The Effects of Pre-Fermentative Treatments on the Aroma of Krstač and Žižak Wines
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Winemaking
2.3. Liquid–Liquid Extraction
2.4. GC/FID-MS Analysis
2.5. Statistical Analysis
2.6. Sensory Analysis
3. Results and Discussion
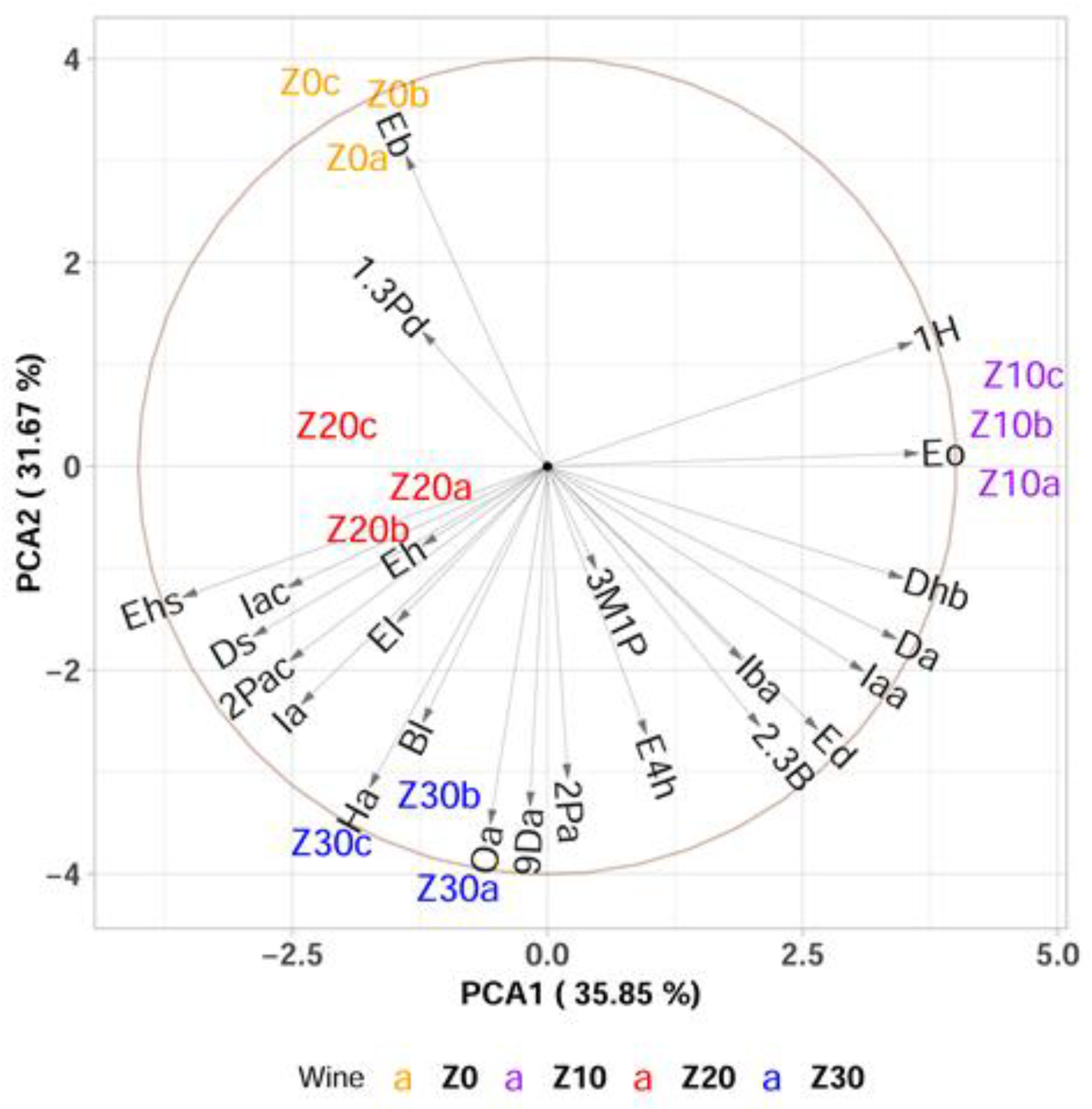
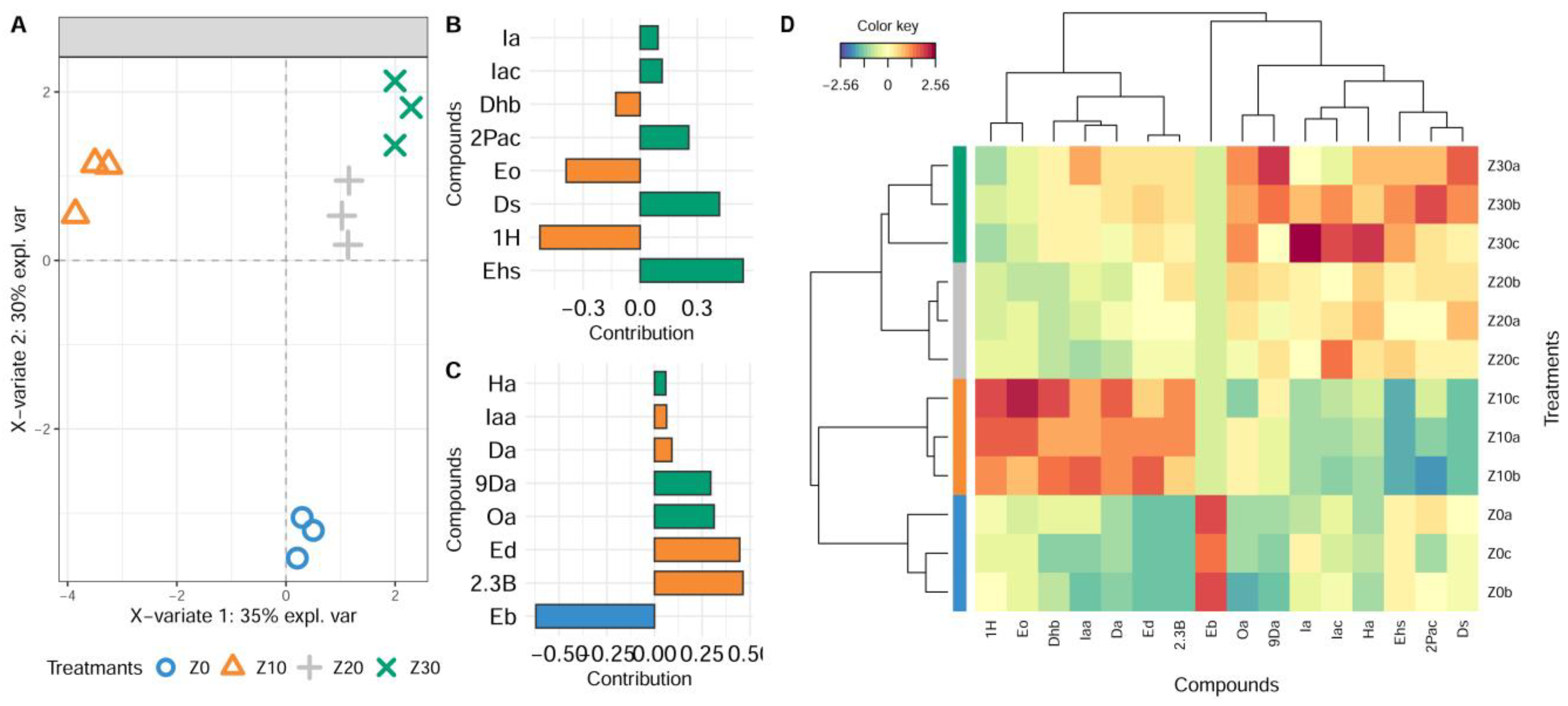
3.1. Effect of Pre-Fermentative Static Settling on the Aroma of Wine Produced from Krstač and Žižak Variety
PCA and Multivariate sPLS-DA
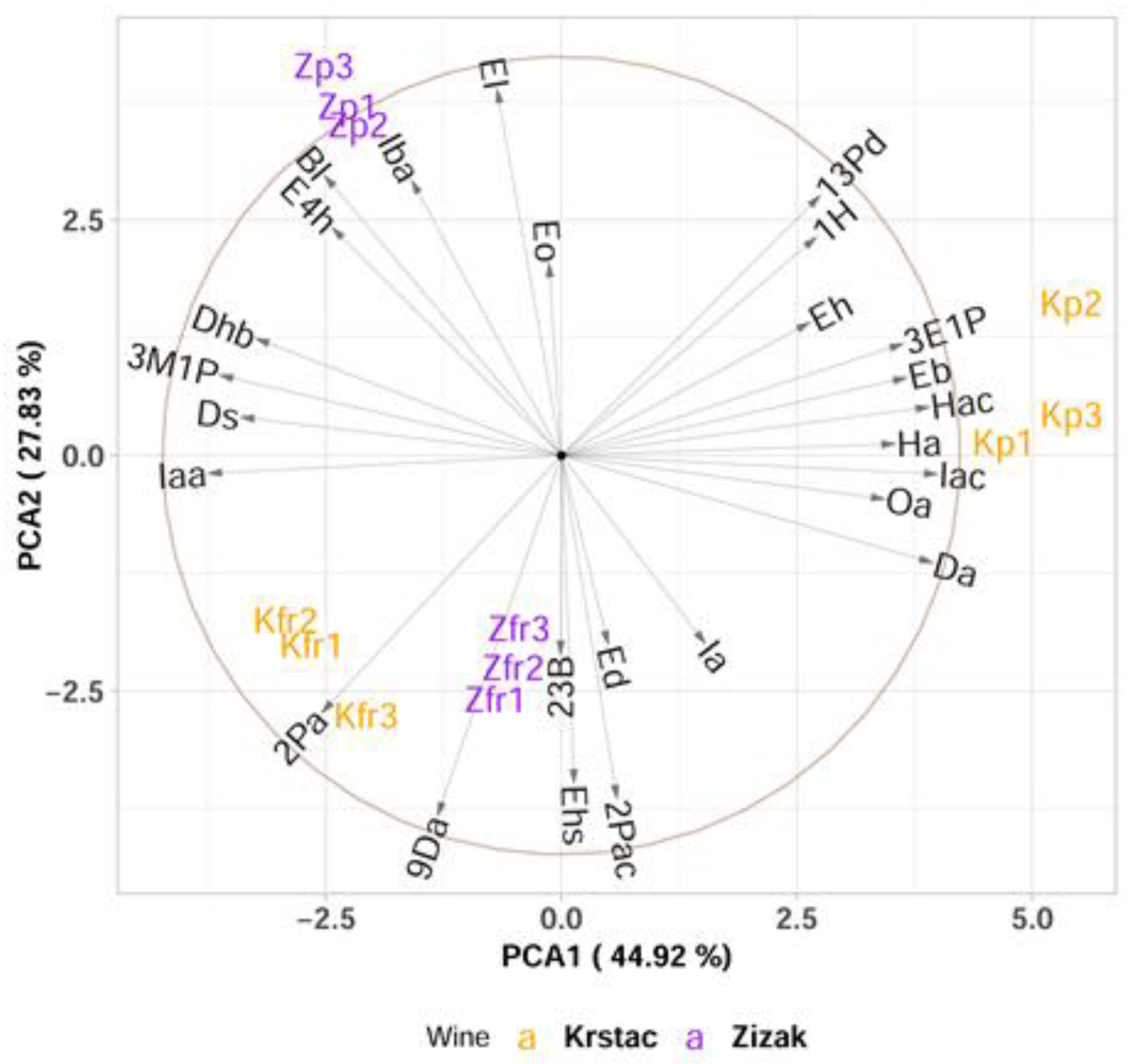
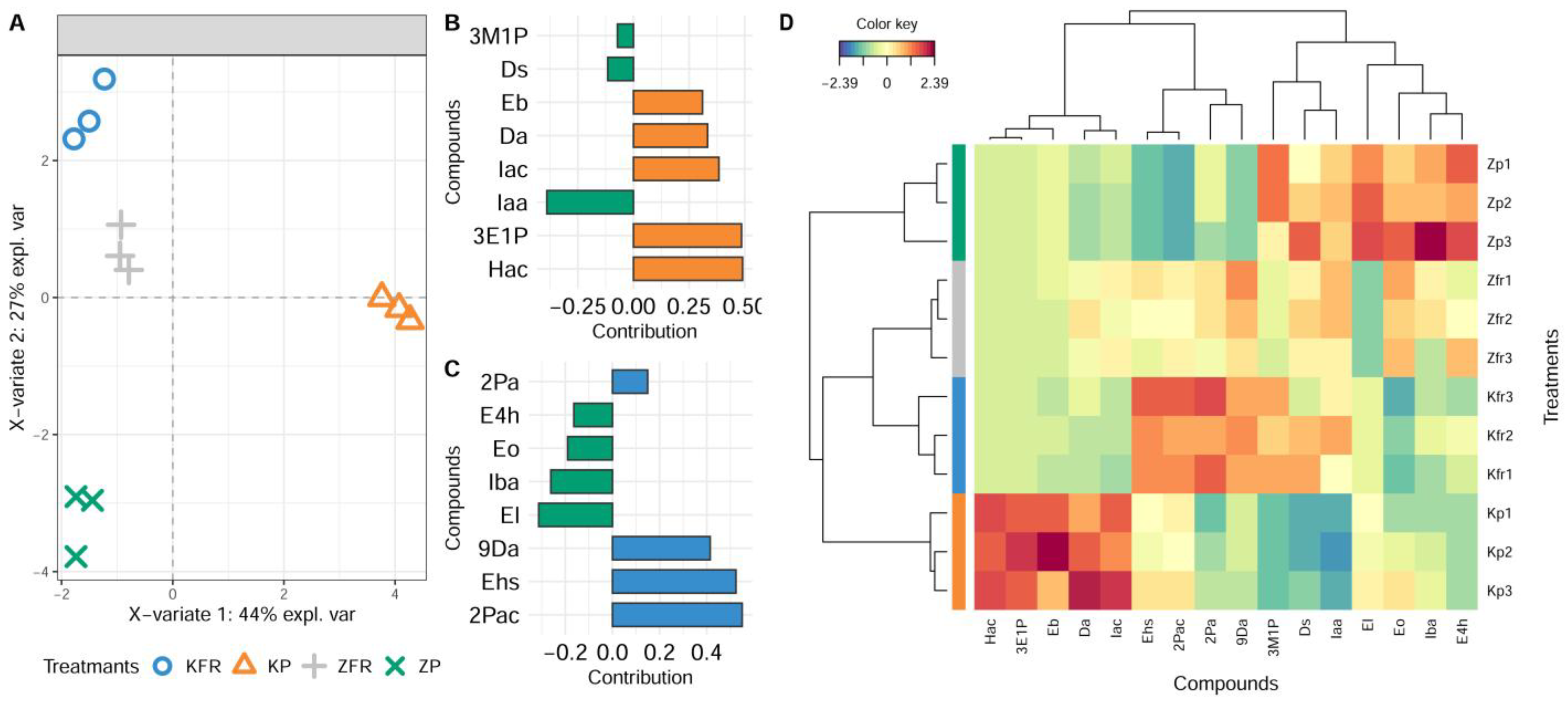
3.2. Effect of Pressing Pressure on the Aroma of Wine Produced from Krstač and Žižak Varieties
PCA and Multivariate sPLS-DA
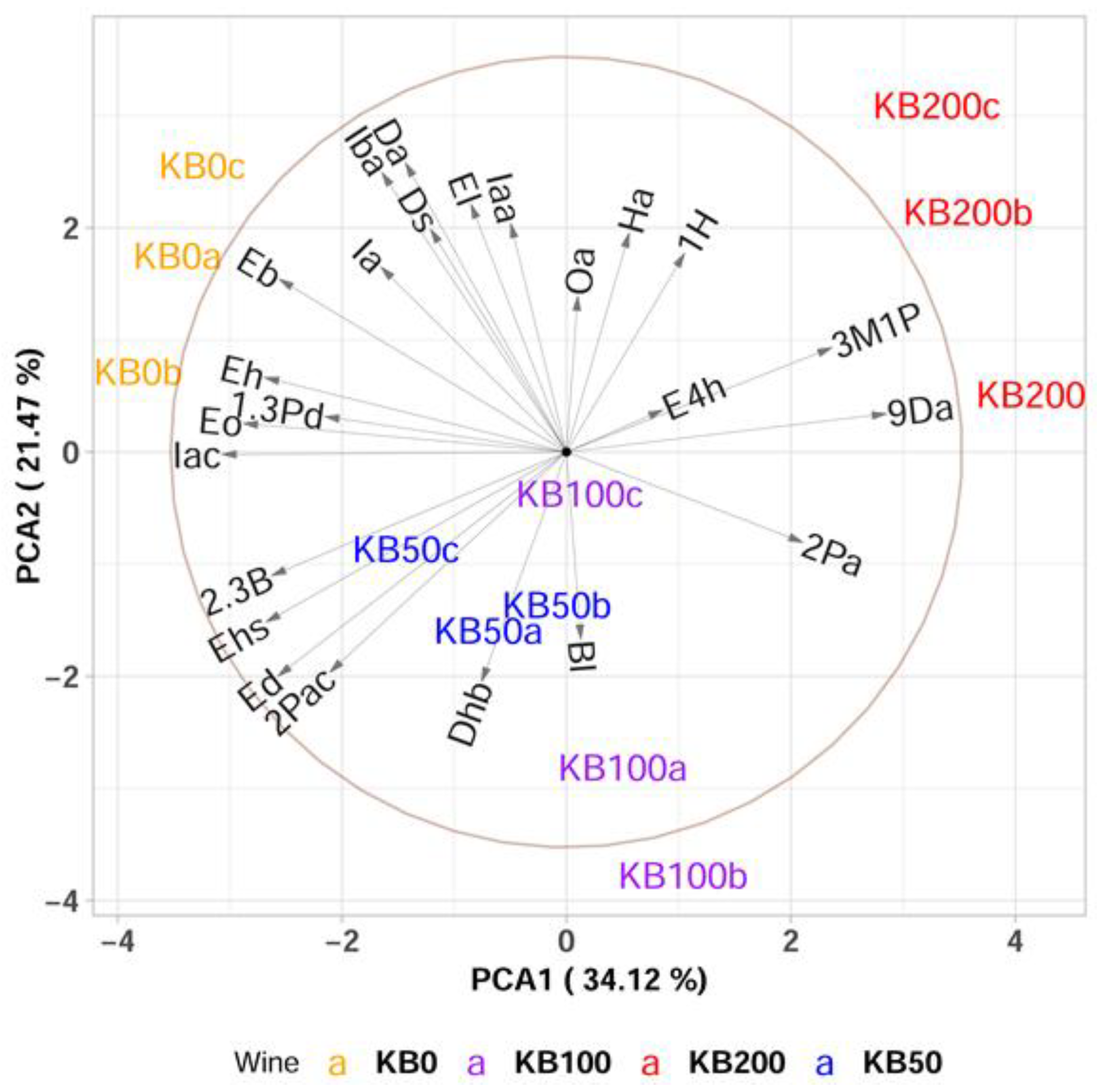
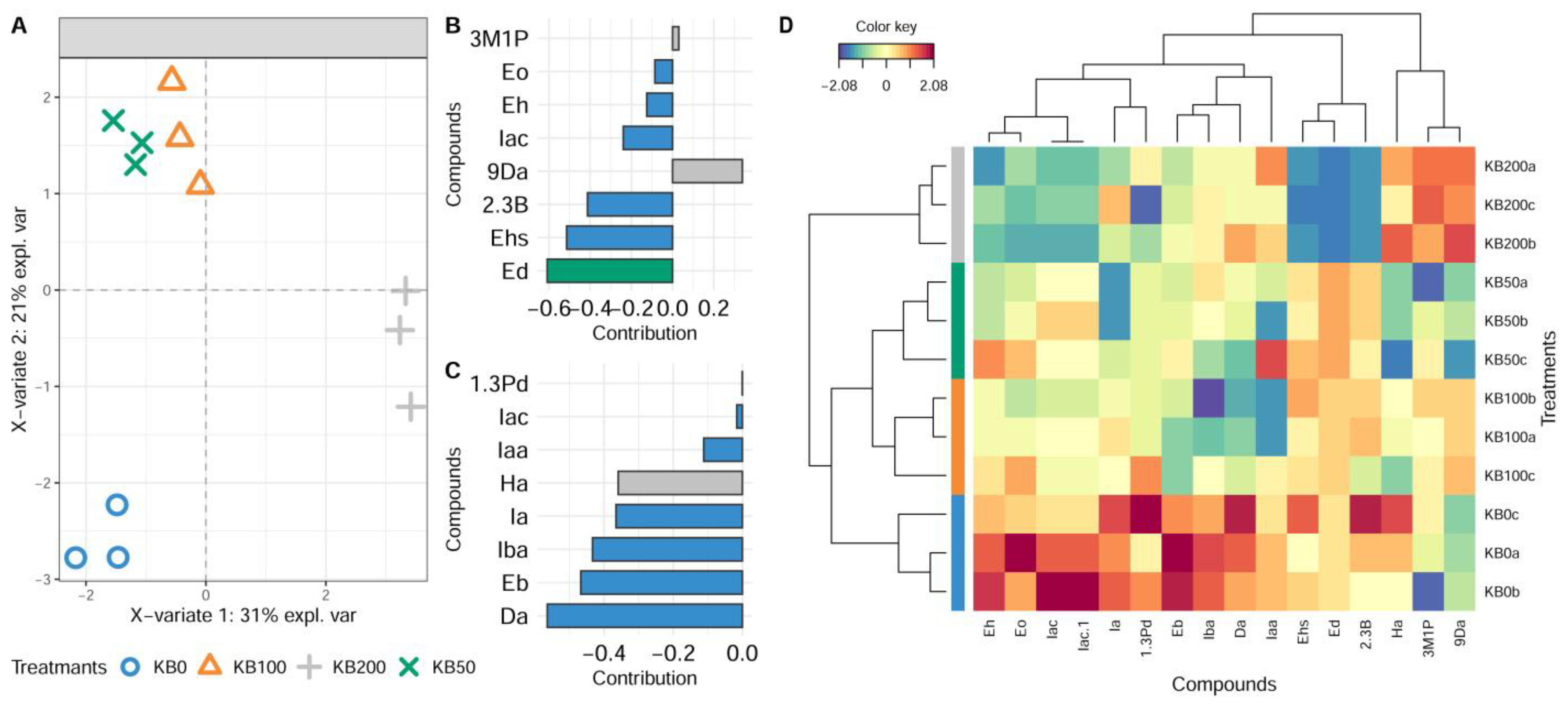
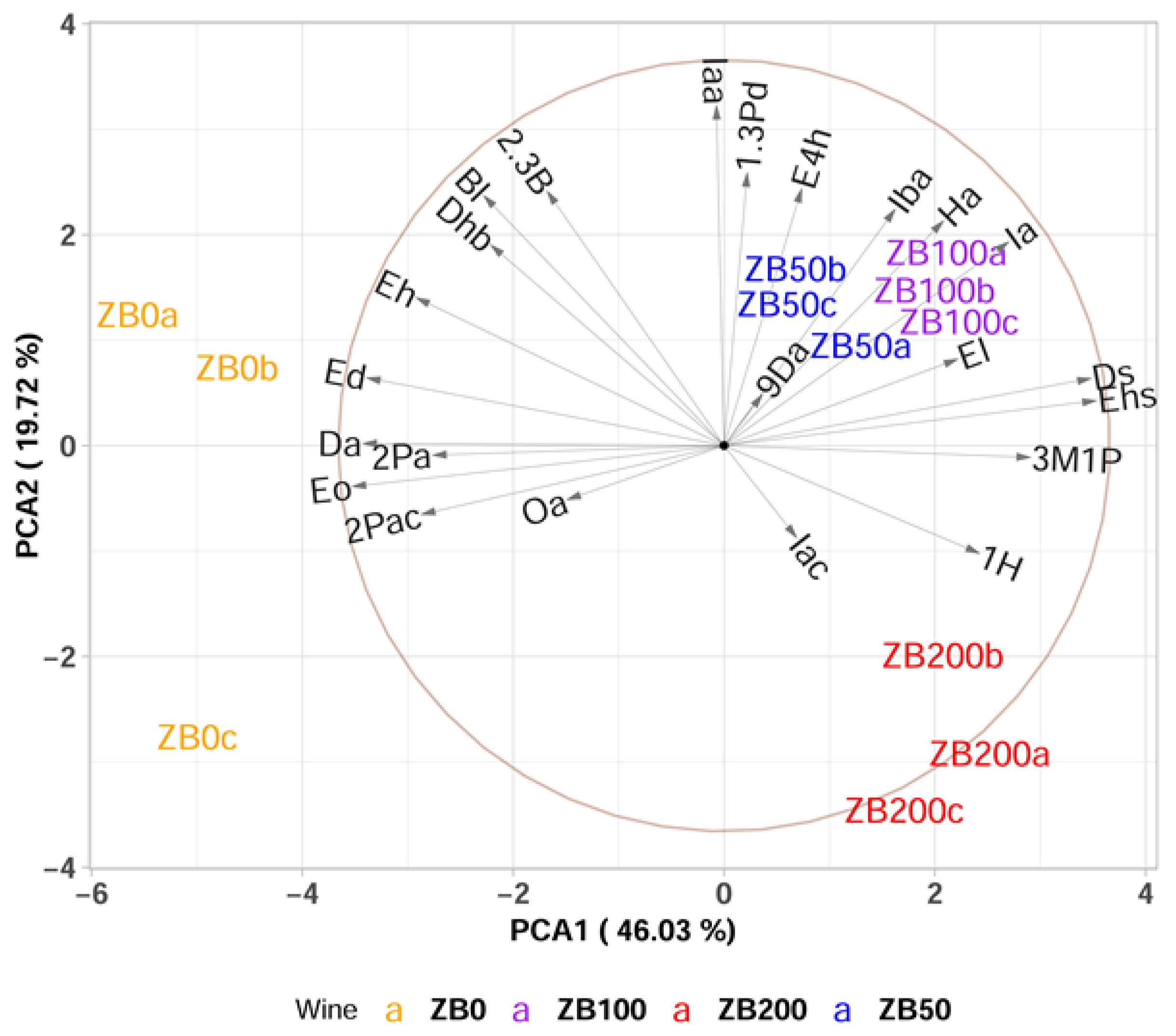
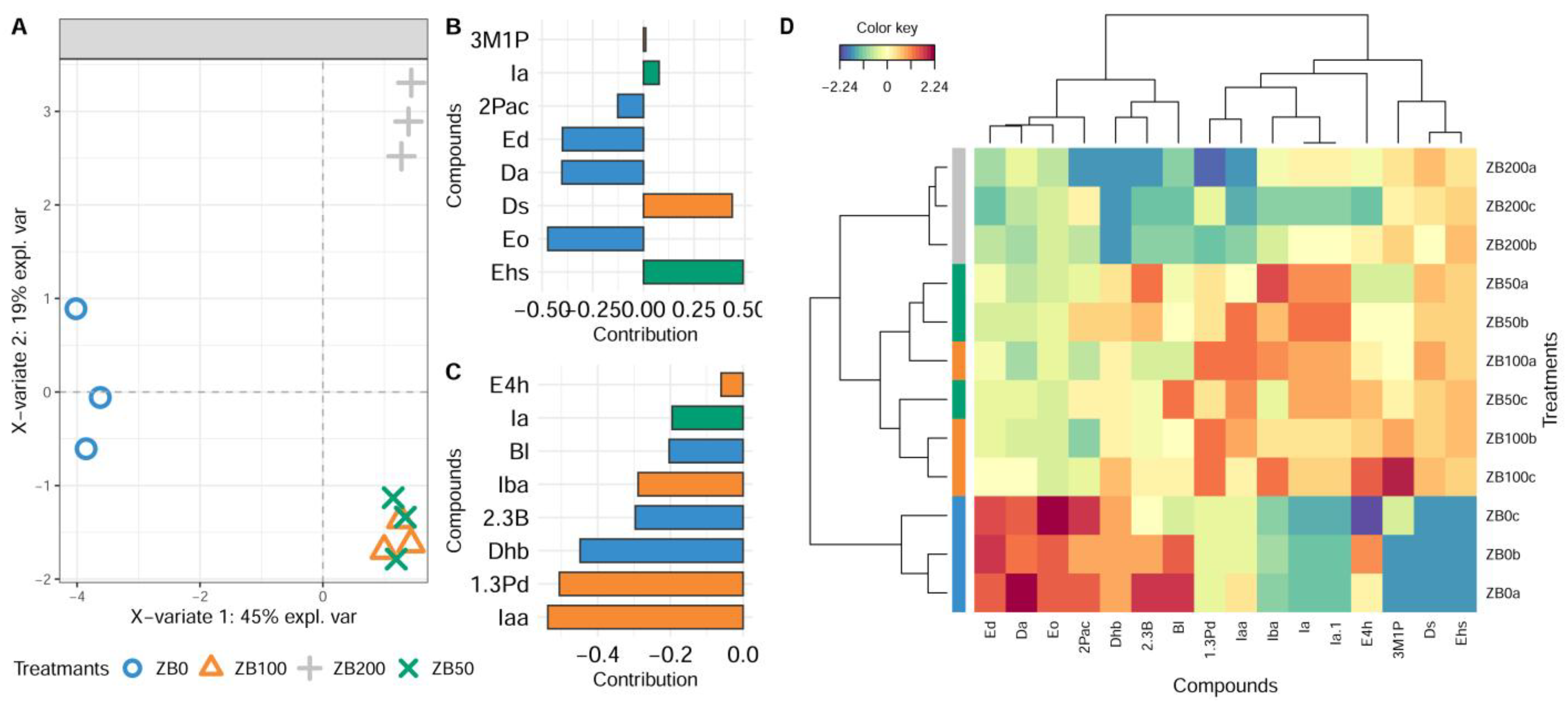
3.3. Effect of Bentonite Addition to the Must on the Aroma of Wines Produced from Krstač and Žižak Varieties
PCA and Multivariate sPLS-DA
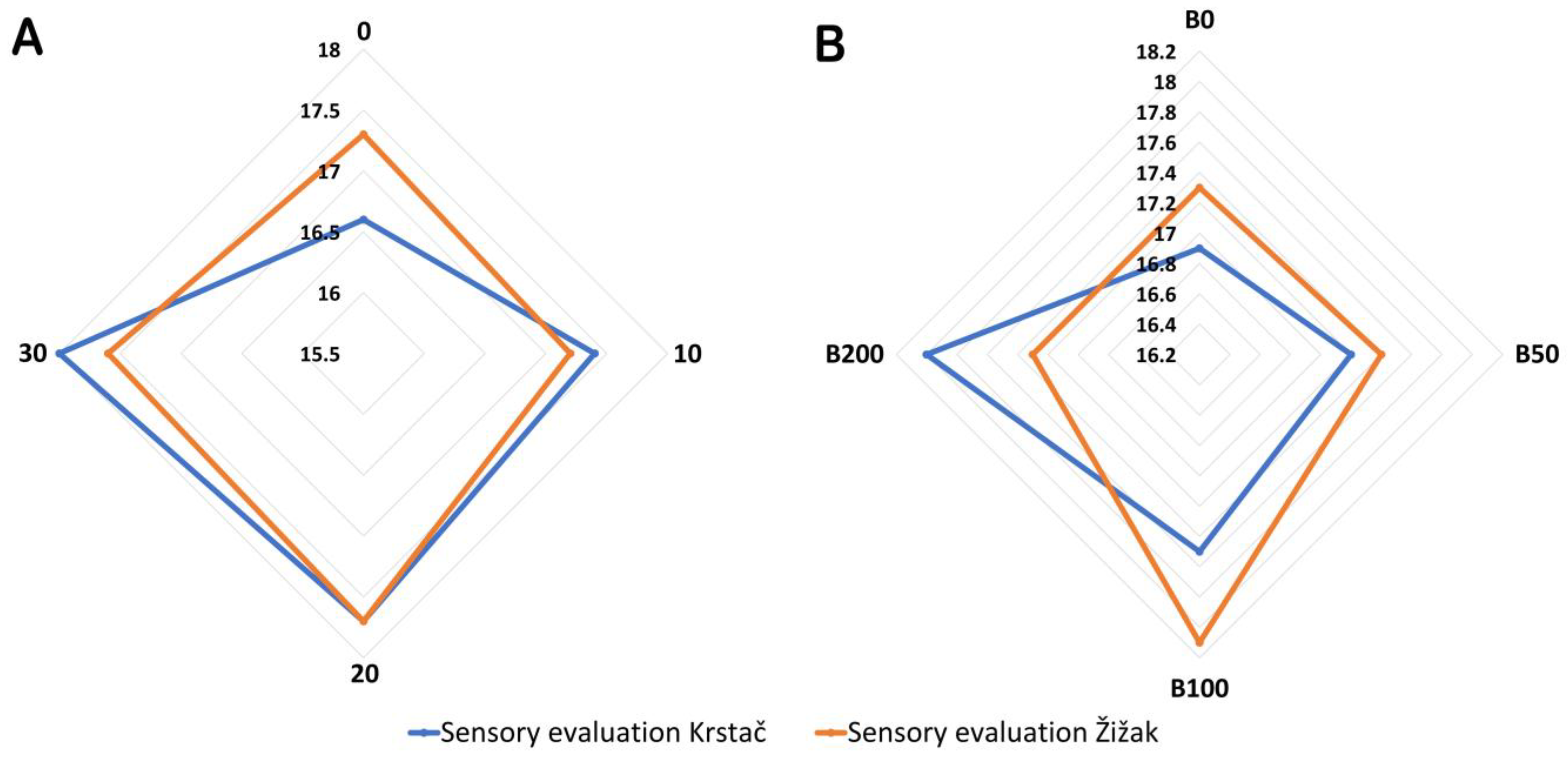
3.4. Sensory Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sánchez-Palomo, E.; Alonso-Villegas, R.; González-Viñas, M.A. Characterisation of free and glycosidically bound aroma compounds of La Mancha Verdeja white wines. Food Chem. 2015, 173, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Radeka, S.; Bestulić, E.; Rossi, S.; Orbanić, F.; Bubola, M.; Plavša, T.; Lukić, I.; Jeromel, A. Effect of different vinification techniques on the sensory profile of Malvazija Istarska wines. Fermentation 2023, 9, 676. [Google Scholar] [CrossRef]
- Maggu, M.; Winz, R.; Kilmartin, P.A.; Trought, M.C.; Nicolau, L. Effect of skin contact and pressure on the composition of Sauvignon Blanc must. J. Agric. Food Chem. 2007, 55, 10281–10288. [Google Scholar] [CrossRef] [PubMed]
