Impact of Neem Cake on In Vitro Ruminal Fermentation, Gas Production Kinetics, and Enteric Greenhouse Gas Emissions in Finishing Beef Cattle Diets
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics, Experimental Designs, and Chemical Analysis
2.2. Ruminal Fluid Collection and Buffer Solution Preparation
2.3. In Vitro Gas Production
2.4. Enteric Carbon Dioxide and Methane
2.5. Statistical Analysis
3. Results
3.1. Effects of Neem Cake Inclusion on Rumen In Vitro Fermentation Kinetics and Characteristics
3.2. Effects of Neem Cake Inclusion on Enteric Methane and Carbon Dioxide Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duffield, T.F.; Merrill, J.K.; Bagg, R.N. Meta-analysis of the effects of monensin in beef cattle on feed efficiency, body weight gain, and dry matter intake. J. Anim. Sci. 2012, 90, 4583–4592. [Google Scholar] [PubMed]
- Ahvanooei, M.R.R.; Norouzian, M.A.; Piray, A.H.; Vahmani, P.; Ghaffari, M.H. Effects of monensin supplementation on rumen fermentation, methane emissions, nitrogen balance, and metabolic responses of dairy cows: A systematic review and dose-response meta-analysis. J. Dairy Sci. 2024, 107, 607–624. [Google Scholar]
- Bezerra, W.G.A.; Horn, R.H.; Silva, I.N.G.; Teixeira, R.S.C.; Lopes, E.S.; Albuquerque, Á.H.; Cardoso, W.C. Antibióticos no setor avícola: Uma revisão sobre a resistência microbiana. Arq. Zootecnia 2017, 66, 301–307. [Google Scholar]
- Subapriya, R.; Nagini, S. Medicinal properties of neem leaves: A review. Curr. Med. Chem.-Anti-Cancer Agents 2005, 5, 149–156. [Google Scholar] [CrossRef]
- Islas, J.F.; Acosta, E.; G-Buentello, Z.; Delgado-Gallegos, J.L.; Moreno-Treviño, M.G.; Escalante, B.; Moreno-Cuevas, J.E. An overview of Neem (Azadirachta indica) and its potential impact on health. J. Funct. Foods 2020, 74, 104171. [Google Scholar] [CrossRef]
- Martinez, S.S. O Nim–Azadirachta Indica–Natureza, Usos Múltiplos, Produção; IAPAR: Londrina, Brazil, 2002. [Google Scholar]
- Nath, K.; Rajagopal, S.; Garg, A.K. Water-washed neem (Azadirachta indica juss) seed kernel cake as a cattle feed. J. Agric. Sci. 1983, 101, 323–326. [Google Scholar] [CrossRef]
- Aruwayo, A. Neem (Azadirachta indica) Seed Cake/Kernel as Protein Source in Ruminants Feed. Am. J. Exp. Agric. 2013, 3, 482–494. [Google Scholar] [CrossRef]
- Gowda, S.K.; Sastry, V.R.B. Neem seed cake in animal seeding-scope and limitations-Review. Asian-Australas. J. Anim. Sci. 2000, 13, 720–728. [Google Scholar] [CrossRef]
- Gupta, A.; Ansari, S.; Gupta, S.; Narwani, M.; Gupta, M.; Singh, M.; Manali Singh, C. Therapeutics role of neem and its bioactive constituents in disease prevention and treatment. J. Pharmacogn. Phytochem. 2019, 8, 680–691. [Google Scholar]
