Abstract
The aim of this research was to obtain an apple juice with a strong antioxidant effect through the fermentation of mixed lactic acid bacteria. Lactobacillus plantarum and Lactobacillus casei were selected and combined as fermentation strains from six strains of lactic acid bacteria. The effects of the amount of inoculated lactic acid bacteria, the fermentation temperature, and the fermentation time on the antioxidant capacity and sensory score of apple juice were investigated. The optimal technological conditions were as follows: an inoculation amount of 3%, a fermentation temperature of 37 °C, and a fermentation time of 37 h, which were determined through single-factor experiments and the Box–Behnken design. Under these conditions, the DPPH scavenging ability of the fermented apple juice was 62.31%, and the sensory score was 91.33. HS-SPME-GC-MS analysis showed that there were 39 and 46 main aroma compounds in apple juice and fermented apple juice, respectively. Fermentation increased the number of viable lactic acid bacteria, enhanced the polyphenol content, and enhanced the sensory score. A comparative evaluation of scavenging activity and total reducing power revealed that the antioxidant capacity of fermented apple juice significantly exceeded that of unfermented apple juice. Generally, mixed lactic acid bacteria fermentation enhanced antioxidant activities, while also presenting better aroma flavors and improved overall acceptability.
1. Introduction
Apple (Malus pumila Mill.) is one of the most widely consumed fruits globally and has always been popular among consumers due to its richness in nutrients and sweet flavor [1,2]. Despite the substantial production of apples, the majority of them are currently used for fresh consumption with limited processing, meaning that fewer product category does not adequately meet consumer expectations [3,4]. Probiotics are defined as a group of live micro-organisms that confer health benefits to the host [5]. Lactic acid bacteria (LAB) comprise a significant proportion of these micro-organisms. LAB are extensively utilized in the food industry to produce various dairy products and fermented foods [6]. Currently, dairy products are still the main source of probiotics, but many people suffer from a lack of probiotic intake due to lactose intolerance, milk protein allergy, or their own choice of a dairy-free diet. Fruit and vegetable juices are regarded as viable substitutes [7]. It has been demonstrated that LAB can utilize fruit and vegetable juices as fermentation substrates for a variety of physiological activities, thereby enhancing their flavor and increasing their functional activities [8], such as their antioxidant [9], hypoglycemic [10,11], and antimicrobial [12] activities.
In recent years, research on fermented fruit and vegetable juices has increased, but available products remain limited, with excessive focus placed on taste optimization at the expense of functional development, therefore failing to meet consumer demands. Apples contain diverse nutrients, including sugars, organic acids, polyphenols, and vitamins, which provide excellent substrates for LAB fermentation [13,14]. LAB fermentation not only retains apple juice nutrients but also generates bioactive compounds beneficial to human health [15]. Park et al. [16] found that the fermentation of cabbage apple juice with Lactobacillus plantarum ameliorated obesity and hyperlipidemia in rats. Sun et al. [17] noted that mixed strain fermentation improved the viable counts and quality of the products, emphasizing the synergistic effect of LAB. Almond juice fermented with mixed strains showed many advantages in terms of changes in its chemical composition and its organoleptic properties compared to fermentation with a single strain [18].
The objective of this study was to develop antioxidant-rich apple juice through mixed LAB fermentation. Six LAB strains were screened for their suitability in apple juice fermentation. Key process parameters, including inoculum amount, fermentation temperature, and duration, were optimized. Subsequently, fermented apple juice was compared with unfermented juice to evaluate the impacts of fermentation on viable bacterial counts, polyphenol and flavonoid contents, sensory quality, antioxidant activity, and volatile compound profiles.
2. Materials and Methods
2.1. Reagents and Materials
Fresh Red Fuji Apples were provided by Shandong Zhongyi Modern Wisdom Agriculture Co., Ltd. (Zibo, China). Lactobacillus plantarum JYLP-002, Lactobacillus acidophilus JYLA-191, Lactobacillus casei LC-12, Lactobacillus paracasei JLPE-176, and Lactobacillus fermentum JYLR-005 were provided by Shandong Zhongke Jiayi Bio-engineering Co., Ltd. (Weifang, China). Enterococcus faecalis was obtained from the Agriculture Microorganism Resources Preservation Utilization Center (AMCC) of Shandong Agricultural University (Taian, China). The MRS broth medium and MRS medium were provided by Qingdao Haibo Biotechnology Co., Ltd. (Qingdao, China). Gallic acid, Folin–Ciocalteu reagent, DPPH, and ABTS reagent were provided by Beijing Solarbio Technology Co., Ltd. (Beijing, China). Anhydrous ethanol, anhydrous sodium carbonate, sodium hydroxide, and sodium bicarbonate were purchased from Tianjin Kaitong Chemical Reagent Co., Ltd. (Tianjin, China).
2.2. Preparation of Apple Juice
Fresh red Fuji apples were placed in 0–4 °C cold storage before the start of the experiment to maximize the maintenance of fruit quality. The apples were selected to remove the pest-infested, rotten, and mechanically damaged fruits, followed by washing, crushing, juicing, and filtration to obtain fresh apple juice. A total of 0.1% sodium erythorbate was added to prevent browning and 10% sodium bicarbonate was used to adjust the pH of the apple juice, which was then kept at 85 °C for 15 min for pasteurization.
2.3. Preparation of Seed Liquid
The fermentation agent was prepared according to the method of Ran et al. [19]. The strain was kept in glycerol tubes, removed from the refrigerator, inoculated into MRS medium, and incubated at 37 °C for 24 h for activation, which was repeated 2 times. The activated cultures were centrifuged, and the precipitate was resuspended in sterile water, diluted to about 107 CFU/mL, and prepared for use.
2.4. Screening of LAB
The different activated lactic acid bacteria strains were inoculated into a conical flask containing 50 mL sterile apple juice with an inoculation amount of 3% (v/v), placed in a constant temperature incubator at 37 °C for fermentation, and samples were taken at 12 h intervals for determination. The viable bacterial count was determined by the method of Li et al. [20]. The total acid content was determined according to the Chinese National standard GB 12456-2021 [21]. The polyphenol content was determined according to the method of Li et al. [22], using the Folin–Ciocalteu colorimetry method. The DPPH scavenging ability was determined according to the method of Wang et al. [23]. The ABTS scavenging ability was determined according to the method of Abdel et al. [24]. The sensory scoring was conducted with reference to the method of Raju et al. [25] and Ren et al. [26]. The sensory evaluation consisted of 10 experts in food science and engineering: five men and five women aged between 25 and 50 years old. Sensory analysis was performed through quantitative descriptive analysis, and the products were rated in five aspects: color, aroma, taste, tissue condition, and overall acceptability, with each component accounting for 20 points, with the scoring standard shown in Table S1.
2.5. Optimization of Apple Juice Fermentation
2.5.1. Strains Formulation Experimental Design
The strain proportions were designed with reference to Cong et al. [27] and Lan et al. [28]; the screened L. plantarum and L. casei were mixed at ratios of 1:1, 1:2, 1:3, 2:1, and 3:1. The mixed cultures (3% v/v) were inoculated into sterilized apple juice and fermented at 37 °C for 24 h. The polyphenol content, DPPH scavenging activity, and sensory scores were analyzed.
2.5.2. Single-Factor Experimental Design
Single-factor experiments were conducted with three factors, strain inoculum (from 1% to 9%), fermentation temperature (from 28 to 40 °C), and fermentation time (from 12 to 60 h) as variables, in order to optimize the process conditions of apple juice fermentation by determining the polyphenol content, the scavenging rate of DPPH radicals, and the sensory scores of the fermented apple juice.
2.5.3. Box–Behnken Design (BBD)
The fermentation parameters were optimized by a three-factor three-level test based on a single-factor experiment. There were 17 groups of experiments, and each group of experiments was repeated three times independently. The apple juice fermentation process was optimized by response surface test analysis using the DPPH radical scavenging rate and the sensory score. The experimental design is shown in Table 1.

Table 1.
Box–Behnken design factors and horizontal design.
2.6. Volatile Compound Analysis (HS-SPME-GC-MS)
The composition and relative content of the volatile compounds were analyzed by the HS-SPME-GC-MS system [29], referencing the method of Sun et al. [30] but with minor adjustments. A total of 2 mL of the sample was placed into a 20 mL headspace vial and 0.5 g NaCl was added to promote the volatilization of the components. The headspace vials were incubated at 45 °C for 30 min, and a 65 μm SPME device was inserted into the headspace for 30 min. The sample was then analyzed by GC-MS. At first, the oven temperature program was 40 °C, which was held for 3 min; then, the temperature increased to 120 °C at a rate of 4 °C/min; and then increased to 240 °C at a rate of 6 °C/min and was held for 9 min. Unknowns were identified by matching their mass spectral libraries and retention indices. By matching with the NIST22 library, a similarity of >85% was considered credible. The relative content of each volatile component was determined using the peak area normalization method, which calculated the percentage of the peak area to the sum of the peak areas of all components.
2.7. Physicochemical Analysis
Viable counts, total acids, total phenolics, and sensory scores were determined according to the method described above. The total flavonoid content was determined as described by Yang et al. [31].
2.8. Determination of Antioxidant Activity In Vitro
2.8.1. Total Reducing Power Assay
The total reducing power of apple juice was determined using the Prussian blue method [32]. An appropriate apple juice sample solution was mixed with a phosphate-buffer solution and a potassium ferricyanide solution. The mixture was reacted at 50 °C for 20 min, quickly cooled, and trifluoroacetic acid was added and centrifuged at 3000 rpm for 10 min. Next, the supernatant was mixed with deionized water and ferric chloride solution, underwent a reaction for 10 min, and the absorbance was measured at 700 nm. Deionized water was used as a blank and ascorbic acid was used as a control.
2.8.2. DPPH Scavenging Ability
The DPPH scavenging ability was determined according to the method described by Yang [31]. DPPH was dissolved in 95% ethanol to form 0.1 mmol/L DPPH solution. An appropriate amount of apple juice sample solution was mixed with DPPH for 30 min in the dark, and the absorbance was measured at 517 nm. Ascorbic acid was employed as the positive control. The scavenging ability was calculated according to the following formula:
where A1 is the test sample solution with the DPPH solution and A0 is deionized water with the DPPH solution.