- Alti-Palacios, L.; Martίnez, J.; Teixeira, J.A.C.; Cȃmara, J.S.; Perestrelo, R. Influence of cold pre-fermentation maceration on the Volatilomic pattern and aroma of white wines. Foods 2023, 12, 1135. [Google Scholar] [CrossRef]
- Burin, V.M.; Caliari, V.; Bordignon-Luiz, M.T. Nitrogen compounds in must and volatile profile of white wine: Influence of clarification process before alcoholic fermentation. Food Chem. 2016, 202, 417–425. [Google Scholar] [CrossRef]
- Seabrook, A.; van der Westhuzen, T. Fining during fermentation focus on white and rosé. Advantages of fining in must rather than wine on aroma and colour. Wine Vitic. J. 2018, 33, 30–33. [Google Scholar]
- Parish-Virtue, K.; Herbst-Johnstone, M.; Bouda, F.; Fedrizzi, B.; Deed, R.C.; Kilmartin, P.A. Aroma and sensory profiles of Sauvignon Blanc wines from commercially produced free run and pressed juices. Beverages 2021, 7, 29. [Google Scholar] [CrossRef]
- Vázques-Pateiro, I.; Mirás-Avalos, J.M.; Falqué, E. Influence of must clarification technique on the volatile composition of Albariño and Treixadure wines. Molecules 2022, 27, 810. [Google Scholar] [CrossRef]
- Madžgalj, V.; Petrović, A.; Čakar, U.; Maraš, V.; Sofrenić, I.; Tešević, V. The influence of different enzymatic preparations and skin contact time on aromatic profile of wines produced from autochthonous grape varieties Krstač and Žižak. J. Serb. Chem. Soc. 2023, 88, 11–23. [Google Scholar] [CrossRef]
- Casalta, E.; Salmon, J.M.; Picou, C.; Sablayrolles, J.M. Grape Solids: Lipid composition and role during alcoholic fermentation under enological conditions. Am. J. Enol. Vitic. 2019, 70, 147–154. [Google Scholar] [CrossRef]
- Mierczynska-Vasilev, A.; Smith, P.A. Current state of knowledge and challenges in wine clarification. Aus. J. Grape Wine Res. 2015, 21, 615–626. [Google Scholar] [CrossRef]
- Armada, L.; Falqué, E. Repercussion of the clarification treatment agents before the alcoholic fermentation on volatile composition of white wines. Eur. Food Res. Technol. 2007, 225, 553–558. [Google Scholar] [CrossRef]
