Exploring the Phytochemical Profiles, and Antioxidant and Antimicrobial Activities of the Hydroethanolic Grape Pomace Extracts from Two Romanian Indigenous Varieties
Abstract
1. Introduction
2. Materials and Methods
2.1. Grape Pomace Samples
2.2. Extraction Procedure
2.3. Evaluation of Phenolic Profile and Content
2.3.1. The Total Phenolic Content (TPC)
2.3.2. Total Flavonoid Content (TFC)
2.3.3. Total Anthocyanin Content (TAC)
2.3.4. UHPLC Analysis
2.4. DPPH Antioxidant Assay
2.5. Antibacterial Activity
2.5.1. Microorganisms
2.5.2. Plate Screening of Antimicrobial Activity of the Extract Samples
2.5.3. Microdilution Technique
2.6. Statistical Analysis
3. Results
3.1. Phytochemical Profile
3.1.1. Determination of Total Phenolic, Flavonoid, and Anthocyanin Content
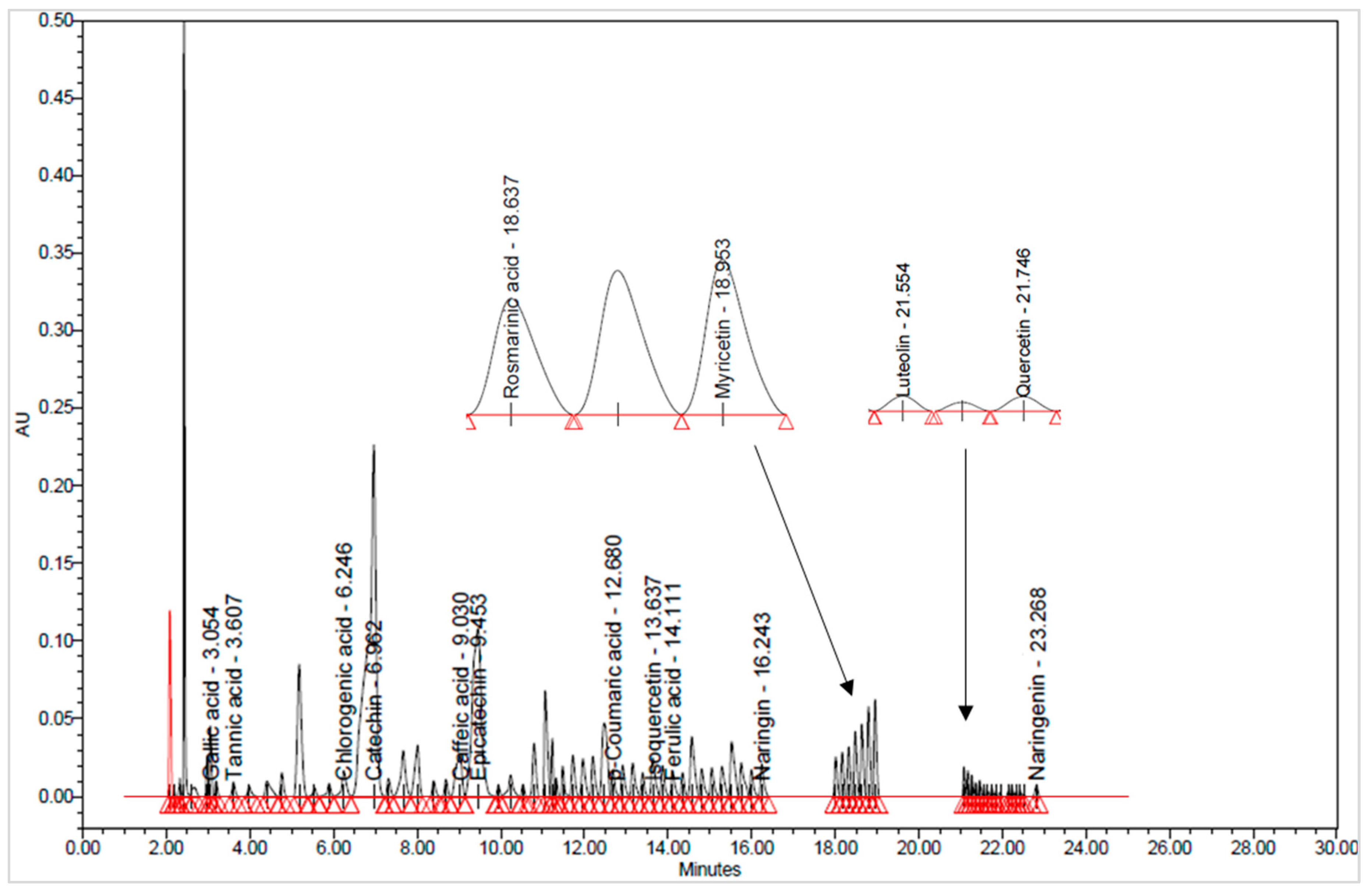
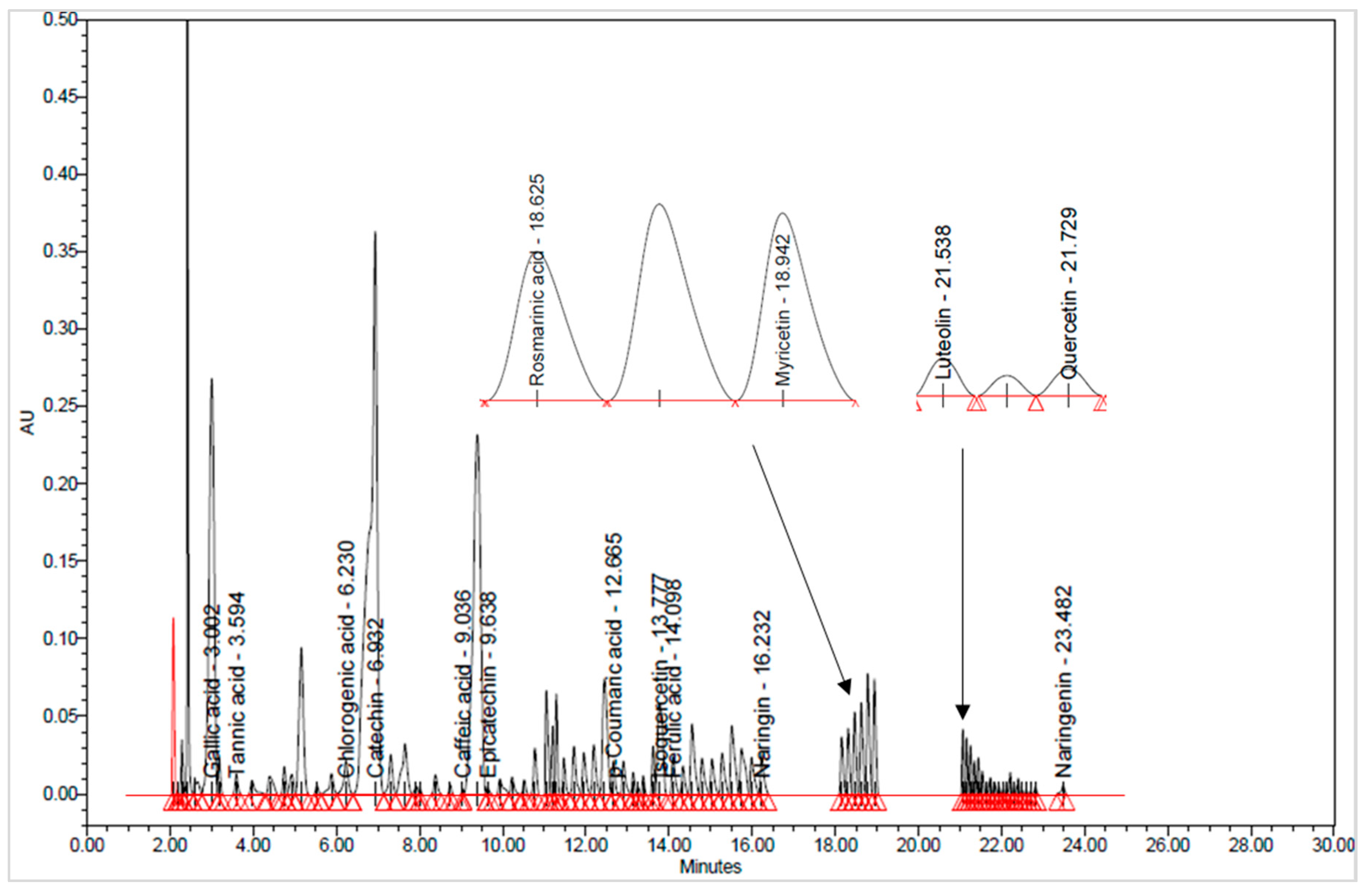
3.1.2. Bioactive Compounds Identification through UHPLC Analysis
3.2. Antioxidant Activity
3.3. Antimicrobial Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QCL (accessed on 6 July 2024).
- OIV. STATE of the World Vine and Wine Sector in 2023. Available online: https://www.oiv.int/sites/default/files/2024-04/OIV_STATE_OF_THE_WORLD_VINE_AND_WINE_SECTOR_IN_2023.pdf (accessed on 6 July 2024).
- Ministry of Agriculture and Rural Development—Romania. Available online: https://www.madr.ro/horticultura/viticultura-vinificatie.html (accessed on 20 August 2024).
- Soceanu, A.; Dobrinas, S.; Sirbu, A.; Manea, N.; Popescu, V. Economic aspects of waste recovery in the wine industry. A multidisciplinary approach. Sci. Total Environ. 2021, 759, 143543. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, C.; Friant, P.; Choné, X.; Tregoat, O.; Koundouras, S.; Dubourdieu, D. Influence of climate, soil, and cultivar on terroir. Am. J. Enol. Vitic. 2004, 55, 207–217. [Google Scholar] [CrossRef]
- De Oliveira, J.B.; Egipto, R.; Laureano, O.; de Castro, R.; Pereira, G.E.; Ricardo-da-Silva, J.M. Climate effects on physicochemical composition of syrah grapes at low and high altitude sites from tropical grown regions of Brazil. Food Res. Int. 2019, 121, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Muhlack, R.A.; Potumarthi, R.; Jeffery, D.W. Sustainable wineries through waste valorisation: A review of grape marc utilisation for value-added products. Waste Manag. 2018, 72, 99–118. [Google Scholar] [CrossRef]
- Lyas, T.; Chowdhary, P.; Chaurasia, D.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Sustainable green processing of grape pomace for the production of value-added products: An overview. Environ. Technol. Innov. 2021, 23, 101592. [Google Scholar]
- Frîncu, M.; Dumitrache, C.; Petre, A.C.; Andrei, M.O.Ț.; Teodorescu, R.I.; Bărbulescu, D.I.; Tudor, V.; Matei, F. Physico-chemical characterization of some sources of grape marc from Pietroasa vineyard. AgroLife Sci. J. 2023, 12, 81–86. [Google Scholar] [CrossRef]
- Niculescu, V.-C.; Ionete, R.-E. An Overview on Management and Valorisation of Winery Wastes. Appl. Sci. 2023, 13, 5063. [Google Scholar] [CrossRef]
- Constantin, O.E.; Stoica, F.; Rațu, R.N.; Stănciuc, N.; Bahrim, G.E.; Râpeanu, G. Bioactive components, applications, extractions, and health benefits of winery by-products from a circular bioeconomy perspective: A review. Antioxidants 2024, 13, 100. [Google Scholar] [CrossRef]
- Tomoiagă, L.L.; Iliescu, M.L.; Răcoare, H.S.; Botea, V.; Sîrbu, A.D.; Puşcă, G.; Chedea, V.S. Grape pomance generation from grape cultivars cultivated in Târnave vineyards in the framework of the climate change. Rom. J. Hortic. 2020, 1, 81–88. [Google Scholar] [CrossRef]
- Olejar, K.J.; Fedrizzi, B.; Kilmartin, P.A. Influence of harvesting technique and maceration process on aroma and phenolic attributes of sauvignon blanc wine. Food Chem. 2015, 183, 181–189. [Google Scholar] [CrossRef]
- Geană, E.I.; Ionete, R.E.; Niculescu, V.; Artem, V.; Ranca, A. Changes in polyphenolic content of berry skins from different red grapes cultivars during ripening. Smart Energy Sustain. Environ. 2016, 19, 95. [Google Scholar]
- Tchouakeu Betnga, P.F.; Poggesi, S.; Darnal, A.; Longo, E.; Rudari, E.; Boselli, E. Terroir dynamics: Impact of vineyard and canopy treatment with chitosan on anthocyanins, phenolics, and volatile and sensory profiles of Pinot noir wines from south tyrol. Molecules 2024, 29, 1916. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; Garcia-Viguera, C. Natural bioactive compounds from winery by-products as health promoters: A review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef]
