Abstract
Soy (tofu) whey is one of the two side-streams from tofu processing, which has been shown to be suitable for microbial growth. In this work, probiotic Bifidobacterium animalis subsp. lactis Bl-04 and B94 were used to ferment soy whey with different supplements to explore the possibility of harnessing Bifidobacterium fermentation to upcycle soy whey. Soy whey was supplemented with different supplements (control, CN; glucose, G; glucose + cysteine, GC; glucose + cysteine + yeast extract, GCY) and inoculated with either B. lactis Bl-04 or B94. Growth, substrate utilization, and metabolic products were monitored before and after fermentation. Bl-04 managed to grow in all four media, while B94 needed cysteine to grow. The contents of sucrose, cysteine, methionine, and succinic acid decreased in the fermented samples. Acetic and lactic acids were produced in fermented soy whey ranging from 0.49–2.66 g/L and 0.58–2.88 g/L, respectively, with vitamin B12 at 2.06–4.56 μg/L. Increases in isoflavone aglycones (0.19–25.05 mg/L) and iron (0.03–0.12 mg/L) were observed. The PCA analysis of volatiles showed a distinct clustering due to short-chain fatty acids (acetic, butyric, and isovaleric acid), 2,3-butanedione (diacetyl), H2S, and 3-methylthiophene. Overall, the selection of suitable bifidobacterial strains and supplements for soy whey fermentation can open avenues to upcycle soy whey.
1. Introduction
Plant-based diets and their different forms have been increasingly adopted in the West. This trend has seen significant growth due to increased concerns about animal product consumption and their detrimental carry-over effect on health and the environment [1]. With a strong worldwide interest in plant-based foods, there has been an increase in vegans from 4 million in 2014 to nearly 20 million in 2017 in the USA alone. The global market for plant-based meat alternatives is projected to reach a high of USD 3.5 billion by 2026 [2]. The adoption of a plant-based diet has been shown to have beneficial effects that include a lower incidence of colon cancer and type 2 diabetes mellitus [3]. Yet, there are concerns about deficiencies in vitamin B12, iron, and amino acid quality when an array of plant-based products is not being consumed [3].
Tofu is a product made from soybeans that is known for being plant-based and its multitude of health benefits [4]. Tofu production is essentially standardized worldwide. Simply put, soymilk is prepared from soybeans and is then heated before curdling by an added coagulant, after which soy whey is pressed out from the curd (tofu). With the increase in interest in plant-based diets, the production of tofu worldwide will inevitably increase in tandem. The problem addressed in this study is two-facetted: one from the production of tofu (liquid side-stream) and the other from the adoption of a plant-based diet.
During the production of tofu, a massive amount of soy whey is produced, where 9 parts of soy whey to 1 part of tofu are produced [5]. This side-stream is the first problem that poses a threat to the environment due to its nutrient content (carbohydrates (8.5 g/L), proteins (1.33–8.2 g/L), fats (3.9–10 g/L), and minerals (3.9–4.6 g/L)) [6]. As a result, attempts to use valorization of soy whey to produce products such as kefir and kombucha have been made [7,8]. The majority of biovalorization strategies employ fermentation to improve flavor and produce beneficial metabolites [9]. Other biovalorization strategies include nisin and prebiotic production and nutrient recovery [6].
The second problem is associated with the adoption of a plant-based diet, where individuals need to consume a variety of protein-rich foods to supplement their diet to obtain sufficient essential amino acids, while possibly still lacking some micronutrients such as iron and vitamin B12 [10]. Bifidobacterial fermentation has been shown to contribute to not only vitamin B12 and amino acid formation but also the synthesis of short-chain fatty acids (SCFA) and SCFA precursors such as lactic acids [11,12].
Vitamin B12 is mainly found in animal products or as a by-product of bacterial fermentation. Bacillus megaterium and Propionibacterium shermanii are the main microorganisms used in the production of vitamin B12 for the supplement industry [13]. However, bifidobacteria have also been shown to be capable of anabolizing vitamin B12 [12]. B12 production in bacteria commonly occurs through the de novo or salvage pathway, where the former can either be aerobic or anaerobic and the latter needs an ATP-driven ABC transporter to transport cobinamide [14]. Bifidobacteria produce lactic acid and acetic acid through the bifidus shunt where two glucose molecules are converted to three acetic acid and two lactic acid molecules. Finally, genomic analysis has predicted the capability of bifidobacteria to produce all 20 amino acids, with glycerate-3-phosphate being an intermediate for serine biosynthesis or 2-oxoglutarate for proline biosynthesis [11].