- Tipu, M.A.; Akhtar, M.S.; Anjum, M.I.; Raja, M.L. New Dimension of Medicinal Plants As Animal Feed. Pakistan Vet. J 2006, 26, 144–148. [Google Scholar]
- Sarkar, S.; Singh, R.P.; Bhattacharya, G. Exploring the role of Azadirachta indica (neem) and its active compounds in the regulation of biological pathways: An update on molecular approach. 3 Biotech 2021, 11, 178. [Google Scholar] [CrossRef]
- Reddy, I.V.; Neelima, P. Neem (Azadirachta indica): A review on medicinal Kalpavriksha. Int. J. Econ. Plants 2022, 9, 59–63. [Google Scholar] [CrossRef]
- Akanmu, A.M.; Hassen, A. The use of certain medicinal plant extracts reduced in vitro methane production while improving in vitro organic matter digestibility. Anim. Prod. Sci. 2018, 58, 900–908. [Google Scholar] [CrossRef]
- Akanmu, A.M.; Hassen, A.; Adejoro, F.A. Gas production, digestibility and efficacy of stored or fresh plant extracts to reduce methane production on different substrates. Animals 2020, 10, 146. [Google Scholar] [CrossRef]
- El-zaiat, H.M.; Elshafie, E.I.; Al-Marzooqi, W.; Al-Dughaishi, K. Effects of Neem (Azadirachta indica) Leaf Powder Supplementation on Rumen Fermentation, Feed Intake, Apparent Digestibility and Performance in Omani Sheep. Animals 2022, 12, 3146. [Google Scholar] [CrossRef]
- Wylie, M.R.; Merrell, D.S. The Antimicrobial Potential of the Neem Tree Azadirachta indica. Front. Pharmacol. 2022, 13, 891535. [Google Scholar] [CrossRef]
- Jack, A.A.; Adewumi, M.K.; Adegbeye, M.J.; Ekanem, D.E.; Salem, A.Z.M.; Faniyi, T.O. Growth-promoting effect of water-washed neem (Azadirachta indica A. Juss) fruit inclusion in West African dwarf rams. Trop. Anim. Health Prod. 2020, 52, 3467–3474. [Google Scholar] [CrossRef]
- Yang, W.Z.; Laurain, J.; Ametaj, B.N. Neem oil modulates rumen fermentation properties in a continuous cultures system. Anim. Feed Sci. Technol. 2009, 149, 78–88. [Google Scholar] [CrossRef]
- Patra, A.K.; Kamra, D.N.; Agarwal, N. Effect of plant extracts on in vitro methanogenesis, enzyme activities and fermentation of feed in rumen liquor of buffalo. Anim. Feed Sci. Technol. 2006, 128, 276–291. [Google Scholar] [CrossRef]
- NASEM. National Academy of Sciences, Engineering, and Medicine: Nutrient Requirements of Beef Cattle, 8th Revised ed.; National Academies Press: Washington, DC, USA, 2016. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Official Methods of Analysis; Association of Official Analytical Chemists, Inc.: Washington, DC, USA, 1990. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; AOAC: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Yáñez-Ruiz, D.R.; Bannink, A.; Dijkstra, J.; Kebreab, E.; Morgavi, D.P.; O’Kiely, P.; Reynolds, C.K.; Schwarm, A.; Shingfield, K.J.; Yu, Z.; et al. Design, implementation and interpretation of in vitro batch culture experiments to assess enteric methane mitigation in ruminants—A review. Anim. Feed Sci. Technol. 2016, 216, 1–18. [Google Scholar] [CrossRef]