- Iacopini, P.; Baldi, M.; Storchi, P.; Sebastiani, L. Catechin, epicatechin, quercetin, rutin and resveratrol in red grape: Content, in vitro antioxidant activity and interactions. J. Food Compos. Anal. 2008, 21, 589–598. [Google Scholar] [CrossRef]
- Guaita, M.; Bosso, A. Polyphenolic characterization of grape skins and seeds of four italian red cultivars at harvest and after fermentative maceration. Foods 2019, 8, 395. [Google Scholar] [CrossRef]
- Troilo, M.; Difonzo, G.; Paradiso, V.M.; Summo, C.; Caponio, F. Bioactive compounds from vine shoots, grape stalks, and wine lees: Their potential use in agro-food chains. Foods 2021, 10, 342. [Google Scholar] [CrossRef]
- Caponio, G.R.; Minervini, F.; Tamma, G.; Gambacorta, G.; De Angelis, M. Promising application of grape pomace and its agri-food valorization: Source of bioactive molecules with beneficial effects. Sustainability 2023, 15, 9075. [Google Scholar] [CrossRef]
- Stalikas, C.D. Extraction, separation, and detection methods for phenolic acids and flavonoids. J. Sep. Sci. 2007, 30, 3268–3295. [Google Scholar] [CrossRef]
- Ćujić, N.; Šavikin, K.; Janković, T.; Pljevljakušić, D.; Zdunić, G.; Ibrić, S. Optimization of polyphenols extraction from dried chokeberry using maceration as traditional technique. Food Chem. 2016, 194, 135–142. [Google Scholar] [CrossRef]
- Aires, A. Phenolics in foods: Extraction, analysis and measurements. In Phenolic Compounds—Natural Sources, Importance and Applications; Soto-Hernandez, M., Palma-Tenango, M., Del Rosario Garcia-Mateos, M., Eds.; IntechOpen: London, UK, 2017; pp. 61–88. [Google Scholar]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Soxhlet extraction of phenolic compounds from Vernonia cinereal leaves and its antioxidant activity. J. Appl. Res. Med. Aromat. Plants 2018, 11, 12–17. [Google Scholar]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Ju, Z.Y.; Howard, L.R. Effects of solvent and temperature on pressurized liquid extraction of anthocyanins and total phenolics from dried red grape skin. J. Agric. Food Chem. 2003, 51, 5207–5213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Pintać, D.; Majkić, T.; Torović, L.; Orčić, D.; Beara, I.; Simin, N.; Mimica–Dukić, N.; Lesjak, M. Solvent selection for efficient extraction of bioactive compounds from grape pomace. Ind. Crops Prod. 2018, 111, 379–390. [Google Scholar] [CrossRef]
- Rodrigues, R.P.; Sousa, A.M.; Gando-Ferreira, L.M.; Quina, M.J. Grape pomace as a natural source of phenolic compounds: Solvent screening and extraction optimization. Molecules 2023, 28, 2715. [Google Scholar] [CrossRef]
- Castro, L.E.N.; Sganzerla, W.G.; Silva, A.P.G.; John, O.D.; Barroso, T.L.C.T.; Rostagno, M.A.; Forster-Carneiro, T. Sustainable extraction methods for the recovery of polyphenolic compounds from grape pomace and its biological properties: A comprehensive review. Phytochem. Rev. 2024, 1–28. [Google Scholar] [CrossRef]
- Melo, F.d.O.; Ferreira, V.C.; Barbero, G.F.; Carrera, C.; Ferreira, E.d.S.; Umsza-Guez, M.A. Extraction of bioactive compounds from wine lees: A systematic and bibliometric review. Foods 2024, 13, 2060. [Google Scholar] [CrossRef]
- Corrales, M.; Toepfl, S.; Butz, P.; Knorr, D.; Tauscher, B. Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: A comparison. Innov. Food Sci. Emerg. Technol. 2008, 9, 85–91. [Google Scholar] [CrossRef]
- Comuzzo, P.; Marconi, M.; Zanella, G.; Querzè, M. Pulsed electric field processing of white grapes (cv. Garganega): Effects on wine composition and volatile compounds. Food Chem. 2018, 264, 16–23. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Nobre, C.; Rodrigues, R.M.; Genisheva, Z.; Botelho, C.; Teixeira, J.A. Extraction of phenolic compounds from grape pomace using ohmic heating: Chemical composition, bioactivity and bioaccessibility. Food Chem. 2024, 436, 137780. [Google Scholar] [CrossRef]
- Bhunia, A.K. General mechanism of pathogenesis. In Foodborne Microbial Pathogens; Springer: Berlin/Heidelberg, Germany, 2018; pp. 87–115. [Google Scholar]
- Todd, E. Food-borne disease prevention and risk assessment. Int. J. Environ. Res. Public Health 2020, 17, 5129. [Google Scholar] [CrossRef] [PubMed]
- Lencova, S.; Svarcova, V.; Stiborova, H.; Demnerova, K.; Jencova, V.; Hozdova, K.; Zdenkova, K. Bacterial biofilms on polyamide nanofibers: Factors influencing biofilm formation and evaluation. ACS Appl. Mater. Interfaces 2021, 13, 2277–2288. [Google Scholar] [CrossRef] [PubMed]
