Abstract
The Jerez (Sherry) vinegars, including Vinagre de Jerez, Reserva, and Gran Reserva, are crafted from Sherry wines and are protected under the Denomination of Origin in Spain. The aim of this study was to (i) characterize the physicochemical properties and composition; (ii) investigate the impact of the aging process on color properties, phenolics, volatile compounds, and sensorial profiles; and (iii) find a marker for tracing the authenticity of non-aged (Apto) and aged Jerez vinegars. The phenolic components were identified through LC-MS/MS, whereas the volatile compounds were examined using the GC-MS/MS technique. As the aging progressed, a decrease was observed in the levels of flavonol and phenolic acids, with anthocyanin components being undetectable in non-aged and aged samples. In the Gran Reserva variety, 2-methylbutyl acetate, acetic acid, and ethanol emerged as the predominant volatile substances. The presence of oaklactone and ethyl butanoate components served as marker substances to authenticate the Gran Reserva. Additionally, alterations in color properties were noted, marked by a decrease in yellow content and an increase in the red component depending on aging. Furthermore, novel sensory descriptors, such as vanilla, clove, woody, and nutty notes, and winy character emerged in the samples with prolonged aging.
1. Introduction
Wine vinegar is an enological product that is highly appreciated by the consumer, and it has different varieties depending on the raw materials used and the preparation process. Jerez (also known as Sherry) wine vinegar is one of the most well-known products of this type in the world []. Jerez vinegar (JV) is a very valuable and high-quality product produced in the Vinagre de Jerez Denomination of Origin (DO) (geographical indication of origin “Vinagre de Jerez”) region in the southwest of Spain, protected by Spain and Europe, and of primary importance in the region of origin []. Besides JV, the other wine vinegars produced and marketed under a specific Protected Designation of Origin (PDO) are “Vinagre de Condado de Huelva” and “Vinagre de Montilla-Moriles” []. JV, produced under geographically indicated regions, is obtained from Sherry wines produced from Palomino, Pedro Ximenez, and/or Muscatel grapes produced by following the traditional aging method called “Soleras and Criaderas” [].
Jerez vinegars have an extraordinary organoleptic property (bright, highly aromatic, and rich) because of the traditional production technique. This special production technique includes a dynamic aging system. In this method, Jerez vinegars are aged in wooden barrels with an oxidative process simultaneously with a slow acetification process. As aging and acetification occur simultaneously in oak barrels, major changes in composition and sensory properties are observed throughout the process. Meanwhile, depending on the aging period in barrels, Jerez vinegars are called “Vinagre de Jerez (VJ)” for at least 6 months to 2 years, “Reserva” for at least 2 years to 10 years, and “Gran Reserva” for at least 10 years and more []. Analyzing the chemical changes that occur in vinegars with such a long aging process and evaluating their effects on sensory properties are also particularly important in terms of studying and revealing the effects of acetification and oxidation processes in vinegar.
In the analysis of aged vinegars, volatile compounds serve as significant indicators of their distinctive properties []. In this context, to characterize the olfactory impact of odorants, gas chromatography–mass spectrometry–olfactometry (GC-MS-O) has been studied for just the “Reserva” category (aged up to two years) [], for Shanxi aged vinegar [], and for Italian balsamic vinegar []. On the other hand, the volatile compounds and aroma composition have been analyzed from the “VJ” type (aged up to six months) using the techniques of headspace sorptive extraction (HSSE)-GC-MS, liquid–liquid extraction GC-MS (LLE-GC-MS), and GC-FID by several pieces of research [,].
Moreover, volatile and aroma compositions, anthocyanin profiles of vinegar during fermentation, and the aging process in particular remain unknown []. Compared to volatile component analysis, anthocyanin analysis studies in vinegar are less common. Anthocyanin composition was analyzed in Cabernet Sauvignon wine vinegar [], Condado de Huelva, and Montilla Moriles [] by means of liquid chromatography coupled to a diode array detector.
On the other hand, colorimetric characteristics and sensorial profiles of aged vinegars are another component for identifying aged vinegars. Especially the sensorial profile of aged vinegars is affected highly by the fermentation process, oxidation process, and the structure of storage containers, such as different kinds of wood casks [,,,,,,]. In addition, colorimetric features from the physicochemical qualities of aged vinegars show a significant change in terms of visual color because of the transformation of bioactive compounds [,,,,]. As a result, during the microbiological and aging processes of wine vinegars, they undergo various influences and reactions, resulting in a new product with a transformed character. As mentioned earlier, some of these changes have been examined in a few Jerez samples, specifically looking at volatile compounds in vinegars aged for 6 months and 2 years. However, essential bioactive components such as anthocyanins, additional physicochemical properties (colorimetric), and the sensory profile of both non-aged and aged Jerez vinegars remain unexplored in the literature despite their importance for thorough characterization.
Consequently, the objectives of the study were (i) to determine the content and composition of coloring agents (red, yellow, and blue), anthocyanins, flavonol, volatile compounds, and sensory profiles in non-aged (Apto) and all types of Jerez vinegars (VJ, Reserva, and Gran Reserva) aged for different years in oak barrels; (ii) to show the effect of acetification and the aging process on other colorimetric characteristics (color intensity, color density, and tonality), phenolics, volatile compounds, and sensory profiles; and (iii) to propose a new approach to the authentication of Jerez vinegars by following new distinctive markers of aged vinegars.
2. Materials and Method
2.1. Reagents and Chemicals
Methanol, acetonitrile (all LC-MS grade, ≥99.9%), sodium chloride and 1-hexanol (all reagent grade, 98%) for chromatographic analysis were purchased from Panreac Applichem (Darmstadt, Germany). Formic acid (LC-MS grade, ≥99.9%) was supplied from VWR Chemicals BDH (Leuven, Belgium). Anthocyanins: Cyanidin-3-O-glucoside, delphinidin-3-O-glucoside, malvidin-3-O-glucoside, pelargonidin-3-O-glucoside, peonidin-3-O-glucoside, petunidin-3-O-glucoside, cyanidin-3,5-di-O-glucoside, delphinidin-3,5-di-O-glucoside, malvidin-3,5-di-O-glucoside, pelargonidin-3,5-di-O-glucoside, peonidin-3,5-di-O-glucoside; malvidin-3-O-galactoside, cyanidin-3-O-rutinoside, cyanidin-3-O-arabinoside, and pelargonidin-3-O-rutinoside; flavonol: myricetin-3-O-glucoside, quercetin-3-O-glucoside, quercetin-3-O-galactoside, quercetin-3-O-rutinoside, quercetin-3-O-glucuronide, and quercetin-3-O-glucopyranoside; flavone: luteolin-7-O-glucoside; phenolic acid: caffeic acid, neochlorogenic acid, chlorogenic acid, and gallic acid; flavanone: eriotricin, hesperidin, cynarine, and rutin; and stilbene: (E)-resveratrol were purchased from Cymit Química (Barcelona, Spain).