DPPH scavenging ability (%) = [(A0 − A1)/A0] × 100
2.8.3. ABTS Scavenging Ability
The free radical scavenging activity of ABTS was determined according to the method reported by Garzón [33]. Appropriate samples were mixed with ABTS solution in a 96-well plate and reacted in the dark for 10 min, and the absorbance value was measured at 734 nm; all operations were performed at room temperature. Ascorbic acid was employed as the positive control. The scavenging ability was calculated according to the following formula:
where A1 is the test sample solution with the ABTS solution and A0 is deionized water with the ABTS solution
ABTS scavenging ability (%) = [(A0 − A1)/A0] × 100
2.9. Data Analysis
All experiments (batches) were repeated three times. The results were expressed by the mean ± standard deviation. Statistical analysis was performed using SPSS 26.0 (Chicago, IL, USA); p < 0.05 indicated statistical significance. The charts were created by Microsoft Excel and Origin 2022 software (OriginLab, Northampton, MA, USA).
3. Results and Discussion
3.1. The Effects of Fermentation of Different LAB on Apple Juice
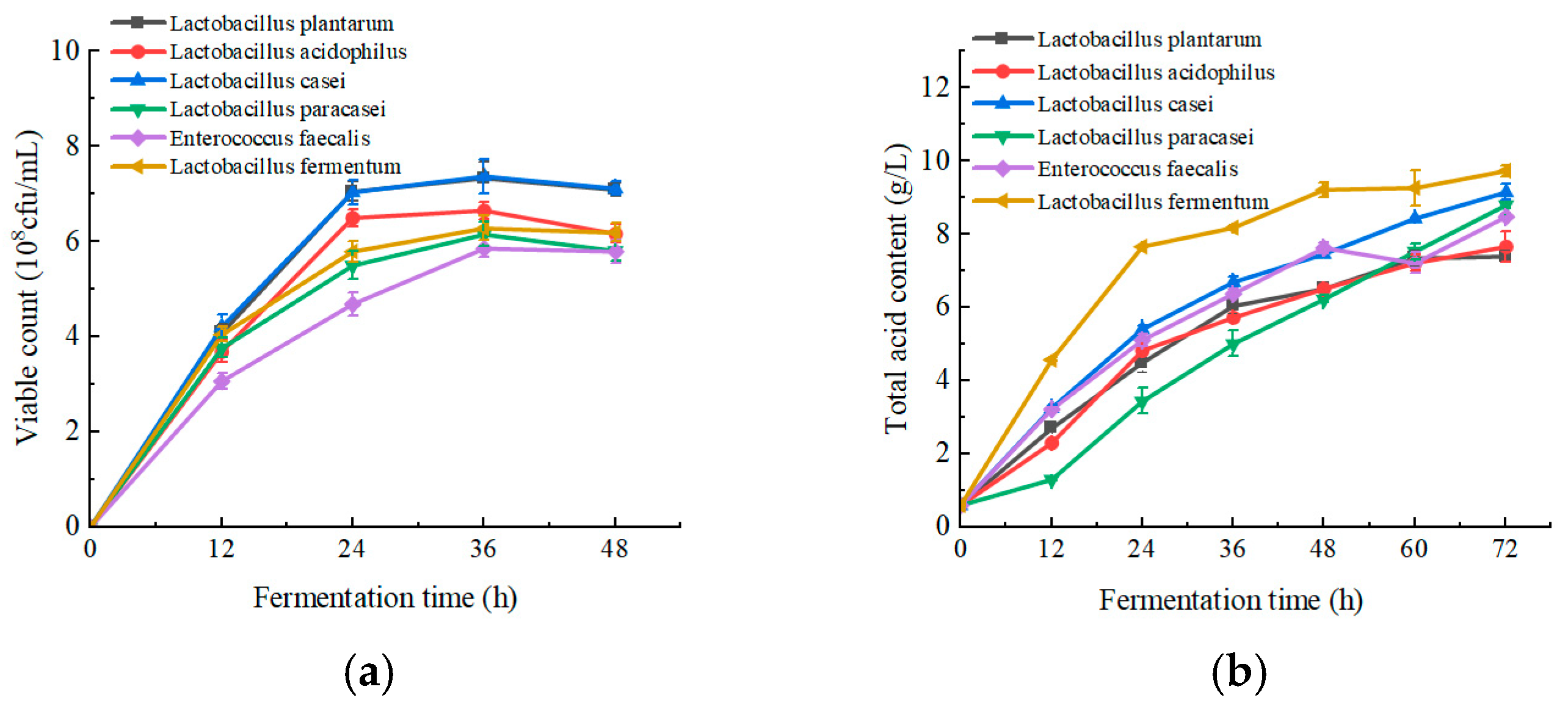
Viable count is an important evaluation index for measuring the degree of fermentation of LAB [34]. From Figure 1a, it can be seen that the viable count initially increased and then leveled off in apple juice. L. plantarum and L. casei grew better in apple juice, with both viable counts reaching 7 × 108 CFU/mL, which is consistent with the results of the viable counts of different LAB in apple juice fermentation in the study by Ruoyu Zheng [35].
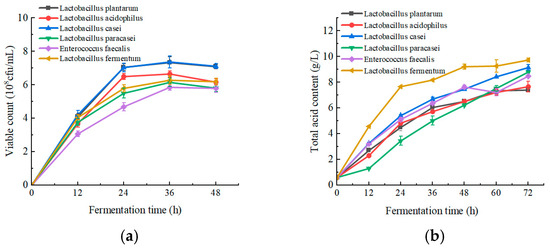
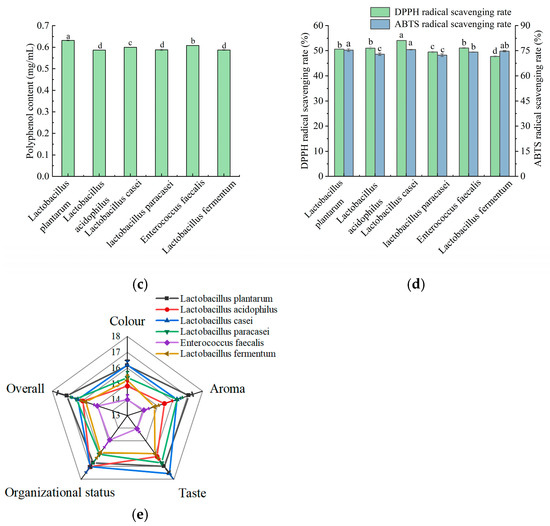
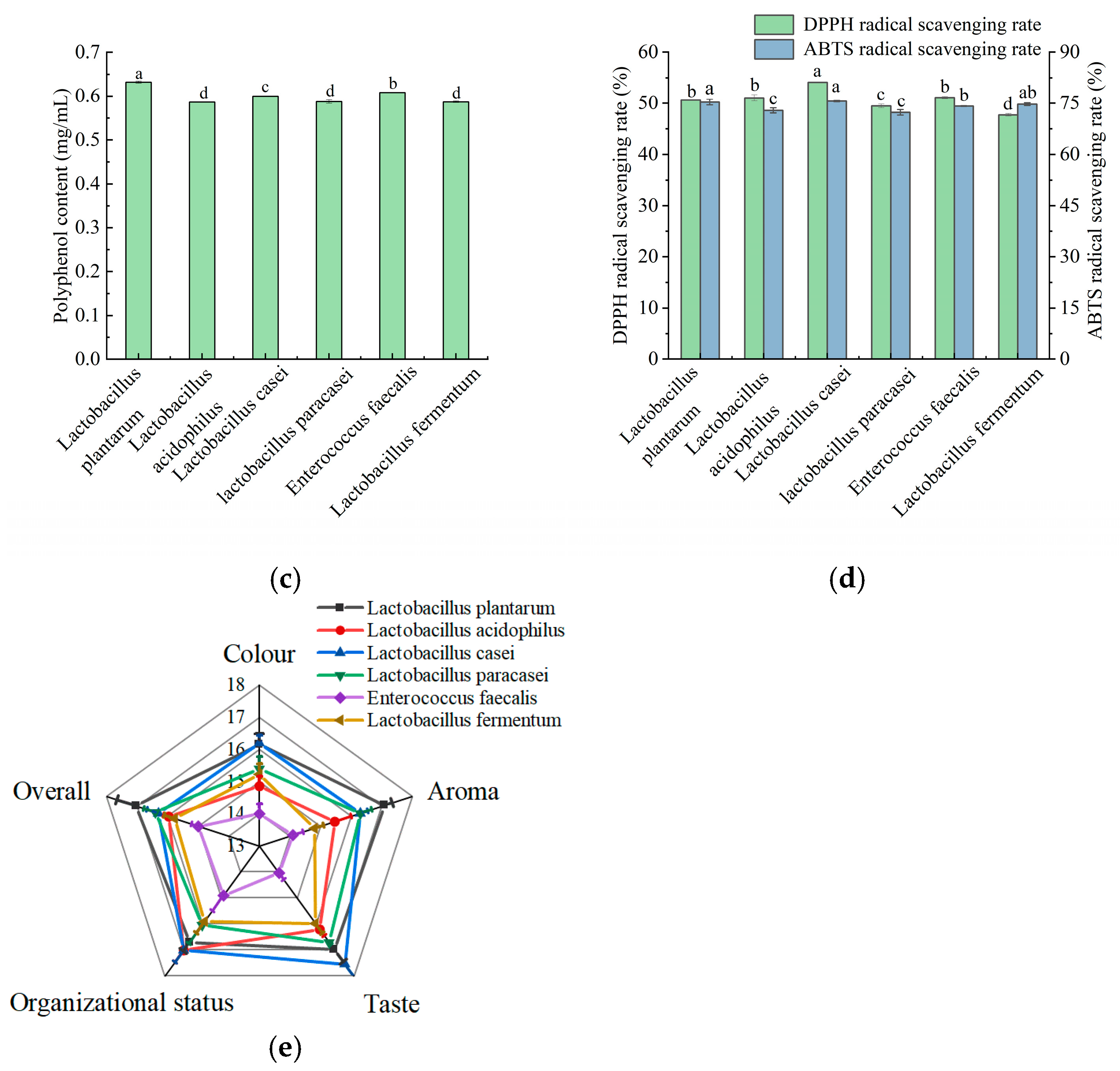
Figure 1.
