Impact of Heat Treatment on Hard Cider Enriched with Cryo-Concentrated Apple Must: Microbiological Profile, Functional Properties, and Storage Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Methods
2.2.1. Apple Juice Processing
2.2.2. Cryo-Concentration of Apple Must
2.2.3. Hard Cider Processing and Formulation
2.3. Analysis
2.3.1. Microbiological Monitoring
2.3.2. Quantification of Sugars and Glycerol
2.3.3. Physicochemical Analysis
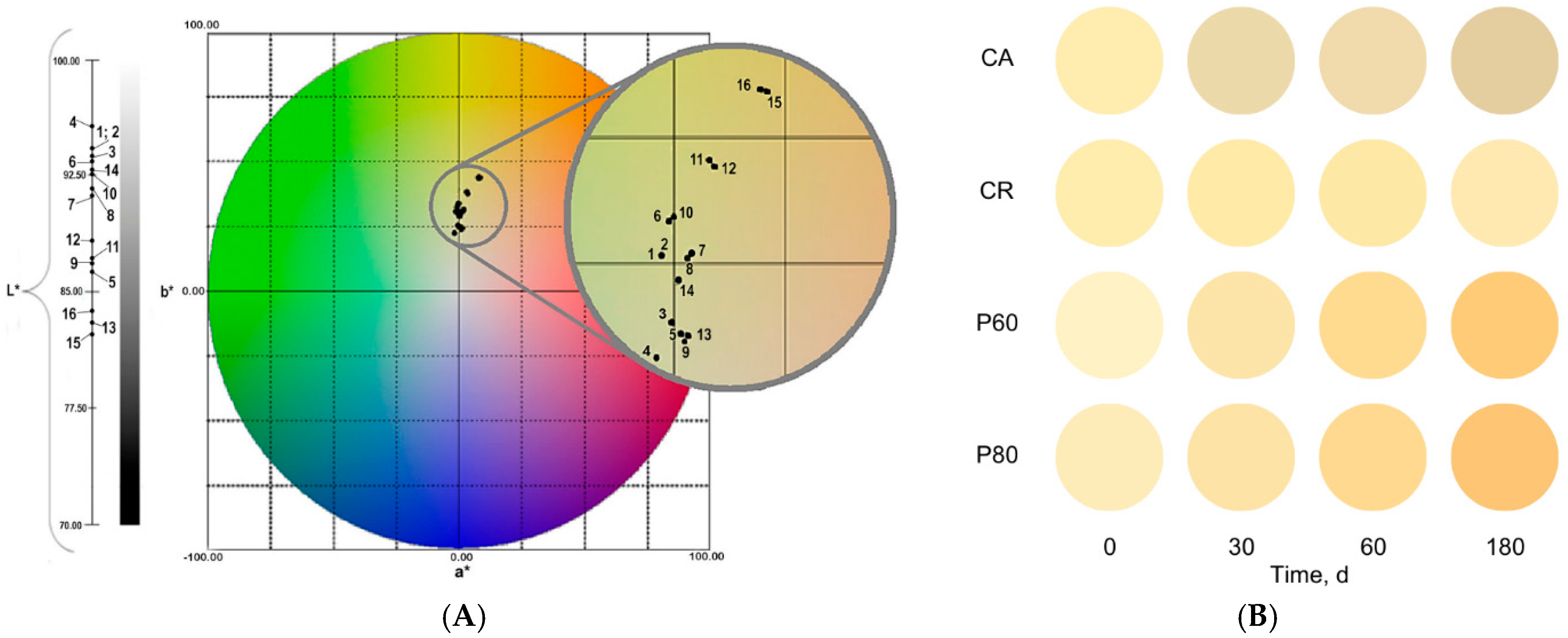
2.3.4. Colorimetric Analysis
2.3.5. Phenolic Composition
2.3.6. In Vitro Antioxidant Capacity
2.3.7. Statistical Analysis
3. Results and Discussion
3.1. Microbiological Aspects
3.2. Physicochemical Composition
3.3. Phenolic Composition and Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bedriñana, R.P.; Madrera, R.R.; Lobo, A.P. Production of New Ciders: Chemical and Sensory Profiles. In Natural Products in Beverages: Botany, Phytochemistry, Pharmacology and Processing; Mérillon, J.-M., Riviere, C., Lefèvre, G., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–43. ISBN 978-3-031-04195-2. [Google Scholar]
- Zuriarrain-Ocio, A.; Zuriarrain, J.; Etxebeste, O.; Dueñas, M.T.; Berregi, I. Evolution of Main Polyphenolics during Cidermaking. LWT—Food Sci. Technol. 2022, 167, 113798. [Google Scholar] [CrossRef]
- Picchi, M.; Domizio, P.; Wilson, M.; Santos, J.; Orrin, F.; Zanoni, B.; Canuti, V. Chemical Characterization, Sensory Definition and Prediction Model of the Cider Dryness from New York State Apples. Foods 2023, 12, 2191. [Google Scholar] [CrossRef] [PubMed]
- Villière, A.; Arvisenet, G.; Bauduin, R.; Le Quéré, J.M.; Sérot, T. Influence of Cider-Making Process Parameters on the Odourant Volatile Composition of Hard Ciders. J. Inst. Brew. 2015, 121, 95–105. [Google Scholar] [CrossRef]
- Alberti, A.; Santos, T.P.M.; Zielinski, A.A.F.; Santos, C.M.E.; Braga, C.M.; Demiate, I.M.; Nogueira, A. Impact on Chemical Profile in Apple Juice and Cider Made from Unripe, Ripe and Senescent Dessert Varieties. LWT—Food Sci. Technol. 2016, 65, 436–443. [Google Scholar] [CrossRef]
- Cousin, F.J.; Le Guellec, R.; Schlusselhuber, M.; Dalmasso, M.; Laplace, J.-M.; Cretenet, M. Microorganisms in Fermented Apple Beverages: Current Knowledge and Future Directions. Microorganisms 2017, 5, 39. [Google Scholar] [CrossRef]
