Oh, Y.J.; Kim, T.S.; Moon, H.W.; Lee, S.Y.; Lee, S.Y.; Ji, G.E.; Hwang, K.T. Lactobacillus plantarum PMO 08 as a Probiotic Starter Culture for Plant-Based Fermented Beverages. Molecules 2020, 25, 5056. [Google Scholar] [CrossRef]
- Wu, Y.; Li, S.; Tao, Y.; Li, D.; Han, Y.; Show, P.L.; Wen, G.; Zhou, J. Fermentation of blueberry and blackberry juices using Lactobacillus plantarum, Streptococcus thermophilus and Bifidobacterium bifidum: Growth of probiotics, metabolism of phenolics, antioxidant capacity in vitro and sensory evaluation. Food Chem. 2021, 348, 129083. [Google Scholar] [CrossRef]
- Kim, D.-O.; Lee, K.W.; Lee, H.J.; Lee, C.Y. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J. Agric. Food Chem. 2002, 50, 3713–3717. [Google Scholar] [CrossRef]
- Kim, D.-O.; Chun, O.K.; Kim, Y.J.; Moon, H.-Y.; Lee, C.Y. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef]
- Lee, S.-S.; Kim, H.-G.; Park, E.-H.; Kim, K.J.; Bang, M.-H.; Kim, G.; Jeon, H.-J.; Lee, C.-G.; Shin, M.-C.; Kim, D.-O.; et al. Antioxidant and anti-inflammatory effects in lipopolysaccharide-induced THP-1 cells of coumarins from the bark of Hesperethusa crenulata R. Appl. Biol. Chem. 2021, 64, 90. [Google Scholar] [CrossRef]
- Nosal, B.M.; Sakaki, J.R.; Kim, D.O.; Chun, O.K. Impact of coffee preparation on total phenolic content in brewed coffee extracts and their contribution to the body’s antioxidant status. Food Sci. Biotechnol. 2022, 31, 1081–1088. [Google Scholar] [CrossRef]
- Nam, T.G.; Kim, D.O.; Eom, S.H. Effects of light sources on major flavonoids and antioxidant activity in common buckwheat sprouts. Food Sci. Biotechnol. 2018, 27, 169–176. [Google Scholar] [CrossRef]
- Ministry of Food and Drug Safety (MFDS). Functional Food Functional Ingredient Probiotics Safety Evaluation Guide. Available online: https://www.mfds.go.kr/brd/m_1060/view.do?seq=15100&srchFr=&srchTo=&srcWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=32 (accessed on 5 January 2025).
- Masood, M.I.; Qadir, M.I.; Shirazi, J.H.; Khan, I.U. Beneficial effects of lactic acid bacteria on human beings. Crit. Rev. Microbiol. 2011, 37, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Sionek, B.; Szydłowska, A.; Küçükgöz, K.; Kołożyn-Krajewska, D. Traditional and New Microorganisms in Lactic Acid Fermentation of Food. Fermentation 2023, 9, 1019. [Google Scholar] [CrossRef]
- Khushboo; Karnwal, A.; Malik, T. Characterization and selection of probiotic lactic acid bacteria from different dietary sources for development of functional foods. Front. Microbiol. 2023, 14, 1170725. [Google Scholar] [CrossRef]
- Rault, A.; Bouix, M.; Beal, C. Fermentation pH influences the physiological-state dynamics of Lactobacillus bulgaricus CFL1 during pH-controlled culture. Appl. Environ. Microbiol. 2009, 75, 4374–4381. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.M.B.; Mousavi Khaneghah, A.; Barba, F.J.; Nemati, Z.; Sohrabi Shokofti, S.; Alizadeh, F. Fermented sweet lemon juice (Citrus limetta) using Lactobacillus plantarum LS5: Chemical composition, antioxidant and antibacterial activities. J. Funct. Foods 2017, 38, 409–414. [Google Scholar] [CrossRef]
- Low, R.H.P.; Baba, A.S.; Aboulfazli, F. Effects of Different Levels of Refined Cane Sugar and Unrefined Coconut Palm Sugar on the Survivability of Lactobacillus acidophilus in Probiotic Ice Cream and its Sensory and Antioxidant Properties. Food Sci. Technol. Res. 2015, 21, 857–862. [Google Scholar] [CrossRef][Green Version]
- Glaasker, E.; Tjan, F.S.; Ter Steeg, P.F.; Konings, W.N.; Poolman, B. Physiological response of Lactobacillus plantarum to salt and nonelectrolyte stress. J. Bacteriol. 1998, 180, 4718–4723. [Google Scholar] [CrossRef]
- Ding, X.; Qian, F.; Mu, G.; Tuo, Y. Optimization of medium composition of Lactobacillus plantarum Y44 using Plackett-Burman and Box-Behnken designs. Prep. Biochem. Biotechnol. 2023, 53, 1058–1066. [Google Scholar] [CrossRef]
- FAO/WHO Joint FAO/WHOWorking Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food. 2002. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/382476b3-4d54-4175-803f-2f26f3526256/content (accessed on 20 January 2023).