- Menke, K.H.; Steingass, H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Tagliapietra, F.; Cattani, M.; Bailoni, L.; Schiavon, S. In vitro rumen fermentation: Effect of headspace pressure on the gas production kinetics of corn meal and meadow hay. Anim. Feed Sci. Technol. 2010, 158, 197–201. [Google Scholar] [CrossRef]
- Tagliapietra, F.; Cattani, M.; Hansen, H.H.; Hindrichsen, I.K.; Bailoni, L.; Schiavon, S. Metabolizable energy content of feeds based on 24 or 48h in situ NDF digestibility and on in vitro 24h gas production methods. Anim. Feed Sci. Technol. 2011, 170, 182–191. [Google Scholar] [CrossRef]
- Schofield, P.; Pitt, R.E.; Pell, A.N. Kinetics of fiber digestion from in vitro gas production. J. Anim. Sci. 1994, 72, 2980–2991. [Google Scholar] [CrossRef]
- Chaney, A.L.; Marbach, E.P. Modified reagents for determination of urea and ammonia. Clin. Chem. 1962, 8, 130–132. [Google Scholar] [CrossRef]
- Tagliapietra, F.; Cattani, M.; Hansen, H.H.; Bittante, G.; Schiavon, S. High doses of vitamin E and vitamin C influence in vitro rumen microbial activity. Anim. Feed Sci. Technol. 2013, 183, 210–214. [Google Scholar] [CrossRef]
- Oh, J.; Hristov, A.N. Effects of plant-derived bio-active compounds on rumen fermentation, nutrient utilization, immune response, and productivity of ruminant animals. Acs Symp. Ser. 2016, 1218, 167–186. [Google Scholar] [CrossRef]
- Elghandour, M.M.Y.; Vallejo, L.H.; Salem, A.Z.M.; Mellado, M.; Camacho, L.M.; Cipriano, M.; Olafadehan, O.A.; Olivares, J.; Rojas, S. Moringa oleifera leaf meal as an environmental friendly protein source for ruminants: Biomethane and carbon dioxide production, and fermentation characteristics. J. Clean. Prod. 2017, 165, 1229–1238. [Google Scholar] [CrossRef]
- Roychoudhury, R. Neem Products. In Ecofriendly Pest Management for Food Security; Omkar, Ed.; Academic Press: Cambridge, MA, USA; Elsevier: London, UK, 2016; pp. 545–562. [Google Scholar] [CrossRef]
- Kumar, G.H.; Vidya Priyadarsini, R.; Vinothini, G.; Vidjaya Letchoumy, P.; Nagini, S. The neem limonoids azadirachtin and nimbolide inhibit cell proliferation and induce apoptosis in an animal model of oral oncogenesis. Investig. New Drugs 2010, 28, 392–401. [Google Scholar] [CrossRef]
- Linda, J.; Okon, E.-O. Comparative Study of the Phytochemical Properties of Jatropha curcas and Azadirachta indica Plant Extracts. J. Poisonous Med. Plants Res. 2014, 2, 20–24. [Google Scholar]
- Gupta, P.; Zaidi, A.H.; Manna, S.K. Suppression of IKK, but not activation of p53 is responsible for cell death mediated by naturally occurring oxidized tetranortriterpenoid. J. Cell. Biochem. 2018, 119, 6828–6841. [Google Scholar] [CrossRef] [PubMed]
