1. Introduction
As the Federal Cabinet of Germany incorporated the agreed-upon measures of the Paris Climate Agreement within the Climate Action Plan 2050 [
1], which outlines ambitious energy-related objectives, it prompted a comprehensive optimization of the wastewater management sectors to reduce energy demand and enhance energy production. Furthermore, the guidelines of the National Bioeconomy Strategy of the German Federal Government [
2] foster climate-neutral advancements through biobased technologies and biogenic resources in order to establish a sustainable, circular economy. In this context, discussions regarding innovative biobased technologies for nutrient removal such as mainstream deammonification arise [
3,
4,
5].
Microalgae, which use light energy to convert inorganic carbons, such as carbon dioxide and bicarbonate, into high-energy biomass [
6,
7], are regarded as a potential alternative to conventional activated sludge systems currently used for municipal wastewater treatment. For their growth, they require not only carbon but also nitrogen and phosphorus in dissolved forms [
8], as present in wastewater. In parallel with the potential substitution of the nitrification/denitrification process and the associated energy demand, the produced algal biomass can be converted into biogas or biofuels, significantly improving the energy balance of wastewater treatment plants (WWTP) [
9,
10,
11,
12,
13,
14].
Although previous studies from as early as the 1950s [
15] have reported that wastewater serves as a suitable medium for algal cultivation, the separation of biomass from the remediated water remains a substantial challenge in terms of feasibility and reliability until today [
16].
Reported removal rates for common separation technologies used with microalgae bacteria biomass (MBB) range from 92.47% to 99% for coagulation and flocculation [
17,
18,
19,
20,
21], from 95.7% to 99% for flotation [
20,
22], 90% to 99% for membrane filtration [
23,
24], and around 95% in the case of a decanter centrifugation [
20]. The specific energy demands of these technologies may vary. Of the mentioned technologies, coagulation and flocculation have the lowest energy demand with 0.07 to 0.1 kWh·m
−3 [
20,
25] and decanter centrifugation has the highest with 8.0 kWh·m
−3 [
26]. The approximate 30% contribution of microalgae harvesting to the total production cost [
26,
27] highlights the economic importance of further innovation in this production step for advancing the technical readiness level of algal biotechnology in wastewater treatment. In contrast, pile cloth media filtration (PCMF) used during tertiary phosphorus removal was able to reach comparable removal rates of 75% to 95% but with a considerably lower energy demand reported as lower than 0.0068 kWh·m
−3 [
28]. PCMF seems promising as an alternative to other technologies more commonly used for microalgae harvesting, with effluent concentrations of total suspended solids (TSS) lower than 2 mg·L
−1. Considering discharge thresholds from wastewater treatment, TSS removal rates play a vital role in the technological applicability of separation technologies and are therefore regarded as a key parameter in the evaluation of such technologies. This is the case because MBB has to be removed as completely as possible from the effluent, since previous steps are only used to accumulate nutrients and pollutants in the biomass, which still remains in the wastewater. Only after a separation step, where the accumulated nutrients and pollutants are removed in the form of MBB, can the wastewater be considered cleaned.
PCMF is a surface filtration variant based on cloth filtration that uses 3D woven filter media for the separation of solids [
29]. PCMF can either be operated under free flow conditions or as a pressurized system. Typically, pile cloth media (PCM) is either mounted on a drum or a disc, resulting in the two designs of drum or disc filter. The free-flow design has an influent and effluent weir, which decouples the machine hydraulically from upstream and downstream processes. PCMF is an outside–in filtration process, meaning that influent water flows through the fully submerged filter construction from the outside of the PCM to the inside of the filter construction, whereby solids are retained. Separated solids are retained by the PCM and form a filter cake that additionally improves the removal efficiency. With increasing solids retention by the PCM material, the hydraulic resistance increases, resulting in an increase in the water level or differential pressure. If the water level or differential pressure exceeds a certain threshold, a filter cleaning cycle is triggered. During filter cleaning, the drum or disc is rotated alongside a static filter cleaning system, which reverses the flow direction (inside-out cleaning) and thereby removes the retained solids on the PCM. The filter cleaning system consists of a suction lip mounted on a suction bar, which is connected to a filter cleaning pump. No externally supplied backwash water is required for filter cleaning, and backwash water can normally be recirculated, for instance, to a primary clarifier. The filtration process is not interrupted during filter cleaning.
Currently, the application of the PCMF technology revolves around the removal of TSS and the chemical oxygen demand (COD) [
30] of phosphorus and microplastics in WWTP effluent [
28]. It can also act as a treatment step in micropollutant removal [
31], primary filtration, and primary effluent filtration during wastewater treatment [
32]; as a treatment step in water reuse systems [
33]; as well as in pre-treatment for the removal of algae and TSS in desalination processes [
34]. In these cases, PCMF has proven to be a reliable and energy-efficient separation method but was not previously applied for the separation of microalgae as a part of the wastewater treatment process. This study aims to close this gap and provide a report on the application of PCMF for the separation of microalgae during wastewater treatment as well as give recommendations for the dimensioning of a large-scale PCMF step for microalgae removal.