- Xiong, Z.R.; Chen, A.; Jiang, G.Z.; Lewis, A.G.; Sislak, C.D.; Cobo, M.; Worobo, R.W.; Gibney, P.A. Evaluation of Foodborne Pathogen Die-off in Back-Sweetened Wine and Apple Cider Models. J. Food Prot. 2021, 84, 1023–1032. [Google Scholar] [CrossRef]
- Calugar, P.C.; Coldea, T.E.; Salanță, L.C.; Pop, C.R.; Pasqualone, A.; Burja-Udrea, C.; Zhao, H.; Mudura, E. An Overview of the Factors Influencing Apple Cider Sensory and Microbial Quality from Raw Materials to Emerging Processing Technologies. Processes 2021, 9, 502. [Google Scholar] [CrossRef]
- Turk, M.F.; Vorobiev, E.; Baron, A. Improving Apple Juice Expression and Quality by Pulsed Electric Field on an Industrial Scale. LWT—Food Sci. Technol. 2012, 49, 245–250. [Google Scholar] [CrossRef]
- Del Vecchio, H.W.; Dayharsh, C.A.; Baselt, F.C. Thermal Death Time Studies on Beer Spoilage Organisms–I. Proceedings. Annu. Meet.-Am. Soc. Brew. Chem. 1951, 9, 45–50. [Google Scholar] [CrossRef]
- Valliere, B.; Harkins, S. A Preliminary Evaluation to Establish Bath Pasteurization Guidelines for Hard Cider. Beverages 2020, 6, 24. [Google Scholar] [CrossRef]
- Reid, M.S.; Padfield, C.A.S.; Watkins, C.B.; Harman, J.E. Starch Iodine Pattern as a Maturity Index for Granny Smith Apples 1. Comparison with Flesh Firmness and Soluble Solids Content. N. Z. J. Agric. Res. 1982, 25, 229–237. [Google Scholar] [CrossRef]
- Los, P.R.; Braga, C.M.; Carvalho, J.R.F.; Simões, D.R.S.; Nogueira, A. Application of Sensory Analyses in the Development of a New Apple Cider. Rev. Bras. Tecnol. Agroind. 2017, 10, 2150–2168. [Google Scholar] [CrossRef]
- da Silva, N.; Junqueira, V.C.A.; de Arruda Silveira, N.F.; Taniwaki, M.H.; Gomes, R.A.R.; Okazaki, M.M. Manual de Métodos de Análise Microbiológica de Alimentos e Água; Editora Blucher: São Paulo, Brazil, 2018; ISBN 9788521212256. [Google Scholar]
- Santos, T.P.M.; Alberti, A.; Judacewski, P.; Zielinski, A.A.F.; Nogueira, A. Effect of Sulphur Dioxide Concentration Added at Different Processing Stages on Volatile Composition of Ciders. J. Inst. Brew. 2018, 124, 261–268. [Google Scholar] [CrossRef]
- Latimer, G.W., Jr. (Ed.) Official Methods of Analysis of AOAC International, 20th ed.; AOAC International: Rockville, MD, USA, 2016; Volume 2. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Cerri, M.L.; Gomes, T.A.; Carraro, M.d.M.; Wojeicchowski, J.P.; Demiate, I.M.; Lacerda, L.G.; Alberti, A.; Nogueira, A. Assessing the Impact of Simultaneous Co-Fermentation on Malolactic Bioconversion and the Quality of Cider Made with Low-Acidity Apples. Fermentation 2023, 9, 1017. [Google Scholar] [CrossRef]