Bifidobacteria are potential probiotics that have been extensively studied for their health-promoting capabilities and as such, they have been utilized in numerous supplemental and food products. Studies have shown that bifidobacteria, being one of the dominant members of the gut microflora, can confer physiological and clinical benefits such as prevention of diarrhea, reduction in oral-fecal gut transit time, and immune system stimulation [15,16]. B. animalis subsp. lactis, compared to its human origin counterpart B. longum subsp. longum, is less sensitive to stressful environments, such as bile, acid, and oxygen, which can help it survive better through the oral-fecal gut route in addition to post fermentation acidification and long term storage [17], making it a good candidate for fermentation of soy whey. Therefore, the objective of this study is to understand the growth and metabolic activities of B. lactis Bl-04 and B94 in soy whey and biofortify it with micronutrients such as vitamin B12. The result of this study will enhance our understanding of B. lactis and its fermentation characteristics in minimally supplemented soy whey. The results of this study can open avenues for a beverage or functional ingredient development from an upcycled side-stream raw ingredient.
2. Materials and Methods
2.1. Bifidobacterial Cultures
Pure frozen dried cultures of B. animalis subsp. lactis Bl-04 (Danisco, Copenhagen, Denmark), and B. animalis subsp. lactis LATFI B94 (Lallemand, Montreal, QC, Canada) were sub-cultured twice statically in Man, Rogosa, and Sharpe (MRS) (Oxoid Ltd., Hampshire, UK) broth supplemented with 0.05% cysteine HCl (Sigma-Aldrich, St. Louis, MO, USA) (MRSC) for 24 h at 37 °C. Following the pre-culturing, the cell pellets were washed twice with 0.85% (w/v) saline solution before being resuspended in unsupplemented soy whey, serving as pre-cultures for the final fermentation.
2.2. Soy (Tofu) Whey Preparation
Soy whey was made in a food processing laboratory based on the method reported by Chua et al. (2018) [5]. To begin, soybeans were soaked for 16 h in a 1:6 (w/v) deionized water ratio. Soybeans were then strained and blended using a blender with deionized water in a 1:6 dry weight-to-volume ratio to obtain a slurry. Soymilk was filtered through a cheesecloth to remove the pulp. Deionized water (1:2 (w/v)) was used to wash the pulp, and the flow-through was pooled together with the soymilk. The soymilk was then brought to a boil and held for 5 min. The soymilk was cooled down to 87 °C before 2% (soybean dry weight) gypsum (calcium sulfate, Home Brew Ohio, Sandusky, United States) suspended in water in a 10% ratio (w/v) was added to it. The soymilk and gypsum mixture was well stirred and left to curdle for 15 min before being hydraulically pressed to separate the soy whey from the tofu. Soy whey was split into four equal portions, of which one was left as unsupplemented (CN) and the others were supplemented with 2% (w/v) glucose (G), 2% (w/v) glucose + 0.05% (w/v) cysteine-HCl (GC), or 2% (w/v) glucose + 0.05% (w/v) cysteine-HCl + 0.25% yeast extract (GCY) (Oxoid Ltd., Hampshire, United Kingdom). The soy whey was pasteurized at 90 °C for 30 min, and potato dextrose agar was used to verify the effectiveness of the pasteurization.
2.3. Fermentation
Duplicate laboratory scale fermentations were conducted twice. The pre-culture was inoculated into the respective soy whey at a 1% (v/v) inoculation ratio to obtain an initial cell count of ~5 log CFU/mL. Aliquots of 40 mL or 10 mL were then transferred to 50 mL or 15 mL centrifuge tubes, respectively. Incubation was at 37 °C for the 48 h fermentation period before the 15 mL tubes were then transferred to 4 or 25 °C aerobic storage. Sampling was conducted at 0 h, 6 h, 12 h, 24 h, 36 h, and 48 h using the 40 mL aliquots and at 1 week, 2 weeks, 3 weeks, and 4 weeks using the 10 mL aliquots. pH measurement was performed with a pH meter (827 pH Lab, Metrohm, Herisau, Switzerland), °Brix with a refractometer (RX-5000α, ATAGO, Tokyo, Japan), and cell count with pour plating on MRSC agar incubated for 48 h at 37 °C in an anaerobic jar with AnaeroGen™ (Oxoid Ltd., Hampshire, UK).