- Maia, M.S.; Domingos, M.M.; de Sao Jose, J.F.B. Viability of Probiotic Microorganisms and the Effect of Their Addition to Fruit and Vegetable Juices. Microorganisms 2023, 11, 1335. [Google Scholar] [CrossRef]
- Pereira, A.L.F.; Almeida, F.D.L.; de Jesus, A.L.T.; da Costa, J.M.C.; Rodrigues, S. Storage Stability and Acceptance of Probiotic Beverage from Cashew Apple Juice. Food Bioprocess. Technol. 2012, 6, 3155–3165. [Google Scholar] [CrossRef]
- Santos Filho, A.L.d.; Freitas, H.V.; Rodrigues, S.; Abreu, V.K.G.; de Oliveira Lemos, T.; Gomes, W.F.; Narain, N.; Pereira, A.L.F. Production and stability of probiotic cocoa juice with sucralose as sugar substitute during refrigerated storage. Lebensm.-Wiss. Technol. 2019, 99, 371–378. [Google Scholar] [CrossRef]
- Kaprasob, R.; Kerdchoechuen, O.; Laohakunjit, N.; Sarkar, D.; Shetty, K. Fermentation-based biotransformation of bioactive phenolics and volatile compounds from cashew apple juice by select lactic acid bacteria. Process Biochem. 2017, 59, 141–149. [Google Scholar] [CrossRef]
- Kareena, A.; Siripongvutikorn, S.; Usawakesmanee, W.; Wichienchot, S. In Vitro evaluation of probiotic bacteria and yeast growth, pH changes and metabolites produced in a pure culture system using protein base products with various added carbon sources. Food Sci. Technol. 2022, 42, e18321. [Google Scholar] [CrossRef]
- Kim, D.; Oh, I. Development of fermented beverage with citrus fruit extract using probiotics: Impact on antioxidant activity and in vitro digestibility. Appl. Biol. Chem. 2024, 67, 23. [Google Scholar] [CrossRef]
- Yang, W.; Liu, J.; Zhang, Q.; Liu, H.; Lv, Z.; Zhang, C.; Jiao, Z. Changes in nutritional composition, volatile organic compounds and antioxidant activity of peach pulp fermented by lactobacillus. Food Biosci. 2022, 49, 101894. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, R.; Wang, W.; Sun, T.; Han, X.; Ai, M.; Huang, S. Study on Nutritional Characteristics, Antioxidant Activity, and Volatile Compounds in Non-Saccharomyces cerevisiae–Lactiplantibacillus plantarum Co-Fermented Prune Juice. Foods 2025, 14, 1966. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- He, Z.; Zhang, H.; Wang, T.; Wang, R.; Luo, X. Effects of Five Different Lactic Acid Bacteria on Bioactive Components and Volatile Compounds of Oat. Foods 2022, 11, 3230. [Google Scholar] [CrossRef]