2. Materials and Methods
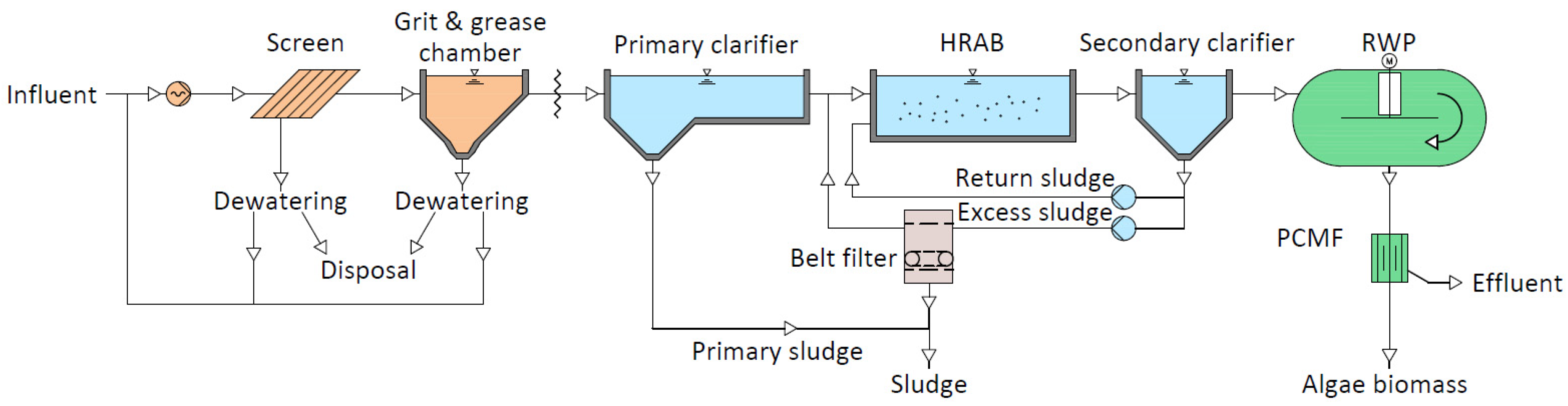
The PCMF was operated as part of a pilot-scale WWTP employing microalgae for the removal of nitrogen and phosphorus at a WWTP in the center of the German federal state Hesse (50.528615, 8.896596). The pilot plant was supplied with mechanically pretreated wastewater from a conventional municipal WWTP. The setup consisted of a primary clarifier, a high-rate aeration basin (HRAB) for COD removal, and a raceway pond (RWP) as a cultivation system for microalgae for biological phosphorus and nitrogen removal.
Figure 1 shows the process scheme of the pilot plant.
The HRAB was solely constructed for COD removal and was not equipped with extensive nutrient removal processes beyond activated sludge production. Processes such as enhanced biological phosphorus removal and denitrification were suppressed by constant aeration. Nitrification was inhibited by reducing the sludge age down to 4–5 days, hence necessitating growth rates lower than those commonly observed for nitrifiers. This allowed for a reduced reactor volume compared to conventional activated sludge processes, thus decreasing the demand for oxygenation. Despite constant aeration, this leads to a lower energy demand for aeration and preserves high nutrient concentrations for downstream microalgae cultivation. The nutrient-rich wastewater was then fed to the RWP after the activated sludge was removed in a secondary clarifier and recycled into the HRAB. The average wastewater composition after this step was 80 mg·L−1 COD, 29.5 mg·L−1 Ntot, 3.28 mg·L−1 Ptot, and 35 mg·L−1 TSS.
The RWP was constructed with a volume of 3 m
3 at a water depth of 0.3 m. A heterogenous culture of different microalgae and bacteria strains was cultivated in an open pond system fed with real wastewater, since the perpetuation of a monoculture was deemed impossible in the context of constant exposure to contamination with various species [
35,
36]. During operation, the microalgae strains
Pediastrum boryanum,
Scenedesmus acutus, Scenedesmus caudata-acueolatus, and
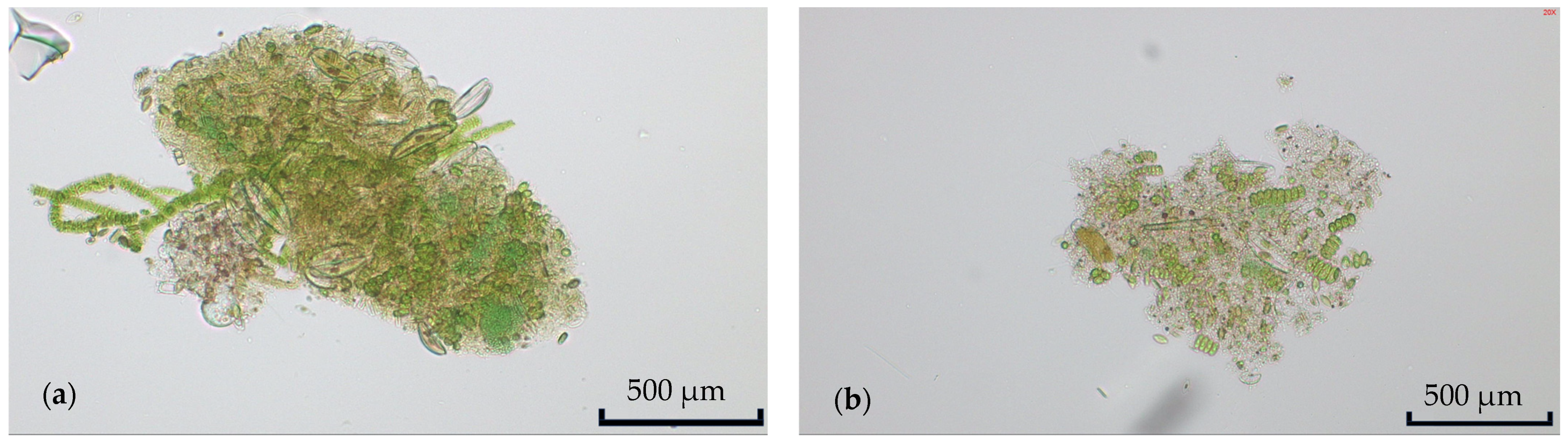
Chlorella vulgaris were identified as generally dominant in the culture. Images of the microalgae culture were taken at least once per week using an optical microscope (Panthera U by MOTICEUROPE, S.L.U., Barcelona, Spain). To determine a basic culture composition, two random samples were taken during this time, and the microalgae strain and macroinvertebrate taxonomy were visually identified through the observation of morphological variations under light microscopy. The prevalence of microalgae strains in these representative samples was determined by counting.
