Proposal for a Conceptual Biorefinery for the Conversion of Waste into Biocrude, H2 and Electricity Based on Hydrothermal Co-Liquefaction and Bioelectrochemical Systems
Abstract
1. Introduction
2. Consumption and Energy Demand
3. Biomass and Residues
4. Sugarcane and Malt Bagasse
5. Sewage Sludge
6. Microalgae
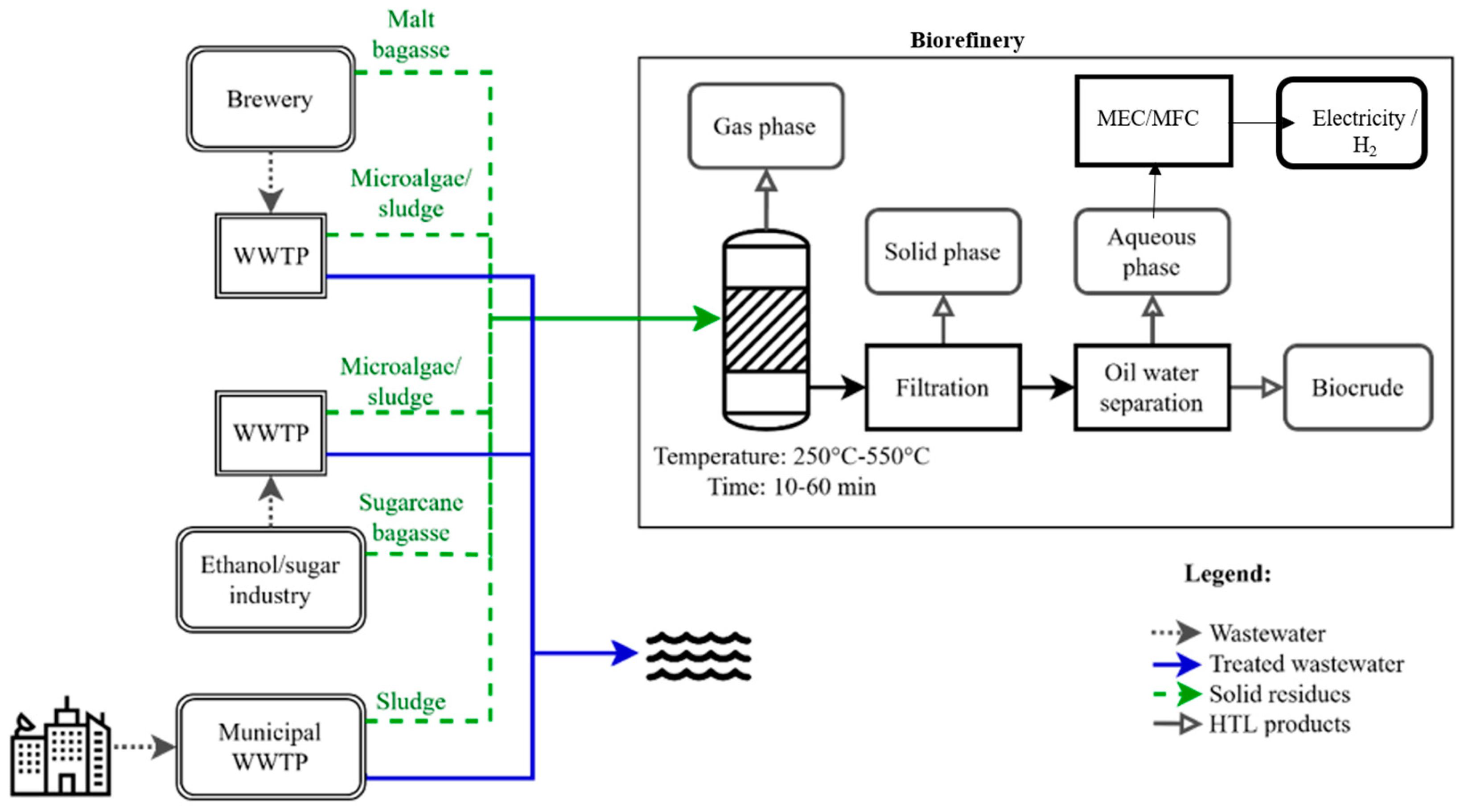
7. Hydrothermal Co-Liquefaction Process and Biocrude as Product
8. Aqueous Phase from HTL and Co-HTL
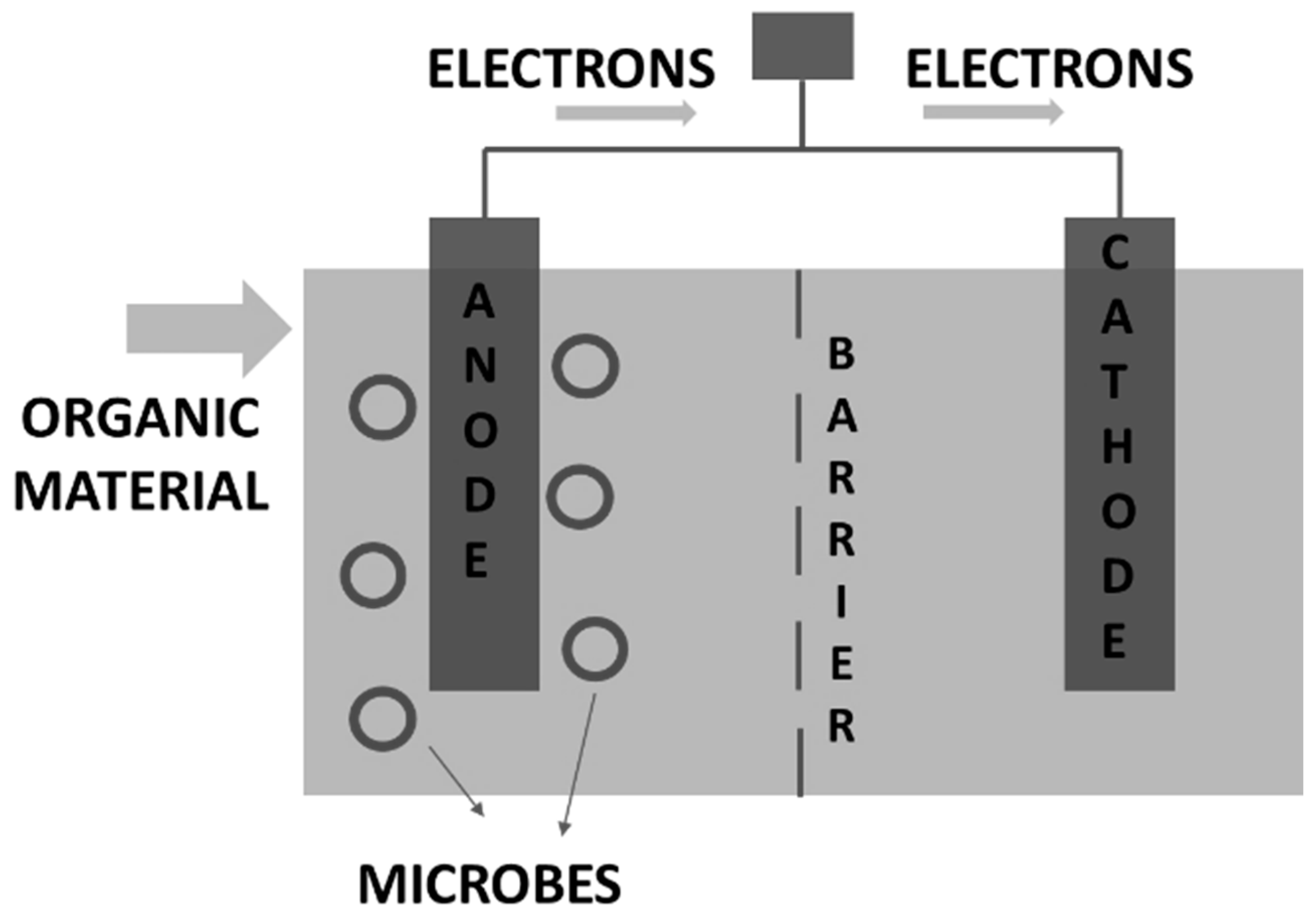
9. Challenges and Prospects in HTL-AP Valorization Technologies
10. Conclusions and Future Prospects
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, Q.; Wang, X.; Cong, X. How Does Foreign Direct Investment Affect CO2 Emissions in Emerging Countries? New Findings from a Nonlinear Panel Analysis. J. Clean. Prod. 2020, 249, 119422. [Google Scholar] [CrossRef]
- Lei, R.; Feng, S.; Lauvaux, T. Country-Scale Trends in Air Pollution and Fossil Fuel CO2 emissions during 2001-2018: Confronting the Roles of National Policies and Economic Growth. Environ. Res. Lett. 2020, 16, 014006. [Google Scholar] [CrossRef]
- Chandel, A.K.; Forte, M.B.S.; Gonçalves, I.S.; Milessi, T.S.; Arruda, P.V.; Carvalho, W.; Mussatto, S.I. Brazilian Biorefineries from Second Generation Biomass: Critical Insights from Industry and Future Perspectives. Biofuels Bioprod. Biorefining 2021, 15, 1190–1208. [Google Scholar] [CrossRef]
- Rosendahl, L. Direct Thermochemical Liquefaction for Energy Applications; Woodhead Publishing: Sawston, UK, 2017; pp. 1–356. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, S.; Cao, B.; Hu, Y.; Abomohra, A.E.F.; Wang, Q.; Qian, L.; Liu, L.; Liu, X.; He, Z.; et al. Optimization of Hydrothermal Co-Liquefaction of Seaweeds with Lignocellulosic Biomass: Merging 2nd and 3rd Generation Feedstocks for Enhanced Bio-Oil Production. Energy 2019, 173, 413–422. [Google Scholar] [CrossRef]
- Chen, W.T.; Zhang, Y.; Zhang, J.; Schideman, L.; Yu, G.; Zhang, P.; Minarick, M. Co-Liquefaction of Swine Manure and Mixed-Culture Algal Biomass from a Wastewater Treatment System to Produce Bio-Crude Oil. Appl. Energy 2014, 128, 209–216. [Google Scholar] [CrossRef]
- Biller, P.; Ross, A.B. Potential Yields and Properties of Oil from the Hydrothermal Liquefaction of Microalgae with Different Biochemical Content. Bioresour. Technol. 2011, 102, 215–225. [Google Scholar] [CrossRef]
- Yang, J.; (Sophia) He, Q.; Yang, L. A Review on Hydrothermal Co-Liquefaction of Biomass. Appl. Energy 2019, 250, 926–945. [Google Scholar] [CrossRef]
- Pedersen, T.H.; Grigoras, I.F.; Hoffmann, J.; Toor, S.S.; Daraban, I.M.; Jensen, C.U.; Iversen, S.B.; Madsen, R.B.; Glasius, M.; Arturi, K.R.; et al. Continuous Hydrothermal Co-Liquefaction of Aspen Wood and Glycerol with Water Phase Recirculation. Appl. Energy 2016, 162, 1034–1041. [Google Scholar] [CrossRef]
- Population Division, United Nations. World Population Prospects. Available online: https://population.un.org/wpp/ (accessed on 9 July 2024).
- Ritchie, H.; Rodés-Guirao, L.; Mathieu, E.; Gerber, M.; Ortiz-Ospina, E.; Hasell, J.; Roser, M. Population Growth. Our World in Data. 2024. Available online: https://ourworldindata.org/population-growth (accessed on 9 July 2024).
- Sustainable Consumption and Production. Available online: https://www.un.org/sustainabledevelopment/sustainable-consumption-production/ (accessed on 9 July 2024).
- Ahmed, Z.; Ahmad, M.; Murshed, M.; Vaseer, A.I.; Kirikkaleli, D. The Trade-off between Energy Consumption, Economic Growth, Militarization, and CO2 Emissions: Does the Treadmill of Destruction Exist in the Modern World? Environ. Sci. Pollut. Res. 2022, 29, 18063–18076. [Google Scholar] [CrossRef]