2.2. Vinegar Samples
Four traditional Jerez wine vinegar samples non-aged (young) and aged at different periods (VJ (Crianza), Reserva, and Gran Reserva) were kindly provided by a Jerez vinegar producer in the Jerez de la Frontera district of Cádiz, Spain. The Jerez vinegar was obtained from Sherry wine produced with Palomino grapes. All of the samples were provided in duplicate (from two batches of the same kind of vinegar).
All Jerez vinegar samples can be produced only using the origin of Sherry wines under the Protected Designation of Origin (PDO) and European system with the application of different aging periods defined by the PDO. Jerez vinegar is aged in American oak wooden casks according to a process known as the “Criadera y Solera” system, and the analyzed samples were aged using this technique. According to the PDO, the aging period of Sherry wine vinegars must be processed as follows: (i) VJ (Crianza) vinegars must have an aging period of at least 6 months to 2 years with residual alcohol <3% (v/v) and total acidity >70 g/L acetic acid; (ii) the other sample, Reserva vinegar, can have a range from 2 to 10 years for the aging period with residual alcohol <3% (v/v) and total acidity >70 g/L acetic acid; and for the last Jerez vinegar, (iii) Gran Reserva must be aged for more than 10 years with less than 3% (v/v) residual alcohol and total acidity >80 g/L acetic acid.
2.3. Anthocyanins and the Other Polyphenol Compositions (LC-MS/MS)
Vinegar samples were diluted (1:1, w/v) by the addition of an extractant composed of methanol/water/formic acid (80:19.9:0.1, v/v). After that, diluted vinegar samples were filtered through a 0.45 µm pore size membrane filter before injection in the LC-MS/MS system. Anthocyanins and polyphenol composition analyses were performed on a Shimadzu LC-MS/MS 8050 triple quadrupole mass spectrometer (Shimadzu Corporation, Kyoto, Japan) equipped with an electrospray ionization (ESI) as the source operating in negative and positive modes. The method of LC-MS/MS analysis was carried out according to the procedure performed in an article by Uysal et al. []. The mobile phase for anthocyanins and polyphenols consisted of two solvents: (i) Solvent A, water/formic acid (99.9:0.1, v/v), and (ii) Solvent B, acetonitrile/formic acid (99.9:0.1, v/v). Anthocyanin compounds were eluted under the following conditions: 0.4 mL min−1 flow rate and 50 °C, isocratic conditions for 2 min with 95% A, from 2 to 10 min linear gradient of 5% to 95% acetonitrile with 0.1% formic acid (B); isocratic conditions with solvent B continued between 10 and 11 min and then returned to initial conditions of 95% A for 1 min and isocratic conditions with 95% of 1% aqueous formic acid for 4 min followed by washing and reconditioning the column. On the other hand, the elution conditions of polyphenol compounds were as follows: 0.4 mL min−1 flow rate and 40 °C, isocratic conditions for 2 min with 90% A, from 2 to 17 min linear gradient of 10% to 100% acetonitrile with 0.1% formic acid (B), and then returned to initial conditions of 90% A for 4 min, followed by washing and reconditioning the column. The sample volume injected was 10 µL both anthocyanin and polyphenol compounds. Two MS experiments were implemented for anthocyanins in positive mode and for polyphenols in negative mode before and after fragmentation. The MS conditions were as follows: Analyses were performed with full scan mode, and data-dependent MS scanning from m/z 100 to 1800, via collision-induced fragmentation experiments carried out using argon as the collision gas. The capillary voltage was 4.0 kV. The capillary temperature was set to 300 °C, and the source heater temperature was 250 °C, while the desolvation gas flow rate was 160 L/h. Characterization of the single components was performed considering retention time and the accurate molecular masses. In addition, concentrations of the individual compounds were determined by external calibration with corresponding standard compounds. The stock solution of all individual standards was prepared with the extractant in the range of concentrations of 0.1, 0.3, 0.5, 0.8, and 1 mg/L. The limit of detection (LOD) and limit of quantification (LOQ) values for the present chromatographic method were determined at a signal-to-noise ratio (S/N) of 3 and 10, respectively. The analysis for anthocyanin and polyphenols was performed in duplicate for each sample.
2.4. Volatile Analysis by Gas Chromatography–Mass Spectrometry (GC-MS)
The volatile substances of vinegar samples were determined by using a gas chromatography (GC-2030)–mass spectrometer (GCMS-TQ8040 NX, Shimadzu Corporation, Kyoto, Japan) coupled with the head space solid-phase microextraction (HS-SPME) technique. Volatile analysis was performed with a procedure described in one study [] with a small modification. Before analyzing, samples were prepared briefly as follows: A 10 mL aliquot of the vinegar sample (10 mL) was transferred into a 20 mL vial, followed by the addition of 100 μL of 1-hexanol as internal standard. The sample was saturated by adding 1 g of sodium chloride). After that, the vial was tightly capped with a polypropylene cap PTFE/silicone septum. The sample was extracted at a constant temperature of 40 °C for 40 min with agitation (250 rpm) in an AOC-6000 Plus autosampler (Shimadzu Corporation, Kyoto, Japan) with SPME capability. Separation and identification of analytes were performed with both InertCap Pure-WAX (30 m × 0.25 mm × 0.25 μm) and Sapiens X5MS (30 m × 0.25 mm × 0.25 μm) capillary columns (Teknokroma, Barcelona, Spain). Following extraction, volatile analytes were desorbed from DVB/CAR/PDMS fiber coating in the injection port of the GC at 210 °C (Pure-WAX) and at 230 °C (X5MS) for 1 min in 1:10 split mode. The condition of column temperature for both columns was adjusted as follows: initial temperature of 50 °C, held for 1 min, followed by increases to 100 °C at a rate of 2 °C/min, increasing by 3 °C/min to 180 °C, and increases from 180 to 230 °C at a rate of 20 °C/min, and then this temperature was held constant for 5 min. Helium was used as the carrier gas at 0.6 mL/min. The MS conditions of the Pure-WAX column were as follows: capillary direct interface temperature, 240 °C; ion source temperature, 210 °C; ionization mode was positive; and m/z scan range, 40–400 amu. The differences in the injection port and MS conditions of the other column were as follows: capillary direct interface temperature, 280 °C, and ion source temperature, 230 °C.
Identification of the compounds was performed based on the retention index, comparison of EI mass spectra, and C6–C40 alkane (Sigma-Aldrich, Steinheim, Germany) mixing. Each volatile compound was determined by using the relative area to the internal standard (1-hexanol, 1000 mg/L) of each compound. The relative concentration of an analyte = peak area of compound × concentration of internal standard/peak area of the internal standard. The analysis of each sample was performed in triplicate.
2.5. Chromatic Characteristics of Sherry Vinegars
The chromatic characteristics of (i) tonality, (ii) color intensity, and (iii) color density in the Jerez vinegar samples were examined according to the procedure described in []. Absorbance measurements were taken using a UV–visible spectrophotometer (UV-1280, Shimadzu Corporation, Kyoto, Japan). Colorimetric properties of vinegar samples were measured at wavelengths of 420 nm (yellow components), 520 nm (red components), and 620 nm (blue components). From these measurements, the color intensity (IC), tonality (T), and color density (D) were calculated with the following formulas:
IC = A420 + A520 + A620
T = A420/A520
D = A420 + A520
The Glories color index percentages of yellow (Y %), red (R %), and blue (B %) of the vinegars were calculated using the data obtained at 420, 520, and 620 nm []. The measurements of colorimetric properties were performed in triplicate.