Effect of fermentation of different LAB on viable count, total acid content, polyphenol content, DPPH and ABTS scavenging capacity, and sensory scores of apple juice. (a) Viable count; (b) total acid content; (c) polyphenol content; (d) DPPH and ABTS scavenging capacity; and (e) sensory scores. Lowercase letters indicate significant differences (p < 0.05).
LAB produce acids during fermentation, which increases the total acid content. Therefore, determining the total acid content during apple juice fermentation can reflect the growth of lactobacilli [36]. As shown in Figure 1b, the total acid content of apple juice increased during the 72 h of fermentation, and reached about tenfold or more at the end of fermentation. Among them, L. fermentum was the most acid-producing, followed by L. casei and L. plantarum.
Polyphenol is an important quality factor in apples and has various functions, such as being anti-inflammatory, antioxidant, and regulating color change and flavor in fruit [8]. As shown in Figure 1c, the highest polyphenol content in apple juice was fermented by L. plantarum, followed by E. faecalis.
The in vitro antioxidant capacity of apple juice was evaluated using DPPH and ABTS radical scavenging assays. As shown in Figure 1d, the highest DPPH free radical scavenging activity was observed in apple juice fermented by L. casei. Meanwhile, the highest ABTS free radical scavenging rate was achieved in apple juice fermented by L. casei, L. plantarum, and L. fermentum, and there was no significant difference among the three. The results of the two radical scavenging rates were not identical, which was also observed in the determination of the antioxidant capacity of different medicinal plants by Perera et al. [37]. This may be due to the different electronic structures and reactivities of the two radicals, which react differently with the different functional groups of the compounds in apple juice [38].
Sensory quality is a crucial factor in food products and directly influences consumers’ purchasing decisions [39]. As shown in Figure 1e, apple juice fermented by L. plantarum had the highest scores in the three areas of color, aroma, and overall acceptability. However, apple juice fermented by L. casei had the highest taste score. The apple juice fermented with L. acidophilus and L. casei scored higher in terms of tissue status. Overall, L. plantarum and L. casei exhibited the highest sensory scores, followed by L. paracasei and L. acidophilus.
In summary, apple juice fermented by L. plantarum exhibited the highest bacterial viability, polyphenol content, and sensory scores. The apple juice fermented by L. casei exhibited the highest free radical scavenging rate and improved palatability. L. plantarum and L. casei showed higher microbial viabilities than the other strains, which is consistent with our findings [40]. Therefore, L. plantarum and L. casei were selected as the strains for the mixed fermentation of apple juice for subsequent experiments.
3.2. Optimization of Apple Juice Fermented by LAB
3.2.1. Strains Formulation Experiments
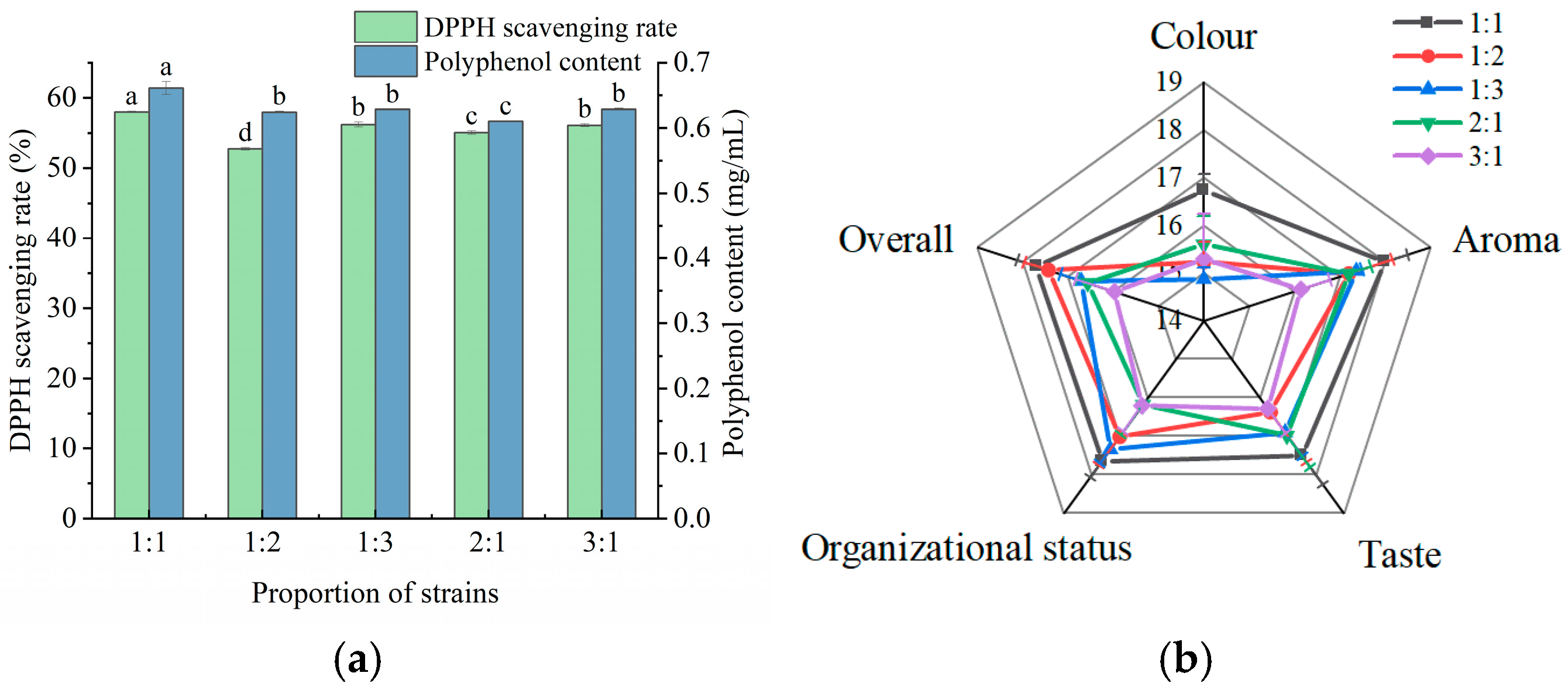
The fermentation flavor, texture, and functional activity of the composite strains were superior to those of the individual strains [34]; therefore, L. plantarum and L. casei were compounded in different ratios for apple juice fermentation. As shown in Figure 2a, the antioxidant capacity and polyphenol content of apple juice were highest when the ratio of the strains was 1:1. At this ratio, the apple juice achieved a DPPH scavenging rate of 58.02%, a polyphenol content of 0.661 mg/mL, and a sensory score of 87.57 (Figure 2b). Therefore, a compound ratio of L. plantarum and L. casei of 1:1 was selected for subsequent experiments.
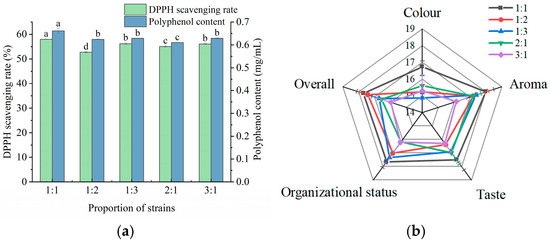
Figure 2.
Effect of strain ratio on polyphenol content, DPPH scavenging rate, and sensory scores of fermented apple juice. (a) Polyphenol content and DPPH scavenging rate; and (b) sensory scores. Lowercase letters indicate significant differences (p < 0.05).
3.2.2. Single Factor Experiments
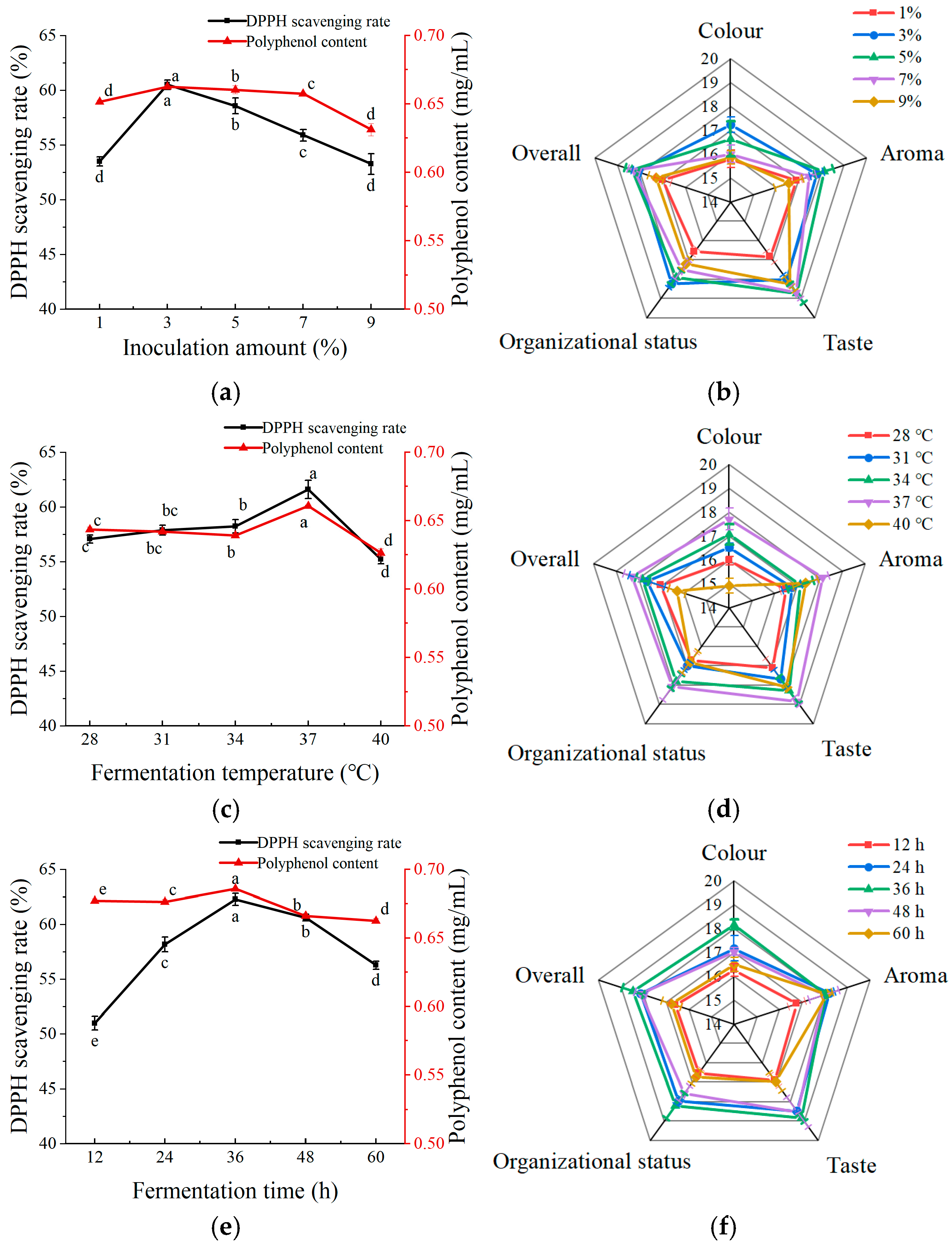
As shown in Figure 3a, the highest polyphenol content and DPPH scavenging rate occurred at an inoculation amount of 3%, within the analyzed range of 1%–9%. The color, aroma, taste, tissue morphology, and overall acceptability of the fermented apple juice were optimal at the levels of 3% and 5% inoculum (Figure 3b). When the inoculum amount was too large or too small, the fermented apple juice had poor sensory scores. Taken together, the inoculation amount of the composite strain was set to 3%.