- Destek, M.A.; Sinha, A. Renewable, Non-Renewable Energy Consumption, Economic Growth, Trade Openness and Ecological Footprint: Evidence from Organisation for Economic Co-Operation and Development Countries. J. Clean. Prod. 2020, 242, 118537. [Google Scholar] [CrossRef]
- United Nations. Goal 7—Ensure Access to Affordable, Reliable, Sustainable and Modern Energy for All; United Nations: New York, NY, USA.
- United Nations. Climate Change; United Nations: New York, NY, USA.
- World—Total Including LUCF: Greenhouse Gas (GHG) Emissions—Climate Watch. Available online: https://www.climatewatchdata.org/ghg-emissions?end_year=2021&start_year=1990 (accessed on 9 July 2024).
- Global Bioenergy Statistics 2021. Available online: https://www.worldbioenergy.org/news/640/47/Global-Bioenergy-Statistics-2021/ (accessed on 9 July 2024).
- Ritchie, H.; Rosado, P.; Roser, M. Energy Production and Consumption. Our World in Data 2024. Available online: https://ourworldindata.org/energy-production-consumption (accessed on 9 July 2024).
- Alonso, D.M.; Bond, J.Q.; Dumesic, J.A. Catalytic Conversion of Biomass to Biofuels. Green Chem. 2010, 12, 1493–1513. [Google Scholar] [CrossRef]
- Akhtar, J.; Amin, N.A.S. A Review on Process Conditions for Optimum Bio-Oil Yield in Hydrothermal Liquefaction of Biomass. Renew. Sustain. Energy Rev. 2011, 15, 1615–1624. [Google Scholar] [CrossRef]
- Zmierczak, W.W.; Miller, J.D. Processes for Catalytic Conversion of Lignin to Liquid Bio-Fuels. MX2007013672A, 2 May 2006. [Google Scholar]
- Khan, S.A.; Rashmi; Hussain, M.Z.; Prasad, S.; Banerjee, U.C. Prospects of Biodiesel Production from Microalgae in India. Renew. Sustain. Energy Rev. 2009, 13, 2361–2372. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, C. (Charles); Champagne, P. Overview of Recent Advances in Thermo-Chemical Conversion of Biomass. Energy Convers Manag 2010, 51, 969–982. [Google Scholar] [CrossRef]
- Huang, H.; Yuan, X.; Zeng, G.; Wang, J.; Li, H.; Zhou, C.; Pei, X.; You, Q.; Chen, L. Thermochemical Liquefaction Characteristics of Microalgae in Sub- and Supercritical Ethanol. Fuel Process. Technol. 2011, 92, 147–153. [Google Scholar] [CrossRef]
- Zou, S.; Wu, Y.; Yang, M.; Li, C.; Tong, J. Thermochemical Catalytic Liquefaction of the Marine Microalgae Dunaliella Tertiolecta and Characterization of Bio-Oils. Energy Fuels 2009, 23, 3753–3758. [Google Scholar] [CrossRef]
- Xiu, S.; Shahbazi, A. Bio-Oil Production and Upgrading Research: A Review. Renew. Sustain. Energy Rev. 2012, 16, 4406–4414. [Google Scholar] [CrossRef]
- Bioenergy—Energy: Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/energy/bioenergy/en/ (accessed on 9 July 2024).
- Demirbas, A. Ayhan Competitive Liquid Biofuels from Biomass. Appl. Energy 2011, 88, 17–28. [Google Scholar] [CrossRef]
- Peterson, A.A.; Vogel, F.; Lachance, R.P.; Fröling, M.; Antal, M.J.; Tester, J.W. Thermochemical Biofuel Production in Hydrothermal Media: A Review of Sub- and Supercritical Water Technologies. Energy Environ. Sci 2008, 1, 32–65. [Google Scholar] [CrossRef]
- Zhang, Q.; Chang, J.; Wang, T.; Xu, Y. Review of Biomass Pyrolysis Oil Properties and Upgrading Research. Energy Convers. Manag. 2007, 48, 87–92. [Google Scholar] [CrossRef]
- Kandasamy, S.; Zhang, B.; He, Z.; Bhuvanendran, N.; EL-Seesy, A.I.; Wang, Q.; Narayanan, M.; Thangavel, P.; Dar, M.A. Microalgae as a Multipotential Role in Commercial Applications: Current Scenario and Future Perspectives. Fuel 2022, 308, 122053. [Google Scholar] [CrossRef]
- Saladini, F.; Patrizi, N.; Pulselli, F.M.; Marchettini, N.; Bastianoni, S. Guidelines for Emergy Evaluation of First, Second and Third Generation Biofuels. Renew. Sustain. Energy Rev. 2016, 66, 221–227. [Google Scholar] [CrossRef]
- Energy Technology Perspectives 2008—Analysis—IEA. Available online: https://www.iea.org/reports/energy-technology-perspectives-2008 (accessed on 9 July 2024).