- Almansour, A.M.; Alhadlaq, M.A.; Alzahrani, K.O.; Mukhtar, L.E.; Alharbi, A.L.; Alajel, S.M. The Silent Threat: Antimicrobial-resistant pathogens in food-producing animals and their impact on public health. Microorganisms 2023, 11, 2127. [Google Scholar] [CrossRef] [PubMed]
- Mulchandani, R.; Wang, Y.; Gilbert, M.; Van Boeckel, T.P. Global trends in antimicrobial use in food-producing animals: 2020 to 2030. PLoS Glob. Public Health 2023, 3, e0001305. [Google Scholar] [CrossRef]
- Naghavi, M.; Mestrovic, T.; Gray, A.; Hayoon, A.G.; Swetschinski, L.R.; Aguilar, G.R.; Weaver, N.D.; Ikuta, K.S.; Chung, E.; Wool, E.E.; et al. Global burden associated with 85 pathogens in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Infect. Dis. 2024, 24, 868–895. [Google Scholar] [CrossRef]
- Badea, F.; Diguta, C.F.; Matei, F. The use of lactic acid bacteria and their metabolites to improve the shelf life of perishable fruits and vegetables. Sci. Bull. Ser. F Biotechnol. 2022, XXVI, 117–125. [Google Scholar]
- Utoiu, E.; Oancea, A.; Stanciuc, A.M.; Stefan, M.L.; Toma, A.; Moraru, A.; Diguta, C.F.; Matei, F.; Cornea, C.P.; Oancea, F. Prebiotic content and probiotic effect of kombucha fermented pollen. AgroLife Sci. J. 2018, 7, 149–156. [Google Scholar]
- Kouadio, N.J.; Zady, A.L.O.; Kra, K.A.S.; Diguță, F.C.; Niamke, S.; Matei, F. In Vitro Probiotic Characterization of Lactiplantibacillus plantarum Strains Isolated from Traditional Fermented Dockounou Paste. Fermentation 2024, 10, 264. [Google Scholar] [CrossRef]
- Pristavu, M.C.; Diguță, C.; Coulibaly, W.H.; Youte Fanche, S.A.; Dopcea, G.; Matei, F. Review of postbiotics as new health promoters. AgroLife Sci. J. 2022, 11, 142–152. [Google Scholar] [CrossRef]
- Ma, L.; Tu, H.; Chen, T. Postbiotics in Human Health: A Narrative Review. Nutrients 2023, 15, 291. [Google Scholar] [CrossRef]
- Yan, R.; Zeng, X.; Shen, J.; Wu, Z.; Guo, Y.; Du, Q.; Tu, M.; Pan, D. New clues for postbiotics to improve host health: A review from the perspective of function and mechanisms. J. Sci. Food Agric. 2024, 104, 6376–6387. [Google Scholar] [CrossRef] [PubMed]
- Răducu, A.L.; Popa, A.; Boiu-Sicuia, O.A.; Israel-Roming, F.; Cornea, C.P.; Jurcoane, S. Antimicrobial activity of camelina oil and hydroalcoholic seed extracts. Rom. Biotecnol. Lett. 2021, 26, 2355–2360. [Google Scholar] [CrossRef]
- Roleira, F.M.F.; Tavares-Da-Silva, E.J.; Varela, C.L.; Costa, S.C.; Silva, T.; Garrido, J.; Borges, F. Plant Derived and Dietary Phenolic Antioxidants: Anticancer Properties. Food Chem. 2015, 183, 235–258. [Google Scholar] [CrossRef]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Caponio, G.R.; Lippolis, T.; Tutino, V.; Gigante, I.; De Nunzio, V.; Milella, R.A.; Gasparro, M.; Notarnicola, M. Nutraceuticals: Focus on anti-inflammatory, anti-cancer, antioxidant properties in gastrointestinal tract. Antioxidants 2022, 11, 1274. [Google Scholar] [CrossRef]
- Lobiuc, A.; Pavăl, N.E.; Mangalagiu, I.I.; Gheorghiță, R.; Teliban, G.C.; Amăriucăi-Mantu, D.; Stoleru, V. Future Antimicrobials: Natural and Functionalized Phenolics. Molecules 2023, 28, 1114. [Google Scholar] [CrossRef]
- Li, S.; Jiang, S.X.; Jia, W.T.; Guo, T.M.; Wang, F.; Li, J.; Yao, Z.L. Natural antimicrobials from plants: Recent advances and future prospects. Food Chem. 2023, 432, 137231. [Google Scholar] [CrossRef] [PubMed]
- Arlet, A.C.I.; Tociu, M.; Balanuca, B.; Israel-Roming, F. Extraction and evaluation of total phenolics content from red corn bran. Sci. Bull. Ser. F Biotechnol. 2023, 27, 60–67. [Google Scholar]
- Baroi, A.M.; Toma, D.I.; Alin, D.I.N.; Vizitiu, D.E.; Fierascu, I.; Fierascu, R.C. Grapevine plant waste utilization in nanotechnology. AgroLife Sci. J. 2024, 13, 203–216. [Google Scholar]
- Cerchezan, G.; Israel-Roming, F. Correlation of the chemical parameters with the sensorial properties of wine. Sci. Bull. Ser. F Biotechnol. 2024, XXVIII, 95–104. [Google Scholar]
- Hornedo-Ortega, R.; Reyes González-Centeno, M.; Chira, K.; Jourdes, M.; Teissedre, P.-L. Phenolic compounds of grapes and wines: Key compounds and implications in sensory perception. In Winemaking—Stabilization, Aging Chemistry and Biochemistry, 1st ed.; Câmara, J., Ed.; Nova Science Publisher: Hauppauge, NY, USA; IntechOpen; London, UK, 2020. [Google Scholar]