Figure 2 shows two exemplary microscopy images of the RWP microalgae culture.
Figure 1.
Process scheme of the “AlgA” pilot plant for wastewater treatment consisting of a mechanical treatment step (orange), an activated sludge treatment step with a primary and secondary clarifier, a high-rate aeration basin (HRAB) for COD removal (blue), a microalgae treatment step utilizing a raceway pond (RWP) for biomass cultivation as well as nitrogen and phosphorus removal, and a pile cloth media filtration (PCMF) for biomass separation (green) (modified from: [
37]).
Figure 1.
Process scheme of the “AlgA” pilot plant for wastewater treatment consisting of a mechanical treatment step (orange), an activated sludge treatment step with a primary and secondary clarifier, a high-rate aeration basin (HRAB) for COD removal (blue), a microalgae treatment step utilizing a raceway pond (RWP) for biomass cultivation as well as nitrogen and phosphorus removal, and a pile cloth media filtration (PCMF) for biomass separation (green) (modified from: [
37]).
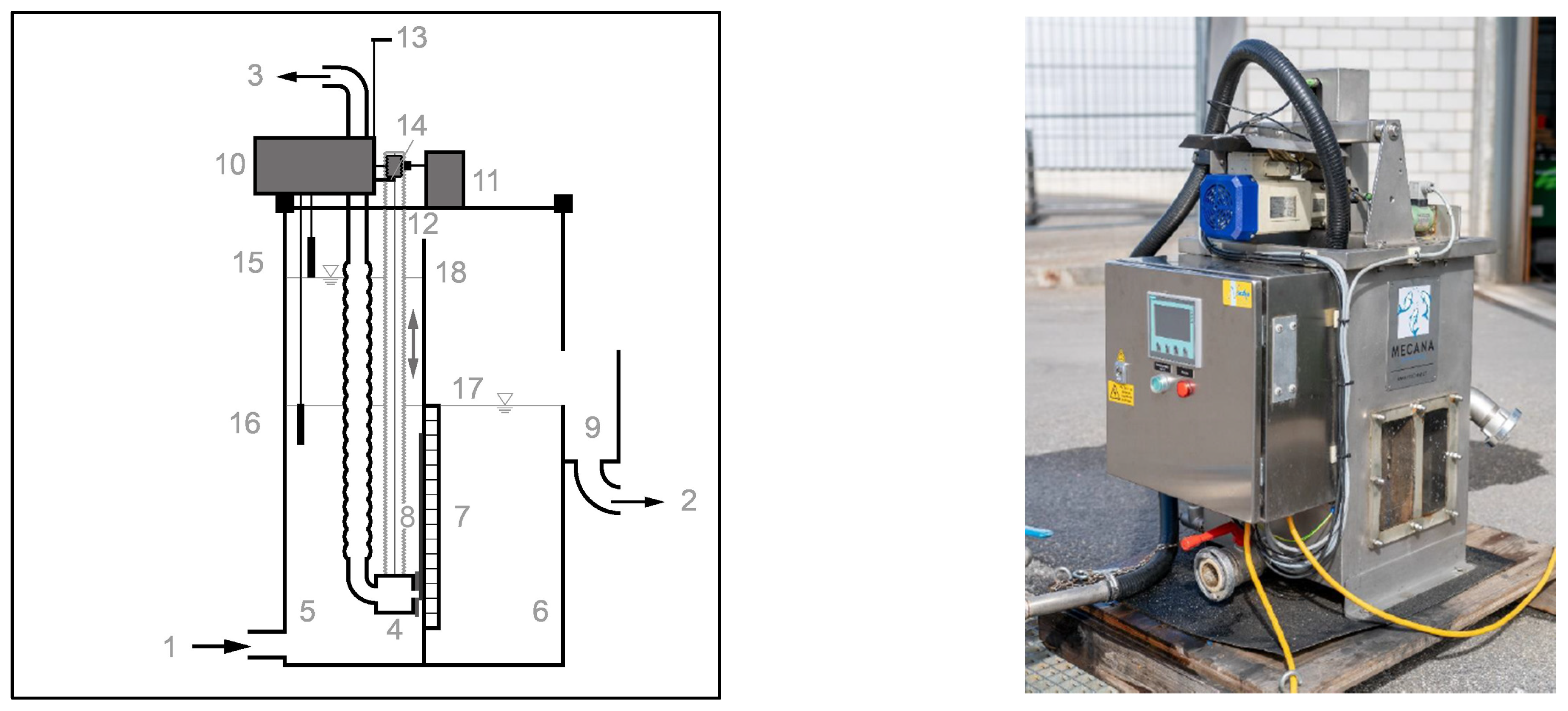
The PCMF comprises the last step in this process scheme, separating the microalgae bacteria biomass from the RWP effluent before discharge to a water body. The installed mini plate PCMF (Mecana AG, Reichenburg, Switzerland) had a variable filter surface area and exchangeable PCM regulating the filter active area; this is in contrast to the previously described setup of full-scale PCMFs as disc filters. During operation, filter masks with openings sized 0.01 m
2 and 0.04 m
2 were used. The filter utilized a two-chamber system with a fixed PCM as the separating pile layer in between for filtration, as depicted in
Figure 3. In this setup, the PCM remains static during filter cleaning, and a movable suction lip removes retained solids. During the studies, a plain version of the suction lip was investigated.