- World Food and Agriculture—Statistical Yearbook 2021; FAO: Rome, Italy, 2021.
- Bolognesi, S.; Bernardi, G.; Callegari, A.; Dondi, D.; Capodaglio, A.G. Biochar Production from Sewage Sludge and Microalgae Mixtures: Properties, Sustainability and Possible Role in Circular Economy. Biomass Convers. Biorefin. 2021, 11, 289–299. [Google Scholar] [CrossRef]
- Peter, A.P.; Khoo, K.S.; Chew, K.W.; Ling, T.C.; Ho, S.H.; Chang, J.S.; Show, P.L. Microalgae for Biofuels, Wastewater Treatment and Environmental Monitoring. Environ. Chem. Lett. 2021, 19, 2891–2904. [Google Scholar] [CrossRef]
- Conab—Boletim Da Safra de Cana-de-Açúcar. Available online: https://www.conab.gov.br/info-agro/safras/cana/boletim-da-safra-de-cana-de-acucar (accessed on 9 July 2024).
- Annual Ethanol Production. Available online: https://ethanolrfa.org/markets-and-statistics/annual-ethanol-production (accessed on 9 July 2024).
- Sugar: World Markets and Trade—USDA Foreign Agricultural Service. Available online: https://fas.usda.gov/data/sugar-world-markets-and-trade-05232024 (accessed on 9 July 2024).
- Hofsetz, K.; Silva, M.A. Brazilian Sugarcane Bagasse: Energy and Non-Energy Consumption. Biomass Bioenergy 2012, 46, 564–573. [Google Scholar] [CrossRef]
- Conab—Portal de Informações Agropecuária. Available online: https://portaldeinformacoes.conab.gov.br/produtos-360.html (accessed on 9 July 2024).
- Empresa de Pesquisa Energética Balanço Energético Nacional 2021. Available online: https://www.epe.gov.br/pt/publicacoes-dados-abertos/publicacoes/balanco-energetico-nacional-2021 (accessed on 9 July 2024).
- de Moraes Rocha, G.J.; Nascimento, V.M.; Gonçalves, A.R.; Silva, V.F.N.; Martín, C. Influence of Mixed Sugarcane Bagasse Samples Evaluated by Elemental and Physical–Chemical Composition. Ind. Crops Prod. 2015, 64, 52–58. [Google Scholar] [CrossRef]
- Iwase, C.H.T.; Piacentini, K.C.; Giomo, P.P.; Čumová, M.; Wawroszová, S.; Běláková, S.; Minella, E.; Rocha, L.O. Characterization of the Fusarium Sambucinum Species Complex and Detection of Multiple Mycotoxins in Brazilian Barley Samples. Food Res. Int. 2020, 136, 109336. [Google Scholar] [CrossRef]
- Foreign Agricultural Service Barley Explorer. Available online: https://ipad.fas.usda.gov/cropexplorer/cropview/commodityView.aspx?cropid=0430000 (accessed on 9 July 2024).
- Helm, C.V.; de Francisco, A. Chemical Characterization of Brazilian Hulless Barley Varieties, Flour Fractionation, and Protein Concentration. Sci. Agric. 2004, 61, 593–597. [Google Scholar] [CrossRef]
- Farzaneh, V.; Ghodsvali, A.; Bakhshabadi, H.; Zare, Z.; Carvalho, I.S. The Impact of Germination Time on the Some Selected Parameters through Malting Process. Int. J. Biol. Macromol. 2017, 94, 663–668. [Google Scholar] [CrossRef]
- Kok, Y.J.; Ye, L.; Muller, J.; Ow, D.S.W.; Bi, X. Brewing with Malted Barley or Raw Barley: What Makes the Difference in the Processes? Appl. Microbiol. Biotechnol. 2019, 103, 1059–1067. [Google Scholar] [CrossRef]
- Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Bimetallic Catalysts for Upgrading of Biomass to Fuels and Chemicals. Chem. Soc. Rev. 2012, 41, 8075–8098. [Google Scholar] [CrossRef]
- Cai, J.; He, Y.; Yu, X.; Banks, S.W.; Yang, Y.; Zhang, X.; Yu, Y.; Liu, R.; Bridgwater, A.V. Review of Physicochemical Properties and Analytical Characterization of Lignocellulosic Biomass. Renew. Sustain. Energy Rev. 2017, 76, 309–322. [Google Scholar] [CrossRef]
- Ahmed Baloch, H.; Nizamuddin, S.; Siddiqui, M.T.H.; Mubarak, N.M.; Dumbre, D.K.; Srinivasan, M.P.; Griffin, G.J. Sub-Supercritical Liquefaction of Sugarcane Bagasse for Production of Bio-Oil and Char: Effect of Two Solvents. J. Environ. Chem. Eng. 2018, 6, 6589–6601. [Google Scholar] [CrossRef]
- Sangjan, A.; Ngamsiri, P.; Klomkliang, N.; Wu, K.C.W.; Matsagar, B.M.; Ratchahat, S.; Liu, C.G.; Laosiripojana, N.; Sakdaronnarong, C. Effect of Microwave-Assisted Wet Torrefaction on Liquefaction of Biomass from Palm Oil and Sugarcane Wastes to Bio-Oil and Carbon Nanodots/Nanoflakes by Hydrothermolysis and Solvothermolysis. Renew. Energy 2020, 154, 1204–1217. [Google Scholar] [CrossRef]
- Ramirez, J.A.; Rainey, T.J. Comparative Techno-Economic Analysis of Biofuel Production through Gasification, Thermal Liquefaction and Pyrolysis of Sugarcane Bagasse. J. Clean. Prod. 2019, 229, 513–527. [Google Scholar] [CrossRef]
- Santos, M.; Jiménez, J.J.; Bartolomé, B.; Gómez-Cordovés, C.; Del Nozal, M.J. Variability of Brewer’s Spent Grain within a Brewery. Food Chem. 2003, 80, 17–21. [Google Scholar] [CrossRef]
- Mello, L.R.P.F.; Mali, S. Use of Malt Bagasse to Produce Biodegradable Baked Foams Made from Cassava Starch. Ind. Crops Prod. 2014, 55, 187–193. [Google Scholar] [CrossRef]
- Mendes, J.F.; Norcino, L.B.; Manrich, A.; Pinheiro, A.C.M.; Oliveira, J.E.; Mattoso, L.H.C. Development, Physical-Chemical Properties, and Photodegradation of Pectin Film Reinforced with Malt Bagasse Fibers by Continuous Casting. J. Appl. Polym. Sci. 2020, 137, 49178. [Google Scholar] [CrossRef]
- Zhu, L.; Huo, S.; Qin, L. A Microalgae-Based Biodiesel Refinery: Sustainability Concerns and Challenges. Int. J. Green Energy 2015, 12, 595–602. [Google Scholar] [CrossRef]
- Qadir, M.; Drechsel, P.; Jiménez Cisneros, B.; Kim, Y.; Pramanik, A.; Mehta, P.; Olaniyan, O. Global and Regional Potential of Wastewater as a Water, Nutrient and Energy Source. Nat. Resour. Forum 2020, 44, 40–51. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations AQUASTAT—FAO’s Global Information System on Water and Agriculture. Available online: https://www.fao.org/aquastat/en/ (accessed on 9 July 2024).