- Techakanon, C.; Sirimuangmoon, C. The Effect of Pasteurization and Shelf Life on the Physicochemical, Microbiological, Antioxidant, and Sensory Properties of Rose Apple Cider during Cold Storage. Beverages 2020, 6, 43. [Google Scholar] [CrossRef]
- Manoochehri, H.; Hosseini, N.F.; Saidijam, M.; Taheri, M.; Rezaee, H.; Nouri, F. A Review on Invertase: Its Potentials and Applications. Biocatal. Agric. Biotechnol. 2020, 25, 101599. [Google Scholar] [CrossRef]
- Garai, G.; Dueñas, M.T.; Irastorza, A.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V. Biogenic Amines in Natural Ciders. J. Food Prot. 2006, 69, 3006–3012. [Google Scholar] [CrossRef] [PubMed]
- Juhart, J.; Medic, A.; Jakopic, J.; Veberic, R.; Hudina, M.; Stampar, F. Use of HPLC-MS to Determine the Loss of Metabolites in Apple Juices under Different Storage Conditions. Foods 2023, 12, 2822. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Gramza-Michałowska, A. Enzymatic Browning in Apple Products and Its Inhibition Treatments: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 5038–5076. [Google Scholar] [CrossRef]
- Murtaza, A.; Iqbal, A.; Marszałek, K.; Iqbal, M.A.; Ali, S.W.; Xu, X.; Pan, S.; Hu, W. Enzymatic, Phyto-, and Physicochemical Evaluation of Apple Juice under High-Pressure Carbon Dioxide and Thermal Processing. Foods 2020, 9, 243. [Google Scholar] [CrossRef]
- Yan, S.; Luo, Y.; Zhou, B.; Ingram, D.T. Dual Effectiveness of Ascorbic Acid and Ethanol Combined Treatment to Inhibit Browning and Inactivate Pathogens on Fresh-Cut Apples. LWT—Food Sci. Technol. 2017, 80, 311–320. [Google Scholar] [CrossRef]
- Vegara, S.; Martí, N.; Mena, P.; Saura, D.; Valero, M. Effect of Pasteurization Process and Storage on Color and Shelf-Life of Pomegranate Juices. LWT—Food Sci. Technol. 2013, 54, 592–596. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Soliva-Fortuny, R.; Hernández-Jover, T.; Martín-Belloso, O. Carotenoid and Phenolic Profile of Tomato Juices Processed by High Intensity Pulsed Electric Fields Compared with Conventional Thermal Treatments. Food Chem. 2009, 112, 258–266. [Google Scholar] [CrossRef]