- Doulia, D.; Anagnos, E.K.; Liapis, K.S.; Klimentzos, D.A. Effect of clarification process on the removal of pesticide residues in red wine and comporison with white wine. J. Environ. Sci. Health 2018, 53, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Vernhet, A.; Bes, M.; Bouissou, D.; Carrillo, S.; Brillouet, J.-M. Characterization of suspended solids in thermo-treated red musts. Oeno One 2016, 50, 9–21. [Google Scholar] [CrossRef]
- Huang, D.; Fan, W.; Dai, R.; Lu, Y.; Liu, Y.; Song, Y.; Qin, Y.; Su, Y. Impact of must clarification treatments on chemical and sensory profiles of kiwifruit wine. npj Sci. Food 2024, 8, 40. [Google Scholar] [CrossRef]
- Ayestarán, B.M.; Ancín, M.C.; García, A.M.; González, A.; Garrido, J.J. Influence of prefermentation clarification on nitrogenous content of musts and wines. J. Agric. Food Chem. 1995, 43, 476–482. [Google Scholar] [CrossRef]
- Diéguez, S.C.; Lois, L.C.; Gόmez, E.F.; Luisa, M.; De la Peña, G. Aromatic composition of the Vitis vinifera grape Albarino. Lebensm. Wiss. Technol. 2003, 36, 585–590. [Google Scholar] [CrossRef]
- Lambri, M.; Colangelo, D.; Dordoni, R.; Torchio, F.; De Faveri, D.M. Innovations in the use of bentonite in oenology: Interactions with grape and wine proteins, colloids, polyphenols and aroma compounds. In Grape and Wine Biotechnology; Morata, A., Loira, I., Eds.; Intech: London, UK, 2016; Chapter 18; pp. 381–400. [Google Scholar] [CrossRef]
- Puig-Deu, M.; López-Tamames, E.; Buxaderas, S.; Torre-Boronat, M.C. Quality of base and sparkling wines as influenced by the type of fining agent added pre-fermentation. Food Chem. 1999, 66, 35–42. [Google Scholar] [CrossRef]
- Salazar, F.N.; Marangon, M.; Labbé, M.; Lira, E.; Rodrίguez-Bencomo, J.J.; López, F. Comparative study of sodium bentonite and sodium-activated bentonite fining during white wine fermentation: Its effect on protein content, protein stability, lees volume, and volatile compounds. Eur. Food Res.Technol. 2017, 243, 2043–2054. [Google Scholar] [CrossRef]