- Agência Nacional de Águas. Atlas Esgotos: Despoluição de Bacias Hidrográficas; Agência Nacional de Águas: Brasília, Brazil, 2020. [Google Scholar]
- United Nations Progress on Wastewater Treatment (SDG Target 6.3). Available online: https://www.sdg6data.org/en/indicator/6.3.1 (accessed on 9 July 2024).
- von Sperling, M. Urban Wastewater Treatment in Brazil; Inter-American Development Bank: Washington, DC, USA, 2016. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, Z.; Dai, M.; Fang, S.; Liao, Y.; Yu, Z.; Ma, X. Co-Pyrolysis Kinetics of Sewage Sludge and Bagasse Using Multiple Normal Distributed Activation Energy Model (M-DAEM). Bioresour. Technol. 2018, 259, 173–180. [Google Scholar] [CrossRef] [PubMed]
- De Abreu, A.H.M.; Leles, P.S.D.S.; Alonso, J.M.; Abel, E.L.D.S.; De Oliveira, R.R. Characterization of Sewage Sludge Generated in Rio de Janeiro, Brazil, and Perspectives for Agricultural Recycling. Semin. Cienc. Agrar. 2017, 38, 2433–2448. [Google Scholar] [CrossRef]
- Huang, H.-J.; Yuan, X.-Z.; Zhu, H.-N.; Li, H.; Liu, Y.; Wang, X.-L.; Zeng, G.-M. Comparative Studies of Thermochemical Liquefaction Characteristics of Microalgae, Lignocellulosic Biomass and Sewage Sludge. Energy 2013, 56, 52–60. [Google Scholar] [CrossRef]
- Rahman, T.; Jahromi, H.; Roy, P.; Adhikari, S.; Hassani, E.; Oh, T.S. Hydrothermal Liquefaction of Municipal Sewage Sludge: Effect of Red Mud Catalyst in Ethylene and Inert Ambiences. Energy Convers. Manag. 2021, 245, 114615. [Google Scholar] [CrossRef]
- Andreoli, C.V.; Von Sperling, M.; Fernandes, F. Lodo de Esgotos: Tratamento e Disposição Final; Portal Regional da BVS: Brazil, 2001; 483p. [Google Scholar]
- Bahadar, A.; Bilal Khan, M. Progress in Energy from Microalgae: A Review. Renew. Sustain. Energy Rev. 2013, 27, 128–148. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial Applications of Microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, R.; Banerjee, S.; Arora, A. Recent Advances in the Integrated Biorefinery Concept for the Valorization of Algal Biomass through Sustainable Routes. Biofuels Bioprod. Biorefining 2021, 15, 879–898. [Google Scholar] [CrossRef]
- Toor, S.S.; Rosendahl, L.; Rudolf, A. Hydrothermal Liquefaction of Biomass: A Review of Subcritical Water Technologies. Energy 2011, 36, 2328–2342. [Google Scholar] [CrossRef]
- Brindhadevi, K.; Anto, S.; Rene, E.R.; Sekar, M.; Mathimani, T.; Thuy Lan Chi, N.; Pugazhendhi, A. Effect of Reaction Temperature on the Conversion of Algal Biomass to Bio-Oil and Biochar through Pyrolysis and Hydrothermal Liquefaction. Fuel 2021, 285, 119106. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from Microalgae—A Review of Technologies for Production, Processing, and Extractions of Biofuels and Co-Products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Duan, P.; Savage, P.E. Upgrading of Crude Algal Bio-Oil in Supercritical Water. Bioresour. Technol. 2011, 102, 1899–1906. [Google Scholar] [CrossRef]
- Valdez, P.J.; Nelson, M.C.; Wang, H.Y.; Lin, X.N.; Savage, P.E. Hydrothermal Liquefaction of Nannochloropsis Sp.: Systematic Study of Process Variables and Analysis of the Product Fractions. Biomass Bioenergy 2012, 46, 317–331. [Google Scholar] [CrossRef]
- Palomino, A.; Godoy-Silva, R.D.; Raikova, S.; Chuck, C.J. The Storage Stability of Biocrude Obtained by the Hydrothermal Liquefaction of Microalgae. Renew. Energy 2020, 145, 1720–1729. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, C.; Yang, X.; Tang, X.; Zhang, C.; Yang, X. Optimizing Process of Hydrothermal Liquefaction of Microalgae via Flash Heating and Isolating Aqueous Extract from Bio-Crude. J. Clean. Prod. 2020, 258, 120660. [Google Scholar] [CrossRef]
- Masoumi, S.; Boahene, P.E.; Dalai, A.K. Biocrude Oil and Hydrochar Production and Characterization Obtained from Hydrothermal Liquefaction of Microalgae in Methanol-Water System. Energy 2021, 217, 119344. [Google Scholar] [CrossRef]
- Zhou, Y.; Schideman, L.; Yu, G.; Zhang, Y. A Synergistic Combination of Algal Wastewater Treatment and Hydrothermal Biofuel Production Maximized by Nutrient and Carbon Recycling. Energy Environ. Sci. 2013, 6, 3765–3779. [Google Scholar] [CrossRef]
- Mishra, S.; Mohanty, K. Co-HTL of Domestic Sewage Sludge and Wastewater Treatment Derived Microalgal Biomass—An Integrated Biorefinery Approach for Sustainable Biocrude Production. Energy Convers. Manag. 2020, 204, 112312. [Google Scholar] [CrossRef]
- Roberts, G.W.; Fortier, M.O.P.; Sturm, B.S.M.; Stagg-Williams, S.M. Promising Pathway for Algal Biofuels through Wastewater Cultivation and Hydrothermal Conversion. Energy Fuels 2013, 27, 857–867. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, L.; Qi, Y. Enhancing the Productivity of Microalgae Cultivated in Wastewater toward Biofuel Production: A Critical Review. Appl. Energy 2015, 137, 282–291. [Google Scholar] [CrossRef]
- Watanabe, M.M.; Isdepsky, A. Biocrude Oil Production by Integrating Microalgae Polyculture and Wastewater Treatment: Novel Proposal on the Use of Deep Water-Depth Polyculture of Mixotrophic Microalgae. Energies 2021, 14, 6992. [Google Scholar] [CrossRef]
- Farooq, W.; Lee, Y.C.; Ryu, B.G.; Kim, B.H.; Kim, H.S.; Choi, Y.E.; Yang, J.W. Two-Stage Cultivation of Two Chlorella sp. Strains by Simultaneous Treatment of Brewery Wastewater and Maximizing Lipid Productivity. Bioresour. Technol. 2013, 132, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Santana, H.; Cereijo, C.R.; Teles, V.C.; Nascimento, R.C.; Fernandes, M.S.; Brunale, P.; Campanha, R.C.; Soares, I.P.; Silva, F.C.P.; Sabaini, P.S.; et al. Microalgae Cultivation in Sugarcane Vinasse: Selection, Growth and Biochemical Characterization. Bioresour. Technol. 2017, 228, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Walter, V.; Hornung, U.; Dahmen, N. Hydrothermal Liquefaction of Chlorella vulgaris and Nannochloropsis gaditana in a Continuous Stirred Tank Reactor and Hydrotreating of Biocrude by Nickel Catalysts. Fuel Process. Technol. 2019, 191, 168–180. [Google Scholar] [CrossRef]