The pilot plant was continuously operated for 18 months. During this time, the PCMF was investigated under varying climate conditions and volumetric filtration rates and with three different PCMs. Water temperature in the RWP, air temperature, and the photosynthetically active radiation (PAR) were measured with environmental sensors (Sensolyt 700 IQ/SET (WTW, Weilheim, Germany), R-PT100-I (otom Group, Bräunlingen, Germany), and 6017.0000 (Theodor Friedrichs & Co., Schenefeld, Germany), respectively). Climate parameters were measured as part of data on microalgae growth and nutrient removal but impacted the PCMF only indirectly by influencing the biomass concentration in the PCMF influent. More relevant to the removal were differences in the pilot plant operation between summer and winter. The hydraulic retention time (HRT) of wastewater in the RWP was changed depending on the weather conditions and microalgae growth. During the summer months (May to October), a high biological activity with low doubling times was observed, and accordingly, a low HRT was chosen. During the winter months (November to April) more time was necessary for the microalgae to multiply, and a higher HRT was chosen to prevent a microalgae bacteria biomass (MBB) wash out. Under summer conditions (May to October), the RWP was fed with 600 L·d−1 of wastewater and 300 L·d−1 during the winter, resulting in HRTs of 5 and 10 days, respectively, and an influent into the PCMF of an equivalent amount.
Over the course of 18 months, two different OptiFiber
® PCM types, Microfiber (Fiber diameter ≥5 μm–<10 μm) and Ultrafiber (Fiber diameter <5 μm), were investigated in the studies, resulting in three different PCMs (PES-14, UF-10, UFS-9). PCMs have a multidimensional structure consisting of a filter-active, fluidizable pile layer and a non-filter-active backing. The backing serves as a support for the pile layer. The pile layer consists of multiple superimposed filaments or fibers woven into the backing. There is no defined pore size for PCMs by definition.
Table 1 shows the characteristics of the PCMs used in this study. After successful operation with PES-14 for 12 months, the PCM was changed to use the remaining operation time to test two other PCMs (UF-10 and UFS-9) and potentially improving the separation efficiency of the system.
During operation, the concentration of TSS [mg·L−1] was measured three times per week with 0.45 µm cellulose–acetate membrane filters (type AC04550BL, Hahnemühle FineArt GmbH, Dassel, Germany) according to DIN 38,409 at three different steps in the treatment process:
- -
TSSin—as influent concentration
- -
TSSeff—as effluent concentration
- -
TSSbw—as backwash water concentration
The energy demand used to calculate the number of filter cleaning cycles and as a basis for scale-up calculations was automatically measured by the PLC used for automation and data management with a Simatic ET 200SP AI Energy Meter 400VAC ST (Siemens AG, Munich, Germany).
During the 18 months of the operation of the pilot plant, a log book was kept, and noteworthy events, like clogged pipes and defective equipment, were reported. This log was used to identify irregularities caused by malfunctions of upstream processes, which cannot be attributed to the functionality of the PCMF. Consequently, data associated with such irregularities were excluded from the analysis.
The PCMF removal efficiency was calculated from the TSS concentrations in the influent and in the effluent of the filtration step using Equation (1):
where TSS
in is the concentration of TSS in the influent to the filtration step in [mg·L
−1], and TSS
eff is the concentration of TSS in the effluent in [mg·L
−1].
The filtration velocity was calculated as the quotient of the influent flow into the PCMF step and the available filtration area using Equation (2):
where Q is the daily amount of water in the influent to the PCMF in [m
3·d
−1], and A is the available filtration area in [m
2].
PCMF itself has a low energy demand. Energy demand for full-scale drum and disc filters ranges from <0.3 to 20 Wh·m
−3, depending on the frequency of the filter cleaning and settled sludge removal. For disc filters specifically, the energy demand ranges from 0.3 Wh·m
−3 with a filter cleaning interval of every two hours to up to 15 Wh·m
−3 in the case of continuous filter cleaning [
29]. In a gravity flow system, the PCMF consumes energy only during biomass extraction when filter cleaning is triggered. The pilot plant did not have a system for removing settled sludge, so that the energy demand can be directly related to filter cleaning. Therefore, the measured energy demand could be used to calculate the number of filter cleaning cycles per day using Equation (3):
where n
FC is the number of daily filter cleaning cycles, e
tot,d stands for the energy demand per day, and e
FC is the energy demand of a single filter-cleaning cycle.
In this study, the solids loading rate (SLR) describes the amount of TSS per filtration area and hour under which a PCM was operated. SLR was calculated using Equation (4):
where TSS
in is the concentration of TSS in the influent to the filtration step in [mg·L
−1], and v is the filtration velocity in [m·h
−1].
Measured energy demands during the operation of the pilot plant are hardly comparable to the energy demand of large-scale operations, as shown in
Table 1, due to the economics of scale. Instead, the measured energy demands, TSS concentrations, and PCM characteristics were used to dimension a large-scale PCMF unit with energy demand values based on empirical data of PCMF components like pumps and drive. As a basis for the dimensioning, a WWTP with a size of 10,000 PE (population equivalent) was assumed, as well as an average wastewater composition for this size in Germany. Larger WWTPs are unlikely to be fitted with an RWP, due to the required area for the wastewater treatment of multiple hectares. This potential RWP was assumed to be operated in a way that assured nutrient removal throughout the year, resulting in fluctuating HRTs, depending on the biomass growth rates, while maintaining a fixed TSS concentration of MBB.