2.6. Descriptive Sensory Analysis with Trained Personnel
Nine panelists consisting of five females and four males, aged between 28 and 60 years, assessed Jerez vinegar samples at the facilities of the Food Quality and Safety (CSA) research group of Universidad Miguel Hernández de Elche (UMH). The descriptors (attributes) and questionnaire have been created according to the Foundation of Organization of Evaluation Conformity and Food Certification (OECCA, Cádiz, Spain) [] and a study described in an article by Tesfaye et al. []. While samples of 20 mL of vinegar diluted three times with water were served in a non-transparent standard wine-tasting cup for odor analysis (detection of volatile compounds with vinegar outside the mouth), samples of 20 mL of vinegar diluted five times with water were also served for flavor analysis [combination of odor, aroma (detection of volatile compounds with vinegar inside the mouth), basic tastes, and chemical feeling factors] and global attributes. On the other hand, for appearance analysis, samples were served in transparent cups. Sensorial analyses were performed in room conditions (18–20 °C) and under white light. The samples were coded with 3-digit numbers and served one by one with the appropriate questionnaire and randomly, waiting for 5 min between samples. For palate cleansing, water and unsalted crackers were offered to the panelists between the samples. The attributes of sensorial evaluation were as follows: odor (odor intensity, vinegar ID, winy character, raisin, ethyl acetate (chemical), alcohol/liquor, woody, fruity, spicy, vanilla, clove, toasted, nuts, and leather/old); flavor (odor intensity, vinegar ID, winy character, raisin, ethyl acetate (chemical), alcohol/liquor, woody, fruity, spicy, vanilla, clove, toasted, nuts, and leather/old); basic tastes (sweetness, sourness, and bitterness); chemical sensations (astringent and pungent); global attributes (aftertaste); appearance (color and untuoso (texture)); and defects (dirty (sucio), bacteria, cheese, and sawdust (wood shavings)). Panelists used a 0 to 10 scale for assessment, where 10 is extremely high intensity and 0 is extremely low intensity or unnoticeable.
2.7. Statistical Analysis
All experiments were performed in triplicate, and the results are presented as mean ± standard deviation (SD). Statistical analysis was performed using XLSTAT Premium version 2016 (Addingsoft, Barcelona, Spain). The one-way analysis of variance (ANOVA) test was applied to examine the significant differences in chromatic characteristics, anthocyanins, polyphenols, and volatile compounds of the vinegar samples. Then, Tukey’s multiple range tests were applied for the post hoc test, where the effect is commonly considered significant if the resulting p-value is below 0.05.
3. Results and Discussion
3.1. Phenolic Compounds
The anthocyanin, t-resveratrol (trans-stilbene), flavanones, flavonols, and phenolic acid compounds were determined using the positive mode of ESI, which has a mode of flavylium cations under acidic conditions. Table 1 shows the phenolic compounds identified and quantified in the vinegar samples. In anthocyanin analysis, fifteen different derivatives of major mono-anthocyanin compounds were analyzed by LC-MS/MS, but only six forms of these compounds, which are peonidin-3,5-di-O-glucoside, cyanidin-3-O-rutinoside, pelargonidin-3-O-glucoside, peonidin-3-O-glucoside, malvidin-3-O-glucoside, and malvidin-3-O-galactoside, could be detected with a trace amount in some of the vinegar samples. However, as can be seen from the table, none of the anthocyanin compounds were found even in trace amounts in Gran Reserva samples.

Table 1.
Retention time, mass spectral characteristics, and concentration of anthocyanins and other polyphenols (µg/L) present in Jerez vinegars.
Grapes are the unique source of these compounds. On the other hand, anthocyanins are not stable substances and can be degraded under many kinds of processes. First, the vinification process of wine production promotes several reactions between anthocyanins and other molecules, such as pyruvic acid, vinyl phenol, acetone, and glyoxylic acid, leading to a decrease in free anthocyanin contents and copolymerization reactions between tannins and anthocyanins [,]. Second, the acetification process of Sherry wine to a vinegar product [] was observed with a decrease (~50%) in the content of monomeric anthocyanins during the acetification of red wine. In many studies, the acetification process was related to a decrease in the total phenolic content of 13%. Surprisingly, most of the individual phenolic compounds, the anthocyanins, showed a much higher decrease of ~50% due to low oxidizable tannins contributing to the total phenols. Moreover, it was stated that the concentration of the monomeric phenols might be reduced by oligo- and polymerization [,,], yielding products that still contribute to the total phenol content. Therefore, these mechanisms might explain why vinegar samples include a lower content of anthocyanins than wine products. Obviously, while these processes are taking place, there are many conditions, such as temperature, pH, ethanol content, and the presence of oxygen, that can affect the degradation of anthocyanins. Although four anthocyanin compounds were found in trace amounts in the Apto sample as seen in the table, this result contributed to the reduction in anthocyanin compounds during the acetification process. This can be explained by the fact that Apto also contains lower amounts of anthocyanins. Another raw substance obtained from grapes is t-resveratrol. This component was detected only in the VJ sample with a content of 25.04 µg L−1. This result can be explained by the acetification process of red wine vinegar and the loss of t-resveratrol in wood. Additionally, this may be due to polymerization and precipitation [,].
A statistically significant decrease and difference was found for flavonol and flavone compounds of the Apto to Gran Reserva samples depending aging period, for instance, quercetin-3-O-glucoside (from 153.89 µg/L to ND), quercetin-3-O-glucuronide (1806.09 to 61.44 µg/L), quercetin-3-O-galactoside (239.92 µg/L to ND), quercetin-3-O-glucopyranoside (from 79.71 µg/L to ND), and luteolin-7-O-glucoside (from 7.28 to 1.77 µg/L), which have been identified for the first time in Jerez samples. It can be stated that there is a significant effect of aging on flavonol compounds even in wood stored for at least six months (VJ). It can be concluded that some reactions between phenolic molecules reduce the flavonoid content of the samples depending on aging time, which can be explained by some reactions taking place between phenolic molecules. One of these reactions may occur by the polycondensation mechanism between flavonoid and tannin compounds and lead to the loss of monomeric flavonoid compounds []. Moreover, gallic acid and caffeic acid, among the phenolic acid derivatives, showed a significant decrease in the content of the VJ and Gran Reserva samples from 539.01 to 177.05 µg/L and 992.92 to 92.73 µg/L, respectively. The decrease in these compounds might be explained by the second mechanism, which is the oxidation process during the aging period []. Moreover, the total phenolic compound content shows a sharp decrease depending on the aging period in the sample from Apto (3713.56 µg/L) to Gran Reserva (361.69 µg/L). A similar result was also obtained for aged balsamic vinegar samples with reduced phenolic compound content from 1500.6 to 1321.4 mg/kg []. On the other hand, an increase, although not significant, in the gallic content of Apto and Vinagre de Jerez samples was shown (from 421.84 to 539.01 µg/L) during the six-month aging period in a wood cask. This phenomenon is explained by the hydrolysis of gallic tannins to free gallic during the aging period. In a study, some phenolic compounds were analyzed in VJ and Reserva samples, and a similar increasing trend was obtained in the content of gallic acid []. Besides gallic acid, a similar trend was also detected for the caffeic acid component (907.27 to 992.92 µg/L) between Apto and VJ samples. In another study, the concentration of gallic acid was found to increase after 360 days of aging, and the increase in caffeic acid concentration in the first year was explained by the hydrolysis of their corresponding tartaric esters []. Additionally, eriotricin and hesperidin, from flavanone compounds, were detected for the first time in some Jerez samples. While eriotricin was determined as 65.82 and 84.67 µg/L in Apto and Reserva samples, hesperidin was detected as 65.05 µg/L in only Reserva.