- Xu, D.; Guo, S.; Liu, L.; Lin, G.; Wu, Z.; Guo, Y.; Wang, S. Heterogeneous Catalytic Effects on the Characteristics of Water-Soluble and Water-Insoluble Biocrudes in Chlorella Hydrothermal Liquefaction. Appl. Energy 2019, 243, 165–174. [Google Scholar] [CrossRef]
- Salam, K.A.; Velasquez-Orta, S.B.; Harvey, A.P. A Sustainable Integrated in Situ Transesterification of Microalgae for Biodiesel Production and Associated Co-Product-a Review. Renew. Sustain. Energy Rev. 2016, 65, 1179–1198. [Google Scholar] [CrossRef]
- Huang, H.J.; Yuan, X.Z. Recent Progress in the Direct Liquefaction of Typical Biomass. Prog. Energy Combust. Sci. 2015, 49, 59–80. [Google Scholar] [CrossRef]
- Durak, H.; Genel, S. Catalytic Hydrothermal Liquefaction of Lactuca Scariola with a Heterogeneous Catalyst: The Investigation of Temperature, Reaction Time and Synergistic Effect of Catalysts. Bioresour. Technol. 2020, 309, 123375. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Li, B.; Liu, Z.; Zhang, Y.; Lu, H. Hydrothermal Liquefaction for Algal Biorefinery: A Critical Review. Renew. Sustain. Energy Rev. 2014, 38, 933–950. [Google Scholar] [CrossRef]
- Vardon, D.R.; Sharma, B.K.; Scott, J.; Yu, G.; Wang, Z.; Schideman, L.; Zhang, Y.; Strathmann, T.J. Chemical Properties of Biocrude Oil from the Hydrothermal Liquefaction of Spirulina Algae, Swine Manure, and Digested Anaerobic Sludge. Bioresour. Technol. 2011, 102, 8295–8303. [Google Scholar] [CrossRef]
- Tian, C.; Liu, Z.; Zhang, Y.; Li, B.; Cao, W.; Lu, H.; Duan, N.; Zhang, L.; Zhang, T. Hydrothermal Liquefaction of Harvested High-Ash Low-Lipid Algal Biomass from Dianchi Lake: Effects of Operational Parameters and Relations of Products. Bioresour. Technol. 2015, 184, 336–343. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Kishore, N.; Gu, S. A Review on Hydrothermal Liquefaction of Biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- Jena, U.; McCurdy, A.T.; Warren, A.; Summers, H.; Ledbetter, R.N.; Hoekman, S.K.; Seefeldt, L.C.; Quinn, J.C. Oleaginous Yeast Platform for Producing Biofuels via Co-Solvent Hydrothermal Liquefaction. Biotechnol. Biofuels 2015, 8, 167. [Google Scholar] [CrossRef]
- Demirbaş, A. Mechanisms of Liquefaction and Pyrolysis Reactions of Biomass. Energy Convers. Manag. 2000, 41, 633–646. [Google Scholar] [CrossRef]
- Biswas, B.; Bisht, Y.; Kumar, J.; Yenumala, S.R.; Bhaskar, T. Effects of Temperature and Solvent on Hydrothermal Liquefaction of the Corncob for Production of Phenolic Monomers. Biomass Convers. Biorefin. 2022, 12, 91–101. [Google Scholar] [CrossRef]
- Biswas, B.; Arun Kumar, A.; Bisht, Y.; Krishna, B.B.; Kumar, J.; Bhaskar, T. Role of Temperatures and Solvents on Hydrothermal Liquefaction of Azolla Filiculoides. Energy 2021, 217, 119330. [Google Scholar] [CrossRef]
- Zhu, Z.; Rosendahl, L.; Toor, S.S.; Yu, D.; Chen, G. Hydrothermal Liquefaction of Barley Straw to Bio-Crude Oil: Effects of Reaction Temperature and Aqueous Phase Recirculation. Appl. Energy 2015, 137, 183–192. [Google Scholar] [CrossRef]
- Sahoo, A.; Saini, K.; Jindal, M.; Bhaskar, T.; Pant, K.K. Co-Hydrothermal Liquefaction of Algal and Lignocellulosic Biomass: Status and Perspectives. Bioresour. Technol. 2021, 342, 125948. [Google Scholar] [CrossRef] [PubMed]
- Gai, C.; Li, Y.; Peng, N.; Fan, A.; Liu, Z. Co-Liquefaction of Microalgae and Lignocellulosic Biomass in Subcritical Water. Bioresour. Technol. 2015, 185, 240–245. [Google Scholar] [CrossRef]
- Sintamarean, I.M.; Pedersen, T.H.; Zhao, X.; Kruse, A.; Rosendahl, L.A. Application of Algae as Cosubstrate to Enhance the Processability of Willow Wood for Continuous Hydrothermal Liquefaction. Ind. Eng. Chem. Res. 2017, 56, 4562–4571. [Google Scholar] [CrossRef]
- Karagöz, S.; Bhaskar, T.; Muto, A.; Sakata, Y. Comparative Studies of Oil Compositions Produced from Sawdust, Rice Husk, Lignin and Cellulose by Hydrothermal Treatment. Fuel 2005, 84, 875–884. [Google Scholar] [CrossRef]
- Li, Q.; Yuan, X.; Hu, X.; Meers, E.; Ong, H.C.; Chen, W.H.; Duan, P.; Zhang, S.; Lee, K.B.; Ok, Y.S. Co-Liquefaction of Mixed Biomass Feedstocks for Bio-Oil Production: A Critical Review. Renew. Sustain. Energy Rev. 2022, 154, 111814. [Google Scholar] [CrossRef]
- Elliott, D.C. Historical Developments in Hydroprocessing Bio-Oils. Energy Fuels 2007, 21, 1792–1815. [Google Scholar] [CrossRef]
- López Barreiro, D.; Gómez, B.R.; Ronsse, F.; Hornung, U.; Kruse, A.; Prins, W. Heterogeneous Catalytic Upgrading of Biocrude Oil Produced by Hydrothermal Liquefaction of Microalgae: State of the Art and Own Experiments. Fuel Process. Technol. 2016, 148, 117–127. [Google Scholar] [CrossRef]
- Xu, D.; Lin, G.; Guo, S.; Wang, S.; Guo, Y.; Jing, Z. Catalytic Hydrothermal Liquefaction of Algae and Upgrading of Biocrude: A Critical Review. Renew. Sustain. Energy Rev. 2018, 97, 103–118. [Google Scholar] [CrossRef]
- Yang, J.H.; Shin, H.Y.; Ryu, Y.J.; Lee, C.G. Hydrothermal Liquefaction of Chlorella vulgaris: Effect of Reaction Temperature and Time on Energy Recovery and Nutrient Recovery. J. Ind. Eng. Chem. 2018, 68, 267–273. [Google Scholar] [CrossRef]
- Faeth, J.L.; Savage, P.E. Effects of Processing Conditions on Biocrude Yields from Fast Hydrothermal Liquefaction of Microalgae. Bioresour. Technol. 2016, 206, 290–293. [Google Scholar] [CrossRef]
- Xiu, S.; Shahbazi, A.; Wallace, C.W.; Wang, L.; Cheng, D. Enhanced Bio-Oil Production from Swine Manure Co-Liquefaction with Crude Glycerol. Energy Convers. Manag. 2011, 52, 1004–1009. [Google Scholar] [CrossRef]
- Leng, L.; Li, J.; Yuan, X.; Li, J.; Han, P.; Hong, Y.; Wei, F.; Zhou, W. Beneficial Synergistic Effect on Bio-Oil Production from Co-Liquefaction of Sewage Sludge and Lignocellulosic Biomass. Bioresour. Technol. 2018, 251, 49–56. [Google Scholar] [CrossRef]
- Jasiūnas, L.; Pedersen, T.H.; Toor, S.S.; Rosendahl, L.A. Biocrude Production via Supercritical Hydrothermal Co-Liquefaction of Spent Mushroom Compost and Aspen Wood Sawdust. Renew. Energy 2017, 111, 392–398. [Google Scholar] [CrossRef]
- Dandamudi, K.P.R.; Muppaneni, T.; Sudasinghe, N.; Schaub, T.; Holguin, F.O.; Lammers, P.J.; Deng, S. Co-Liquefaction of Mixed Culture Microalgal Strains under Sub-Critical Water Conditions. Bioresour. Technol. 2017, 236, 129–137. [Google Scholar] [CrossRef]