| Analytical Parameters | Dry Cider | Cryo-Concentrate Apple Must | Hard Cider with Cryo-Concentrate |
|---|---|---|---|
| Total sugar (g/100 mL) | 0.56 ± 0.01 | 52.2 ± 0.3 | 4.48 ± 0.01 |
| Sucrose (g/100 mL) | <LOD | 8.3 ± 0.1 | 0.17 ± 0.01 |
| Glucose (g/100 mL) | <LOD | 13.2 ± 0.1 | 1.39 ± 0.01 |
| Fructose (g/100 mL) | 0.56 ± 0.01 | 30.6 ± 0.1 | 2.91 ± 0.01 |
| Glycerol (g/100 mL) | 0.38 ± 0.01 | <LOD | 0.29 ± 0.01 |
| Ethanol (% v/v) | 6.75 ± 0.07 | - | 4.6 ± 0.1 |
| Density (g/cm) | 0.99319 | 1.21458 | 1.01446 |
| pH | 3.79 ± 0.01 | 3.23 ± 0.02 | 3.55 ± 0.01 |
| Total acidity (g MA/L) | 3.51 ± 0.01 | 28.11 ± 0.02 | 4.81 ± 0.05 |
| Volatile acidity (g AC/L) | 1.0 ± 0.2 | 0.6 ± 0.2 | 0.9 ± 0.1 |
| TFC (mg CAE/L) | 496 ± 8 | 5440 ± 80 | 870 ± 26 |
| FRAP (µmol TE/L) | 734 ± 6 | 11,996 ± 214 | 1911 ± 30 |
| DPPH (µmol TE/L) | 546 ± 44 | 4755 ± 85 | 1217 ± 17 |
| ABTS (µmol TE/L) | 1061 ± 60 | 13,024 ± 97 | 2923 ± 134 |
| L* | 91.07 ± 0.01 | 80.90 ± 0.03 | 94.0 ± 0.3 |
| a* | −0.71 ± 0.01 | 12.62 ± 0.02 | −1.13 ± 0.03 |
| b* | 39.30 ± 0.10 | 74.60 ± 0.09 | 30.6 ± 0.1 |
| °Hue | 88.70 ± 0.01 | 80.40 ± 0.03 | 92.11 ± 0.05 |
| Chroma | 39.30 ± 0.10 | 75.66 ± 0.08 | 30.6 ± 0.1 |
| Sample | Storage Days | Molds/Yeasts (Cells/mL) | Lactic Acid Bacteria (Cells/mL) |
|---|---|---|---|
| CA | 0 | 2.7 × 104 | 2.5 × 105 |
| 30 | 1.1 × 106 | <1.0 × 102 | |
| 60 | 1.0 × 105 | 1.9 × 105 | |
| 120 | 4.7 × 104 | 8.4 × 105 | |
| 180 | 4.5 × 103 | 8.9 × 106 | |
| CR | 0 | 2.7 × 104 | 2.5 × 105 |
| 30 | 6.0 × 105 | 4.0 × 105 | |
| 60 | 5.0 × 105 | 5.7 × 104 | |
| 120 | 2.1 × 105 | 4.2 × 104 | |
| 180 | 2.2 × 103 | 2.7 × 105 | |
| P60 | 0 | 3.7 × 103 | presence (<4.0 × 101) |
| 30 | 1.3 × 103 | <1 × 102 | |
| 60 | 1.8 × 103 | presence (<4.0 × 102) | |
| 120 | 1.1 × 103 | presence (<4.0 × 102) | |
| 180 | 5.2 × 102 | presence (<4.0 × 102) | |
| P80 | 0 | 1.9 × 103 | presence (<4.0 × 101) |
| 30 | presence (<4.0 × 102) | <1 × 102 | |
| 60 | 1.6 × 103 | presence (<4.0 × 102) | |
| 120 | 9.5 × 102 | presence (<4.0 × 102) | |
| 180 | 3.3 × 101 | presence (<4.0 × 102) |
| Sample | Storage Day | Total Sugar | Sucrose | Glucose | FRU | GLY | Density (kg/m3) | Ethanol (% v/v) | pH | Total Acidity | Volatile Acidity | °Hue | Chroma |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CA | 0 | 4.68 a ± 0.01 | 0.60 a ± 0.01 | 1.39 a ± 0.01 | 2.91 a ± 0.01 | 0.29 i ± 0.01 | 1014.5 d | 4.6 fg ± 0.1 | 3.55 d ± 0.01 | 0.48 b ± 0.05 | 0.08 c ± 0.01 | 92.1 a ± 0.1 | 30.6 e ± 0.1 |