Although the effect of aging on anthocyanins is not seen much, it is observed that it has a significant effect on other phenolic components. Major flavonol and phenolic acid compounds show statistical differences according to the aging process. In conclusion, as a notable study, many phenolic compounds from Jerez samples were identified and determined in this study. As a result of this analysis, the impact of aging on these compounds through a series of chemical interactions revealed that some chemical transformations occur between free and polymerized forms of anthocyanins, flavonol, and phenolic acids.
3.2. Volatile Compounds
Volatile compounds of vinegar samples were identified and quantified using GC-MS/MS by applying the HS-SPME technique. A total of 62 volatile compounds could be quantified by using DB-Wax and DB-5 columns, and the results are shown in Table 2. As can be seen from the table, 24 volatile compounds could be found for both columns, while the remaining 27 and 11 volatile compounds were identified only in the DB-Wax and DB-5 columns, respectively. Thus, it can be said that most of the compounds can be determined with the DB-Wax column. These volatile compounds are classified into seven chemical families: esters, acids, alcohols, aldehydes, ketones, lactones, phenolic compounds, and others (not in any specific family). The total contents of these chemical groups according to the samples are given in Figure 1.

Table 2.
Volatile compounds identified in Jerez vinegars (mg/L).
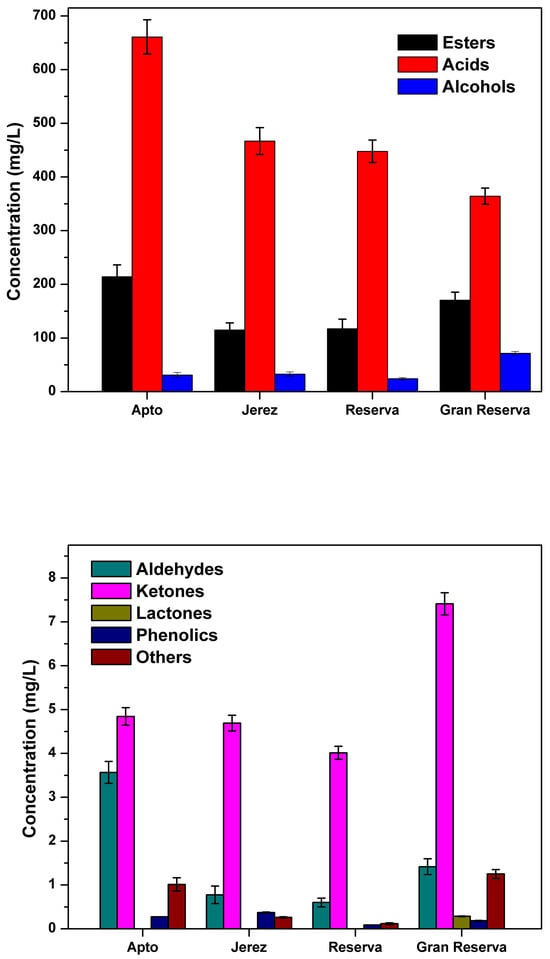
Figure 1.
Total concentration of chemical families of esters, acids, alcohols, aldehydes, ketones, lactones, phenolics, and others found in Jerez vinegar samples.
Acids and esters constitute the main volatile substances in the samples and show a notable change during the aging process. A decrease in the acid content by approximately half was observed due to aging (from Apto, 661.02, to Gran Reserva, 364.24 mg/L). A slight decrease in the amount of ester was detected from Apto, 214.09, to VJ, 115.06 mg/L, and then an increase again (from 115.06 to 170.43 mg/L, Gran Reserva) was found during the long aging process. Esters are the most diverse compounds with 31 substances, among the volatile compound families, and they give off flower, fruity, and rose-like odors. Ethyl acetate, phenethyl acetate, and ethyl octanoate were found to be the most abundant components in the Apto sample with the amount of 37.87, 23.64, and 15.20 mg/L, respectively. Esters are mostly produced during the fermentation process of vinegars by turning off ethanol and acid production [,]. Ethyl acetate, the main ester component, may be formed by the condensation of ethanol and acetic acid with the loss of water and was found in the range of 60–1451 mg/L in aged Jerez samples []. Ethyl acetate was also found in other types of aged vinegars, namely, Zhenjiang and Shanxi vinegar in increasing concentrations with aging [,]. However, in this study, ethyl acetate exhibited a declining profile with a content of 37.87 to 24.54 mg/L in the Gran Reserva sample, and this result can be explained because of volatilization or due to the change in esterification balance []. Some specific components have been identified for the first time in Jerez samples and can be used as a marker to authenticate the Gran Reserva sample, which are ethyl butanoate (with the aroma of banana and apple), 0.623 mg/L, and ethyl heptanoate, 0.190 mg/L. During post-processing of vinegar (aging), these compounds may be produced from the esterification process between ethanol-butanoic acid and ethanol-heptanoic acid. On the other side, (Z)-3-hexenol acetate was identified for the first time with an amount of 0.090 mg/L. Additionally, it can be accepted as an authenticator compound for the Reserva sample. A similar condensation reaction between acetic acid and hexanol can be stated in the formation of this compound during the aging period.
Secondly, as can be seen from the table, the most abundant chemical family in terms of quantity are acids with seven components. The most dominant component was obtained in acids and all chemical substances for acetic acid with a varying concentration of 634.95–352.84 mg/L from Apto to Gran Reserva samples, respectively. For acetic acid, a main flavor product of the acetification process of vinegar, a sharp decline in its content can be explained due to evaporation and condensation reactions with ethanol []. Moreover, a similar decrease in the concentration of acetic acid was observed during the aging of traditional balsamic vinegar in oak barrels []. The second main component is isovaleric acid with a content of 23.12 mg/L and 25.35 mg/L in the samples of Apto and Jerez, respectively. A quantity of 9.567 mg/L was quantified in Gran Reserva. Due to this observed decrease, it is believed that isovaleric acid is involved in the esterification reactions of short-chain carboxylic esters during the aging process []. As can be seen from Figure 1, during the aging time, it can be implied that all of these factors, including vaporization, esterification, and condensation reactions, play a significant role in the decrease in the content of acids.