- Jin, B.; Duan, P.; Xu, Y.; Wang, F.; Fan, Y. Co-Liquefaction of Micro- and Macroalgae in Subcritical Water. Bioresour. Technol. 2013, 149, 103–110. [Google Scholar] [CrossRef]
- Ellersdorfer, M. Hydrothermal Co-Liquefaction of Chlorella vulgaris with Food Processing Residues, Green Waste and Sewage Sludge. Biomass Bioenergy 2020, 142, 105796. [Google Scholar] [CrossRef]
- Xu, D.; Wang, Y.; Lin, G.; Guo, S.; Wang, S.; Wu, Z. Co-Hydrothermal Liquefaction of Microalgae and Sewage Sludge in Subcritical Water: Ash Effects on Bio-Oil Production. Renew. Energy 2019, 138, 1143–1151. [Google Scholar] [CrossRef]
- Yang, J.; He, Q.S.; Niu, H.; Astatkie, T.; Corscadden, K.; Shi, R. Statistical Clarification of the Hydrothermal Co-Liquefaction Effect and Investigation on the Influence of Process Variables on the Co-Liquefaction Effect. Ind. Eng. Chem. Res. 2020, 59, 2839–2848. [Google Scholar] [CrossRef]
- Cui, Z.; Greene, J.M.; Cheng, F.; Quinn, J.C.; Jena, U.; Brewer, C.E. Co-Hydrothermal Liquefaction of Wastewater-Grown Algae and Crude Glycerol: A Novel Strategy of Bio-Crude Oil-Aqueous Separation and Techno-Economic Analysis for Bio-Crude Oil Recovery and Upgrading. Algal Res. 2020, 51, 102077. [Google Scholar] [CrossRef]
- Seiple, T.E.; Skaggs, R.L.; Fillmore, L.; Coleman, A.M. Municipal Wastewater Sludge as a Renewable, Cost-Effective Feedstock for Transportation Biofuels Using Hydrothermal Liquefaction. J. Environ. Manag. 2020, 270, 110852. [Google Scholar] [CrossRef]
- Watson, J.; Wang, T.; Si, B.; Chen, W.T.; Aierzhati, A.; Zhang, Y. Valorization of Hydrothermal Liquefaction Aqueous Phase: Pathways towards Commercial Viability. Prog. Energy Combust. Sci. 2020, 77, 100819. [Google Scholar] [CrossRef]
- Leng, L.; Zhang, W.; Leng, S.; Chen, J.; Yang, L.; Li, H.; Jiang, S.; Huang, H. Bioenergy Recovery from Wastewater Produced by Hydrothermal Processing Biomass: Progress, Challenges, and Opportunities. Sci. Total Environ. 2020, 748, 142383. [Google Scholar] [CrossRef]
- Chen, K.; Lyu, H.; Hao, S.; Luo, G.; Zhang, S.; Chen, J. Separation of Phenolic Compounds with Modified Adsorption Resin from Aqueous Phase Products of Hydrothermal Liquefaction of Rice Straw. Bioresour. Technol. 2015, 182, 160–168. [Google Scholar] [CrossRef]
- Lee, I.G.; Ihm, S.K. Hydrogen Production by SCWG Treatment of Wastewater from Amino Acid Production Process. Ind. Eng. Chem. Res. 2010, 49, 10974–10980. [Google Scholar] [CrossRef]
- Xia, R.; Na, D.; Zhang, Y.; Baoming, L.; Zhidan, L.; Haifeng, L. Nitrogen and Phosphorous Adsorption from Post-Hydrothermal Liquefaction Wastewater Using Three Types of Zeolites. Int. J. Agric. Biol. Eng. 2015, 8, 86–95. [Google Scholar] [CrossRef]
- Chen, H.; Wan, J.; Chen, K.; Luo, G.; Fan, J.; Clark, J.; Zhang, S. Biogas Production from Hydrothermal Liquefaction Wastewater (HTLWW): Focusing on the Microbial Communities as Revealed by High-Throughput Sequencing of Full-Length 16S RRNA Genes. Water Res. 2016, 106, 98–107. [Google Scholar] [CrossRef]
- Alvares, A.B.C.; Diaper, C.; Parsons, S.A. Partial Oxidation by Ozone to Remove Recalcitrance from Wastewaters—A Review. Environ. Technol. 2001, 22, 409–427. [Google Scholar] [CrossRef]
- Shende, A.; Nan, W.; Kodzomoyo, E.; Shannon, J.; Nicpon, J.; Shende, R.; Shende, A.; Nan, W.; Kodzomoyo, E.; Shannon, J.; et al. Evaluation of Aqueous Product from Hydrothermal Liquefaction of Cardboard as Bacterial Growth Medium: Co-Liquefaction of Cardboard and Bacteria for Higher Bio-Oil Production. J. Sustain. Bioenergy Syst. 2017, 7, 51–64. [Google Scholar] [CrossRef]
- Leng, L.; Li, J.; Wen, Z.; Zhou, W. Use of Microalgae to Recycle Nutrients in Aqueous Phase Derived from Hydrothermal Liquefaction Process. Bioresour. Technol. 2018, 256, 529–542. [Google Scholar] [CrossRef]
- Jena, U.; Vaidyanathan, N.; Chinnasamy, S.; Das, K.C. Evaluation of Microalgae Cultivation Using Recovered Aqueous Co-Product from Thermochemical Liquefaction of Algal Biomass. Bioresour. Technol. 2011, 102, 3380–3387. [Google Scholar] [CrossRef]
- Lyu, H.; Chen, K.; Yang, X.; Younas, R.; Zhu, X.; Luo, G.; Zhang, S.; Chen, J. Two-Stage Nanofiltration Process for High-Value Chemical Production from Hydrolysates of Lignocellulosic Biomass through Hydrothermal Liquefaction. Sep. Purif. Technol. 2015, 147, 276–283. [Google Scholar] [CrossRef]
- Shanmugam, S.R.; Adhikari, S.; Shakya, R. Nutrient Removal and Energy Production from Aqueous Phase of Bio-Oil Generated via Hydrothermal Liquefaction of Algae. Bioresour. Technol. 2017, 230, 43–48. [Google Scholar] [CrossRef]
- McGinn, P.J.; Park, K.C.; Robertson, G.; Scoles, L.; Ma, W.; Singh, D. Strategies for Recovery and Recycling of Nutrients from Municipal Sewage Treatment Effluent and Hydrothermal Liquefaction Wastewaters for the Growth of the Microalga Scenedesmus Sp. AMDD. Algal Res. 2019, 38, 101418. [Google Scholar] [CrossRef]
- Mazur, Z.M. The Co-Cultivation of Rice and Algae to Improve Process Economics for Algal Biofuel Production. Ph.D. Thesis, University of Illinois at Urbana-Champaign, Champaign, IL, USA, 2016. [Google Scholar]
- Nelson, M.; Zhu, L.; Thiel, A.; Wu, Y.; Guan, M.; Minty, J.; Wang, H.Y.; Lin, X.N. Microbial Utilization of Aqueous Co-Products from Hydrothermal Liquefaction of Microalgae Nannochloropsis Oculata. Bioresour. Technol. 2013, 136, 522–528. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, C.; Rao, Y.; Jing, Y.; Luo, G.; Zhang, S. Methane Potentials of Wastewater Generated from Hydrothermal Liquefaction of Rice Straw: Focusing on the Wastewater Characteristics and Microbial Community Compositions. Biotechnol Biofuels 2017, 10, 140. [Google Scholar] [CrossRef]
- Si, B.; Watson, J.; Aierzhati, A.; Yang, L.; Liu, Z.; Zhang, Y. Biohythane Production of Post-Hydrothermal Liquefaction Wastewater: A Comparison of Two-Stage Fermentation and Catalytic Hydrothermal Gasification. Bioresour. Technol. 2019, 274, 335–342. [Google Scholar] [CrossRef]