| 30 | <LOD | <LOD | <LOD | <LOD | 0.44 b ± 0.01 | 993.5 h | 7.4 b ± 0.1 | 3.98 ab ± 0.04 | 0.31 c ± 0.01 | 0.20 a ± 0.01 | 87.9 bc ± 0.1 | 24.4 h ± 0.2 | |
| 60 | <LOD | <LOD | <LOD | <LOD | 0.43 c ± 0.01 | 993.8 h | 7.1 c ± 0.2 | 4.01 a ± 0.03 | 0.3 c ± 0.1 | 0.08 c ± 0.00 | 88.7 b ± 0.1 | 23.8 hi ± 0.1 | |
| 120 | <LOD | <LOD | <LOD | <LOD | 0.44 b ± 0.01 | 993.7 h | 7.4 b ± 0.2 | 3.98 ab ± 0.01 | 0.3 c ± 0.1 | 0.09 c ± 0.00 | 87.9 bc ± 0.1 | 24.1 h ± 0.2 | |
| 180 | <LOD | <LOD | <LOD | <LOD | 0.45 a ± 0.01 | 993.7 h | 7.7 a ± 0.1 | 3.95 b ± 0.02 | 0.3 c ± 0.1 | 0.11 b ± 0.02 | 87.2 c ± 0.0 | 24.3 h ± 0.1 | |
| CR | 0 | 4.68 a ± 0.01 | 0.60 a ± 0.01 | 1.39 a ± 0.01 | 2.91 a ± 0.01 | 0.29 i ± 0.01 | 1014.5 d | 4.6 fg ± 0.1 | 3.55 d ± 0.01 | 0.48 b ± 0.05 | 0.08 c ± 0.01 | 92.1 a ± 0.1 | 30.6 e ± 0.2 |
| 30 | 4.08 b ± 0.03 | <LOD | 1.27 b ± 0.01 | 2.81 b ± 0.02 | 0.31 g ± 0.01 | 1013.8 d | 4.9 f ± 0.1 | 3.63 c ± 0.01 | 0.49 b ± 0.05 | 0.06 cd ± 0.01 | 90.7 ab ± 0.1 | 33.4 d ± 0.1 | |
| 60 | 3.86 c ± 0.01 | <LOD | 1.19 c ± 0.01 | 2.66 c ± 0.01 | 0.33 e ± 0.01 | 1012.0 e | 5.0 f ± 0.1 | 3.67 c ± 0.02 | 0.48 b ± 0.03 | 0.08 c ± 0.00 | 90.2 ab ± 0.1 | 33.7 d ± 0.2 | |
| 120 | 3.53 cd ± 0.01 | <LOD | 1.06 c ± 0.01 | 2.59 d ± 0.03 | 0.32 ef ± 0.01 | 1011.6 f | 5.9 e ± 0.1 | 3.65 c ± 0.02 | 0.49 b ± 0.02 | 0.08 c ± 0.02 | 89.7 b ± 0.3 | 31.2 e ± 0.2 | |
| 180 | 3.46 d ± 0.03 | <LOD | 0.93 d ± 0.01 | 2.53 d ± 0.03 | 0.31 g ± 0.01 | 1011.2 g | 6.7 d ± 0.1 | 3.63 c ± 0.03 | 0.5 ab ± 0.1 | 0.06 cd ± 0.01 | 89.3 b ± 0.3 | 28.7 f ± 0.6 | |
| P60 | 0 | 4.65 a ± 0.03 | 0.60 a ± 0.01 | 1.26 b ± 0.01 | 2.74 bc ± 0.03 | 0.35 d ± 0.01 | 1014.5 d | 4.7 ef ± 0.1 | 3.55 d ± 0.02 | 0.49 b ± 0.05 | 0.12 b ± 0.02 | 92.1 a ± 0.2 | 22.5 i ± 0.1 |
| 30 | 4.34 b ± 0.03 | 0.54 ab ± 0.01 | 1.15 c ± 0.02 | 2.64 c ± 0.01 | 0.30 h ± 0.01 | 1014.8 c | 4.50 f ± 0.01 | 3.63 c ± 0.01 | 0.48 b ± 0.07 | 0.07 c ± 0.01 | 87.6 c ± 0.1 | 30.5 e ± 0.2 | |
| 60 | 4.41 ab ± 0.04 | 0.40 bc ± 0.01 | 1.24 b ± 0.01 | 2.69 c ± 0.04 | 0.30 h ± 0.01 | 1014.8 c | 4.7 ef ± 0.1 | 3.64 c ± 0.01 | 0.48 b ± 0.07 | 0.06 cd ± 0.01 | 85.3 d ± 0.2 | 38.3 b ± 0.4 | |