In the case of alcohols, it has been observed that certain components detected in very small amounts decrease over time, such as α-terpineol and linalool (not found in Gran Reserva), while some main components found in higher amounts significantly increase, namely, ethanol (from 3.573 to 41.39 mg/L) and amyl alcohol (from 13.35 to 16.06 mg/L). Additionally, as can be seen from Figure 1, there is a significant sharp increase in the number of alcohols in the Gran Reserva sample. The aging period can have an impact on the ethanol content in certain types of vinegar. In one study, ethanol content was not detected in Sherry vinegar aged for 6 months []. In another study, the concentration of ethanol was found to show a declining profile during the aging period of traditional balsamic vinegar []. However, in this study, the statistically significant increase in the content of ethanol may have resulted from the hydrolysis of ethyl acetate into ethanol, resulting in the amount of ethyl acetate.
Four components, namely, acetaldehyde, valeraldehyde, furfural, and benzaldehyde, can be identified and quantified in low amounts in aldehydes. During the aging process, aldehydes exhibited a decreasing trend from Apto to Reserva (from 3.567 to 0.601 mg/L), followed by a modest increase from Reserva (0.601 mg/L) to Gran Reserva (1.417 mg L−1), as depicted in Figure 1. A statistically significant difference was observed for benzaldehyde. Initially, a decrease from 2.168 to 0.327 mg/L (between Apto and Jerez) was noted, followed by an increase to 0.495 mg/L in the Gran Reserva sample observed throughout the aging period. An increase in benzaldehyde between VJ and Gran Reserva samples may be explained by oxidation reactions and the decarboxylation of phenylalanine and methionine []. Benzaldehyde is also stated as one of the main flavor aldehyde components in many vinegars, such as Chinese traditional bran vinegar (Cupei), Italian balsamic vinegar, and Shanxi aged vinegar [,,]. A change in the concentration of acetaldehyde was found from 0.098 to 0.288 mg/L, which is an intermediary and is produced by ethanol oxidation, resulting in flavor differences []. One of the aromatic wood compounds, furfural, was found to have a content of 0.164 and 0.304 mg/L for Reserva and Gran Reserva, respectively. It may be extracted from the oak barrels during the aging process, and, additionally, furfural comes from the degradation of monosaccharides resulting from the partial hydrolysis of hemicellulose []. Like aldehydes, four ketone substances could be detected in all samples. While acetoin was the most abundant compound (3.036 mg/L) in the Apto sample, 2-acetylpropane was found to be the predominant compound in Gran Reserva, with its level increasing from 1.239 to 3.365 mg/L during the aging process. As in aldehydes, ketone substances exhibited a low decrease between Apto and Reserva samples but then a high increase in Gran Reserva samples, as shown in Figure 1. Over the aging time, the reason for the increase in the number of ketones can be explained by the Maillard reaction, that is, Strecker degradation [].
Oaklactone, identified for the first time in the Jerez vinegars, was exclusively detected in the Gran Reserva sample (0.286 mg/L). Its presence serves as an indicator of long aging in traditional wooden barrels and shares similarities with compounds extracted, such as whisky lactones [,]. Additionally, this component can be used as a marker for Gran Reserva samples. 2-Ethylphenol, a volatile phenol, was found at a content of 0.275 for the Apto sample and 0.183 mg/L for the Gran Reserva sample. Additionally, other compounds identified in different categories included 2-methyl-1,3-dioxane (0.253 mg/L), linalool 3,7-oxide (0.074 mg/L), and 2,4,5-trimethyl-1,3-dioxolane (0.924 mg/L) in the Gran Reserva samples.
As a result, the total content of volatile compounds exhibited a decreasing trend in the range of 915.72 to 616.60 mg/L from the Apto to Gran Reserva samples. This decline is indicative of various processes such as oxidation, esterification, wood extraction, hydrolysis, and fermentation playing a significant role during this period. In a previous study by Morales et al. [], 18 volatile compounds were identified in Sherry wine vinegars aged up to 24 months using gas chromatography coupled with a flame ionization detector (GC-FID). In the current study, the GC-HSPME fiber method was utilized, enabling the capture and identification of even minor volatile components in all samples.
This study identified an additional forty-four volatile compounds, totaling sixty-two volatile components in all samples, including the detection of these substances in Gran Reserva. While prior research extensively studied aroma components in aged vinegars to understand their complexity, certain unidentified volatile components remained undiscovered until this examination. The methodology utilized in this study facilitated the quantification of sixty-two volatile substances using two distinct types of columns, providing new insights into the comprehensive volatile profile of all Jerez vinegars.
3.3. Chromatic Characteristics
Absorbance measurements (A420, A520, and A620), color intensity (CI), tonality (T), color density (D), and yellow (%), red (%), and blue (%) percentages of vinegar samples were taken using UV-VIS spectroscopy. The values of all chromatic parameters of vinegar samples are shown in Table 3. The table illustrates a notable difference in color composition between non-aged and aged samples. The Apto sample exhibited the highest yellow pigment content (73%) but the lowest levels of red (21%) and blue pigments (6%) compared to other samples. Conversely, the Reserva sample displayed the lowest yellow pigment content (60%) and the highest levels of red (29%) and blue pigments (11%). These changes can be attributed to the increase in absorbance values at 420 nm (from 0.879 to 2.049) and at 520 nm (from 0.257 to 0.784), indicating an overall increase in color concentrations. However, the rise in red components may have proportionally exceeded that of yellow components during aging, resulting in this observed difference. The change in the color for these absorbance measurements might be related to Maillard and caramelization reactions and finalized with browning agents [,]. In a study on Sherry vinegar, absorbance measurements were taken at 470 nm to assess the impact of aging time. A simultaneous increase in absorbance values was observed, which was attributed to the oxidation of polyphenolic compounds during the aging process []. Furthermore, an increase in the blue pigment was observed in both Apto and aged samples, with percentages of 6% and 11% for Apto and Gran Reserva, respectively. The change in absorbance values at 420 nm, 520 nm, and 620 nm from Apto to Reserva indicated an overall increase in all CI values (from 1.210 to 4.676) and D values (from 1.136 to 4.164). In contrast, there was a decline in the T values of non-aged and aged samples, decreasing from 3.419 to 2.616 between the Apto and VJ samples. The increase in the T value may be attributed to the rise in red pigment and a decrease in yellow pigment values. The color characteristics of wine vinegar originate from grapes and the fermented product of wine. Grapes are rich in anthocyanins, tannins, and resveratrol compounds, which contribute to their unique color. However, as detailed in the phenolic section of the results, mono-anthocyanins were not detected even in the non-aged vinegar sample due to various reactions that occur during the acetification process, leading to the formation of anthocyanin derivatives. Furthermore, the aging process facilitates copolymerization reactions between tannins and anthocyanin derivatives, resulting in the loss of these monomeric compounds [].

Table 3.
The chromatic characteristics of Jerez vinegars are determined by Glories method.
Notably, even though there is a statistical difference in the yellow (60% to 62%) and red color pigments (29% to 27%) during long-term aging and storage, the change in absorbance values at 420 nm (from 2.825 to 2.786) and 520 nm (from 1.339 to 1.190) is less pronounced for Reserva and Gran Reserva samples, respectively.