- Li, Y.; Tarpeh, W.A.; Nelson, K.L.; Strathmann, T.J. Quantitative Evaluation of an Integrated System for Valorization of Wastewater Algae as Bio-Oil, Fuel Gas, and Fertilizer Products. Environ. Sci. Technol. 2018, 52, 12717–12727. [Google Scholar] [CrossRef]
- Ramos-Tercero, E.A.; Bertucco, A.; Brilman, D.W.F. Process Water Recycle in Hydrothermal Liquefaction of Microalgae To Enhance Bio-Oil Yield. Energy Fuels 2015, 29, 2422–2430. [Google Scholar] [CrossRef]
- Shen, R.; Liu, Z.; He, Y.; Zhang, Y.; Lu, J.; Zhu, Z.; Si, B.; Zhang, C.; Xing, X.H. Microbial Electrolysis Cell to Treat Hydrothermal Liquefied Wastewater from Cornstalk and Recover Hydrogen: Degradation of Organic Compounds and Characterization of Microbial Community. Int. J. Hydrogen Energy 2016, 41, 4132–4142. [Google Scholar] [CrossRef]
- Shen, R.; Jiang, Y.; Ge, Z.; Lu, J.; Zhang, Y.; Liu, Z.; Ren, Z.J. Microbial Electrolysis Treatment of Post-Hydrothermal Liquefaction Wastewater with Hydrogen Generation. Appl. Energy 2018, 212, 509–515. [Google Scholar] [CrossRef]
- Hidalgo, D.; Tommasi, T.; Cauda, V.; Porro, S.; Chiodoni, A.; Bejtka, K.; Ruggeri, B. Streamlining of Commercial Berl Saddles: A New Material to Improve the Performance of Microbial Fuel Cells. Energy 2014, 71, 615–623. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Adhami, A.; Darvari, S.; Zirepour, A.; Oh, S.E. Microbial Fuel Cell as New Technology for Bioelectricity Generation: A Review. Alex. Eng. J. 2015, 54, 745–756. [Google Scholar] [CrossRef]
- Hu, W.J.; Niu, C.G.; Wang, Y.; Zeng, G.M.; Wu, Z. Nitrogenous Heterocyclic Compounds Degradation in the Microbial Fuel Cells. Process Saf. Environ. Prot. 2011, 89, 133–140. [Google Scholar] [CrossRef]
- Liu, Z.; He, Y.; Shen, R.; Zhu, Z.; Xing, X.H.; Li, B.; Zhang, Y. Performance and Microbial Community of Carbon Nanotube Fixed-Bed Microbial Fuel Cell Continuously Fed with Hydrothermal Liquefied Cornstalk Biomass. Bioresour. Technol. 2015, 185, 294–301. [Google Scholar] [CrossRef]
- Toczyłowska-Mamińska, R.; Szymona, K.; Kloch, M. Bioelectricity Production from Wood Hydrothermal-Treatment Wastewater: Enhanced Power Generation in MFC-Fed Mixed Wastewaters. Sci. Total Environ. 2018, 634, 586–594. [Google Scholar] [CrossRef]
- Shen, R.; Lu, J.; Zhu, Z.; Duan, N.; Lu, H.; Zhang, Y.; Liu, Z. Effects of Organic Strength on Performance of Microbial Electrolysis Cell Fed with Hydrothermal Liquefied Wastewater. Int. J. Agric. Biol. Eng. 2017, 10, 206–217. [Google Scholar] [CrossRef]
- Sheng, L.; Wang, X.; Yang, X. Prediction Model of Biocrude Yield and Nitrogen Heterocyclic Compounds Analysis by Hydrothermal Liquefaction of Microalgae with Model Compounds. Bioresour. Technol. 2018, 247, 14–20. [Google Scholar] [CrossRef]
- Swetha, A.; ShriVigneshwar, S.; Gopinath, K.P.; Sivaramakrishnan, R.; Shanmuganathan, R.; Arun, J. Review on Hydrothermal Liquefaction Aqueous Phase as a Valuable Resource for Biofuels, Bio-Hydrogen and Valuable Bio-Chemicals Recovery. Chemosphere 2021, 283, 131248. [Google Scholar] [CrossRef]
- Wang, Z.; Watson, J.; Wang, T.; Yi, S.; Si, B.; Zhang, Y. Enhancing Energy Recovery via Two Stage Co-Fermentation of Hydrothermal Liquefaction Aqueous Phase and Crude Glycerol. Energy Convers. Manag. 2021, 231, 113855. [Google Scholar] [CrossRef]
| Proximate Analysis | (wt.%) |
|---|---|
| Moisture Content | 82.4 ± 1.2 |
| Volatile Matter | 52.9 ± 0.7 |
| Fixed Carbon by Difference | 17.3 ± 2.92 |
| Elemental Composition (wt.%): | |
| C | 33.1 ± 0.3 |
| H | 5.5 ± 0.1 |
| N | 5.0 ± 0.1 |
| O | 25.9 ± 0.1 |
| Species | Proteins (%) | Carbohydrates (%) | Lipids (%) |
|---|---|---|---|
| Anabaena cylindrica | 43–56 | 25–30 | 4–7 |
| Aphanizomenon flosaquae | 62 | 23 | 3 |
| Chlamydomonas rheinhardii | 48 | 17 | 21 |
| Chlorella pyrenoidosa | 57 | 26 | 2 |
| Chlorella vulgaris | 51–58 | 12–17 | 14–22 |
| Dunaliella salina | 57 | 32 | 6 |
| Euglena gracilis | 39–61 | 14–18 | 14–20 |
| Porphyridium cruentum | 28–39 | 40–57 | 9–14 |
| Scenedesmus obliquus | 50–56 | 10–17 | 12–14 |
| Spirogyra sp. | 6–20 | 33–64 | 11–21 |
| Arthrospira maxima | 60–71 | 13–16 | 6–7 |
| Spirulina platensis | 46–63 | 8–14 | 4–9 |
| Process | Biomass | Mixture Ratio | Dry Biomass/Solvent (w/v) | Temp. (°C) | Time (min) | HTL Biocrude Yield | Ref. |
|---|---|---|---|---|---|---|---|
| HTL | Chlorella vulgaris | - | 20% | 300 | 5 | 40% | Yang et al. [109] |
| 325 | 5 | 42% | |||||
| 350 | 5 | 43% | |||||
| 300 | 10 | 49% | |||||
| 325 | 10 | 47% | |||||
| 350 | 10 | 46% | |||||
| 300 | 30 | 48% | |||||
| 325 | 30 | 44% | |||||
| 350 | 30 | 41% | |||||
| HTL | Chlorella vulgaris | - | 15% | 350 | 60 | 29% | Faeth e Savage [110] |
| Neochloris oleoabundans | 34% | ||||||
| Botryococcus braunii | 40% | ||||||
| Nannochloropsis sp. | 38% | ||||||
| HTL | Sugarcane bagasse | - | - | 240 | 60 | 46.0% | Ahmed Baloch et al. [52] |
| 260 | 49.0% | ||||||
| 280 | 51.8% | ||||||
| HTL | Swine manure | - | 20% | 260 | 15 | 15% | Xiu et al. [111] |
| 280 | 18% | ||||||
| 300 | 21% | ||||||
| 340 | 24% | ||||||
| 360 | 20% | ||||||
| 340 | 5 | 17% | |||||
| 15 | 24% | ||||||
| 30 | 23% | ||||||
| 60 | 21% | ||||||
| 90 | 12.5% | ||||||
| HTL and co-HTL | Rice straw/sewage sludge | 8/0 | 8% | 300 | 20 | 22.74% | Leng, Li and Yuan et al. [112] |
| 6/2 | 27.39% | ||||||
| 5/3 | 31.33% | ||||||
| 4/4 | 32.45% | ||||||
| 3/5 | 29.92% | ||||||
| 2/6 | 22.78% | ||||||
| 0/8 | 23.67% | ||||||
| Wood sawdust/sewage sludge | 8/0 | 26.73% | |||||
| 6/2 | 32.02% | ||||||
| 5/3 | 28.80% | ||||||
| 4/4 | 27.63% | ||||||
| 3/5 | 26.48% | ||||||
| 2/6 | 39.46% | ||||||
| 0/8 | 23.67% | ||||||
| HTL and co-HTL | Spent mushroom compost/aspen wood sawdust | 1/0 | 20% | 400 | 15 | 35.05% | Jasiūnas et al. [113] |
| 1/0 | 47.85% | ||||||
| 2/1 | 23.00% | ||||||
| 1/1 | 21.23% | ||||||
| ½ | 17.90% | ||||||
| 1/3 | 15.52% | ||||||
| 0/1 | 20.65% | ||||||
| HTL and co-HTL | Cyanidioschyzon merolae/Galdieria sulphuraria | 1/0 | 20% | 150 | 30 | 2.6% | Dandamudi et al. [114] |
| 200 | 4.4% | ||||||
| 250 | 16.4% | ||||||
| 300 | 18.8% | ||||||
| 0/1 | 150 | 0.5% | |||||
| 200 | 2.4% | ||||||
| 250 | 8.2% | ||||||