- Sophia, J.; Kowshik, J.; Dwivedi, A.; Bhutia, S.K.; Manavathi, B.; Mishra, R.; Nagini, S. Nimbolide, a neem limonoid inhibits cytoprotective autophagy to activate apoptosis via modulation of the PI3K/Akt/GSK-3β signalling pathway in oral cancer. Cell Death Dis. 2018, 9, 1087. [Google Scholar] [CrossRef] [PubMed]
- de Passos, M.S.; de Carvalho Junior, A.R.; Boeno, S.I.; das Virgens, L.d.L.G.; Calixto, S.D.; Ventura, T.L.B.; Lassounskaia, E.; Braz-Filho, R.; Vieira, I.J.C. Terpenoids isolated from Azadirachta indica roots and biological activities. Rev. Bras. Farmacogn. 2019, 29, 40–45. [Google Scholar] [CrossRef]
- Owens, F.N.; Basalan, M. Ruminal Fermentation. In Rumenology; Millen, D.D., De Beni Arrigoni, M., Pacheco, R.D.L., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 63–102. [Google Scholar] [CrossRef]
- Allen, M.S. Relationship between fermentation acid production in the rumen and the requirement for physically effective fiber. J. Dairy Sci. 1997, 80, 1447–1462. [Google Scholar] [CrossRef]
- Agarwal, N.; Kewalramani, N.; Kamra, D.N.; Agarwal, D.K.; Nath, K. Effect of water extracts of neem (Azadirachta indica) on the activity of hydrolytic enzymes of mixed rumen bacteria from buffalo. J. Sci. Food Agric. 1991, 57, 147–150. [Google Scholar] [CrossRef]
- Roman-Garcia, Y.; Mitchell, K.E.; Denton, B.L.; Lee, C.; Socha, M.T.; Wenner, B.A.; Firkins, J.L. Conditions stimulating neutral detergent fiber degradation by dosing branched-chain volatile fatty acids. II: Relation with solid passage rate and pH on neutral detergent fiber degradation and microbial function in continuous culture. J. Dairy Sci. 2021, 104, 9853–9867. [Google Scholar] [CrossRef]
- Apajalahti, J.; Vienola, K.; Raatikainen, K.; Holder, V.; Moran, C.A. Conversion of branched-chain amino acids to corresponding isoacids-an in vitro tool for estimating ruminal protein degradability. Front. Vet. Sci. 2019, 6, 311. [Google Scholar] [CrossRef]
- Fagundes, G.M.; Benetel, G.; Santos, K.C.; Welter, K.C.; Melo, F.A.; Muir, J.P.; Bueno, I.C.S. Tannin-rich plants as natural manipulators of rumen fermentation in the livestock industry. Molecules 2020, 25, 2943. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Singh, B.; Dawra, R.K. Effect of tannin-rich leaves of oak (Quercus incana) on various microbial enzyme activities of the bovine rumen. Br. J. Nutr. 1988, 60, 287–296. [Google Scholar]
- Faniyi, T.O.; Prates, Ê.R.; Adegbeye, M.J.; Adewumi, M.K.; Elghandour, M.M.M.Y.; Salem, A.Z.M.; Ritt, L.A.; Zubieta, A.S.; Stella, L.; Ticiani, E.; et al. Prediction of biogas and pressure from rumen fermentation using plant extracts to enhance biodigestibility and mitigate biogases. Environ. Sci. Pollut. Res. 2019, 26, 27043–27051. [Google Scholar] [CrossRef]