- Karami, S.; Rahimi, M.; Babaei, A. An Overview on the antioxidant, anti-inflammatory, antimicrobial and anti-cancer activity of grape extract. Biomed. Res. Clin. Prac. 2018, 3, 1–4. [Google Scholar] [CrossRef][Green Version]
- Chedea, V.S.; Tomoiaga, L.L.; Macovei, S.O.; Magureanu, D.C.; Iliescu, M.L.; Bocsan, I.C.; Buzoianu, A.D.; Vosloban, C.M.; Pop, R.M. Antioxidant/pro-oxidant actions of polyphenols from grapevine and wine by-products-base for complementary therapy in ischemic heart diseases. Front. Cardiovasc. Med. 2021, 8, 750508. [Google Scholar] [CrossRef]
- Chedea, V.S.; Macovei, Ș.O.; Bocsan, I.C.; Măgureanu, D.C.; Levai, A.M.; Buzoianu, A.D.; Pop, R.M. Grape pomace polyphenols as a source of compounds for management of oxidative stress and inflammation—A possible alternative for non-steroidal anti-inflammatory drugs? Molecules 2022, 27, 6826. [Google Scholar] [CrossRef] [PubMed]
- Bocsan, I.C.; Măgureanu, D.C.; Pop, R.M.; Levai, A.M.; Macovei, Ș.O.; Pătrașca, I.M.; Chedea, V.S.; Buzoianu, A.D. Antioxidant and anti-inflammatory actions of polyphenols from red and white grape pomace in ischemic heart diseases. Biomedicines 2022, 10, 2337. [Google Scholar] [CrossRef]
- Radulescu, C.; Buruleanu, L.C.; Nicolescu, C.M.; Olteanu, R.L.; Bumbac, M.; Holban, G.C.; Simal-Gandara, J. Phytochemical profiles, antioxidant and antibacterial activities of grape (Vitis vinifera L.) seeds and skin from organic and conventional vineyards. Plants 2020, 9, 1470. [Google Scholar] [CrossRef] [PubMed]
- Luchian, C.E.; Cotea, V.V.; Vlase, L.; Toiu, A.M.; Colibaba, L.C.; Răschip, I.E.; Nadăş, G.; Gheldiu, A.M.; Tuchiluş, C.; Rotaru, L. Antioxidant and antimicrobial effects of grape pomace extracts. BIO Web Conf. 2019, 15, 04006. [Google Scholar] [CrossRef]
- Cotoras, M.; Vivanco, H.; Melo, R.; Aguirre, M.; Silva, E.; Mendoza, L. In vitro and in vivo evaluation of the antioxidant and prooxidant activity of phenolic compounds obtained from grape (Vitis vinifera) pomace. Molecules 2014, 19, 21154–21167. [Google Scholar] [CrossRef] [PubMed]
- Louli, V.; Ragoussis, N.; Magoulas, K. Recovery of phenolic antioxidants from wine industry by-products. Bioresour. Technol. 2004, 92, 201–208. [Google Scholar] [CrossRef]
- Krasteva, D.; Ivanov, Y.; Chengolova, Z.; Godjevargova, T. Antimicrobial Potential, Antioxidant Activity, and Phenolic Content of Grape Seed Extracts from Four Grape Varieties. Microorganisms 2023, 11, 395. [Google Scholar] [CrossRef]
- Baydar, N.G.; Özkan, G.; Saǧdiç, O. Total Phenolic Contents and Antibacterial Activities of Grape (Vitis vinifera L.) Extracts. Food Control 2004, 15, 335–339. [Google Scholar] [CrossRef]
- Chedea, V.S.; Braicu, C.; Chirila, F.; Ober, C.; Socaciu, C. Antibacterial action of an aqueous grape seed polyphenolic extract. Afr. J. Biotechnol. 2011, 10, 6276–6280. [Google Scholar]
- Tseng, A.; Zhao, Y. Effect of different drying methods and storage time on the retention of bioactive compounds and antibacterial activity of wine grape pomace (Pinot Noir and Merlot). J. Food Sci. 2012, 77, H192–H201. [Google Scholar] [CrossRef] [PubMed]
- Sanhueza, L.; Tello, M.; Vivanco, M.; Mendoza, L.; Wilkens, M. Relation between antibacterial activity against food transmitted pathogens and total phenolic compounds in grape pomace extracts from Cabernet Sauvignon and Syrah Varieties. Adv. Microbiol. 2014, 04, 225–232. [Google Scholar] [CrossRef]
- Friedman, M. Antibacterial, antiviral, and antifungal properties of wines and winery byproducts in relation to their flavonoid content. J. Agric. Food Chem. 2014, 62, 6025–6042. [Google Scholar] [CrossRef]
- Silva, A.; Silva, V.; Igrejas, G.; Gaivão, I.; Aires, A.; Klibi, N.; Dapkevicius, M.d.L.; Valentão, P.; Falco, V.; Poeta, P. Valorization of Winemaking By-Products as a novel source of antibacterial properties: New strategies to fight antibiotic resistance. Molecules 2021, 26, 2331. [Google Scholar] [CrossRef]
- da Silva, W.P.; Lopes, G.V.; Ramires, T.; Kleinubing, N.R. May phenolics mitigate the antimicrobial resistance in foodborne pathogens? Curr. Opin. Food Sci. 2023, 25, 101107. [Google Scholar]
- Sateriale, D.; Forgione, G.; Di Rosario, M.; Pagliuca, C.; Colicchio, R.; Salvatore, P.; Paolucci, M.; Pagliarulo, C. Vine-Winery Byproducts as Precious Resource of Natural Antimicrobials: In Vitro Antibacterial and Antibiofilm Activity of Grape Pomace Extracts against Foodborne Pathogens. Microorganisms 2024, 12, 437. [Google Scholar] [CrossRef]