- Torres, C.A.; Romero, L.A.; Diaz, R.I. Quality and sensory attributes of apple and quince leathers made without preservatives and with enhanced antioxidant activity. LWT—Food Sci. Technol. 2015, 62, 996–1003. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, J.; Fan, L.; Qin, Z.; Chen, Q.; Zhao, L. Antioxidant properties of a vegetable-fruit beverage fermented with two Lactobacillus plantarum strains. Food Sci. Biotechnol. 2018, 27, 1719–1726. [Google Scholar] [CrossRef] [PubMed]
- Badshah, S.L.; Faisal, S.; Muhammad, A.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Antiviral activities of flavonoids. Biomed. Pharmacother. 2021, 140, 111596. [Google Scholar] [CrossRef] [PubMed]
- Rathod, N.B.; Elabed, N.; Punia, S.; Ozogul, F.; Kim, S.K.; Rocha, J.M. Recent Developments in Polyphenol Applications on Human Health: A Review with Current Knowledge. Plants 2023, 12, 1217. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, C.; Ni, D.; Yang, Y.; Tian, J.; Li, Y.; Chen, S.; Ye, X.; Wang, L. Effects of Fermentation on Bioactivity and the Composition of Polyphenols Contained in Polyphenol-Rich Foods: A Review. Foods 2023, 12, 3315. [Google Scholar] [CrossRef]
- Bühlmann, C.H.; Mickan, B.S.; Tait, S.; Batstone, D.J.; Mercer, G.D.; Bahri, P.A. Lactic acid from mixed food waste fermentation using an adapted inoculum: Influence of pH and temperature regulation on yield and product spectrum. J. Clean. Prod. 2022, 373, 133716. [Google Scholar] [CrossRef]
- Zapasnik, A.; Sokolowska, B.; Bryla, M. Role of Lactic Acid Bacteria in Food Preservation and Safety. Foods 2022, 11, 1283. [Google Scholar] [CrossRef]
- Anumudu, C.K.; Miri, T.; Onyeaka, H. Multifunctional Applications of Lactic Acid Bacteria: Enhancing Safety, Quality, and Nutritional Value in Foods and Fermented Beverages. Foods 2024, 13, 3714. [Google Scholar] [CrossRef]