| 300 | 14.0% | ||||||
| 4/1 | 300 | 25.5% | |||||
| 1/1 | 16.5% | ||||||
| ¼ | 13.0% | ||||||
| 4/1 | 15 | 20.0% | |||||
| 30 | 26.0% | ||||||
| 45 | 24.0% | ||||||
| 60 | 23.0% | ||||||
| Co-HTL | Spirulina platensis/Entermorpha prolifera | 1/1 | - | 300 | 40 | 17% | Jin et al. [115] |
| 330 | 19% | ||||||
| 360 | 21% | ||||||
| 340 | 20 | 30% | |||||
| 40 | 22% | ||||||
| 60 | 20% | ||||||
| 120 | 20% | ||||||
| HTL and co-HTL | Chlorella vulgaris/sewage sludge | 0/1 | 10% | 350 | 15 | 12% | Ellersdorfer [116] |
| 1/1 | 16.4% | ||||||
| Chlorella vulgaris/green waste | 0/1 | 4.4% | |||||
| 1/1 | 10.9% | ||||||
| Chlorella vulgaris/food waste | 0/1 | 18.2% | |||||
| 1/1 | 17.1% | ||||||
| Chlorella vulgaris/grease residue | 0/1 | 76.3% | |||||
| 1/1 | 48.1% | ||||||
| Chlorella vulgaris | - | 18.3% | |||||
| HTL and co-HTL | Mixed-culture algal biomass from wastewater pond/swine manure | 1/0 | 25% | 300 | 60 | 26.5% | Chen et al. [6] |
| 3/1 | 25.8% | ||||||
| 1/1 | 22% | ||||||
| 1/3 | 35.7% | ||||||
| 0/1 | 39% | ||||||
| HTL and co-HTL | Chlorella sp./sewage sludge | 1/0 | 10% | 340 | 30 | 21.5% | Xu et al. [117] |
| 1/3 | 24.5% | ||||||
| 1/1 | 26.8% | ||||||
| 3/1 | 23.5% | ||||||
| 0/1 | 23% | ||||||
| HTL and co-HTL | Chlorella sp./sawdust | 3/1 | 10% | 270 | 10 | 27% | Yang et al. [118] |
| 320 | 35% | ||||||
| 1/1 | 270 | 22% | |||||
| 320 | 28% | ||||||
| 1/3 | 270 | 19% | |||||
| 320 | 25% | ||||||
| 0/1 | 270 | 25.3% | |||||
| 320 | 21.1% | ||||||
| Chlorella sp./spent coffee grounds | 3/1 | 270 | 29% | ||||
| 320 | 34% | ||||||
| 1/1 | 270 | 29% | |||||
| 320 | 37.2% | ||||||
| 1/3 | 270 | 25.0% | |||||
| 320 | 30% | ||||||
| 0/1 | 270 | 23.2% | |||||
| 320 | 25.9% | ||||||
| Chlorella sp. | - | 270 | 24.1% | ||||
| 320 | 34.9% |
| Method for AP Valorization | Main Results | Reference |
|---|---|---|
| Separation of organics | Through the extraction of phenolic compounds from rice straw HTL-AP, the total phenolic compound content in the aqueous solution increased from 18% to 78%. | Chen et al. [123] |
| Through the separation of rice straw HTL-AP, the residue was fractionated into glucose concentrate, monophenol and cyclopentenone concentrate, and acetic acid permeate. | Lyu et al. [131] | |
| Separation of inorganics | Through the collection of N and P from HTL-AP as struvite, 99% of the P and 40–100% of the ammonium nitrogen could be separated. | Shanmugam, Adhikari and Shakya [132] |
| Phosphate recovery up to 75% was achieved from microalgae HTL-AP using struvite. | Mcginn et al. [133] | |
| Algae cultivation | Promising results for the co-production of food and energy through the utilization of HTL-AP as a medium for the growth of algae and rice co-culture | Mazur [134] |
| Microbe cultivation | Escherichia coli and Pseudomonas putida grown using 10–40 vol.% AP from liquefaction of algae | Nelson et al. [135] |
| Anaerobic fermentation | A methane yield of 314 mL CH4/g COD was obtained from rice straw HTL-AP. | Chen et al. [136] |
| A hydrogen yield of 29.3 mL/g COD and a methane yield of 254.3 mL/g COD were achieved from cornstalk HTL-AP using two-stage fermentation. | Si et al. [137] | |
| Hydrothermal gasification | Through catalytic hydrothermal gasification of wastewater–algal biomass HTL-AP, 98.2 ± 0.4% of the COD and 97.2 ± 0.4% of the TOC were removed. | Li et al. [138] |
| Recycling | The production of biocrude oil rose from 14% to 42% following six consecutive cycles of microalgae HTL-AP recycling. | Ramos-Tercero, Bertucco and Brilman [139] |
| Dispositive | Main Results | Reference |
|---|---|---|
| MFC | Through the operation of a fixed-bed MFC constructed with carbon nanotubes using HTL-AP derived from cornstalk, a power density of 680 mW/m3 and a COD removal rate exceeding 80% were achieved. | Liu et al. [145] |
| The power generated in an MFC fed with raw industrial wastewater from wood hydrothermal treatment was 70 mW/m2, and it increased to 360 mW/m2 when municipal wastewater was introduced into the reactor. | Toczyłowska-Mamińska, Szymona and Kloch [146] | |
| MEC | MEC converted furfural, HMF, dimethyl phthalate, and diethyl phthalate from cornstalk HTL-AP, achieving a hydrogen production rate of 3.92 mL/L/d. | Shen et al. [140] |
| The conversion of swine manure HTL-AP in a two-chamber fixed-bed MEC resulted in over 90% removal of organics and a hydrogen production rate of 168.01 ± 7.01 mL/L/d. | Ruixia et al. [147] | |
| When treating hydrothermal liquefied wastewater in an MEC, a COD removal of up to 83.84% was achieved, with a maximum hydrogen production rate of 3.92 mL/Ld. | Sheng, Wang and Yang [148] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bassoli, S.C.; Cardozo, M.H.A.d.L.; Naves, F.L.; Lamas-Samanamud, G.; Amaral, M.d.S. Proposal for a Conceptual Biorefinery for the Conversion of Waste into Biocrude, H2 and Electricity Based on Hydrothermal Co-Liquefaction and Bioelectrochemical Systems. Fermentation 2025, 11, 162. https://doi.org/10.3390/fermentation11040162
Bassoli SC, Cardozo MHAdL, Naves FL, Lamas-Samanamud G, Amaral MdS. Proposal for a Conceptual Biorefinery for the Conversion of Waste into Biocrude, H2 and Electricity Based on Hydrothermal Co-Liquefaction and Bioelectrochemical Systems. Fermentation. 2025; 11(4):162. https://doi.org/10.3390/fermentation11040162
Chicago/Turabian StyleBassoli, Sara Cangussú, Matheus Henrique Alcântara de Lima Cardozo, Fabiano Luiz Naves, Gisella Lamas-Samanamud, and Mateus de Souza Amaral. 2025. "Proposal for a Conceptual Biorefinery for the Conversion of Waste into Biocrude, H2 and Electricity Based on Hydrothermal Co-Liquefaction and Bioelectrochemical Systems" Fermentation 11, no. 4: 162. https://doi.org/10.3390/fermentation11040162
APA StyleBassoli, S. C., Cardozo, M. H. A. d. L., Naves, F. L., Lamas-Samanamud, G., & Amaral, M. d. S. (2025). Proposal for a Conceptual Biorefinery for the Conversion of Waste into Biocrude, H2 and Electricity Based on Hydrothermal Co-Liquefaction and Bioelectrochemical Systems. Fermentation, 11(4), 162. https://doi.org/10.3390/fermentation11040162