3.4. Sensory Analysis
Jerez vinegar is officially renowned for its amber hue with mahogany undertones, offering subtle notes of nuts, wood, and a distinct acetic aroma. With prolonged aging, it develops intricate hints of vanilla, nuts, and matured wood. The Gran Reserva variant stands out for its intense aroma rich in nutty and spicy notes []. While certain characteristics have been acknowledged by regulators, this study delves into previously unexplored sensory descriptors, leading to the development of precise lexicons, some of which were refined in prior works []. These terminologies were further tailored to suit each vinegar variety, aiding in effectively characterizing the sensory profile of all Jerez vinegars using descriptive analysis. The sensory profile of Jerez vinegars, encompassing appearance (color and texture), aroma, flavor (sweetness, sourness, bitterness, pungency, and other specific descriptors), overall impression (aftertaste), and qualification (liking), including a total of 38 attributes, is presented in Table 4. In addition, consumer preferences were assessed under the qualification category. Table 4 reveals that in the appearance category, the darkest color, scoring 9.55 points, was observed in the Reserva samples. This outcome aligns with the color characteristics, indicating a high intensity of red pigments. Additionally, the non-aged sample secured the highest vinegar ID scores for odor (8.00) and flavor (8.05), likely due to its potentially higher acetic acid content in volatile compounds. During aging, many attributes showed a meaningful change in the odor feature. These differences mainly with an increasing quantity were observed in the attributes of winy character (from 1.27 to 7.16), raisin (0.27 to 4.27), liquor (0.11 to 2.38), woody (from 0 to 2.88), toasted (from 0 to 2.50), and nuts (from 0 to 0.72) but not fruity (from 6.61 to 4.05). An increase in the attributes of woody, toasted, and nut corresponded to the aging of the samples taking place in wood casks [,]. Although no statistically significant difference was found in the attributes of clove (0 to 0.33), spicy, and leather, distinctions were noted between the Reserva and Gran Reserva samples. Furthermore, no defects were identified in either the odor or taste categories.

Table 4.
Descriptive sensory analysis of “Jerez” vinegars.
Following odor identification, the flavor attributes exhibited a statistically significant change, particularly noted between the Apto and Gran Reserva samples. The highest winy character, scoring 5.06 points, was detected in the Gran Reserva sample. Additionally, woody attributes were tasted in the Gran Reserva sample, scoring 1.43 points. The woody character of the sample may stem from the (Z)-oaklactone compounds derived from the wood casks [,]. Through the aging process, a decrease in fruity taste (from 5.16 to 4.0), sourness (from 8.11 to 5.38), and pungent (from 3.77 to 2.22) profiles was observed between Apto and Gran Reserva samples. The decrease in the sourness profile may be attributed to the mostly acidic contributors, mainly acetic acid substances, in vinegar [,,]. The fruity profile in the Apto sample is attributed to the presence of fresh fruit notes from grapes in the volatile substances of ethyl esters and acetates []. As can be reviewed in volatile compounds, a significant decrease in many ester components, namely, 2-methylbutyl acetate, isobutyl acetate, and ethyl acetate, affects the change in the concentration of fruity notes in the aged samples. By the point of 0.38, Gran Reserva was identified as the most astringent sample akin to its winy character. An increase in the sweetness attribute was observed in both non-aged and Gran Reserva samples, with points ranging from 1.11 to 2.33. Moreover, the rise in alcohol notes in the Gran Reserva samples (1.81) correlates with the increase in alcohol content of volatile compounds like ethanol. In a separate study, certain attributes of the sensorial profile—such as aroma intensity, woody flavor, ethyl acetate, wine character, and pungent sensation—were examined in VJ samples aged up to six months. Consistent results were obtained with heightened winy character, woody notes, and aroma intensity in the Reserva sample aged up to 24 months []. To the best of my knowledge, the sensory profile of Gran Reserva, along with many new lexicons developed specifically for odor and flavor profiles, has been identified and examined in the analysis of Jerez vinegar in this study.
4. Conclusions
Jerez vinegars of varying maturation durations, including both aged (VJ, Reserva, and Gran Reserva) and non-aged varieties, have been subjected to a thorough analysis encompassing physicochemical properties, composition, and sensorial attributes. A unique facet of this investigation involves the discernment of chromatic features in the Jerez vinegar samples, with a focus on the proportional representation of color components, specifically CI and D. Comparative analysis revealed a notable shift in the hue profile, characterized by a diminishing yellow hue and an amplifying red hue across the aging continuum, attributed to the progressive accumulation of Maillard reaction byproducts. Over 40 volatile components present in Apto, VJ, and Reserva vinegar samples have been meticulously identified and quantified. Noteworthy trends emerged within the volatile compounds, with primary compounds such as esters (including ethyl acetate, 2-methylbutyl acetate, and isobutyl acetate), acids (i.e., acetic acid and isovaleric acid), and aldehydes (such as benzaldehyde) exhibiting a decrease in concentration over the aging process. Conversely, alcohols (specifically ethanol), ketones (including acetone and 2-acetylpropane), and lactone compounds displayed an augmentation in levels with prolonged aging, particularly evident in samples subjected to extended maturation periods.
However, the analysis of anthocyanin composition has presented challenges in quantification due to potential reactions occurring during acetification and oxidation processes that result in the loss of anthocyanin. Notably, phenolics have emerged as prominent components, particularly in the chemical family of flavonol substances within the samples. Furthermore, a comprehensive sensorial evaluation has been conducted across all samples, revealing a substantial impact of aging on various sensory descriptors, evidenced by numerous changes in lexicon terms. Noteworthy intensities were observed in woody, nutty, and wine-like characteristics, whereas fruity and vinegar notes displayed a notable decline. In summary, this investigation underscores the noteworthy influence of chemical reactions throughout fermentation and aging stages, shaping the physical and chemical attributes of aged vinegars and contributing significantly to defining the distinctive characteristics of the final product.
Funding
This work was supported partially by The Scientific & Technological Research Council of Turkey (TUBITAK), project number: 124O687 and the APC was funded partially by Istanbul Bilgi University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
Reyhan Selin Uysal thanks Esther Sendra, Ángel A Carbonell-Barrachina, and CIAGRO-UMH for their support during this study.
Conflicts of Interest
The author declares that there are no conflicts of interest.
References
- Palacios, V.; Valcárcel, M.; Caro, I.; Pérez, L. Chemical and biochemical transformations during the industrial process of sherry vinegar aging. J. Agric. Food. Chem. 2002, 50, 4221–4225. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, W.; García-Parrilla, M.; Troncoso, A. Sensory evaluation of Sherry wine vinegar. J. Sens. Stud. 2002, 17, 133–144. [Google Scholar] [CrossRef]
- Council Regulation (EC) No 510/2006 of 20 March 2006 on the Protection of Geographical Indications and Designations of Origin for Agricultural Products and Foodstuffs. 2006. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:093:0012:0025:en:PDF (accessed on 1 September 2024).