2.4. Sugar, Acid, Isoflavone, Amino Acid Analysis
The soy whey samples were first centrifuged at 11,000× g for 10 min before being filtered through a 0.22 µm syringe filter prior to analysis using HPLC. Sugars were analyzed using an Agilent UPLC-RID (Agilent; Santa Clara, CA, USA) with a Supelcogel C-610H (300 × 7.8 mm; Supelco, Bellefonte, PA, USA) column. The mobile phase employed was 0.1% (v/v) sulfuric acid flowing at 0.5 mL/min with both the column and detector set to 35 °C. All sugar analytes (Table 1) were identified and quantified using their retention times and external standard curves (Sigma-Aldrich, St. Louis, MO, USA) with a coefficient of determination of at least 0.98.
The organic acid and isoflavone analyses employed the methodology of [5], using HPLC (Shimadzu, Kyoto, Japan) coupled with a photodiode array (PDA). Supelcogel C-610 H (300 × 7.8 mm; Supelco, Bellefonte, PA, USA) was utilized for organic acid separation at 40 °C. The mobile phase was 0.1% (v/v) sulfuric acid flowing at 0.4 mL/min with the PDA set to 210 nm. All the quantified organic acids in Table 1 were obtained from Sigma-Aldrich and used to develop the external calibration curves for quantification.
Zorbax Eclipse Plus C18 column (150 × 4.6 mm; Agilent Santa Clara, CA, USA) was used for isoflavone separation with a gradient elution and PDA set to 262 nm. The mobile phase employed was 0.1% acetic acid (A) and 0.1% acetic acid in methanol (B). The HPLC grade isoflavone standards were purchased from Wako Pure Chemical Industries, Ltd., and the external calibration curves are developed for quantification or isoflavones listed in Table 2.
The amino acids were analyzed using the MembraPure (Berlin, Germany) pre-set physiological separation program for ARACUS Amino Acid Analyzer. The quantification was undertaken according to the MembraPure single-point calibration factor combined with the physiological amino acid standard (Sigma-Aldrich, St. Louis, MO, USA).
2.5. Antioxidant Capacity Assays
The 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay and oxygen radical absorbance capacity (ORAC) potential of the samples were assayed following the steps outlined by [18]. In brief, the samples were centrifuged at 11,000× g for 10 min before being filtered through a 0.20 µm syringe filter prior to analysis. For the DPPH radical scavenging assay, the soy whey was diluted 100-fold in deionized water, while 160-fold dilution was conducted in a 75 mM phosphate buffer (pH 7.4) for ORAC assay. Both analyses were carried out using a multi-mode reader (Synergy HTX Multi-mode Reader, Agilent, Santa Clara, CA, USA) set at 515 nm for the DPPH radical scavenging assay and fluorescence for ORAC. Results from the readings are expressed as Trolox equivalents per mL of soy whey.
2.6. Vitamin B12 Analysis
For the vitamin B12 analysis, the methodology described in [19] was followed. Briefly, 6 mL of the sample was transferred to a 15-mL centrifuge tube along with 0.1 mL of a 1% KCN solution and 2.5 mL of a 0.4 M sodium acetate (pH 4) solution. The samples were then vortexed and autoclaved at 100 °C for 30 min before being cooled in an ice bath. Prior to extraction, the samples were centrifuged at 11,000× g at 4 °C. Oasis HLB SPE columns (3-cc, 60-mg bed mass, Waters, MA, USA) were conditioned with 2 mL of methanol, followed by equilibration with 2 mL of distilled H2O. The sample was then loaded into the cartridge followed by 2 mL of a 5% (v/v) methanol wash and 1 mL of a 90% methanol elution. The extracted samples were then filtered through a 0.22 µm filter and stored in a 2 mL amber vial before analysis. The sample analysis was conducted using a HPLC (Shimadzu, Kyoto, Japan) coupled with PDA. The Zorbax Eclipse Plus C18 column (150 × 4.6 mm, Agilent, Santa Clara, CA, USA) with a gradient elution of formic acid in water (0.1%) (A) and methanol (B) at a flow rate of 0.25 mL/min at 40 °C with PDA set at 550 nm was employed. The elution gradient was as follows (A:B): 0–3.5 min 100:0, 3.5–11 min 75:25, 11–19 min 65:35, 19–20 min 90:10, 20–35 min 95:5, 35–45 min 95:5. A vitamin B12 (cyanocobalamin) standard was purchased from Sigma-Aldrich (St. Louis, MO, USA) to develop an external calibration curve for quantification.