| 120 | 4.06 b ± 0.01 | 0.30 d ± 0.01 | 1.16 c ± 0.02 | 2.50 d ± 0.01 | 0.26 j ± 0.01 | 1014.9 b | 4.50 f ± 0.02 | 3.63 c ± 0.01 | 0.48 b ± 0.04 | 0.05 d ± 0.01 | 82.5 e ± 0.1 | 40.2 b ± 0.4 | |
| 180 | 3.71 c ± 0.04 | 0.21 e ± 0.01 | 1.08 c ± 0.01 | 2.32 e ± 0.03 | 0.23 k ± 0.01 | 1015.1 b | 4.7 ef ± 0.1 | 3.62 c ± 0.01 | 0.5 ab ± 0.1 | 0.05 d ± 0.01 | 79.7 e ± 0.2 | 44.4 a ± 0.4 | |
| P80 | 0 | 4.63 a ± 0.01 | 0.60 a ± 0.01 | 1.17 c ± 0.01 | 2.71 bc ± 0.01 | 0.31 g ± 0.01 | 1015.0 b | 4.6 fg ± 0.1 | 3.56 d ± 0.02 | 0.49 b ± 0.06 | 0.10 bc ± 0.01 | 90.5 ab ± 0.2 | 25.3 g ± 0.4 |
| 30 | 4.41 ab ± 0.03 | 0.55 ab ± 0.01 | 1.23 b ± 0.01 | 2.65 c ± 0.03 | 0.31 g ± 0.01 | 1015.3 a | 4.6 fg ± 0.1 | 3.63 c ± 0.01 | 0.48 b ± 0.02 | 0.06 cd ± 0.02 | 87.1 c ± 0.1 | 31.2 e ± 0.1 | |
| 60 | 4.48 a ± 0.02 | 0.48 b ± 0.01 | 1.28 b ± 0.01 | 2.80 b ± 0.02 | 0.30 h ± 0.01 | 1015.0 b | 4.6 fg ± 0.2 | 3.64 c ± 0.01 | 0.48 b ± 0.03 | 0.10 bc ± 0.01 | 84.6 d ± 0.1 | 37.9 c ± 0.2 | |
| 120 | 4.34 b ± 0.02 | 0.40 bc ± 0.01 | 1.26 b ± 0.01 | 2.50 d ± 0.01 | 0.28 i ± 0.01 | 1015.0 b | 4.6 fg ± 0.2 | 3.61 c ± 0.01 | 0.5 ab ± 0.1 | 0.08 c ± 0.01 | 81.9 e ± 0.1 | 42.8 ab ± 0.2 | |
| 180 | 4.21 b ± 0.02 | 0.31 d ± 0.01 | 1.25 b ± 0.01 | 2.76 bc ± 0.03 | 0.25 j ± 0.01 | 1015.0 b | 4.6 fg ± 0.1 | 3.58 cd ± 0.02 | 0.51 a ± 0.05 | 0.06 cd ± 0.01 | 79.3 f ± 0.1 | 44.4 a ± 0.2 |
| Samples | Storage Days | Hydroxycinnamic Acid | Flavan-3-Ois | Dihydrochalcones | |||||
|---|---|---|---|---|---|---|---|---|---|
| CQA | CAA | CMA | CAT | EPC | PB2 | PLZ | F2X | ||
| CA | 0 | 11.18 c ± 0.06 | 13.4 d ± 0.2 | 3.27 f ± 0.04 | 10.4 cd ± 0.3 | 16.7 c ±0.5 | 7.8 e ± 0.2 | 7.29 c ± 0.03 | 9.3 b ± 0.4 |
| 30 | 3.77 f ± 0.02 | 15.07 c ± 0.03 | 2.95 g ± 0.02 | 11.47 b ± 0.04 | 16.1 d ± 0.9 | 9.00 c ± 0.07 | 6.46 e ± 0.05 | 7.94 d ± 0.03 | |
| 60 | 1.30 h ± 0.01 | 16.96 b ± 0.04 | 3.59 d ± 0.01 | 11.24 bc ± 0.02 | 14.11 g ± 0.01 | 8.84 c ± 0.04 | 5.28 g ± 0.01 | 7.64 e ± 0.03 | |
| 120 | 0.71 i ± 0.01 | 18.85 ab ± 0.03 | 3.83 d ± 0.01 | 12.18 ab ± 0.04 | 9.45 k± 0.03 | 9.97 c ± 0.05 | 3.3 h ± 0.1 | 5.89 f ± 0.01 | |
| 180 | 0.12 j ± 0.04 | 20.74 a ± 0.04 | 4.07 c ± 0.03 | 13.13 a ± 0.04 | 9.1 k± 0.7 | 11.1 b ± 0.1 | 1.32 i ± 0.04 | 4.14 g ± 0.05 | |
| CR | 0 | 11.2 c ± 0.1 | 13.4 d ± 0.2 | 3.27 f ± 0.04 | 10.4 cd ± 0.3 | 16.72 c ±0.50 | 7.8 e ± 0.2 | 7.29 c ± 0.03 | 9.3 b ± 0.4 |