- Kim, H.R.; Kim, I.D.; Dhungana, S.K.; Kim, M.O.; Shin, D.H. Comparative assessment of physicochemical properties of unripe peach (Prunus persica) and Japanese apricot (Prunus mume). Asian Pac. J. Trop. Biomed. 2014, 4, 97–103. [Google Scholar] [CrossRef]
- Kim, S.M.; Huh, C.K. Isolation and identification of squalene as an antioxidative compound from the fruits of Prunus mume. J. Food Process. Preserv. 2021, 45, e15810. [Google Scholar] [CrossRef]
- Paramithiotis, S.; Stasinou, V.; Tzamourani, A.; Kotseridis, Y.; Dimopoulou, M. Malolactic Fermentation—Theoretical Advances and Practical Considerations. Fermentation 2022, 8, 521. [Google Scholar] [CrossRef]
- Fu, J.; Wang, L.; Sun, J.; Ju, N.; Jin, G. Malolactic Fermentation: New Approaches to Old Problems. Microorganisms 2022, 10, 2363. [Google Scholar] [CrossRef]
- Gu, Q.; Zhang, J.; Lin, B.; Ding, H.; Yan, Q.; Wei, C.; Yao, Y.; Wang, R.; Zou, C. Effects and function of citric acid on fermentation quality and microbial community in sugarcane tops silage with high and low water-soluble carbohydrate content. BMC Plant Biol. 2025, 25, 99. [Google Scholar] [CrossRef]
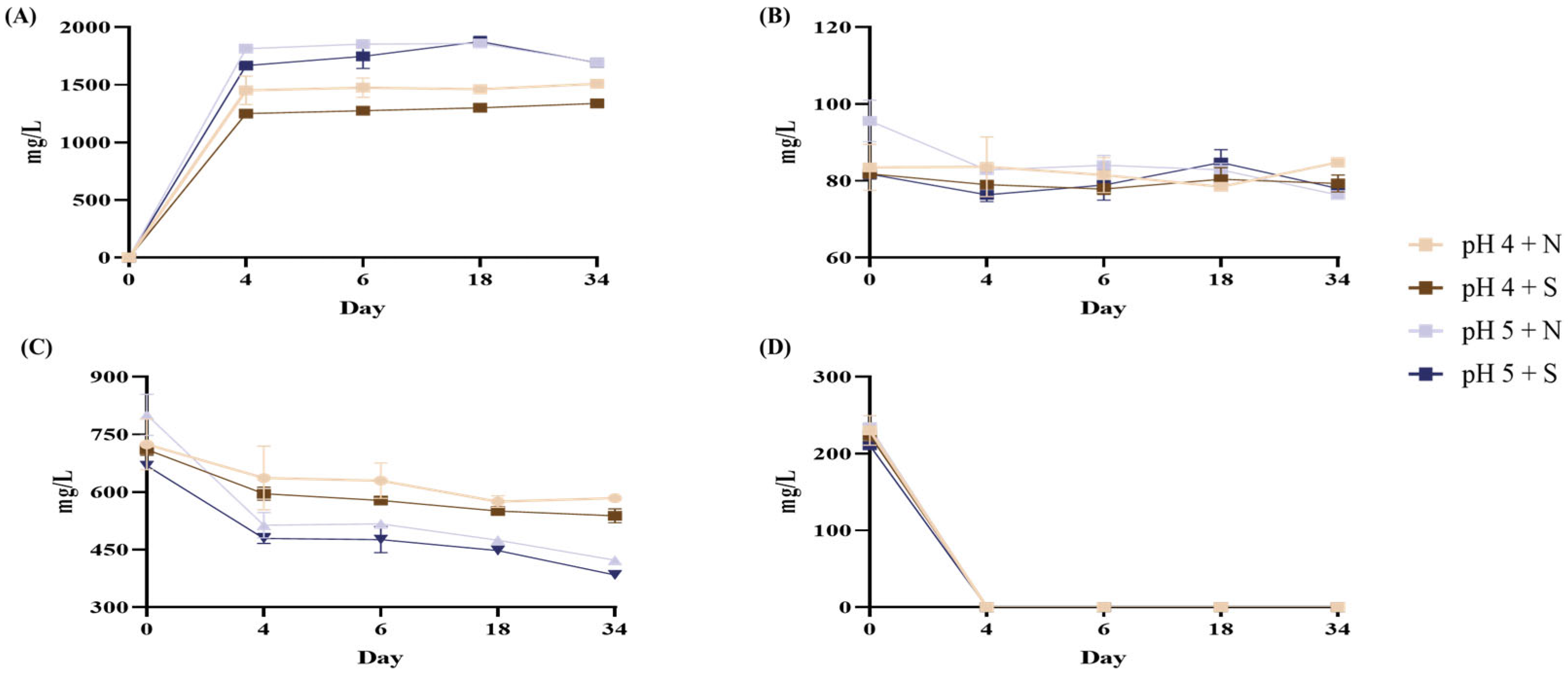
| Organic Acid (mg/L) | MSB Probiotic Beverage | ||||
|---|---|---|---|---|---|
| Day | pH 4 + N | pH 4 + S | pH 5 + X | pH 5 + S | |
| Lactic acid | 0 | ND | ND | ND | ND |
| 4 | 1452.21 ± 123.50 aB | 1251.05 ± 22.93 cC | 1814.32 ± 4.70 bA | 1667.26 ± 28.10 bA | |
| 6 | 1475.99 ± 82.80 aB | 1275.04 ± 3.87 bcC | 1853.79 ± 20.7 aA | 1746.91 ± 104.99 abA | |
| 18 | 1462.64 ± 23.11 aB | 1301.44 ± 9.65 abC | 1859.36 ± 19.49 aA | 1876.43 ± 45.04 aA | |
| 34 | 1508.38 ± 8.84 aB | 1339.93 ± 21.04 aC | 1695.04 ± 9.19 cA | 1691.99 ± 23.99 bA | |
| Oxalic acid | 0 | 83.52 ± 6.02 aB | 81.83 ± 1.09 aB | 95.64 ± 5.41 aA | 81.79 ± 1.32 abB |
| 4 | 83.68 ± 7.73 aA | 79.03 ± 0.79 aA | 82.85 ± 0.96 bcA | 76.38 ± 1.74 bA | |
| 6 | 81.54 ± 4.57 aA | 77.87 ± 1.39 aA | 84.08 ± 2.54 bA | 78.93 ± 3.96 abA | |