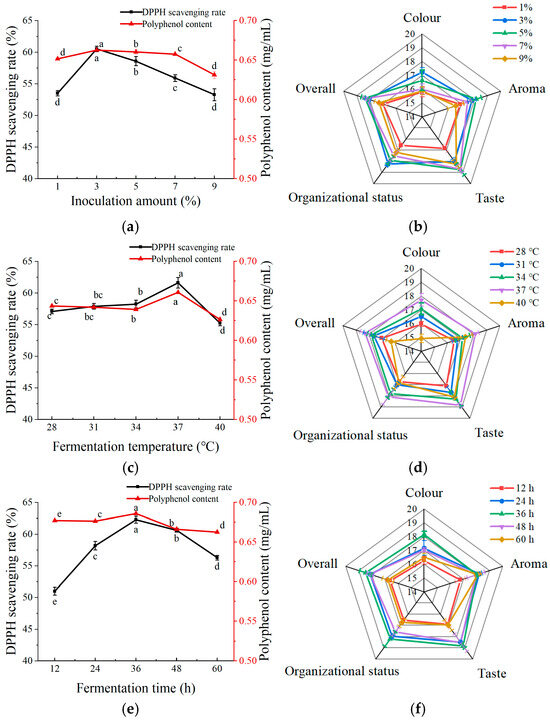
Figure 3.
Effect of different fermentation conditions on apple juice. (a) Effect of inoculum amount on polyphenol content and DPPH scavenging rate of fermented apple juice; (b) effect of inoculum amount on sensory scores of fermented apple juice; (c) effect of fermentation temperature on polyphenol content and DPPH scavenging rate of fermented apple juice; (d) effect of fermentation temperature on sensory scores of fermented apple juice; (e) effect of fermentation time on polyphenol content and DPPH scavenging rate of fermented apple juice; and (f) effect of fermentation time on sensory scores of fermented apple juice. Lowercase letters indicate significant differences (p < 0.05).
In the fermentation temperature range of 28–41 °C, the polyphenol content and DPPH scavenging rate reached their highest at a fermentation temperature of 37 °C (Figure 3c). This may be because the fermentation temperature affects the growth of LAB. As shown in Figure 3d, the color, taste, aroma, and overall acceptability of the fermented apple juice were the highest when the fermentation temperature was 37 °C. Ran et al. [19], using the LAB fermentation of honeysuckle, similarly found that 37 °C was the optimal temperature and the fermented beverage had the highest functional activity and sensory scores. Thus, 37 °C was chosen as the apple juice fermentation temperature for subsequent experiments.
As shown in Figure 3e, the polyphenol content and DPPH scavenging rate increased and then decreased with fermentation time, reaching a maximum after 36 h of apple juice fermentation. This trend may be attributed to the action of LAB, which initially increased the polyphenol content of apple juice and enhanced its antioxidant capacity. However, as fermentation progressed, the rate of polyphenol synthesis became lower than the rate of consumption, resulting in a decline in the polyphenol content and DPPH scavenging activity in the later stages of fermentation. As shown in Figure 3f, the sensory scores of apple juice reached a maximum at 36 h, which could be attributed to the fermentation by LAB that improved the sensory properties of apple juice. However, an extended fermentation duration may result in the accumulation of acids, leading to a decline in flavor intensity. Therefore, 36 h was chosen as the optimal time for apple juice fermentation for subsequent experiments.
3.2.3. Results of Box–Behnken Design (BBD)
According to the Box–Behnken design principle, inoculum amount (A), fermentation temperature (B), and fermentation time (C) were selected as the independent variables. The DPPH scavenging ability and sensory scores of the fermented apple juice were used as response values for the experiments. The fermentation parameters were optimized using a three-factor three-level test based on single-factor experiments. The Box–Behnken design matrix and corresponding results are listed in Table 2.

Table 2.
Box–Behnken design and results.
The analysis of variance (ANOVA) of the Box–Behnken design was carried out by Design-Expert 13.0 software (Stat-Ease Inc., Minneapolis, MN, USA). After regression fitting, the quadratic regression model of the response value on each factor was as follows:
where Y1 was the DPPH scavenging rate (%); Y2 was the sensory score (points); and A, B, and C were the inoculum amount (%), fermentation temperature (°C), and fermentation time (h), respectively.
Y1 = 63.36 − 0.093A − 0.38B − 0.65C + 1.25AB − 2.15AC + 1.55BC − 2.83A2 − 1.19B2 −1.58C2
Y2 = 85.20 + 0.23A + 1.00B + 1.90C + 1.30AB + 0.90AC − 0.80BC − 4.83A2 − 2.13B2 − 3.87C2
As shown in Table 3 and Table 4, both models were significant (p < 0.0001), and the misfit terms were not significant (p > 0.05), indicating that the equations fit the actual data well and explained the variation in responses well. The R2 of the two models were 0.9825 and 0.9838, indicating that the models were well correlated and statistically significant, and could be used to predict and analyze the apple juice fermentation process. Significance tests of the two models showed that B and C in the primary term and AB, AC, BC, A2, B2, and C2 in the secondary term had a significant effect on Y1 (p < 0.05); A and B in the primary term and AB, A2, B2, and C2 in the secondary term had a significant effect on Y2 (p < 0.05).

Table 3.
Analysis of variance based on DPPH radical scavenging rate.

Table 4.
Analysis of variance based on sensory scores.