2.7. Mineral Analysis
Minerals such as cobalt (Co, 238.892 nm), copper (Cu, 324.754 nm), iron (Fe, 238.204 nm), potassium (K, 404.721 nm), magnesium (Mg, 383.231 nm), sodium (Na, 588.995 nm), phosphorous (P, 213.618 nm,), selenium (Se, 196.090 nm), and zinc (Zn, 213.856nm) were analyzed using an ICP-OES (Shimadzu, Kyoto, Japan). The sample preparation included centrifugation at 4000× g for 10 min before filtering through a 0.22 µm syringe filter prior to analysis.
2.8. Volatile Analysis
Gas chromatography coupled with mass spectrometry and flame ionization detector (Agilent; Santa Clara, CA, USA) was employed for the volatile analysis as described by [5] with slight modifications. The whey samples were subjected to pH adjustment to a pH of 2.5 using 1 M HCl before 5 mL was transferred to a 20 mL headspace vial supplemented with 1 g of NaCl. A PAL autosampler (CTC, Basel, Switzerland was employed for 50 min of extraction time at 60 °C using CAR/PDMS SPME fiber (Supelco, Bellefonte, PA, USA) at 250 rpm/min and desorbed at 250 °C for 3 min. Separation was undertaken in an Agilent DF-FFAP (Agilent; Santa Clara, CA, USA) column with helium as the carrier gas at a 1.2 mL/min flowrate. The inlet was set to the splitless mode at 250 °C with an oven program of 50 °C for 5 min, then 5 °C/min to 230 °C, and held for 30 min. All analyses were conducted in the ion mode with the eV and MS source set to 230 °C. NIST08, and the Wiley 275 MS libraries were used in tandem with linear retention indices (LRI) for peak identification.
2.9. Statistical Analysis
R v4.1.0 (R Foundation for Statistical Computing, Vienna, Austria) was primarily used for data manipulation, visualization, statistical analysis, and principal component analysis (PCA). A two-way ANOVA with treatment and supplementation was used to determine the main effects and interactions effect on metabolites (Supplementary Data). When there is a significance in interactions or main effects, Tukey’s HSD post hoc test was performed to assess sample differences with significant differences reported as p < 0.05. Results are reported as mean ± standard deviations, where duplicate trials were undertaken twice independently (n = 4), and instrumental analyses were only conducted with biological replication and a single technical replicate.
3. Results & Discussion
3.1. B. lactis Growth and pH Changes
Tindjau et al. (2023) [19] demonstrated the capability of B. longum BB536 to grow in GC and GCY soy whey. In this study, we monitored the growth and metabolite changes of various supplemented soy whey media fermented by B. lactis strain Bl-04 or B94.
Figure 1 shows the cell count and pH changes of soy whey over 48 h fermentation with B. lactis strains Bl-04 and B94. Strain Bl-04 was able to grow in non-supplemented and supplemented soy whey, while strain B94 was able to grow in GC and GCY supplemented soy whey. Supplementation led to a rapid increase in cell count after 24 h of fermentation for both Bl-04 and B94 by about 1.58 (Bl-04 in GC), 2.48 (Bl-04 in GCY), 2.52 (B94 in GC), and 2.04 log CFU/mL (B94 in GCY). CN and G soy whey were only able to sustain growth for strain Bl-04 by 2.24 (CN) and 2.39 log CFU/mL (G) but not for strain B94. Rozada et al. (2009) [20] hypothesized that cysteine supplementation stimulated metabolism leading to a shorter stationary phase, which could be a necessity for strain B94 growth. As shown in Figure 1b, the trends in pH changes were similar between B94 and Bl-04 samples where the cell counts increased. The drops in pH correspond to lactate and acetate production through the bifidus shunt (Section 3.2) [21]. B94 fermented CN and G samples did not show pH decreases due to their inability to support growth.