- Parish, K.J.; Herbst-Johnstone, M.; Bourdo, F.; Klaere, S.; Fedrizzi, B. Pre-fermentation fining effects on the aroma chemistry of Marlborough Sauvignon blanc press fractions. Food Chem. 2016, 208, 326–335. [Google Scholar] [CrossRef]
- Madžgalj, V.; Živković, N.; Sofrenić, I.; Tešević, V.; Petrović, A. The effects of malolactic fermentation and bentonite treatment on the aroma of wines from autochthonous Krstač and Žižak varieties. Food Feed Res. 2025, 52, 103–119. [Google Scholar] [CrossRef]
- Avram, V.; Floare, C.G.; Hosu, A.; Cimpoiu, C.; Măruţoiu, C.; Moldovan, Z. Characterization of Romanian wines by gas chromatography-mass spectrometry. Anal. Lett. 2014, 48, 1099–1116. [Google Scholar] [CrossRef]
- Veljović, S.; Tomić, N.; Belović, M.; Nikićević, N.; Vukosavljević, P.; Nikšić, M.; Tešević, V. Volatile composition, colour, and sensory quality of spirit-based beverages enriched with medicinal fungus Ganoderma lucidum and herbal extract. Food Technol. Biotechnol. 2019, 57, 408–417. [Google Scholar] [CrossRef]
- Madžgalj, V.; Petrović, A.; Tešević, V.; Anđelković, B.; Sofrenić, I. The influence of different yeast strains and yeast nutrients on the aroma of Krstač and Žižak wines. Maced. J. Chem. Chem. Eng. 2023, 42, 203–214. [Google Scholar] [CrossRef]
- de Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research—R Package Version 1.3-7. 2023. Available online: http://CRAN.R-project.org/package=agricolae (accessed on 21 May 2025).
- R Core Team. R: A Language and Environment for Statistical Computing. Vienna. Austria. 2024. Available online: https://www.R-project.org/ (accessed on 21 May 2025).