The relevant process parameters for the dimensioning were the daily amount of wastewater in the influent (average and maximum) and the expected TSS concentrations. A peak factor for the daily influent and the TSS concentration were not needed, due to the large volume required for microalgae-based wastewater treatment. Influent fluctuations can theoretically be buffered by small changes in the water height in the RWP, thus simplifying the dimensioning and reducing the size of the required PCMF. The relevant PCMF parameters were the chosen PCM, filtration velocity, and the SLR under which the filter would be operated. These values were used to calculate the necessary filter area which in turn dictated the suitable type and amount of PCMF units and therefore the energy demand needed for filtration.
3. Results
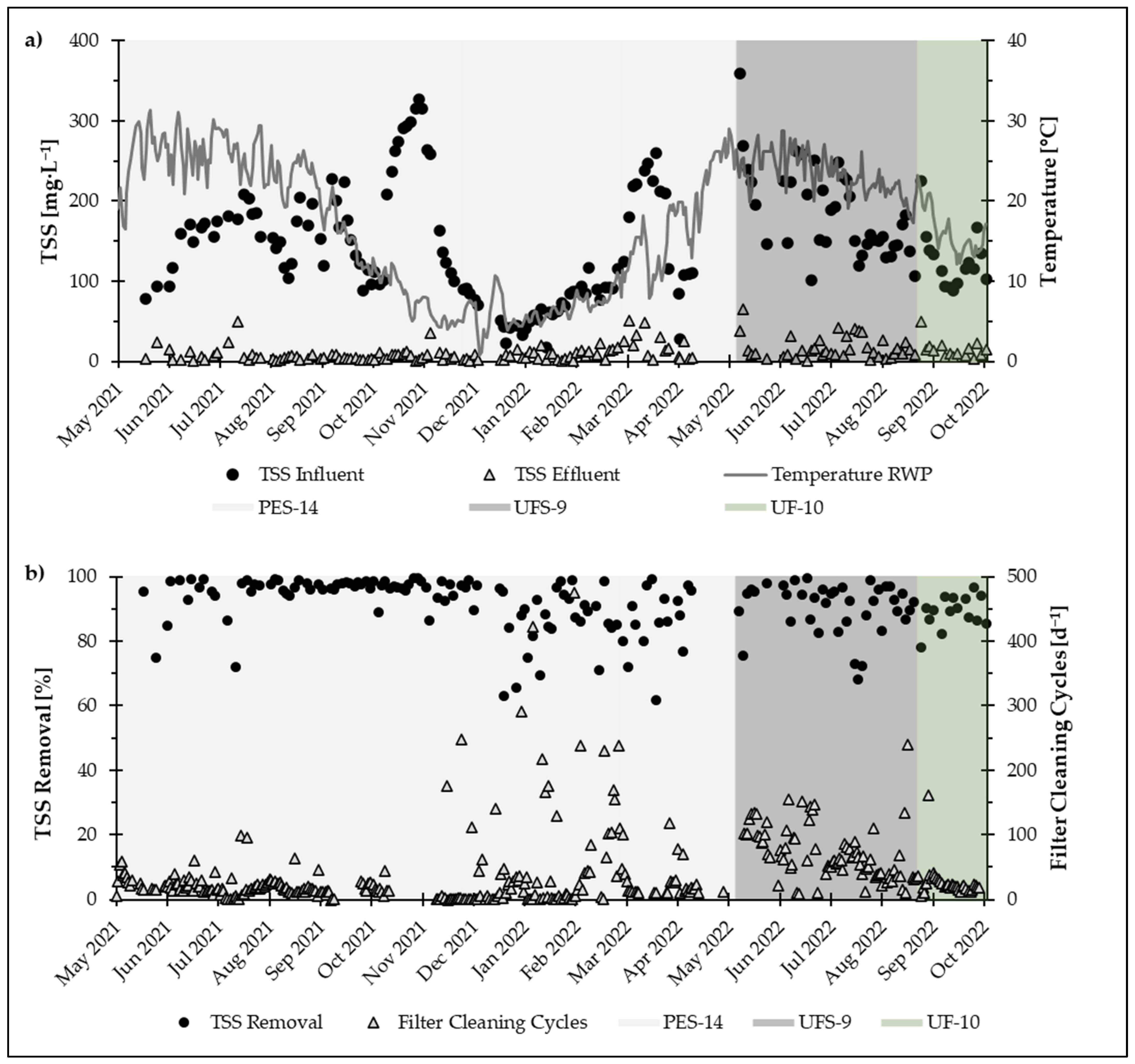
Part (a) of
Figure 4 shows the TSS influent and effluent concentrations observed during PCMF operation. The temperature during this time was also included, as the temperature had a more or less direct impact on the biological activity and therefore on TSS
in. Part (b) depicts the removal efficiencies for the three different PCMs used during the 18 months of operation together with the daily amount of filter cleaning cycles. The operational periods for the different PCMs correspond to the values in
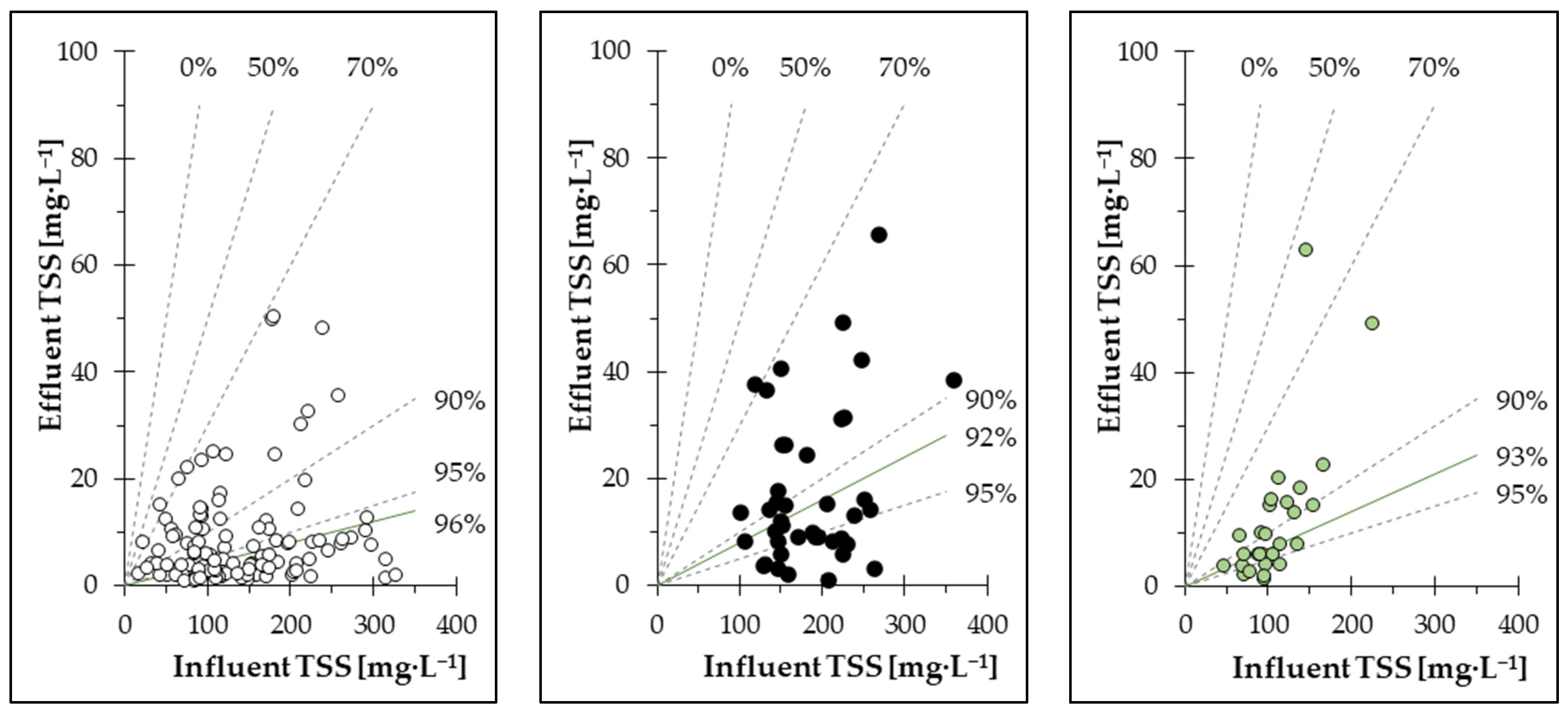
Table 2. The removal rates over 18 months and all PCMs ranged from minimum rates as low as 57% to maximum removal rates of close to 99.9%.
The data show that TSS effluent concentration was relatively constant and was not influenced by the concentration of biomass in the RWP (TSSin) and only partly by high numbers of filter cleaning cycles per day. Therefore, TSS removal is significantly influenced by TSS inflow concentration, while the TSS outflow value remains constant. During the first months of operation, removal efficiencies of 90% up to close to >99% were achieved. This was mainly the case with PCM PES-14, which proved to have reliable performance during the first year of operation. A peak in TSSin in November 2021 was caused by a switch from summer (HRT of 5 days) to winter operation (HRT of 10 days). At the beginning of the second year, a drop in performance was observed. After this point, the removal efficiencies fluctuated between 60% and 99%. This correlated with an increase in filter cleaning cycles and a larger spread between daily values. After this period, the PCM was changed