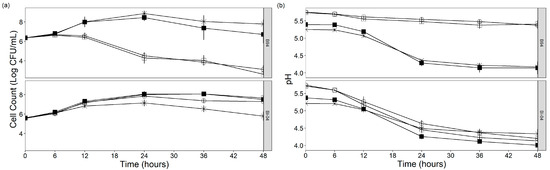
Figure 1.
Changes of (a) cell count, and (b) pH of B. lactis Bl-04 or B94 fermented soy whey. ○ CN = control, □ G = glucose supplement, X GC = glucose and cysteine supplemented, ■ GCY = glucose, cysteine and yeast extract supplemented. (For statistical interpretation of the end of fermentation results, the reader is referred to Table 1).
Cell count and pH were monitored for an additional 28 days after completion of fermentation, as shown in Figure S1. Storage at 25 °C was not able to sustain live cells for 28 days, while at 4 °C, it did to some degree. Bifidobacterial survivability during storage at refrigerated and room temperature was reported before with low temperature assisting in the preservation of microbial populations [20,22]. During storage at 4 °C, Bl-04 had better survivability compared to B94 in all supplemented soy whey media. Interestingly, the Bl-04 cell count was able to stay at around 7 log CFU/mL in CN (7.03 ± 0.36), G (6.92 ± 0.35), and GCY (6.76 ± 0.72). Bl-04 was able to survive better in acidic conditions compared to B94. Acid tolerance in B. lactis has been associated with the induction of H+-ATPase activity [17], which suggests that the ATPase activity is more pronounced in Bl-04 compared to B94 due to its better survivability over four weeks in storage. These conditions achieved the minimum number of viable cell counts for Bl-04 in a probiotic beverage, where the minimum dosage is 109 CFU per serving [23]. The single most striking observation from this data is that B94 was not able to grow in CN and G soy whey, while Bl-04 was able to. A possible explanation to this phenomenon could be due to the presence of a reverse transsulfuration pathway in Bl-04 and not B94. Wada et al. (2021) [24] reported the presence of this pathway in Bifidobacterium that enables the conversion of methionine to cysteine. The presence of this pathway in Bl-04 enables growth in the absence of cysteine. This reinforces the importance of understanding the different strains of B. lactis for different applications.
3.2. Sugar and Organic Acid Changes
The changes in sugars and organic acids are presented in Table 1. The initial glucose content of unsupplemented soy whey was low at 0.78 ± 0.04 g/L. Glucose was added to provide an additional carbon and energy source. Cysteine was added as a source of sulfur, as Bifidobacterium is a sulfur auxotroph [25], and to create a reducing environment for the anaerobic bifidobacteria. Finally, a yeast extract was added to provide extra nitrogen and micronutrient sources for growth. From Table 1, the total sugar utilization was different between strains and supplements with significant interactive effects.
For Bl-04, stachyose, raffinose, glucose, and fructose did not significantly decrease, while sucrose significantly decreased in all four different supplements compared to unfermented soy whey. In the case of B94, a significant decrease was only observed in the sucrose content for GC and GCY supplementation. The inability to utilize stachyose and raffinose while being able to significantly reduce the sucrose after fermentation in soy whey points towards the lack of α-galactosidase and not β-glucosidase or β-galactosidase. This finding is contrary to the report where B. lactis was shown to have specific ATP binding cassette (ABC) transporters to take up α-1,6-galactosides as well as utilize both raffinose and stachyose due to α-galactosidase activity [26]. This lack of enzyme activity could be due to the pH of the fermentation media being well below the optimum bifidobacterial growth pH of 6–7 [22]. In addition to that, the supplementation of yeast extract was found to affect α-galactosidase production, albeit mixed results have been reported [27]. Finally, although raffinose family oligosaccharides (RFO) have been shown to upregulate the gene cluster responsible for ABC transporters in B. lactis, together with the GH_36 subfamily 1, a hydrolase family that encompasses both α-galactosidase and α-glucosidase was reported to be upregulated in the presence of RFO [28], but this is not the case in our study. The minimal changes in glucose and fructose concentrations could be attributed to the hydrolysis of sucrose by β-fructofuranosidaseto its monosaccharides and may reflect a net balance of both monosaccharide formation and utilization.