- Rohart, F.; Gautier, B.; Singh, A.; Lê Cao, K.A. mixOmixs: An R package for ‘omics’ feature selection and multiple data integration. PLoS Comput. Biol. 2017, 13, e1005752. [Google Scholar] [CrossRef] [PubMed]
- Kovačević-Ganić, K.; Staver, M.; Perušić, Đ.; Banović, M.; Komes, D.; Gracin, L. Influence of blending on the aroma of Malvasia istriana wine. Food Technol. Biotech. 2003, 41, 305–314. Available online: https://hrcak.srce.hr/file/180931 (accessed on 5 June 2025).
- Mendes-Ferreira, A.; Barbosa, C.; Lage, P.; Mendes-Faia, A. The impact of nitrogen on yeast fermentation and wine quality. Ciên. Téc. Vitivnic. 2011, 26, 17–32. [Google Scholar]
- Lambrechts, M.G.; Pretorius, I.S. Yeast and its importance to wine aroma—A review. S. Afr. J. Enol. Vitic. 2000, 21, 97–129. [Google Scholar] [CrossRef]
- Sonni, F.; Moore, E.G.; Chinnici, F.; Riponi, C.; Smyth, H.E. Characterisation of Australian Verdelho wines from Queensland Granit Belt region. Food Chem. 2015, 196, 1163–1171. [Google Scholar] [CrossRef]
- Del Barrio-Galán, R.; Valle-Herrero, H.D.; Bueno-Herrera, M.; López-de-la-Cuesta, P.; Pérez-Margariño, S. Volatile and non-volatile characterization of white and rosé wines from different Spanish Protected Designations of Origin. Beverages 2021, 7, 49. [Google Scholar] [CrossRef]
- Burin, V.M.; Gomes, T.M.; Caliari, V.; Rosier, J.P.; Luiz, M.T.B. Establishment of influence the nitrogen content in musts and volatile profile of white wines associated to chemometric tools. Microchem. J. 2015, 122, 20–28. [Google Scholar] [CrossRef]
- Losada, M.M.; Andrés, J.; Cacho, J.; Revilla, E.; López, J.F. Influence of some prefermentative treatments on aroma composition and sensory evaluation of white Godello wines. Food Chem. 2011, 125, 884–891. [Google Scholar] [CrossRef]