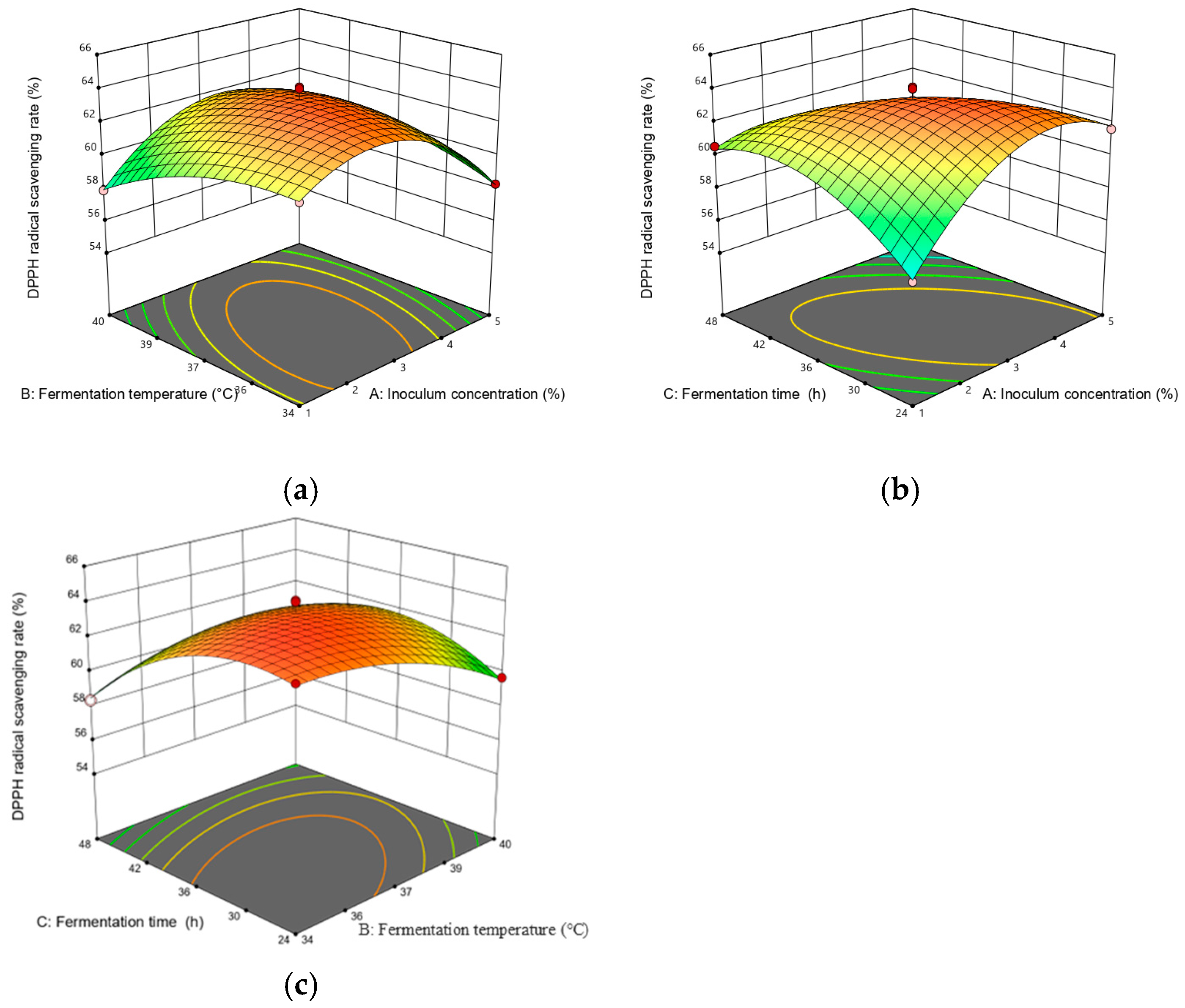
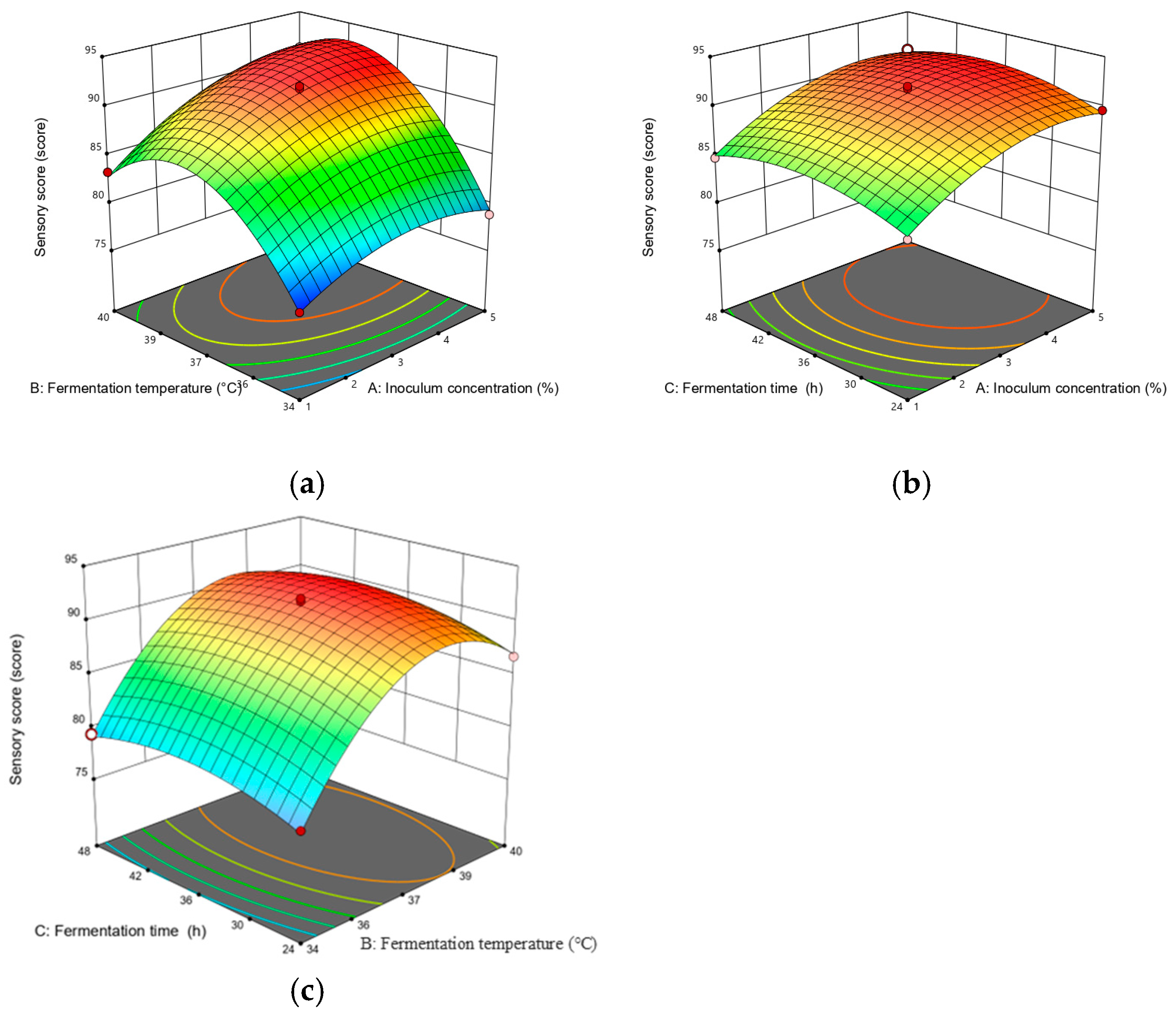
A steeper slope in the response surface plot represented a more significant change in the response value, while a flat slope indicated a less significant effect [22]. Figure 4 illustrates the response surface plots reflecting the interactions between any two variables on DPPH scavenging activity. As shown in Figure 4, the interaction term (AC) had the most significant effect on DPPH scavenging activity, followed by (AB) and (BC). Figure 5 depicts the response surface plots reflecting the interactions between any two variables on the sensory score. As shown in Figure 5, the interaction term (AB) had the most significant effect on the sensory score, while the other interaction terms (AC and BC) were not significant, which is consistent with the ANOVA results presented in Table 3 and Table 4.
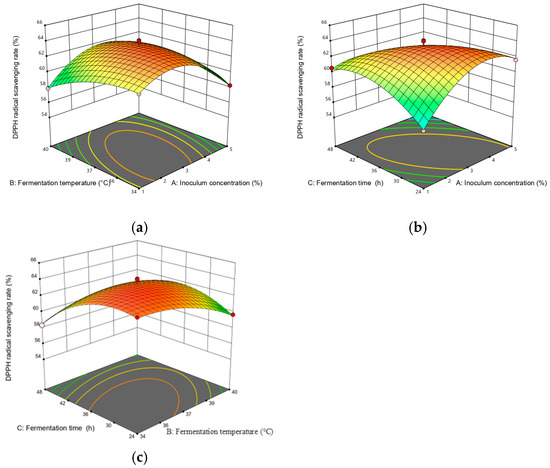
Figure 4.
Response surface plots for interaction between various factors on the DPPH scavenging rate. The two changed variables were (a) inoculum quantity and fermentation temperature; (b) inoculum quantity and fermentation time; and (c) fermentation temperature and fermentation time.
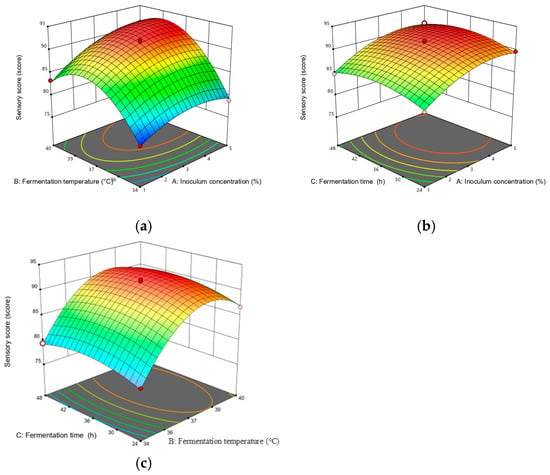
Figure 5.
Response surface plots for interaction between various factors on the sensory score. The two changed variables were (a) inoculum quantity and fermentation temperature; (b) inoculum quantity and fermentation time; and (c) fermentation temperature and fermentation time.
Based on the above results, the optimal process conditions for apple juice fermentation were determined as follows: an inoculum amount of 3.01%, a fermentation temperature of 37.21 °C, and a fermentation time of 36.91 h. Under these conditions, the predicted value of the DPPH scavenging activity was 63.35%, and the predicted value of the sensory score was 92.07. Considering the limitations of that actual operation, the process parameters were modified as follows: an inoculum amount of 3%, a fermentation temperature of 37 °C, and a fermentation time of 37 h. Validation under these adjusted conditions yielded a measured DPPH free radical scavenging rate of 62.31%, with a relative deviation of 1.6% from the predicted value, and a sensory score of 91.33, with a relative deviation of 0.8% from the predicted value. It can be seen that the optimal process conditions obtained by BBD are credible and can be used for the study of the apple juice fermentation process.
3.3. Volatile Composition Analysis (HS-SPME-GC-MS)
3.3.1. Categorization-Based Evaluation of Volatile Compounds
Volatile flavor compounds were detected using HS-SPME-GC-MS. The results are shown in Table S2. A total of fifty-five components were identified in the samples, including nine esters, fourteen alcohols, seven ketones, seven acids, eight olefins, five aldehydes, and five other compounds (Table 5). The number of volatile components in the fermented apple juice was higher than that in the unfermented apple juice. The content of the alcohol, ketone, and acid components increased, while the content of the aldehyde components decreased after fermentation. This may because aldehydes are unstable compounds that can be reduced to alcohols or oxidized to acids in the food matrix by micro-organisms [41]. Moreover, high concentrations of aldehydes can cause off-flavors and affect the overall flavor of apple juice [35]. After fermentation by LAB, the flavor substances in apple juice are significantly altered, especially the balance between esters, ketones, and alcohol compounds, which has an important effect on the flavor of fermented apple juice [26].

Table 5.
Proportion of volatile components in apple juice before and after fermentation.
Fermentation significantly reduced the kinds of and relative content of esters, probably because their volatilization or hydrolysis outweighed their formation [42]. Eight esters were detected in apple juice, of which that with the highest relative content was 2-Methylbutyl acetate (20.73% of total volatiles), presenting blackberry fruit and banana flavors. The lower the level of 2-Methylbutyl acetate, the better the overall apple aroma. The content of 2-Methylbutyl acetate was significantly reduced after apple juice fermentation, and Zhen’s [35] study indicated that as the content of the substance decreased, the overall aroma of the apple improved. Other esters such as ethyl acetate and ethyl caprylate were almost completely consumed during fermentation. Lai et al. [43] found that there was also a decrease in ethyl acetate and other esters during wine fermentation. However, the relative content of hexyl acetate in sweet and fruity aromas increased after apple juice fermentation.