- Draghici-Popa, A.-M.; Buliga, D.-I.; Popa, I.; Tomas, S.T.; Stan, R.; Boscornea, A.C. Cosmetic products with potential photoprotective effects based on natural compounds extracted from waste of the winemaking industry. Molecules 2024, 29, 2775. [Google Scholar] [CrossRef]
- Koutelidakis, A.; Dimou, C. Grape pomace: A challenging renewable resource of bioactive phenolic compounds with diversified health benefits. MOJ Food Process. Technol. 2016, 2, 262–265. [Google Scholar] [CrossRef]
- Lo, S.; Pilkington, L.I.; Barker, D.; Fedrizzi, B. Attempts to create products with increased health-promoting potential starting with pinot noir pomace: Investigations on the process and its methods. Foods 2022, 11, 1999. [Google Scholar] [CrossRef]
- Caponio, G.R.; Noviello, M.; Calabrese, F.M.; Gambacorta, G.; Giannelli, G.; De Angelis, M. Effects of grape pomace polyphenols and in vitro gastrointestinal digestion on antimicrobial activity: Recovery of bioactive compounds. Antioxidants 2022, 11, 567. [Google Scholar] [CrossRef] [PubMed]
- Sinrod, A.J.G.; Shah, I.M.; Surek, E.; Barile, D. Uncovering the promising role of grape pomace as a modulator of the gut microbiome: An in-depth review. Heliyon 2023, 9, e20499. [Google Scholar] [CrossRef] [PubMed]
- Radulescu, C.; Buruleanu, L.C.; Olteanu, R.L.; Nicolescu, C.M.; Bumbac, M.; Gorghiu, L.M.; Nechifor, M.D. Grape by-Products: Potential Sources of Phenolic Compounds for Novel Functional Foods; Intech Open: London, UK, 2023; Available online: https://www.intechopen.com/online-first/88679 (accessed on 4 April 2024).
- Nistor, E.; Dobrei, A.; Dorbei, A.; Bampidis, V.; Ciolac, V. Grape pomace in sheep and dairy cows feeding. J. Hortic. For. 2014, 18, 146–150. [Google Scholar]
- Hassan, Y.I.; Kosir, V.; Yin, X.; Ross, K.; Diarra, M.S. Grape pomace as a promising antimicrobial alternative in feed: A critical review. J. Agric. Food. Chem. 2019, 67, 9705–9718. [Google Scholar] [CrossRef] [PubMed]
- Wittenauer, J.; Mäckle, S.; Sußmann, D.; Schweiggert-Weisz, U.; Carle, R. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia 2015, 101, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Hoss, I.; Rajha, H.N.; El Khoury, R.; Youssef, S.; Manca, M.L.; Manconi, M.; Louka, N.; Maroun, R.G. Valorization of wine-making by-products’ extracts in cosmetics. Cosmetics 2021, 8, 109. [Google Scholar] [CrossRef]
- Ferreira, S.M.; Santos, L. A potential valorization strategy of wine industry by-products and their application in cosmetics—Case study: Grape pomace and grapeseed. Molecules 2022, 27, 969. [Google Scholar] [CrossRef]
- Fernández-Bayo, J.D.; Nogales, R.; Romero, E. Winery vermicomposts to control the leaching of diuron, imidacloprid and their metabolites: Role of dissolved organic carbon content. J. Environ. Sci. Health B 2015, 50, 190–200. [Google Scholar] [CrossRef]
- Domínguez, J.; Martínez-Cordeiro, H.; Álvarez-Casas, M.; Lores, M. Vermicomposting grape marc yields high quality organic biofertiliser and bioactive polyphenols. Waste Manag. Res. 2014, 32, 1235–1240. [Google Scholar] [CrossRef]
- Pękal, A.; Pyrzynska, K. Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Giurescu, I.; Șesan, T.E.; Badju, S.; Lupu, C.; Oancea, F. Preparation of compost from sea buckthorn branches by using a multipurpose Trichoderma strain. Sci. Bull. Ser. F Biotechnol. 2023, 27, 97–104. [Google Scholar]
- Teng, Z.; Jiang, X.; He, F.; Bai, W. Qualitative and Quantitative Methods to Evaluate Anthocyanins. eFood 2020, 1, 339–346. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- CLSI. CLSI Supplement M100S. Performance Standards for Antimicrobial Susceptibility Testing, 26th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2016. [Google Scholar]
- Negro, C.; Aprile, A.; Luvisi, A.; De Bellis, L.; Miceli, A. Antioxidant activity and polyphenols characterization of four monovarietal grape pomaces from Salento (Apulia, Italy). Antioxidants 2021, 10, 1406. [Google Scholar] [CrossRef]
- Muncaciu, M.L.; Zamora Marín, F.; Pop, N.; Babeş, A.C. Comparative polyphenolic content of grape pomace flours from “Fetească Neagră” and “Italian Riesling” cultivars. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 45, 532–539. [Google Scholar] [CrossRef][Green Version]
- Marinelli, V.; Padalino, L.; Nardiello, D.; Del Nobile, M.A.; Conte, A. New approach to enrich pasta with polyphenols from grape marc. J. Chem. 2015, 2015, 34578. [Google Scholar] [CrossRef]