- Kechagia, D.; Paraskevopoulos, Y.; Symeou, E.; Galiotou-Panayotou, M.; Kotseridis, Y. Influence of prefermentative treatments to the major volatile compounds of Assyrtiko wines. J. Agric. Food Chem. 2008, 56, 4555–4563. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, B.; Hory, C.; Bard, M.H.; Taisant, C.; Olsson, A.; Le Fur, Y. Effect of skin contact and settling on the level of the C18:2, C18:3 fatty acids and C6 compounds in Burgundy Chardonnay musts and wines. Food Qual. Prefer. 1995, 6, 35–41. [Google Scholar] [CrossRef]
- Liu, P.T.; Duan, C.Q.; Yan, G.L. Comparing the effects of different unsaturated fatty acids on fermentation performance of Saccharomyces cerevisiae and aroma compounds during red wine fermentation. Molecules 2019, 24, 538. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, M.; Güell, C.; López, F. Industrial wine making: Comparison of must clarification treatments. J. Agric. Food Chem. 1998, 46, 1523–1528. [Google Scholar] [CrossRef]
- Selli, S.; Bagatar, B.; Sen, K.; Kelebek, H. Evaluation of differences in the aroma composition of free-run and pressed neutral grape juices obtained from Emir (Vitis vinifera L.). Chem. Biodivers. 2011, 8, 1776–1782. [Google Scholar] [CrossRef]
- Lu, X.; Yang, C.; Yang, Y.; Peng, B. Analysis of the formation of characteristic aroma compounds by amino acid metabolic pathway during fermentation with Saccharomyces cerevisiae. Molecules 2023, 28, 3100. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Faria, M.; Sá, F.; Barros, F.; Araújo, I.M. C6-alcohols as varietal markers for assessment of wine origin. Anal. Chim. Acta 2006, 563, 300–309. [Google Scholar] [CrossRef]
- Martίnez-Moreno, A.; Toledo-Gil, R.; Bautista-Ortin, A.B.; Gómez-Plaza, E.; Yuste, J.E.; Vallejo, F. Exploring the impact of extended maceration on the volatile compounds and sensory profile of Monastrell red wine. Fermentation 2024, 10, 343. [Google Scholar] [CrossRef]
- Vianna, E.; Ebeler, S.E. Monitoring ester formation in grape juice fermentations using solid phase microextraction coupled with Gas Chromatography-Mass Spectrometry. J. Agric. Food Chem. 2001, 49, 589–595. [Google Scholar] [CrossRef]