- Consejo Regulador del Vino de Jerez (2023, November 7). Sherry Wine. Available online: https://www.sherry.wine/sherry-wine (accessed on 1 September 2024).
- Parrilla, M.G.; Heredia, F.J.; Troncoso, A.M. Sherry wine vinegars: Phenolic composition changes during aging. Food Res. Int. 1999, 32, 433–440. [Google Scholar] [CrossRef]
- Al-Dalali, S.; Zheng, F.; Li, H.; Huang, M.; Chen, F. Characterization of volatile compounds in three commercial Chinese vinegars by SPME-GC-MS and GC-O. LWT 2019, 112, 108264. [Google Scholar] [CrossRef]
- Ríos-Reina, R.; Segura-Borrego, M.P.; Morales, M.L.; Callejón, R.M. Characterization of the aroma profile and key odorants of the Spanish PDO wine vinegars. Food Chem. 2020, 311, 126012. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Xie, J.; Hou, L.; Zhao, M.; Zhao, J.; Cheng, J.; Wang, S.; Sun, B.-G. Aroma constituents in Shanxi aged vinegar before and after aging. J. Agric. Food. Chem. 2016, 64, 7597–7605. [Google Scholar] [CrossRef]
- Corsini, L.; Castro, R.; Barroso, C.G.; Durán-Guerrero, E. Characterization by gas chromatography-olfactometry of the most odour-active compounds in Italian balsamic vinegars with geographical indication. Food Chem. 2019, 272, 702–708. [Google Scholar] [CrossRef]
- Morales, M.L.; Tesfaye, W.; García-Parrilla, M.C.; Casas, J.A.; Troncoso, A.M. Evolution of the aroma profile of sherry wine vinegars during an experimental aging in wood. J. Agric. Food. Chem. 2002, 50, 3173–3178. [Google Scholar] [CrossRef]
- Callejon, R.M.; Morales, M.L.; Ferreira, A.C.S.; Troncoso, A.M. Defining the typical aroma of sherry vinegar: Sensory and chemical approach. J. Agric. Food. Chem. 2008, 56, 8086–8095. [Google Scholar] [CrossRef]
- Cerezo, A.B.; Cuevas, E.; Winterhalter, P.; Garcia-Parrilla, M.C.; Troncoso, A.M. Anthocyanin composition in Cabernet Sauvignon red wine vinegar obtained by submerged acetification. Food Res. Int. 2010, 43, 1577–1584. [Google Scholar] [CrossRef]
- García-Parrilla, M.C.; Cerezo, A.B.; Tesfaye, W.; Troncoso, A.M. Phenolic compounds as markers for the authentication of Sherry vinegars: A foresight for high quality vinegars characterization. In Progress in Authentication of Food and Wine; ACS Publications: Washington, DC, USA, 2011; pp. 201–213. [Google Scholar]
- Gao, Y.; Jo, Y.; Chung, N.; Gu, S.-Y.; Jeong, Y.-J.; Kwon, J.-H. Physicochemical qualities and flavor patterns of traditional Chinese vinegars manufactured by different fermentation methods and aging periods. Prev. Nutr. Food Sci. 2017, 22, 30. [Google Scholar] [CrossRef] [PubMed]
- Giudici, P.; Gullo, M.; Solieri, L.; Falcone, P.M. Technological and microbiological aspects of traditional balsamic vinegar and their influence on quality and sensorial properties. Adv. Food Nutr. Res. 2009, 58, 137–182. [Google Scholar]
- Góamez, M.L.M.; Bellido, B.B.; Tesfaye, W.; Fernandez, R.M.C.; Valencia, D.; Fernandez-Pachón, M.S.; García-Parrilla, M.C.; González, A.M.T. Sensory evaluation of sherry vinegar: Traditional compared to accelerated aging with oak chips. J. Food Sci. 2006, 71, S238–S242. [Google Scholar] [CrossRef]
- Durán-Guerrero, E.; Castro, R.; García-Moreno, M.d.V.; Rodríguez-Dodero, M.d.C.; Schwarz, M.; Guillén-Sánchez, D. Aroma of sherry products: A review. Foods 2021, 10, 753. [Google Scholar] [CrossRef]
- Cejudo-Bastante, M.J.; Durán, E.; Castro, R.; Rodríguez-Dodero, M.C.; Natera, R.; García-Barroso, C. Study of the volatile composition and sensory characteristics of new Sherry vinegar-derived products by maceration with fruits. LWT-Food Sci. Technol. 2013, 50, 469–479. [Google Scholar] [CrossRef]
- González-García, J.A.; Cereceda, M.; Durán-Guerrero, E.; Rodríguez-Dodero, M.C.; Castro, R. Comparative study on the use of seasoned or unseasoned casks made of wood from different origins for the ageing of Sherry vinegar. J. Sci. Food Agric. 2024, 104, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Yao, J.; Zhang, J.; Duan, W.; Zhang, B.; Xie, X.; Xia, M.; Song, J.; Zheng, Y.; Wang, M. Evaluation of nutritional compositions, bioactive compounds, and antioxidant activities of Shanxi aged vinegars during the aging process. J. Food Sci. 2018, 83, 2638–2644. [Google Scholar] [CrossRef]
- Verzelloni, E.; Tagliazucchi, D.; Conte, A. Changes in major antioxidant compounds during aging of traditional balsamic vinegar. J. Food Biochem. 2010, 34, 152–171. [Google Scholar] [CrossRef]
- Durán-Guerrero, E.; Schwarz, M.; Fernández-Recamales, M.Á.; Barroso, C.G.; Castro, R. Characterization and differentiation of Spanish vinegars from jerez and condado de huelva protected designations of origin. Foods 2019, 8, 341. [Google Scholar] [CrossRef]
- Uysal, R.S.; Issa-Issa, H.; Sendra, E.; Carbonell-Barrachina, Á.A. Changes in anthocyanin pigments, trans-resveratrol, and colorimetric characteristics of Fondillón wine and other “Monastrell” wines during the aging period. Eur. Food Res. Technol. 2023, 249, 1821–1831. [Google Scholar] [CrossRef]
- Carbonell-Barrachina, Á.A.; Szychowski, P.J.; Vásquez, M.V.; Hernández, F.; Wojdyło, A. Technological aspects as the main impact on quality of quince liquors. Food Chem. 2015, 167, 387–395. [Google Scholar] [CrossRef] [PubMed]
- OIV. Compendium of international methods of wine and must analysis. In Proceedings of the International Organisation of Vine and Wine, Paris, France, 6 July 2021; p. 196. [Google Scholar]
- Glories, Y. The colour of red wines. Conn. Vigne Vin 1984, 18, 195–217. [Google Scholar]
- OECCA. Ficha de Cata de Vinagres. 2022. Available online: https://www.oecca.es/ (accessed on 1 September 2024).