| 18 | 78.48 ± 1.00 aA | 80.42 ± 2.99 aA | 82.89 ± 1.71 bcA | 84.76 ± 3.39 aA | |
| 34 | 84.83 ± 1.22 aA | 79.31 ± 2.23 aB | 76.33 ± 0.61 cB | 78.01 ± 0.99 abB | |
| Citric acid | 0 | 724.47 ± 66.33 aA | 710.84 ± 14.90 aA | 801.21 ± 53.75 aA | 668.98 ± 5.29 aB |
| 4 | 678.87 ± 41.01 abA | 595.80 ± 17.06 bB | 513.66 ± 32.89 bC | 479.05 ± 13.39 bC | |
| 6 | 629.94 ± 46.16 abA | 578.23 ± 8.74 bcAB | 517.22 ± 5.75 bB | 476.46 ± 34.12 bB | |
| 18 | 575.11 ± 15.04 bA | 550.81 ± 11.71 cdA | 474.98 ± 1.35 bcB | 447.93 ± 11.01 bB | |
| 34 | 584.13 ± 11.13 abA | 538.30 ± 18.16 dB | 422.77 ± 5.33 cC | 384.03 ± 8.69 cD | |
| Malic acid | 0 | 230.31 ± 19.22 aA | 224.15 ± 7.81 aA | 234.71 ± 5.14 aA | 210.96 ± 2.86 aA |
| 4 | ND | ND | ND | ND | |
| 6 | ND | ND | ND | ND | |
| 18 | ND | ND | ND | ND | |
| 34 | ND | ND | ND | ND | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, C.-I.; Gwak, Y.-S.; Nam, D.; Nam, T.G.; Kim, H.-S.; Kim, M.-J. Formulation of a Functional Probiotic Beverage Using Maesil (Prunus mume) Syrup By-Product Fermented by Lactiplantibacillus plantarum KFOM 0042. Fermentation 2025, 11, 368. https://doi.org/10.3390/fermentation11070368
Bae C-I, Gwak Y-S, Nam D, Nam TG, Kim H-S, Kim M-J. Formulation of a Functional Probiotic Beverage Using Maesil (Prunus mume) Syrup By-Product Fermented by Lactiplantibacillus plantarum KFOM 0042. Fermentation. 2025; 11(7):368. https://doi.org/10.3390/fermentation11070368
Chicago/Turabian StyleBae, Chan-Il, Yoon-Soo Gwak, Dasol Nam, Tae Gyu Nam, Hyun-Seok Kim, and Mi-Ju Kim. 2025. "Formulation of a Functional Probiotic Beverage Using Maesil (Prunus mume) Syrup By-Product Fermented by Lactiplantibacillus plantarum KFOM 0042" Fermentation 11, no. 7: 368. https://doi.org/10.3390/fermentation11070368
APA StyleBae, C.-I., Gwak, Y.-S., Nam, D., Nam, T. G., Kim, H.-S., & Kim, M.-J. (2025). Formulation of a Functional Probiotic Beverage Using Maesil (Prunus mume) Syrup By-Product Fermented by Lactiplantibacillus plantarum KFOM 0042. Fermentation, 11(7), 368. https://doi.org/10.3390/fermentation11070368