from PES-14 to UFS-9. The removal efficiency for UFS-9 proved to be satisfactory during its operation with performances ranging from 80% to almost >99%. For a short period of time, the filter appeared to have been overloaded from a high concentration of TSSin, resulting again in an increase in filter cleaning cycles and a drop in removal rates. UF-10 was installed as the final PCM and operated for two months. During this time, removal rates fluctuated again between 80% and close to >99% but were overall satisfactory.
Despite varying rates of TSS removal, every PCM was able to reach a low average TSSeff (9.7 mg·L−1 for PES-14, 12.4 mg·L−1 for UF-10 and 17.7 mg·L−1 for UFS-9) with no noticeable impact of TSSin on TSSeff. Between December 2021 and May 2022, fluctuating TSS removal rates could be observed. This was not caused by a drop in filter performance but rather by a considerably decreased TSSin during the winter months, impacting the calculation of removal rates.
This hypothesis is further supported by the ratio of TSS
in to TSS
eff, as depicted in
Figure 5, which shows the correlation between TSS
in and TSS
eff as separate values for each PCM as well as the median value of all removal rates reached. The largest set of data was available for PES-14, which also had the highest median value for removal rates. It can be observed that despite TSS
in concentrations of sometimes over 300 mg·L
−1, only a few data points were higher than 20 mg·L
−1 for TSS
eff. UF-10 and UFS-9 both had relatively similar median values for removal, which were still close to PES-14, but indicated a higher spread of TSS
eff concentrations. UF-10 was operated with lower influent TSS concentrations than PES-14 or UFS-9, which mainly affected the calculation of the removal rates. Removal efficiencies for UFS-9 were in the same range as UF-10 but were generated under generally higher TSS
in concentrations. UFS-9 had the highest TSS
eff concentrations of all three PCMs and had a higher percentage of data points above 20 mg·L
−1 TSS
eff than PES-14 and UF-10. Different concentrations of TSS
in resulted in different intervals between filter cleanings. The large spread of data points for UFS-9 and the high concentrations for TSS
eff can be explained by a possible overload of the PCM by solids. An overload would have resulted in a high amount of filter cleaning cycles, or even continuous filter cleaning, thus preventing the interlocking of PCM and the formation of a filter active pile layer.