- Xu, Y.; Burton, S.; Kim, C.; Sismour, E. Phenolic compounds, antioxidant, and antibacterial properties of pomace extracts from four Virginia-grown grape varieties. Food Sci. Nutr. 2016, 4, 125–133. [Google Scholar] [CrossRef]
- Gül, H.; Acun, S.; Sen, H.; Nayir, N.; Turk, S. Antioxidant activity, total phenolics and some chemical properties of Öküzgözü and Narince grape pomace and grape seed flour. J. Food Agric. Environ. 2013, 11, 28–34. [Google Scholar]
- Arapitsas, P.; Oliveira, J.; Mattivi, F. Do white grapes really exist? Food Res. Int. 2015, 69, 21–25. [Google Scholar] [CrossRef]
- Chiavaroli, A.; Balaha, M.; Acquaviva, A.; Ferrante, C.; Cataldi, A.; Menghini, L.; Rapino, M.; Orlando, G.; Brunetti, L.; Leone, S.; et al. Phenolic Characterization and Neuroprotective Properties of Grape Pomace Extracts. Molecules 2021, 26, 6216. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Lopez, L.M.; DeWitt, C.A.M. Analysis of phenolic compounds in commercial dried grape pomace by high-performance liquid chromatography electrospray ionization mass spectrometry. Food Sci. Nutr. 2014, 2, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, V.; Buzzanca, C.; Melilli, M.G.; Indelicato, S.; Mauro, M.; Vazzana, M.; Arizza, V.; Lucarini, M.; Durazzo, A.; Bongiorno, D. Polyphenol Characterization and Antioxidant Activity of Grape Seeds and Skins from Sicily: A Preliminary Study. Sustainability 2022, 14, 6702. [Google Scholar] [CrossRef]
- Sochorova, L.; Prusova, B.; Jurikova, T.; Mlcek, J.; Adamkova, A.; Baron, M.; Sochor, J. The Study of Antioxidant Components in Grape Seeds. Molecules 2020, 25, 3736. [Google Scholar] [CrossRef]
- Abouelenein, D.; Mustafa, A.M.; Caprioli, G.; Ricciutelli, M.; Sagratini, G.; Vittori, S. Phenolic and nutritional profiles, and antioxidant activity of grape pomaces and seeds from Lacrima di Morro d’Alba and Verdicchio varieties. Food Biosci. 2023, 53, 102808. [Google Scholar] [CrossRef]
- Sabra, A.; Netticadan, T.; Wijekoon, C. Grape bioactive molecules, and the potential health benefits in reducing the risk of heart diseases. Food Chem. X 2021, 12, 100149. [Google Scholar] [CrossRef]
- Chagas, M.D.S.S.; Behrens, M.D.; Moragas-Tellis, C.J.; Penedo, G.X.M.; Silva, A.R.; Gonçalves-de-Albuquerque, C.F. Flavonols and Flavones as Potential anti-Inflammatory, Antioxidant, and Antibacterial Compounds. Oxid. Med. Cell Longev. 2022, 6, 9966750. [Google Scholar] [CrossRef]
- WHO. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- World Health Organization. 2020 Antibacterial Agents in Clinical and Preclinical Development: An Overview and Analysis. Available online: https://www.who.int/publications/i/item/9789240021303 (accessed on 1 July 2024).
- Corrales, M.; Han, J.H.; Tauscher, B. Antimicrobial properties of grape seed extracts and their effectiveness after incorporation into pea starch films. Int. J. Food Sci. Technol. 2009, 44, 425–433. [Google Scholar] [CrossRef]
- Baydar, N.G.; Sagdic, O.O.E.E.; Ozkan, G.; Cetin, S. Determination of antibacterial effects and total phenolic contents of grape (Vitis vinifera L.) seed extracts. Int. J. Food Sci. Technol. 2006, 41, 799–804. [Google Scholar] [CrossRef]
- Papadopoulou, C.; Soulti, K.; Roussis, I.G. Potential antimicrobial activity of red and white wine phenolic extracts against strains of Staphylococcus aureus, Escherichia coli and Candida albicans. Food Technol. Biotechnol. 2005, 43, 41–46. [Google Scholar]
- Oliveira, D.A.; Salvador, A.A.A.S.; Smânia, E.F.A.; Maraschin, M.; Ferreira, S.R.S. Antimicrobial activity and composition profile of grape (Vitis vinifera) pomace extracts obtained by supercritical fluids. J. Biotechnol. 2013, 164, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure–Activity Relationship) Models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, Q.; Li, H.; Qiu, Z.; Yu, Y. Comparative assessment of the antibacterial efficacies and mechanisms of different tea extracts. Foods 2022, 11, 620. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; de Souza, T.S.P.; Wu, H.; Holland, B.; Dunshea, F.R.; Barrow, C.J.; Suleria, H.A.R. Development of phenolic-rich functional foods by lactic fermentation of grape marc: A review. Food Rev. Int. 2023, 40, 1756–1775. [Google Scholar] [CrossRef]
- Barakat, N.; Bouajila, J.; Beaufort, S.; Rizk, Z.; Taillandier, P.; El Rayess, Y. Development of a new kombucha from grape pomace: The impact of fermentation conditions on composition and biological activities. Beverages 2024, 10, 29. [Google Scholar] [CrossRef]