- Ferreira, I.M.; Pérez-Palacios, M.T. Anthocyanic Compounds and Antioxidant Capacity in Fortified Wines. In Processing and Impact on Antioxidants in Beverages; Elsevier: Amsterdam, The Netherlands, 2014; pp. 3–14. [Google Scholar]
- Escribano-Bailón, T.; Dangles, O.; Brouillard, R. Coupling reactions between flavylium ions and catechin. Phytochemistry 1996, 41, 1583–1592. [Google Scholar] [CrossRef]
- Saucier, C.; Bourgeois, G.; Vitry, C.; Roux, D.; Glories, Y. Characterization of (+)-catechin− acetaldehyde polymers: A model for colloidal state of wine polyphenols. J. Agric. Food. Chem. 1997, 45, 1045–1049. [Google Scholar]
- Andlauer, W.; Stumpf, C.; Fürst, P. Influence of the acetification process on phenolic compounds. J. Agric. Food. Chem. 2000, 48, 3533–3536. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, X.; Yao, Y.; Qu, A.; Ding, K.; Zhao, G.; Liu, S.Q. Insights into the microbiota and driving forces to control the quality of vinegar. LWT 2022, 157, 113085. [Google Scholar] [CrossRef]
- Jiang, Y.; Lv, X.; Zhang, C.; Zheng, Y.; Zheng, B.; Duan, X.; Tian, Y. Microbial dynamics and flavor formation during the traditional brewing of Monascus vinegar. Food Res. Int. 2019, 125, 108531. [Google Scholar] [CrossRef]
- Tesfaye, W.; Morales, M.L.; García-Parrilla, M.C.; Troncoso, A.M. Jerez vinegar. In Vinegars of the World; Springer: Berlin/Heidelberg, Germany, 2009; pp. 179–195. [Google Scholar]
- Al-Dalali, S.; Zheng, F.; Sun, B.; Chen, F. Comparison of aroma profiles of traditional and modern Zhenjiang aromatic vinegars and their changes during the vinegar aging by SPME-GC-MS and GC-O. Food Anal. Methods 2019, 12, 544–557. [Google Scholar] [CrossRef]
- Durán Guerrero, E.; Chinnici, F.; Natali, N.; Marín, R.N.; Riponi, C. Solid-phase extraction method for determination of volatile compounds in traditional balsamic vinegar. J. Sep. Sci. 2008, 31, 3030–3036. [Google Scholar] [CrossRef]
- Chen, T.; Gui, Q.; Shi, J.J.; Zhang, X.Y.; Chen, F.S. Analysis of variation of main components during aging process of Shanxi Aged Vinegar. Acetic Acid Bact. 2013, 2, 31–38. [Google Scholar] [CrossRef]
- Larios, A.; García, H.S.; Oliart, R.M.; Valerio-Alfaro, G. Synthesis of flavor and fragrance esters using Candida antarctica lipase. Appl. Microbiol. Biotechnol. 2004, 65, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Tie, Y.; Zhu, W.; Zhang, C.; Yin, L.; Li, L.; Liu, J. Effect of temperature on chemical compounds of Cupei (precursor of bran vinegar) during in-situ aging and revelation of functional microorganisms in the process. LWT 2023, 182, 114912. [Google Scholar] [CrossRef]
- Hongwen, L.; Xuping, W.; Xiaolan, Y. Influence of different fumigation processes on aroma compounds of Shanxi aged vinegar. Food Sci 2015, 36, 90–94. [Google Scholar]
- Al-Dalali, S.; Zheng, F.; Sun, B.; Rahman, T.; Chen, F. Tracking volatile flavor changes during two years of aging of Chinese vinegar by HS-SPME-GC-MS and GC-O. J. Food Compos. Anal. 2022, 106, 104295. [Google Scholar] [CrossRef]
- Callejón, R.; Torija, M.; Mas, A.; Morales, M.; Troncoso, A. Changes of volatile compounds in wine vinegars during their elaboration in barrels made from different woods. Food Chem. 2010, 120, 561–571. [Google Scholar] [CrossRef]
- Zhu, H.; Falcone, P.M.; Qiu, J.; Ren, C.-Z.; Li, Z.-G. Effect of ageing on rheological properties and quality of Shanxi aged vinegar. IOP Conf. Ser. Earth Environ. Sci. 2020, 615, 012096. [Google Scholar] [CrossRef]
- Bozkurt, H.; Göğüş, F.; Eren, S. Nonenzymic browning reactions in boiled grape juice and its models during storage. Food Chem. 1999, 64, 89–93. [Google Scholar] [CrossRef]
- España. Denominación de Origen de España. Dossier de Informacion.Vinagre de Jerez. Available online: https://www.vinagredejerez.org/noticias/Dossier_de_prensa_Vinagre_de_Jerez.pdf (accessed on 1 September 2024).
- Issa-Issa, H.; Noguera-Artiaga, L.; Sendra, E.; Pérez-López, A.J.; Burló, F.; Carbonell-Barrachina, Á.A.; López-Lluch, D. Volatile composition, sensory profile, and consumers’ acceptance of Fondillón. J. Food Qual. 2019, 2019, 5981762. [Google Scholar] [CrossRef]
- Tarko, T.; Krankowski, F.; Duda-Chodak, A. The impact of compounds extracted from wood on the quality of alcoholic beverages. Molecules 2023, 28, 620. [Google Scholar] [CrossRef]
- García-Moreno, M.V.; Sánchez-Guillén, M.M.; Ruiz de Mier, M.; Delgado-González, M.J.; Rodríguez-Dodero, M.C.; García-Barroso, C.; Guillén-Sánchez, D.A. Use of alternative wood for the ageing of brandy de Jerez. Foods 2020, 9, 250. [Google Scholar] [CrossRef] [PubMed]
- Masson, G.; Guichard, E.; Fournier, N.; Puech, J.-L. Stereoisomers of ß-methyl-γ-octalactone. II. Contents in the wood of French (Quercus robur and Quercus petraea) and American (Quercus alba) oaks. Am. J. Enol. Vitic. 1995, 46, 424–428. [Google Scholar] [CrossRef]
- Piggott, J.; Conner, J.; Melvin, J. The contribution of oak lactone to the aroma of wood-aged wine. In Developments in Food Science; Elsevier: Amsterdam, The Netherlands, 1995; Volume 37, pp. 1695–1702. [Google Scholar]
- Vilela, A. Microbial dynamics in sour–sweet wine vinegar: Impacts on chemical and sensory composition. Appl. Sci. 2023, 13, 7366. [Google Scholar] [CrossRef]
- Salles, C. Acids in foods and perception of sourness. In Handbook of Molecular Gastronomy; CRC Press: Boca Raton, FL, USA, 2021; pp. 7–12. [Google Scholar]
- Junge, J.Y.; Bertelsen, A.S.; Mielby, L.A.; Zeng, Y.; Sun, Y.-X.; Byrne, D.V.; Kidmose, U. Taste interactions between sweetness of sucrose and sourness of citric and tartaric acid among Chinese and Danish consumers. Foods 2020, 9, 1425. [Google Scholar] [CrossRef] [PubMed]
- Lytra, G.; Tempere, S.; Marchand, S.; de Revel, G.; Barbe, J.-C. How do esters and dimethyl sulphide concentrations affect fruity aroma perception of red wine? Demonstration by dynamic sensory profile evaluation. Food Chem. 2016, 194, 196–200. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).