- Benedeti, P.B.; Fonseca, M.A.; Shenkoru, T.; Marcondes, M.I.; Paula, E.M.; Silva, L.G.; Faciola, A.P. Does partial replacement of corn with glycerin in beef cattle diets affect in vitro ruminal fermentation, gas production kinetic, and enteric greenhouse gas emissions? PLoS ONE 2018, 13, e0199577. [Google Scholar] [CrossRef] [PubMed]
- Cattani, M.; Tagliapietra, F.; Maccarana, L.; Hansen, H.H.; Bailoni, L.; Schiavon, S. Technical note: In vitro total gas and methane production measurements from closed or vented rumen batch culture systems. J. Dairy Sci. 2014, 97, 1736–1741. [Google Scholar] [CrossRef] [PubMed]
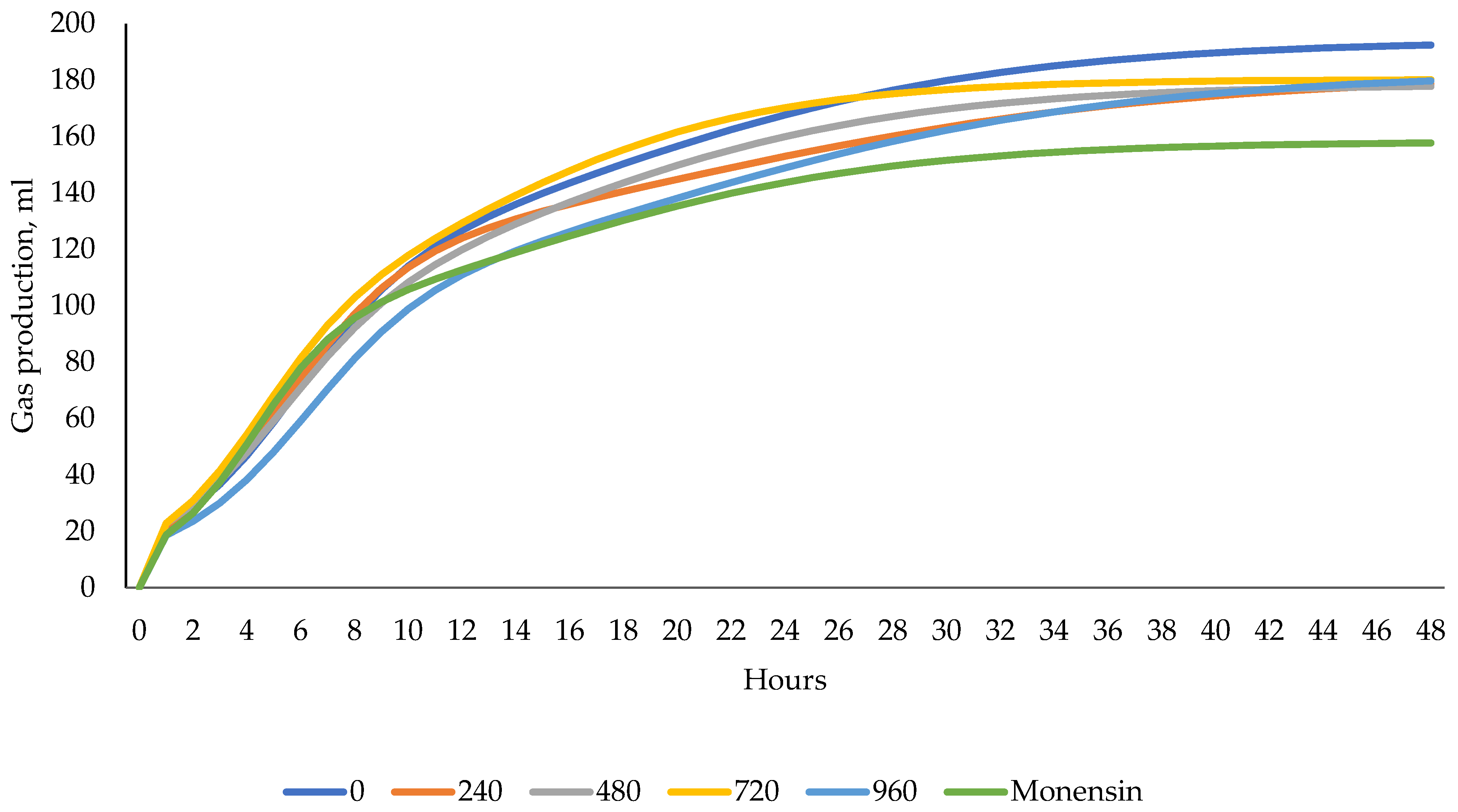
| Item | Neem Cake—Chemical Composition |
|---|---|
| Dry matter, g/kg as fed | 857 |
| Ash, g/kg of DM | 200 |
| Organic matter, g/kg of DM | 800 |
| Crude protein, g/kg of DM | 198 |
| Crude fat, g/kg of DM | 370 |
| Phosphorus, g/kg of DM | 5.87 |
| Calcium, g/kg of DM | 5.51 |
| Magnesium, g/kg of DM | 2.80 |
| Sulfur, g/kg of DM | 4.47 |
| Zinc, mg/kg of DM | 78.7 |
| Copper, mg/kg of DM | 12.6 |
| Manganese, mg/kg of DM | 30.8 |
| Iron, mg/kg of DM | 1846 |
| Item | Basal Diet, g/kg of DM |
|---|---|
| Ingredients | |
| Corn silage | 100 |
| Braquiaria hay | 50.0 |
| Corn finely ground | 690 |
| Soybean meal | 150 |
| Urea | 10.0 |
| Chemical composition | |
| Dry matter, g/kg as fed | 556 |
| Organic matter | 964 |
| Crude protein | 134 |
| Starch | 445 |
| Neutral detergent fiber | 248 |
| Item a | Neem Cake Inclusion, mg/kg DM | Monensin | Pooled SEM | p-Values | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 240 | 480 | 720 | 960 | Treatment b | Linear c | Quadratic c | |||
| V1 | 98.9 | 106 | 86.4 | 79.3 | 88.2 | 81.9 | 11.5 | 0.55 | 0.19 | 0.72 |
| V2 | 95.0 | 75.7 | 92.0 | 101 | 94.7 | 76.3 | 9.77 | 0.31 | 0.43 | 0.62 |
| K1 | 0.11 * | 0.11 * | 0.11 * | 0.14 | 0.11 * | 0.15 | 0.001 | <0.01 | 0.20 | 0.31 |
| K2 | 0.03 | 0.03 | 0.04 | 0.05 | 0.03 | 0.04 | 0.007 | 0.36 | 0.57 | 0.38 |
| L | 1.19 | 0.91 | 0.73 | 0.81 | 1.68 | 1.03 | 0.377 | 0.49 | 0.49 | 0.09 |
| Item | Neem Cake Inclusion, mg/kg DM | Monensin | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 240 | 480 | 720 | 960 | Treatment a | Linear b | Quadratic b | |||
| Gas production 24 h, mL/g DM | 184 | 164 | 163 | 175 | 179 | 155 | 10.1 | 0.28 | 0.97 | 0.13 |
| Gas production 48 h, mL/g DM | 194 | 176 | 173 | 181 | 186 | 158 | 12.5 | 0.40 | 0.78 | 0.26 |
| IVOMD, g/kg | 666 | 634 | 629 | 644 | 652 | 599 | 22.4 | 0.33 | 0.79 | 0.25 |
| pH | 6.36 * | 6.36 * | 6.36 * | 6.35 * | 6.34 * | 6.19 | 0.02 | <0.01 | 0.47 | 0.66 |
| Total VFA, mM | 79.0 * | 80.2 * | 80.9 * | 84.4 | 83.5 ** | 87.9 | 1.26 | <0.01 | <0.01 | 0.78 |
| VFA profile, mol/100 mol | ||||||||||