| Grape Variety | Samples | TPC (mg GAE/g) | TFC (mg RE/g) | TAC (mg cyd-3-gluE/g) |
|---|---|---|---|---|
| Fetească Neagră | FNT | 81.81 ± 3.86 cd | 75.77 ± 5.02 bc | 34.01 ± 2.21 b |
| FNE | 78.61 ± 3.83 c | 75.09 ± 2.93 bc | 36.75 ± 2.66 b | |
| FNS | 93.52 ± 3.06 f | 86.86 ± 7.24 d | 11.18 ± 3.25 a | |
| Tămâioasă Românească | TRT | 45.34 ± 2.33 b | 38.05 ± 3.5 a | 1.69 ± 0.76 a |
| TRE | 27.72 ± 0.04 a | 25.71 ± 0.71 a | 1.06 ± 0.32 a | |
| TRS | 90.43 ± 0.50 ef | 61.01 ± 3.27 b | 1.48 ± 0.56 a |
| No. | Compound Name | Compound Class | λmax (nm) | TR (min) | Grape Pomace Extracts (µg/g) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| FNT | FNE | FNS | TRT | TRE | TRS | |||||
| 1 | GAL | Phenolic acids | 280 | 3.10 | 7.65 ± 0.13 a | 17.82 ± 0.42 c | 26.32 ± 0.46 d | 17.17 ± 0.92 c | 10.96 ± 0.06 b | 34.01 ± 0.02 e |
| 2 | TAN | 280 | 3.44 | 50.04 ± 1.02 d | 73.06 ± 0.28 e | 20.87 ± 0.24 a | 23.24 ± 57 b | 19.99 ± 0.01 a | 34.31 ± 0.36 c | |
| 3 | CLO | 320 | 6.65 | 0.13 ± 0.01 d | 0.16 ± 0.00 e | 0.05 ± 0.00 b | 0.04 ± 0.00 ab | 0.03 ± 0.00 a | 0.07 ± 0.00 c | |
| 4 | CAF | 320 | 9.16 | 0.14 ± 0.01 d | 0.14 ± 0.00 d | 0.09 ± 0.00 b | 0.09 ± 0.00 b | 0.04 ± 0.00 a | 0.11 ± 0.00 c | |
| 5 | COU | 320 | 12.71 | 0.12 ± 0.01 bc | 0.14 ± 0.00 c | 0.10 ± 0.01 b | 0.05 ± 0.00 a | 0.03 ± 0.00 a | 0.17 ± 0.01 d | |
| 6 | FER | 320 | 14.17 | 0.11 ± 0.00 d | 0.11 ± 0.01 d | 0.05 ± 0.00 b | 0.04 ± 0.00 b | 0.02 ± 0.00 a | 0.07 ± 0.00 c | |
| 7 | ROS | 320 | 18.73 | 0.52 ± 0.04 cd | 0.58 ± 0.00 d | 0.29 ± 0.00 b | 0.25 ± 0.01 b | 0.17 ± 0.00 a | 0.45 ± 0.02 c | |
| 8 | CAT | Flavonoid | 280 | 7.09 | 103.38 ± 1.70 c | 48.59 ± 0.58 b | 298.78 ± 0.47 e | 170.41 ± 0.92 d | 19.48 ± 0.71 a | 450.60 ± 0.85 f |
| 9 | EPI | 280 | 9.65 | 197.82 ± 0.85 d | 152.03 ± 0.35 c | 226.63 ± 0.52 e | 117.94 ± 0.57 b | 23.06 ± 0.28 a | 355.00 ± 7.07 f | |
| 10 | NA | 280 | 16.38 | 31.69 ± 0.92 c | 28.16 ± 0.23 b | 48.22 ± 0.46 d | 31.22 ± 0.31 c | 17.32 ± 0.46 a | 61.99 ± 0.01 e | |
| 11 | NAR | 280 | 23.32 | 2.66 ± 0.14 c | 3.06 ± 0.09 d | 0.30 ± 0.00 a | 0.89 ± 0.01 b | 2.60 ± 0.06 c | 1.00 ± 0.01 b | |
| 12 | ISO | 370 | 13.84 | 75.11 ± 1.20 d | 87.42 ± 0.60 e | 10.53 ± 0.30 a | 70.63 ± 0.52 c | 103.22 ± 0.32 f | 22.28 ± 0.40 b | |
| 13 | MYR | 370 | 18.96 | 12.09 ± 0.13 d | 11.72 ± 0.23 c | 1.36 ± 0.03 a | 1.37 ± 0.01 a | 1.04 ± 0.06 a | 2.19 ± 0.04 b | |
| 14 | LUT | 370 | 21.66 | 5.43 ± 0.21 d | 5.78 ± 0.03 d | 1.38 ± 0.01 a | 2.75 ± 0.07 b | 2.63 ± 0.11 b | 3.16 ± 0.14 c | |
| 15 | QUE | 370 | 21.83 | 28.89 ± 0.21 e | 24.50 ± 0.03 d | 3.06 ± 0.08 a | 12.82 ± 0.14 c | 13.34 ± 0.49 c | 5.94 ± 0.08 b | |
| Reference Microorganisms | Fetească Neagră | Tămâioasă Românească | ||||
|---|---|---|---|---|---|---|
| FNT | FNE | FNS | TRT | TRE | TRS | |
| Bacteria | ||||||
| B. cereus ATCC 11778 | ++ | ++ | ++ | ++ | ++ | ++ |
| Ent. faecalis ATCC 29212 | ++ | ++ | ++ | ++ | ++ | ++ |
| Ent. faecium ATCC 6057 | ++ | ++ | ++ | ++ | ++ | ++ |
| Ent. hirae ATCC 10541 | ++ | ++ | ++ | ++ | ++ | ++ |
| L. innocua ATCC 33090 | +++ | +++ | +++ | +++ | +++ | +++ |
| L. ivanovii ATCC 19119 | +++ | +++ | +++ | +++ | +++ | +++ |
| L. monocytogenes ATCC 7644 | +++ | +++ | +++ | +++ | +++ | +++ |
| S. aureus ATCC 33592 MRSA | +++ | +++ | +++ | +++ | +++ | +++ |
| S. aureus ATCC 6538 | +++ | +++ | ++ | +++ | +++ | +++ |
| S. epidermidis ATCC 51625 MRSE | +++ | +++ | +++ | +++ | +++ | +++ |
| S. epidermidis ATCC 12228 | +++ | +++ | +++ | +++ | +++ | +++ |
| S. pyogenes ATCC 19615 | +++ | +++ | +++ | +++ | +++ | ++ |
| R. equi ATCC 6939 | +++ | +++ | +++ | +++ | +++ | +++ |
| E. coli ATCC 8739 | - | - | - | - | - | - |
| P. aeruginosa ATCC 27853 | +++ | +++ | +++ | +++ | +++ | +++ |
| S. enterica Typhimurium ATCC 14028 | - | - | - | - | - | - |
| S. enterica Enteritidis ATCC 13076 | - | - | - | - | - | - |
| S. marcescens ATCC 14756 | +++ | +++ | +++ | ++ | ++ | ++ |
| Fungi | ||||||
| Candida albicans ATCC 10231 | - | - | - | - | - | - |
| C. glabrata ATCC 2001 | - | - | - | - | - | - |
| C. parapsilopsis ATCC 20019 | - | - | - | - | - | - |
| C. tropicalis ATCC 44508 | - | - | - | - | - | - |
| Strains | FNT | FNE | FNS | TRT | TRE | TRS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| B. cereus ATCC 11778 | 8.92 ± 0.00 | 8.92 ± 0.00 | 9.17 ± 0.00 | 9.17 ± 0.00 | 9.75 ± 0.00 | 9.75 ± 0.00 | 7.13 ± 0.00 | 7.13 ± 0.00 | 8.00 ± 0.00 | 8.00 ± 0.00 | 13.30 ± 0.00 | 13.30 ± 0.00 |