Alcohols were the most abundant compounds in apple juice, with 14 being identified, second only to esters in terms of their relative content. Fermentation increased the relative alcohol content. The most common alcohol with the highest substance content in apple juice was 2-methylbutanol (7.50% of total volatiles), which imparts a sweet and tangy aroma [44]. Hexanol, a higher fatty alcohol, has a strong fruity flavor [45]. Isoamyl alcohol is an important alcohol component that produces a strong fruity odor in apple juice [31]. After fermentation, the relative content of these three alcohols increased, along with significant increases in the relative content of 1-nonanol and geraniol (increasing 4.58-fold and 13.84-fold). Fermentation also produced new alcohols (e.g., geraniol and DL-menthol), enriching a variety of volatile components.
Fermentation significantly increased the variety and content of ketones, and a range of new ketones (2-undecanone, 2-tridecanone, and β-damascenone) were produced after fermentation, adding a distinctive fruity and floral aroma to apple juice [46]. The relative aldehyde content decreased significantly after fermentation (reduced by 44.25%) because of their conversion into ketones and acids during fermentation [31]. Acids increased after fermentation, with nonanoic acid being the most abundant acid. Olefins showed very little change after fermentation, with tetradecamethyl cycloheptasiloxane having the highest content, which has an ester flavor [47]. Overall, fermentation created new compounds that positively affected the aroma profile, resulting in a greater diversity of volatile compounds in fermented apple juice.
3.3.2. Clustering and PCA of Volatile Compounds
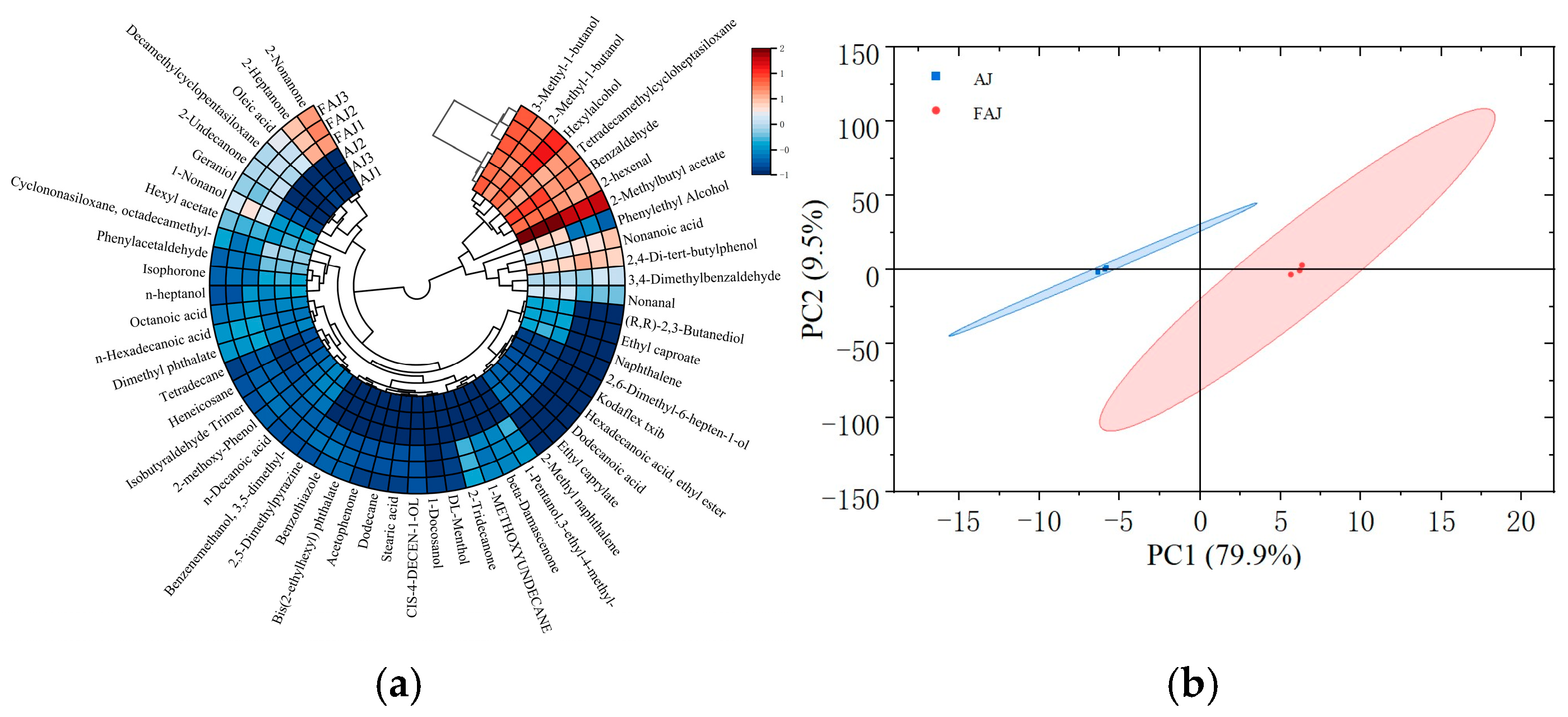
Clustering analysis provided a more intuitive way to analyze the distribution of volatile compounds in the samples based on Euclidean distances [48]. As shown in Figure 6a, the apple juice samples were clearly divided into two groups during cluster analysis. To further visualize the differences between the samples before and after fermentation, a PCA downscaling analysis was conducted. PC1 and PC2 explained 79.9% and 9.5% of the total variance in the samples, respectively, indicating that volatile component information in the samples was sufficiently extracted. Similarly to the cluster analysis results, the PCA categorized the samples into two groups: apple juice and fermented apple juice. As can be seen in Figure 6b, the samples from the two categories were relatively dispersed and belonged to different regions, indicating significant changes in the volatile components of the apple juice before and after fermentation. The specific mechanism by which the volatile components changed is not clear, which can be further studied.
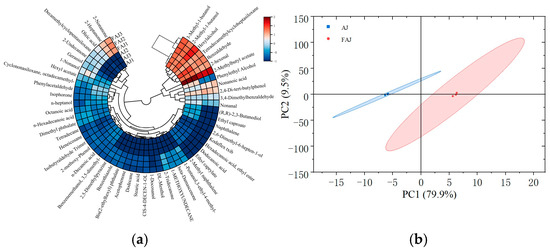
Figure 6.
Clustered heat map and PCA plot of volatile components of apple juice before and after fermentation. (a) Circle heatmap of standardized volatile compounds; and (b) principal component analysis (PCA) of volatile compounds.
3.4. Physicochemical Parameters
From Table 6, it can be seen that the total acid content of apple juice increased after fermentation due to the conversion of sugars to lactic acid during fermentation by LAB [49]. A high concentration of acid inhibits the growth of spoilage bacteria and helps extend the shelf life of apple juice [50]. The viable bacterial count of fermented apple juice reached 7.3 × 108 CFU/mL, which met the requirement of the ‘GB 7101-2022 National Standard for Food Safety Beverages’, which states that the viable bacterial count must be more than 106 CFU/mL [51]. The total phenolic content of apple juice increased significantly after fermentation and the flavonoid content decreased, which may be due to decomposition or conversion to other substances. Li et al. [20] also found this phenomenon when fermenting apple juice with L. plantarum. The sensory scores of the apple juice increased significantly after fermentation by about 33.69% over the raw apple juice. These results suggest that fermentation improves the quality of apple juice, endowing it with better physicochemical properties and sensory characteristics.

Table 6.
Effect of LAB fermentation on the physicochemical properties of apple juice.
3.5. Antioxidant Activity In Vitro
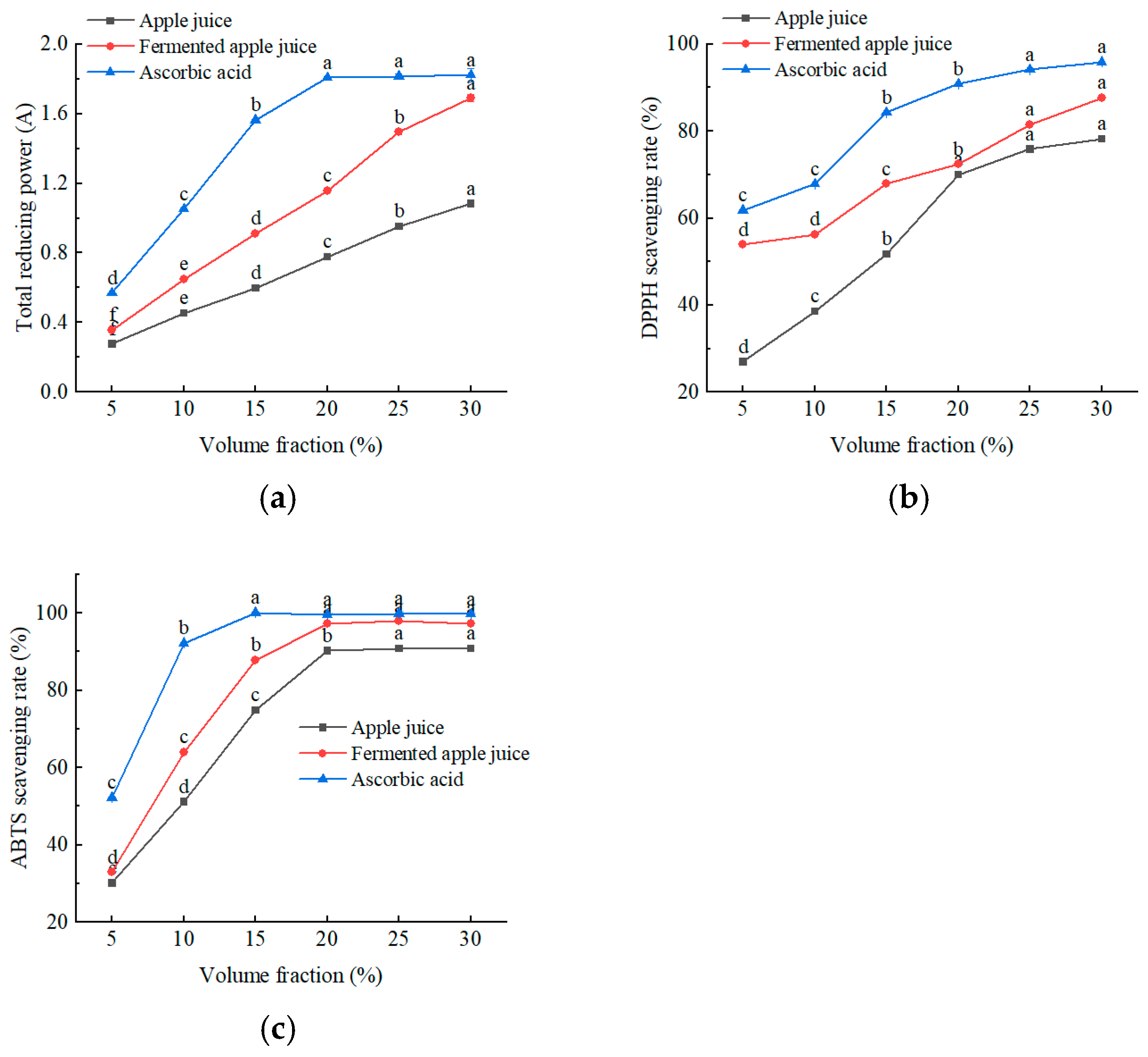
As shown in Figure 7, all samples exhibited total reducing and scavenging capacities for DPPH and ABTS radicals. As can be seen from Figure 7a, the total reducing capacity of the samples increased gradually with increasing concentration, with post-fermentation samples showing a significantly higher capacity than pre-fermentation samples. As shown in Figure 7b and 7c, the IC50 of fermented apple juice for DPPH and ABTS radical scavenging were 2.3% and 7.5%, respectively, which were lower than those of the raw apple juice. Notably, when the concentration of fermented apple juice exceeded 20%, the ABTS radical scavenging capacity was comparable to that of 0.2 mg/mL ascorbic acid. The specific substances that exert antioxidant activity need to be further researched. Overall, the antioxidant capacity of fermented apple juice was significantly higher than that of the unfermented apple juice. The co-fermentation of L. plantarum and L. acidophilus can improve the antioxidant capacity of a honeysuckle beverage, which is consistent with the results of this study [19].