| Acetate | 46.0 | 45.9 | 48.7 ** | 48.9 * | 46.7 | 45.3 | 0.98 | 0.04 | 0.14 | 0.06 |
| Propionate | 28.2 * | 28.6 * | 27.3 * | 25.7 * | 27.8 * | 34.3 | 0.49 | <0.01 | 0.02 | 0.11 |
| Butyrate | 19.3 * | 18.9 * | 18.1 * | 19.0 * | 18.9 * | 15.8 | 0.55 | <0.01 | 0.69 | 0.27 |
| Iso-valerate | 2.04 * | 2.00 * | 1.91 | 2.27 * | 2.31 * | 1.56 | 0.11 | <0.01 | 0.03 | 0.17 |
| Iso-butyrate | 2.10 * | 2.09 * | 1.82 * | 1.87 * | 1.98 * | 1.30 | 0.08 | <0.01 | 0.06 | 0.06 |
| Valerate | 2.41 * | 2.42 * | 2.15 | 2.19 * | 2.31 * | 1.83 | 0.10 | <0.01 | 0.19 | 0.15 |
| BCVFA, mM | 3.28 * | 3.28 * | 3.02 ** | 3.49 * | 3.57 * | 2.51 | 0.15 | <0.01 | 0.09 | 0.12 |
| Acetate:Propionate | 1.64 * | 1.61 * | 1.79 * | 1.91 * | 1.69 * | 1.33 | 0.05 | <0.01 | 0.02 | 0.03 |
| N-NH3, mg/dL | 13.3 | 16.7 * | 20.6 * | 14.5 | 6.28 | 8.73 | 1.99 | <0.01 | 0.02 | <0.01 |
| Item | Neem Cake Inclusion, mg/kg DM | Monensin | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 240 | 480 | 720 | 960 | Treatment a | Linear b | Quadratic b | |||
| CH4, mmol/g OMD | 50.0 * | 55.9 * | 59.9 * | 53.4 * | 54.8 * | 29.7 | 1.54 | <0.01 | 0.14 | <0.01 |
| CO2, mmol/g OMD | 220 * | 231 * | 235 * | 226 * | 221 | 230 | 1.59 | <0.01 | 0.70 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amâncio, B.R.; Silva, T.H.d.; Magnani, E.; Guimarães, J.M.; Marques, V.; Lourenço, A.L.; Paula, E.M.d.; Benedeti, P.D.B.; Branco, R.H. Impact of Neem Cake on In Vitro Ruminal Fermentation, Gas Production Kinetics, and Enteric Greenhouse Gas Emissions in Finishing Beef Cattle Diets. Fermentation 2025, 11, 163. https://doi.org/10.3390/fermentation11040163
Amâncio BR, Silva THd, Magnani E, Guimarães JM, Marques V, Lourenço AL, Paula EMd, Benedeti PDB, Branco RH. Impact of Neem Cake on In Vitro Ruminal Fermentation, Gas Production Kinetics, and Enteric Greenhouse Gas Emissions in Finishing Beef Cattle Diets. Fermentation. 2025; 11(4):163. https://doi.org/10.3390/fermentation11040163
Chicago/Turabian StyleAmâncio, Bruna Roberta, Thiago Henrique da Silva, Elaine Magnani, Jennifer Moreira Guimarães, Victoria Marques, Ana Laura Lourenço, Eduardo Marostegan de Paula, Pedro Del Bianco Benedeti, and Renata Helena Branco. 2025. "Impact of Neem Cake on In Vitro Ruminal Fermentation, Gas Production Kinetics, and Enteric Greenhouse Gas Emissions in Finishing Beef Cattle Diets" Fermentation 11, no. 4: 163. https://doi.org/10.3390/fermentation11040163
APA StyleAmâncio, B. R., Silva, T. H. d., Magnani, E., Guimarães, J. M., Marques, V., Lourenço, A. L., Paula, E. M. d., Benedeti, P. D. B., & Branco, R. H. (2025). Impact of Neem Cake on In Vitro Ruminal Fermentation, Gas Production Kinetics, and Enteric Greenhouse Gas Emissions in Finishing Beef Cattle Diets. Fermentation, 11(4), 163. https://doi.org/10.3390/fermentation11040163