| 30 | 6.41 e ± 0.01 | 9.91 g ± 0.03 | 2.45 h ± 0.01 | 7.06 f ± 0.08 | 10.38 j ± 0.08 | 6.1 f ± 0.4 | 5.22 g ± 0.02 | 6.09 f ± 0.01 | |
| 60 | 9.06 d ± 0.01 | 15.06 c ± 0.04 | 3.87 d ± 0.06 | 10.2 d ± 0.1 | 14.55 fg ± 0.04 | 8.1 d ± 0.1 | 7.4 c ± 0.1 | 8.7 c ± 0.2 | |
| 120 | 6.32 e ± 0.01 | 16.7 b ± 0.3 | 4.28 c ± 0.03 | 9.45 e ± 0.02 | 11.55 ij ± 0.01 | 8.09 d ± 0.07 | 7.15 c ± 0.03 | 7.60 e ± 0.03 | |
| 180 | 3.58 f ± 0.04 | 16.94 b ± 0.05 | 4.69 c ± 0.05 | 8.7 e ± 0.2 | 8.46 l ± 0.06 | 8.1 d ± 0.1 | 6.9 d ± 0.1 | 6.5 f± 0.3 | |
| P60 | 0 | 11.7 b ± 0.4 | 11.9 e ± 0.4 | 2.9 g ± 0.1 | 10.30 d ± 0.03 | 17.26 b ± 0.50 | 8.4 c ± 0.2 | 6.79 e ± 0.02 | 8.49 c ± 0.05 |
| 30 | 10.68 c ± 0.02 | 14.80 cd ± 0.04 | 4.15 c ± 0.03 | 11.1 b ± 0.1 | 18.52 a ± 0.03 | 13.3 a ± 0.2 | 7.86 c ± 0.02 | 9.14 b ± 0.03 | |
| 60 | 8.51 d ± 0.01 | 14.68 cd ± 0.06 | 4.33 c ± 0.03 | 10.65 c ± 0.01 | 14.9 f ± 0.3 | 6.0 f ± 0.2 | 7.3 c ± 0.1 | 9.2 b ± 0.1 | |
| 120 | 5.41 e ± 0.01 | 17.71 b ± 0.05 | 5.86 b ± 0.03 | 11.1 b ± 0.1 | 12.4 i ± 0.5 | 4.8 g ± 0.1 | 7.95 c ± 0.01 | 9.9 a ± 0.1 | |
| 180 | 2.32 g ± 0.03 | 20.74 a ± 0.05 | 7.39 a ± 0.07 | 11.55 b ± 0.01 | 10.9 j ± 0.3 | 3.1 h ± 0.2 | 8.6 b ± 0.1 | 10.6 a ± 0.1 | |
| P80 | 0 | 10.5 c ± 0.3 | 10.2 g ± 0.3 | 2.30 h ± 0.04 | 8.35 e ± 0.3 | 15.5 e ± 0.6 | 8.9 c ± 0.2 | 5.8 f ± 0.3 | 6.41 f ± 0.05 |
| 30 | 13.0 a ± 0.2 | 11.2 ef ± 0.1 | 2.92 g ± 0.02 | 10.1 d ± 0.1 | 15.87 de ± 0.03 | 8.3 cd ± 0.3 | 6.60 e ± 0.03 | 8.71 c ± 0.02 | |
| 60 | 12.0 b ± 0.2 | 12.0 e ± 0.1 | 3.47 d ± 0.01 | 9.4 e ± 0.2 | 13.4 h ± 0.1 | 5.07 g ± 0.06 | 7.11 d ± 0.1 | 8.39 c ± 0.01 | |
| 120 | 12.2 a ± 0.2 | 13.8 d ± 0.3 | 4.64 c ± 0.05 | 10.65 c ± 0.01 | 11.55 ij ± 0.03 | 1.98 i ± 0.02 | 8.44 b ± 0.01 | 9.1 b ± 0.2 | |
| 180 | 12.5 a ± 0.2 | 15.7 c ± 0.2 | 5.82 b ± 0.03 | 11.9 b ± 0.2 | 9.71 k ± 0.03 | <LOQ | 9.77 a ± 0.03 | 9.81 b ± 0.04 | |
| Sample | Storage Days | TPC (mg CAE/L) | DPPH (µmol TE/L) | ABTS (µmol TE/L) | FRAP (µmol TE/L) |
|---|---|---|---|---|---|
| CA | 0 | 870 ab ± 26 | 1217 a ± 17 | 2923 b ± 134 | 1911 b ± 30 |
| 30 | 780 ef ± 26 | 1102 b ± 30 | 3076 a ± 23 | 1862 bc ± 53 | |
| 60 | 810 cde ± 40 | 1123 b ± 17 | 2900 b ± 65 | 1900 bc ± 161 | |
| 120 | 768 efg ± 27 | 839 e ± 42 | 2345 d ± 33 | 1564 fg ± 23 | |
| 180 | 719 gh ± 26 | 556 h ± 7 | 1790 h ± 74 | 1229 i ± 18 | |
| CR | 0 | 870 ab ± 26 | 1217 a ± 17 | 2923 b ± 134 | 1911 b ± 31 |