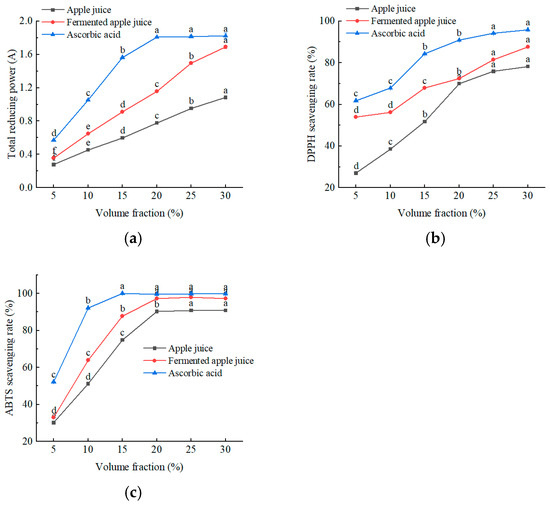
Figure 7.
The scavenging effect of apple juice, fermented apple juice, and ascorbic acid on free radicals and the determination of total reducing power: (a) total reducing power; (b) DPPH; and (c) ABTS. Lowercase letters indicate significant differences (p < 0.05).
4. Conclusions
In this study, L. plantarum and L. casei were mixed for apple juice fermentation. The optimal fermentation conditions were determined by single-factor and BBD experiments. Under the optimal conditions, the DPPH scavenging ability of the fermented apple juice was 62.31%, and the sensory score was 91.33. On this basis, the changes in volatile components, viable bacteria, total acid content, polyphenols, and antioxidant capacity before and after fermentation were studied. The comprehensive data showed that the fermented apple juice was soft in taste; moderate in sweetness and sourness, with both a fruity aroma and fermented flavor; and its antioxidant activity was improved. This research will provide process parameters and a theoretical basis for the industrial production of fermented apple beverages.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation11040161/s1, Table S1: Scoring standard of LAB fermented apple juice; Table S2: Volatile compound contents of apple juice before and after fermentation.
Author Contributions
Conceptualization, X.L., J.Z., W.G. and Z.W.; methodology, X.L., W.G. and Z.W.; software, X.L., L.W. and Y.C.; validation, Z.C., D.W. and N.C.; formal analysis, X.L., W.G. and Z.W.; investigation, J.Z., W.G. and Z.W.; resources, Z.W.; data curation, L.W., Y.C. and Z.W.; writing—original draft preparation, X.L.; writing—review and editing, Z.W., Q.J. and Z.Z.; visualization, X.L., L.W. and Y.C.; supervision, Z.W.; project administration, Z.W.; funding acquisition, Z.W. and W.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Key R&D Program of Shandong Province, China, grant number 2022TZXD008.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data are available within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DPPH | 2,2′-diphenyl-1-picrylhydrazyl |
| ABTS | 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid |
| HS-SPME-GC-MS | Headspace solid-phase micro-extraction and gas chromatography–mass spectrometry |
| PCA | Principal component analysis |
References
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Alves, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Antioxidant and antimicrobial properties of dried Portuguese apple variety (Malus domestica Borkh. cv Bravo de Esmolfe). Food Chem. 2017, 240, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Sun, Y.; Gao, T.; Wu, Y.; Sun, H.; Zhu, Q.; Liu, C.; Zhou, C.; Han, Y.; Tao, Y. Fermentation and Storage Characteristics of “Fuji” Apple Juice Using Lactobacillus acidophilus, Lactobacillus casei and Lactobacillus plantarum: Microbial Growth, Metabolism of Bioactives and in vitro Bioactivities. Front. Nutr. 2022, 9, 833906. [Google Scholar] [CrossRef]
- Dimitrovski, D.; Velickova, E.; Langerholc, T.; Winkelhausen, E. Apple juice as a medium for fermentation by the probiotic Lactobacillus plantarum PCS 26 strain. Ann. Microbiol. 2015, 65, 2161–2170. [Google Scholar] [CrossRef]
- Deng, D.; Li, W. Current situation of apple production and processing in China and development counter-measures. Modern Food 2020, 21, 12–14. [Google Scholar] [CrossRef]
- Siciliano, R.A.; Reale, A.; Mazzeo, M.F.; Morandi, S.; Silvetti, T.; Brasca, M. Paraprobiotics: A New Perspective for Functional Foods and Nutraceuticals. Nutrients 2021, 13, 1225. [Google Scholar] [CrossRef] [PubMed]
- Khushboo; Karnwal, A.; Malik, T. Characterization and selection of probiotic lactic acid bacteria from different dietary sources for development of functional foods. Front. Microbiol. 2023, 14, 1170725. [Google Scholar] [CrossRef]
- Bancalari, E.; Castellone, V.; Bottari, B.; Gatti, M. Wild Lactobacillus casei Group Strains: Potentiality to Ferment Plant Derived Juices. Foods 2020, 9, 314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, X.; Wang, X.; Han, M.; Li, H.; Yue, T.; Wang, Z.; Gao, Z. Effects of fermentation with Lactobacillus fermentum 21828 on the nutritional characteristics and antioxidant activity of Lentinus edodes liquid. J. Sci. Food Agric. 2021, 102, 3405–3415. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, W.; Liu, X.; Shen, W.; Gu, R.; Tang, C. The Antioxidant Activity and Protection of Probiotic Bacteria in the In Vitro Gastrointestinal Digestion of a Blueberry Juice and Whey Protein Fermentation System. Fermentation 2023, 9, 335. [Google Scholar] [CrossRef]
- Mazlan, F.A.; Annuar, M.S.M.; Sharifuddin, Y. Biotransformation ofMomordica charantiafresh juice by Lactobacillus plantarumBET003 and its putative anti-diabetic potential. PeerJ 2015, 3, e1376. [Google Scholar] [CrossRef]
- Li, C.; Ding, Q.; Nie, S.-P.; Zhang, Y.-S.; Xiong, T.; Xie, M.-Y. Carrot Juice Fermented with Lactobacillus plantarum NCU116 Ameliorates Type 2 Diabetes in Rats. J. Agric. Food Chem. 2014, 62, 11884–11891. [Google Scholar] [CrossRef]
- Suriyaprom, S.; Mosoni, P.; Leroy, S.; Kaewkod, T.; Desvaux, M.; Tragoolpua, Y. Antioxidants of Fruit Extracts as Antimicrobial Agents against Pathogenic Bacteria. Antioxidants 2022, 11, 602. [Google Scholar] [CrossRef] [PubMed]
- de Souza Neves Ellendersen, L.; Granato, D.; Bigetti Guergoletto, K.; Wosiacki, G. Development and sensory profile of a probiotic beverage from apple fermented with Lactobacillus casei. Eng. Life Sci. 2012, 12, 475–485. [Google Scholar] [CrossRef]
- Wang, N.; Jiang, S.; Zhang, Z.; Fang, H.; Xu, H.; Wang, Y.; Chen, X. Malus sieversii: The origin, flavonoid synthesis mechanism, and breeding of red-skinned and red-fleshed apples. Hortic. Res. 2018, 5, 70. [Google Scholar] [CrossRef]
- Zhao, L.; Maimaitiyiming, R.; Hong, J.; Wang, L.; Mu, Y.; Liu, B.; Zhang, H.; Chen, K.; Aihaiti, A. Optimization of tomato (Solanum lycopersicum L.) juice fermentation process and analysis of its metabolites during fermentation. Front. Nutr. 2024, 11, 1344117. [Google Scholar] [CrossRef]
- Park, S.; Son, H.-K.; Chang, H.-C.; Lee, J.-J. Effects of Cabbage-Apple Juice Fermented by Lactobacillus plantarum EM on Lipid Profile Improvement and Obesity Amelioration in Rats. Nutrients 2020, 12, 1135. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, H.; Duan, C.; Yan, G. Effects of mixed starters of plant- and wine-derived L. plantarum on hawthorn juice fermentation: Physicochemical properties, phenolic and volatile profiles. Food Biosci. 2023, 56, 103363. [Google Scholar] [CrossRef]
- Bujna, E.; Farkas, N.A.; Tran, A.M.; Dam, M.S.; Nguyen, Q.D. Lactic acid fermentation of apricot juice by mono- and mixed cultures of probiotic Lactobacillus and Bifidobacterium strains. Food Sci. Biotechnol. 2018, 27, 547–554. [Google Scholar] [CrossRef]
- Ran, J.; Tang, Y.; Mao, W.; Meng, X.; Jiao, L.; Li, Y.; Zhao, R.; Zhou, H. Optimization of the fermentation process and antioxidant activity of mixed lactic acid bacteria for honeysuckle beverage. Front. Microbiol. 2024, 15, 1364448. [Google Scholar] [CrossRef]
- Li, Z.; Teng, J.; Lyu, Y.; Hu, X.; Zhao, Y.; Wang, M. Enhanced Antioxidant Activity for Apple Juice Fermented with Lactobacillus plantarum ATCC14917. Molecules 2018, 24, 51. [Google Scholar] [CrossRef]
- GB 12456.35-2021; Food Safety Food Total Acid Determination. Standards Press of China: Beijing, China, 2021.