- Prusova, B.; Humaj, J.; Sochor, J.; Baron, M. Formation, losses, preservation and recovery of aroma compounds in the winemaking process. Fermentation 2022, 8, 93. [Google Scholar] [CrossRef]
- Lambri, M.; Dordoni, R.; Silva, A.; De Faveri, D.M. Effect of bentonite fining on odor-active compounds in two different white wine styles. Am. J. Enol. Vitic. 2010, 61, 225–233. [Google Scholar] [CrossRef]
- Vela, E.; Hernández-Orte, P.; Castro, E.; Ferreira, V.; Lopez, R. Effect of bentonite fining on polyfunctional mercaptans and other volatile compounds in Sauvignon blanc wines. Am. J. Enol. Vitic. 2017, 6, 30–38. [Google Scholar] [CrossRef]
- Lira, E.; Salazar, F.N.; Rodríguez-Bencomo, J.J.; Vincenzi, S.; Curioni, A.; López, F. Effect of using bentonite during fermentation on protein stabilisation and sensory properties of white wine. Int. J. Food Sci. Technol. 2014, 49, 1070–1078. [Google Scholar] [CrossRef]
- Vincenzi, S.; Panighel, A.; Gazzola, D.; Flamini, R.; Curioni, A. Study of combined effect of proteins and bentonite fining on the wine aroma loss. J. Agri. Food Chem. 2015, 63, 2314–2320. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. White Winemaking. In Handbook of Enology, The Microbiology of Wine and Winification; John Wiley and Sons: Chichester, UK, 2000; Volume 1, pp. 397–439. [Google Scholar]
- Lira, E.; Rodríguez-Bencomo, J.J.; Salazar, F.N.; Orriols, I.; Fornos, D.; López, F. Impact of bentonite additions during vinifications on protein stability and volatile compounds of Albariño wines. J. Agric. Food Chem. 2015, 63, 3004–3011. [Google Scholar] [CrossRef]
- Lambri, M.; Dordoni, R.; Silva, A.; De Faveri, D.M. Comparing the impact of bentonite addition for both must clarification and wine fininig on the chemical profile of wine from Chambave Muscat grapes. Int. J. Food Sci. Technol. 2012, 47, 1–12. [Google Scholar] [CrossRef]