The key points of
Figure 4 and
Figure 5 are summarized in
Table 2 together with the filtration velocity of each PCM. The differences in filtration velocity stem from the fact that PES-14 was operated under summer and winter conditions with different influent volumes, while UF-10 and UFS-9 were only operated under summer conditions without changes to water volumes. Judging by the attained TSS
eff concentrations, PES-14 seems to be the most suitable PCM for microalgae separation. In contrast, UFS-9 had the highest TSS
eff concentrations, despite average TSS removal rates. UFS-9 seems therefore slightly more suitable for the separation of microalgae grown under specific conditions. Nevertheless, UFS-9 might be more reliable for a microalgae culture grown under more stable conditions in contrast to what is typically encountered in wastewater treatment. A simple change to improve the TSS
eff might be to increase the filtration area of UFS-9 compared to PES-14 and UF-10, to avoid the hypothesized filter overload. When using Ultrafiber PCM, a larger filter area is therefore required in order to utilize the filter performance of the finer fibers of the PCM.
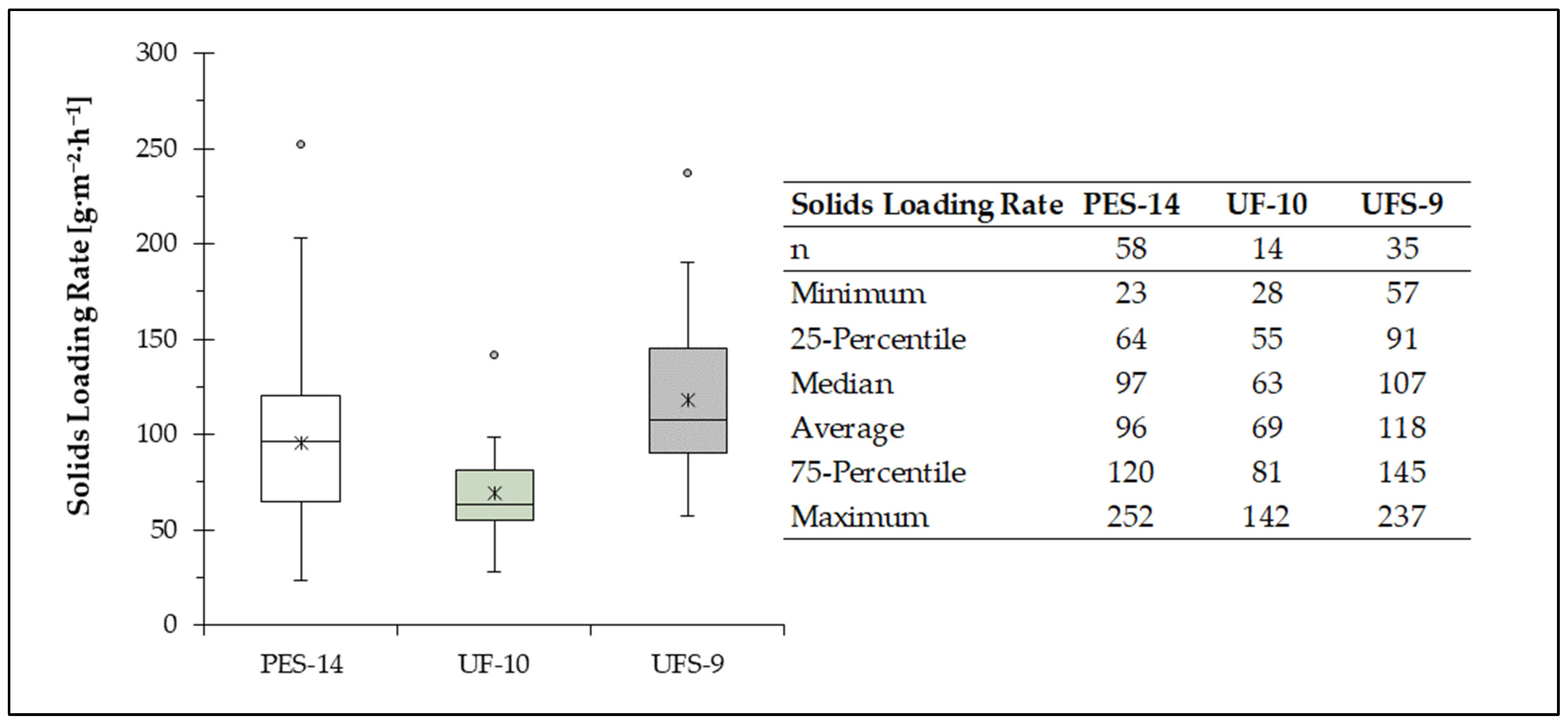
The SLRs under which each PCM was operated are summarized in
Figure 6 as boxplots. SLR is a key parameter for the calculation of filter surface as well as for the choice of PCM. The suspected overload of UFS-9 is supported by the high SLR reached during pilot plant operation. UFS-9 was operated with the highest SLR of all PCMs, with an average value of 118.2 g·m
−2·h
−1, as compared to 95.8 g·m
−2·h
−1 or 69.4 g·m
−2·h
−1 reached with PES-14 und UF-10, respectively. While UFS-9 and PES-14 were operated under a broad range of TSS
in, UF-10 was used on average at lower SLRs.