- You, L.; Shuangjie, F.; Fuxia, H.; Fengqin, W.; Changxin, Z.; Zhaosheng, W. Optimization of extraction of polyphenols from chestnut shell by response surface methodology. IOP Conf. Ser. Earth Environ. Sci. 2021, 791, 012206. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, H.; Wu, A.; Ju, C.; Jiang, J.; Chen, J. Multiple-strain Lactobacillus-fermented soymilk with antioxidant capacity and delicate flavour. Int. J. Food Sci. Technol. 2021, 56, 6052–6061. [Google Scholar] [CrossRef]
- Abdel-Aty, A.M.; Salama, W.H.; Fahmy, A.S.; Mohamed, S.A. Impact of germination on antioxidant capacity of garden cress: New calculation for determination of total antioxidant activity. Sci. Hortic. 2019, 246, 155–160. [Google Scholar] [CrossRef]
- Raju, S.; Deka, S.C. Influence of thermosonication treatments on bioactive compounds and sensory quality of fruit (Haematocarpus validus) juice. J. Food Process. Preserv. 2018, 42, e13701. [Google Scholar] [CrossRef]
- Ren, T.; Yue, T.; Wei, X.; Wang, X.; Yuan, Y. Optimization of probiotic fermentation process for apple pulp and analysis of volatile flavor components before and after fermentation. Food Sci. 2019, 40, 87–93. [Google Scholar]
- Cong, S.; Zhang, X.; Zhao, H.; Sun, M.; Hu, N. Process Optimization and Analysis of Product Quality of Blueberry and Corn Peptide Fermented by Mixed Lactic Acid Bacteria. Fermentation 2024, 10, 454. [Google Scholar] [CrossRef]
- Lan, T.; Lv, X.; Zhao, Q.; Lei, Y.; Gao, C.; Yuan, Q.; Sun, X.; Liu, X.; Ma, T. Optimization of strains for fermentation of kiwifruit juice and effects of mono- and mixed culture fermentation on its sensory and aroma profiles. Food Chem. X 2023, 17, 100595. [Google Scholar] [CrossRef]
- Ma, X.-L.; Wang, X.-C.; Zhang, J.-N.; Liu, J.-N.; Ma, M.-H.; Ma, F.-L.; Lv, Y.; Yu, Y.-J.; She, Y. A study of flavor variations during the flaxseed roasting procedure by developed real-time SPME GC–MS coupled with chemometrics. Food Chem. 2023, 410, 135453. [Google Scholar] [CrossRef]
- Sun, W.; Chen, X.; Bi, P.; Han, J.; Li, S.; Liu, X.; Zhang, Z.; Long, F.; Guo, J. Screening and characterization of indigenous non-Saccharomyces cerevisiae with high enzyme activity for kiwifruit wine production. Food Chem. 2023, 440, 138309. [Google Scholar] [CrossRef]
- Yang, S.; Hou, M.; Tan, W.; Chen, Y.; Li, H.; Song, J.; Wang, X.; Ren, J.; Gao, Z. Lactic acid bacteria sequential fermentation improves viable counts and quality of fermented apple juice via generating two logarithmic phases. Food Chem. 2024, 464, 141635. [Google Scholar] [CrossRef]
- Li, X.; Feng, J.; Lv, J.; Liu, Q.; Liu, X.; Liu, Y.; Xie, S.; Nan, F. Optimization of the preparation process of Spirulina blended liquor and Spirulina fermented wine, analysis of volatile components and in vitro antioxidant study. J. Food Sci. 2024, 89, 7228–7243. [Google Scholar] [CrossRef]
- Garzón, G.A.; Soto, C.Y.; López-R, M.; Riedl, K.M.; Browmiller, C.R.; Howard, L. Phenolic profile, in vitro antimicrobial activity and antioxidant capacity of Vaccinium meridionale swartz pomace. Heliyon 2020, 6, e03845. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sheng, J.; Li, J.; Zhang, P.; Tang, F.; Shan, C. Influence of lactic acid bacteria on physicochemical indexes, sensory and flavor characteristics of fermented sea buckthorn juice. Food Biosci. 2021, 46, 101519. [Google Scholar] [CrossRef]
- Zheng, R. Optimization of Probiotic Fermentation Process of Apple Juice and Changes of Flavor Substances and Functional Components During Fermentation. Bachelor’s Thesis, Shenyang Agricultural University, Shenyang, China, 2020. [Google Scholar]
- Saarisalo, E.; Skyttä, E.; Haikara, A.; Jalava, T.; Jaakkola, S. Screening and selection of lactic acid bacteria strains suitable for ensiling grass. J. Appl. Microbiol. 2007, 102, 327–336. [Google Scholar] [CrossRef]
- Perera, H.D.S.M.; Samarasekera, J.K.R.R.; Handunnetti, S.M.; Weerasena, O.V.D.S.J. In vitro anti-inflammatory and anti-oxidant activities of Sri Lankan medicinal plants. Ind. Crops Prod. 2016, 94, 610–620. [Google Scholar] [CrossRef]
- Chaudhary, N.; Roy, Z.; Bansal, R.; Siddiqui, L. Understanding the Role of Free Radicals and Antioxidant Enzymes in Human Diseases. Curr. Pharm. Biotechnol. 2023, 24, 1265–1276. [Google Scholar] [CrossRef]
- Jürkenbeck, K.; Spiller, A. Importance of sensory quality signals in consumers’ food choice. Food Qual. Prefer. 2021, 90, 104155. [Google Scholar] [CrossRef]
- Chen, C.; Lu, Y.; Yu, H.; Chen, Z.; Tian, H. Influence of 4 lactic acid bacteria on the flavor profile of fermented apple juice. Food Biosci. 2018, 27, 30–36. [Google Scholar] [CrossRef]
- Chen, H.; Xiao, G.; Xu, Y.; Yu, Y.; Wu, J.; Zou, B. High Hydrostatic Pressure and Co-Fermentation by Lactobacillus rhamnosus and Gluconacetobacter xylinus Improve Flavor of Yacon-Litchi-Longan Juice. Foods 2019, 8, 308. [Google Scholar] [CrossRef]
- Zhang, W.; Lao, F.; Bi, S.; Pan, X.; Pang, X.; Hu, X.; Liao, X.; Wu, J. Insights into the major aroma-active compounds in clear red raspberry juice (Rubus idaeus L. cv. Heritage) by molecular sensory science approaches. Food Chem. 2021, 336, 127721. [Google Scholar] [CrossRef]
- Lai, Y.T.; Hou, C.Y.; Lin, S.P.; Lo, Y.C.; Chen, C.H.; Hsieh, C.W.; Lin, H.W.; Cheng, K.C. Sequential culture with aroma-producing yeast strains to improve the quality of Kyoho wine. J. Food Sci. 2023, 88, 1114–1127. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Shi, J.; Ren, X.; Tao, Y.; Ma, F.; Li, R.; Liu, X.; Liu, C. Insights into the aroma profiles and characteristic aroma of ‘Honeycrisp’ apple (Malus × domestica). Food Chem. 2020, 327, 127074. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Liu, G.; Li, B.; Tan, H.; Zheng, R.; Sun, X.; He, F. Influence on the aroma substances and functional ingredients of apple juice by lactic acid bacteria fermentation. Food Biosci. 2022, 51, 102337. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, Y.; Zhou, S.; Li, X.; Tao, Y.; Pan, Y.; Feng, X.; Guo, H.; Chen, P.; Chu, Q. A newly-discovered tea population variety processed Bai Mu Dan white tea: Flavor characteristics and chemical basis. Food Chem. 2024, 446, 138851. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, C.; Su, W.; Tan, R.; Ma, L.; Pan, W.; Li, W. Dynamic effects of ultrasonic treatment on flavor and metabolic pathway of pumpkin juice during storage based on GC–MS and GC-IMS. Food Chem. 2024, 469, 142599. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.R.; Deng, H.; Hu, C.Y.; Zhao, P.T.; Meng, Y.H. Vitality, fermentation, aroma profile, and digestive tolerance of the newly selected Lactiplantibacillus plantarum and Lacticaseibacillus paracasei in fermented apple juice. Front. Nutr. 2022, 9, 1045347. [Google Scholar] [CrossRef]
- Ma, H.; Wang, L.; Yu, H.; Wang, W.; Wu, G.; Qin, G.; Tan, Z.; Wang, Y.; Pang, H. Protease-producing lactic acid bacteria with antibacterial properties and their potential use in soybean meal fermentation. Chem. Biol. Technol. Agric. 2022, 9, 40. [Google Scholar] [CrossRef]
- Fan, Y.; Yang, X.; Hu, C.; Wei, B.; Xu, F.; Guo, Q. Fermentation Performance Evaluation of Lactic Acid Bacteria Strains for Sichuan Radish Paocai Production. Foods 2024, 13, 1813. [Google Scholar] [CrossRef]
- GB 7101-2022; Food Safety Beverages. Standards Press of China: Beijing, China, 2022.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).