- Kumar, Y.; Suhag, R. Impact of fining agents on color, phenolics, aroma, and sensory properties of wine: A review. Beverages 2024, 10, 71. [Google Scholar] [CrossRef]
- Lukić, I.; Lotti, C.; Vrhovsek, U. Evolution of free and bound volatile aroma compounds and phenols during fermentation of Muscat blanc grape juice with and without skins. Food Chem. 2017, 232, 25–35. [Google Scholar] [CrossRef]


| Treatments | Abbreviation (Wine Samples) | Explanation of Treatments |
|---|---|---|
| Static settling | K0, Z0 | Without static settling |
| K10, Z10 | Static settling for 10 h | |
| K20, Z20 | Static settling for 20 h | |
| K30, Z30 | Static settling for 30 h | |
| Pressure | Kfr, Zfr | Free-run juice |
| Kp, Zp | Pressure ~1.8 bar | |
| Bentonite | KB0, ZB0 | Without bentonite |
| KB50, ZB50 | Addition of 50 g/hL bentonite | |
| KB100, ZB100 | Addition of 100 g/hL bentonite | |
| KB200, ZB200 | Addition of 200 g/hL bentonite |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madžgalj, V.; Đorđević, I.; Sofrenić, I.; Petrović, A. The Effects of Pre-Fermentative Treatments on the Aroma of Krstač and Žižak Wines. Fermentation 2025, 11, 577. https://doi.org/10.3390/fermentation11100577
Madžgalj V, Đorđević I, Sofrenić I, Petrović A. The Effects of Pre-Fermentative Treatments on the Aroma of Krstač and Žižak Wines. Fermentation. 2025; 11(10):577. https://doi.org/10.3390/fermentation11100577
Chicago/Turabian StyleMadžgalj, Valerija, Iris Đorđević, Ivana Sofrenić, and Aleksandar Petrović. 2025. "The Effects of Pre-Fermentative Treatments on the Aroma of Krstač and Žižak Wines" Fermentation 11, no. 10: 577. https://doi.org/10.3390/fermentation11100577
APA StyleMadžgalj, V., Đorđević, I., Sofrenić, I., & Petrović, A. (2025). The Effects of Pre-Fermentative Treatments on the Aroma of Krstač and Žižak Wines. Fermentation, 11(10), 577. https://doi.org/10.3390/fermentation11100577