Figure 7 shows the TSS concentrations in the backwash during operation, summarized as boxplots. Average TSS concentrations in the backwash of the filtration step showed only small differences between the three PCMs, with values around 1800 mg·L
−1. This is despite an observed TSS
in range from less than 50 mg·L
−1 to more than 300 mg·L
−1.
A WWTP with 10,000 PE, as was chosen for the exemplary dimensioning of a large-scale PCMF, resulted in an expected average daily influent of 1950 m3·d−1, or 81 m3·h−1, including infiltration water. The maximum daily influent was set at 3117 m3·d−1 or 130 m3·h−1, encompassing the average value and an additional rain event. The MBB concentration in the influent into the PCMF was chosen as a fixed value of 200 mg·L−1. Based on the experiences gathered during pilot plant operation, PES-14 was chosen as the PCM. PES-14 was operated with SLRs regularly ranging from 65 g·m−2·h−1 to 120 g·m−2·h−1. For the calculation, a maximum SLR of 100 g·m−2·h−1 was set, since most values for reliable operation were obtained in this range.
These limiting conditions resulted in a theoretically required filtration area of 260 m2. This filtration area can be provided by three disc filters with 90 m2 of filtration surface each, resulting in a total filtration area of 270 m2, slightly lowering the dimensioning SLR and filtration velocity. This results in an average filtration velocity of 0.3 m/h and a maximum of 0.5 m/h, correlating with the pilot plant operation, and SLRs of 60 g·m−2·h−1 on average and 90 g·m−2·h−1 at maximum. The backwash ranges from 5% to 7% of influent wastewater and TSSeff concentrations of below 10 mg·L−1 are attainable, as shown during pilot plant operation under the same process parameters. The final energy demand considers a pump for filter cleaning, an electrical drive for disc rotation and a sump pump to prevent biomass buildup under the filter discs. The energy demand for disc filters with 90 m2 of filtration surface each is between 7 to 8 Wh·m−3, which corresponds to around 37 Wh·kg TSS−1.
4. Discussion
During operation, three main reasons for decreasing removal efficiencies were identified. One was the mathematical impact of TSSin (Equation (1)) on the removal rates. A drop in TSSin, as was mainly observed during the winter months when biological activity in the RWP was decreased, resulted in worse removal efficiencies, even though TSSeff values remained at a stable level. Additionally, an impact of the frequency of filter cleaning cycles on the removal efficiency was observed. At multiple points during the pilot plant operation, a filter overload and therefore a heightened physical stress on the PCM resulted in multiple cleaning cycles per hour. This might have negatively impacted TSS removal by inhibiting the formation of a filter active pile layer and reducing the removal efficiency, especially for small particles and algae flocs. Lastly, the MBB culture showed visible changes over the operating period, ranging from easily separable flocks to low TSSin concentrations of single cell structures, which were only partially separable during the operation of PES-14 and UF-10. A direct comparison between the applied PCM is in this case hardly expedient, due to the uncontrolled nature of an open pond microalgae culture. This is further aggravated by the fact that the PCMF was only a part of multiple investigations carried out at the pilot plant at the same time. This complicates a direct comparison of all three PCMs, but the lessons learned for full-scale applications of this technology for the separation of microalgae are nonetheless applicable.
As summarized in
Table 3, the energy demand of other MBB removal technologies ranges from 0.07 kWh·m
−3 for coagulation and flocculation to up to 8 kWh·m
−3 for decanter centrifugation. The most commonly used technology for MBB removal is centrifugation due to its reliability and high removal efficiency, despite its high energy demand [
16]. PCMF seems to be a viable alternative to centrifugation with empirically measured removal efficiencies of up to 97% or average TSS
eff concentrations as low as 9.7 mg·L
−1 while having a considerably lower calculated average energy demand of 7 to 8 Wh·m
−3 or 37 Wh·kg TSS than other technologies. When compared to flotation, PCMF achieved slightly lower removal rates of 96% as compared to 99% under optimal conditions but with a decidedly lower energy demand. It can be assumed that PCMF is more reliable than flotation, due to the technology being less impacted by the biological makeup of the influent. The biggest parallels can be drawn between PCMF and coagulation as well as flocculation, due to their low energy demand and both technologies being low maintenance. When compared to an average value for coagulation and flotation, PCMF was able to reach comparable removal rates. The energy demand is in both cases lower than all other technologies, with PCMF having only a tenth of the energy demand of coagulation and flocculation. PCMF has the added advantage of not needing chemicals for operation and separation, thus eliminating further operational costs. A direct comparison to membrane filtration is only partially possible, due to their different fields of application. Looking at the energy demands per treated volume, PCMF is more efficient by a factor of 100. On the other hand, membrane filtration is used in cases where particulates must be removed in the range of under 0.1 µm, which is not possible with PCMF.
A large-scale application of PCMF further reduces the causes of heightened TSSeff concentrations, and lower values seem achievable, as indicated by the median value for TSSeff achieved with a PES-14 of 5.8 mg·L−1. The choice of PCM type itself seems to only marginally influence the concentration of TSS in the effluent in the case of microalgae harvesting. This is due to the properties of the microalgae biomass and its interaction with PCM. All PCMs were able to reach a low TSSeff under normal operation. The choice of PCM only affected operational parameters like the amount of filter cleaning cycles and the energy demand, as well as the probability of a filter overload.
In the case of attained TSS concentrations in the backwash water, PCMF was able to achieve around 1800 mg·L−1 on average. This represented a considerable improvement when compared to the average influent TSS but was also lower than concentrations attainable by centrifugation or membrane filtration.