| 30 | 845 abc ± 52 | 938 d ± 101 | 2959 ab ± 93 | 2075 a ± 45 | |
| 60 | 830 cd ± 11 | 967 cd ± 33 | 2946 ab ± 267 | 1819 bc ± 175 | |
| 120 | 806 cde ± 25 | 754 f ± 28 | 2387 d ± 45 | 1670 e ± 57 | |
| 180 | 783 ef ± 12 | 542 h ± 18 | 1828 g ± 12 | 1506 g ± 81 | |
| P60 | 0 | 875 a ± 15 | 1140 b ± 105 | 3011 ab ± 69 | 1913 b ± 73 |
| 30 | 760 efg ± 31 | 1129 b ± 22 | 2870 e ± 34 | 1764 cde ± 112 | |
| 60 | 770 efg ± 9 | 956 cd ± 41 | 2154 e ± 19 | 1580 fg ± 115 | |
| 120 | 777 ef ± 36 | 788 f ± 28 | 1846 g ± 22 | 1627 f ± 85 | |
| 180 | 788 ef ± 46 | 621 g ± 53 | 1504 i ± 22 | 1700 de ± 33 | |
| P80 | 0 | 860 b ± 10 | 1012 c ± 26 | 2910 b ± 49 | 1819 bcd ± 41 |
| 30 | 813 cde ± 15 | 1118 b ± 55 | 2887 bc ± 71 | 1588 fg ± 15 | |
| 60 | 795 def ± 54 | 1156 ab ± 33 | 2678 d ± 144 | 1554 fg ± 7 | |
| 120 | 769 efg ± 27 | 862 e ± 32 | 2044 f ± 44 | 1389 h ± 15 | |
| 180 | 743 fg ± 9 | 569 h ± 43 | 1410 j ± 10 | 1225 i ± 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carraro, M.d.M.; Sola, I.M.M.S.; Santos, R.D.M.d.; Demiate, I.M.; Alberti, A.; Nogueira, A. Impact of Heat Treatment on Hard Cider Enriched with Cryo-Concentrated Apple Must: Microbiological Profile, Functional Properties, and Storage Stability. Fermentation 2025, 11, 188. https://doi.org/10.3390/fermentation11040188
Carraro MdM, Sola IMMS, Santos RDMd, Demiate IM, Alberti A, Nogueira A. Impact of Heat Treatment on Hard Cider Enriched with Cryo-Concentrated Apple Must: Microbiological Profile, Functional Properties, and Storage Stability. Fermentation. 2025; 11(4):188. https://doi.org/10.3390/fermentation11040188
Chicago/Turabian StyleCarraro, Matheus de Melo, Isabela Maria Macedo Simon Sola, Raul Dias Moreira dos Santos, Ivo Mottin Demiate, Aline Alberti, and Alessandro Nogueira. 2025. "Impact of Heat Treatment on Hard Cider Enriched with Cryo-Concentrated Apple Must: Microbiological Profile, Functional Properties, and Storage Stability" Fermentation 11, no. 4: 188. https://doi.org/10.3390/fermentation11040188
APA StyleCarraro, M. d. M., Sola, I. M. M. S., Santos, R. D. M. d., Demiate, I. M., Alberti, A., & Nogueira, A. (2025). Impact of Heat Treatment on Hard Cider Enriched with Cryo-Concentrated Apple Must: Microbiological Profile, Functional Properties, and Storage Stability. Fermentation, 11(4), 188. https://doi.org/10.3390/fermentation11040188