| Ent. faecalis ATCC 29212 | 8.92 ± 0.00 | 8.92 ± 0.00 | 4.58 ± 0.00 | 4.58 ± 0.00 | 2.44 ± 0.00 | 2.44 ± 0.00 | 3.56 ± 0.00 | 3.56 ± 0.00 | 4.00 ± 0.00 | 4.00 ± 0.00 | 1.66 ± 0.00 | 1.66 ± 0.00 |
| Ent. faecium ATCC 6057 | 2.23 ± 0.00 | 2.23 ± 0.00 | 4.58 ± 0.00 | 4.58 ± 0.00 | 2.44 ± 0.00 | 2.44 ± 0.00 | 7.13 ± 0.00 | 7.13 ± 0.00 | 16.00 ± 0.00 | 16.00 ± 0.00 | 13.30 ± 0.00 | 13.30 ± 0.00 |
| Ent. hirae ATCC 10541 | 4.46 ± 0.00 | 4.46 ± 0.00 | 2.29 ± 0.00 | 2.29 ± 0.00 | 2.44 ± 0.00 | 9.75 ± 0.00 | 3.56 ± 0.00 | 3.56 ± 0.00 | 16.00 ± 0.00 | 16.00 ± 0.00 | 13.30 ± 0.00 | 13.30 ± 0.00 |
| L. ivanovii ATCC 19119 | 2.23 ± 0.00 | 2.23 ± 0.00 | 2.29 ± 0.00 | 2.29 ± 0.00 | 2.44 ± 0.00 | 9.75 ± 0.00 | 1.78 ± 0.00 | 3.56 ± 0.00 | 4.00 ± 0.00 | 4.00 ± 0.00 | 1.66 ± 0.00 | 6.63 ± 0.00 |
| L. monocytogens ATCC 7644 | 2.23 ± 0.00 | 2.23 ± 0.00 | 2.29 ± 0.00 | 2.29 ± 0.00 | 2.44 ± 0.00 | 2.44 ± 0.00 | 3.56 ± 0.00 | 3.56 ± 0.00 | 2.00 ± 0.00 | 2.00 ± 0.00 | 6.63 ± 0.00 | 6.63 ± 0.00 |
| L. innocua ATCC 33090 | 2.23 ± 0.00 | 2.23 ± 0.00 | 2.29 ± 0.00 | 2.29 ± 0.00 | 2.44 ± 0.00 | 2.44 ± 0.00 | 3.56 ± 0.00 | 3.56 ± 0.00 | 4.00 ± 0.00 | 4.00 ± 0.00 | 6.63 ± 0.00 | 6.63 ± 0.00 |
| S. aureus ATCC 33592 MRSA | 1.15 ± 0.00 | 1.15 ± 0.00 | 2.29 ± 0.00 | 2.29 ± 0.00 | 1.22 ± 0.00 | 1.22 ± 0.00 | 3.56 ± 0.00 | 3.56 ± 0.00 | 8.00 ±0.00 | 8.00 ± 0.00 | 1.66 ± 0.00 | 1.66 ± 0.00 |
| S. aureus ATCC 6538 | 4.46 ± 0.00 | 4.46 ± 0.00 | 2.29 ± 0.00 | 2.29 ± 0.00 | 1.22 ± 0.00 | 9.75 ± 0.00 | 1.78 ± 0.00 | 1.78 ± 0.00 | 8.00 ± 0.00 | 8.00 ± 0.00 | 0.83 ± 0.00 | 6.63 ± 0.00 |
| S. epidermidis ATCC 51625 MRSE | 2.23 ± 0.00 | 4.46 ± 0.00 | 1.15 ± 0.00 | 4.58 ± 0.00 | 2.44 ± 0.00 | 1.22 ± 0.00 | 1.78 ± 0.00 | 1.78 ± 0.00 | 4.00 ± 0.00 | 4.00 ± 0.00 | 1.66 ± 0.00 | 3.31 ± 0.00 |
| S. epidermidis ATCC 12228 | 2.23 ± 0.00 | 4.46 ± 0.00 | 2.29 ± 0.00 | 2.29 ± 0.00 | 2.44 ± 0.00 | 1.22 ± 0.00 | 3.56 ± 0.00 | 3.56 ± 0.00 | 8.00 ±0.00 | 8.00 ± 0.00 | 1.66 ± 0.00 | 3.31 ± 0.00 |
| Streptococcus pyogens ATCC 19615 | 0.28 ± 0.00 | 0.56 ± 0.00 | 0.29 ± 0.00 | 0.29 ± 0.00 | 0.31 ± 0.00 | 0.31 ± 0.00 | 0.45 ± 0.00 | 0.45 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 0.41 ± 0.00 | 0.41 ± 0.00 |
| R. equi ATCC 6939 | 8.92 ± 0.00 | 8.92 ± 0.00 | 0.29 ± 0.00 | 0.29 ± 0.00 | 1.22 ± 0.00 | 1.22 ± 0.00 | 0.89 ± 0.00 | 0.89 ± 0.00 | 4.00 ± 0.00 | 4.00 ±0.00 | 1.66 ± 0.00 | 1.66 ± 0.00 |
| P. aeruginosa ATCC 27853 | 4.46 ± 0.00 | 4.46 ± 0.00 | 4.58 ± 0.00 | 4.58 ± 0.00 | 2.44 ± 0.00 | 2.44 ± 0.00 | 3.56 ± 0.00 | 3.56 ± 0.00 | 4.00 ± 0.00 | 4.00 ± 0.00 | 3.31 ± 0.00 | 3.31 ± 0.00 |
| Serratia marcescens ATCC 14756 | 4.46 ± 0.00 | 4.46 ± 0.00 | 2.29 ± 0.00 | 2.29 ± 0.00 | 4.88 ± 0.00 | 4.88 ± 0.00 | 3.56 ± 0.00 | 3.56 ± 0.00 | 8.00 ±0.00 | 8.00 ± 0.00 | 6.63 ± 0.00 | 6.63 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grosu, A.C.; Diguță, F.C.; Pristavu, M.-C.; Popa, A.; Badea, F.; Dragoi Cudalbeanu, M.; Orțan, A.; Dopcea, I.; Băbeanu, N. Exploring the Phytochemical Profiles, and Antioxidant and Antimicrobial Activities of the Hydroethanolic Grape Pomace Extracts from Two Romanian Indigenous Varieties. Fermentation 2024, 10, 470. https://doi.org/10.3390/fermentation10090470
Grosu AC, Diguță FC, Pristavu M-C, Popa A, Badea F, Dragoi Cudalbeanu M, Orțan A, Dopcea I, Băbeanu N. Exploring the Phytochemical Profiles, and Antioxidant and Antimicrobial Activities of the Hydroethanolic Grape Pomace Extracts from Two Romanian Indigenous Varieties. Fermentation. 2024; 10(9):470. https://doi.org/10.3390/fermentation10090470
Chicago/Turabian StyleGrosu, Alexandru Cristian, Filofteia Camelia Diguță, Mircea-Cosmin Pristavu, Aglaia Popa, Florentina Badea, Mihaela Dragoi Cudalbeanu, Alina Orțan, Ioan Dopcea, and Narcisa Băbeanu. 2024. "Exploring the Phytochemical Profiles, and Antioxidant and Antimicrobial Activities of the Hydroethanolic Grape Pomace Extracts from Two Romanian Indigenous Varieties" Fermentation 10, no. 9: 470. https://doi.org/10.3390/fermentation10090470
APA StyleGrosu, A. C., Diguță, F. C., Pristavu, M.-C., Popa, A., Badea, F., Dragoi Cudalbeanu, M., Orțan, A., Dopcea, I., & Băbeanu, N. (2024). Exploring the Phytochemical Profiles, and Antioxidant and Antimicrobial Activities of the Hydroethanolic Grape Pomace Extracts from Two Romanian Indigenous Varieties. Fermentation, 10(9), 470. https://doi.org/10.3390/fermentation10090470