The observed trend of increases in pyruvic acid, α-ketoglutaric acid, lactic acid, and acetic acid was consistent with the increases in cell count and pH reduction. Malic acid and citric acid contents were unaffected by B. lactis fermentation, likely due to the incomplete TCA cycle present in bifidobacteria. The increase in the F6PPK (bifidus) pathway and TCA cycle acids, such as pyruvic and α-ketoglutaric acids, were in line with normal metabolic activities, where carbohydrates were assimilated and broken down through the bifid shunt to pyruvate and acetyl CoA was utilized in the TCA cycle in which α-ketoglutaric acid was catabolized [21]. The decreases in succinic acid in all fermented samples were unexpected as bifidobacteria have been reported to produce succinic acid [29], but this was in line with Tindjau et al. (2023) [19]. Succinic acid is suggested to have been produced due to the necessity of NAD+ regeneration. However, there are other pathways in the central carbon metabolism of bifidobacteria to achieve this, namely through production of lactic acid or ethanol [29]. Acetic acid and lactic acid production is theorized to be produced in a 3:2 molar ratio for every mole of glucose metabolized by bifidobacteria, which is rarely the case in vitro. This could very well explain why the strain Bl-04 acetic acid to lactic acid (A/L) molar ratio was below 1.5, which is a necessity for NAD+ regeneration through the production of lactic acid. However, this was not the case for the strain B94 GC and GCY samples where the A/L molar ratio was 1.61 and 1.55, respectively. The possibility of acetic and lactic acid being produced from pathways involving amino acids or citric acid could also lead to the elevated molar ratio.
The improved acetic acid and lactic acid content was achieved through GCY supplementation. SCFAs such as acetic acid have been researched as therapies for inflammatory bowel disease and regulators of bacterial populations in the gut [30]. Lactic acid, on the other hand, is not an SCFA. However, in the gut, it can be further metabolized by resident butyrate-producing microorganisms through cross-feeding to produce acetate or butyrate and propionate [31].

Table 1.
Physicochemical parameters of soy whey before and after B. lactis Bl-04 or B94 fermentation.
Table 1.
Physicochemical parameters of soy whey before and after B. lactis Bl-04 or B94 fermentation.
| CN | G | GC | GCY | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T 0 h ‡ | T 48 h Bl-04 | T 48 h B94 | T 0 h ‡ | T 48 h Bl-04 | T 48 h B94 | T 0 h ‡ | T 48 h Bl-04 | T 48 h B94 | T 0 h ‡ | T 48 h Bl-04 | T 48 h B94 | |
| °Brix+*@ | 2.01 ± 0.02 g | 1.86 ± 0.03 h | 2.00 ± 0.01 g | 3.73 ± 0.02 d | 3.58 ± 0.02 f | 3.73 ± 0.01 d | 3.77 ± 0.02 cd | 3.65 ± 0.04 e | 3.64 ± 0.02 ef | 4.05 ± 0.04 a | 3.82 ± 0.04 bc | 3.86 ± 0.07 b |
| pH+*@ | 5.72 ± 0.01 a | 4.21 ± 0.05 de | 5.38 ± 0.09 bc | 5.71 ± 0.01 a | 4.14 ± 0.02 ef | 5.41 ± 0.10 b | 5.22 ± 0.00 c | 4.34 ± 0.13 d | 4.18 ± 0.04 def | 5.36 ± 0.02 bc | 4.01 ± 0.0 6f | 4.15 ± 0.18 ef |
| Cell Count Log (CFU/mL)+*@ | 0.00 ± 0.00 e | 7.30 ± 0.10 ab | 3.11 ± 0.40 d | 0.00 ± 0.00 e | 7.67 ± 0.21 a | 2.62 ± 0.07 d | 0.00 ± 0.00 e | 5.8 ± 0.45 c | 7.79 ± 0.44 a | 0.00 ± 0.00 e | 7.51 ± 0.07 ab | 6.71 ± 0.90 b |
| Sugars (g/L) | ||||||||||||
| Stachyose +* | 3.53 ± 0.06 a | 3.78 ± 0.08 a | 3.76 ± 0.08 a | 3.35 ± 0.25 a | 3.38 ± 0.39 a | 3.54 ± 0.10 a | 3.31 ± 0.13 a | 3.66 ± 0.11 a | 3.46 ± 0.32 a | 3.42 ± 0.28 a | 3.6 ± 0.06 a | 3.66 ± 0.24 a |
| Raffinose + | 0.87 ± 0.03 ab | 0.79 ± 0.04 b | 0.88 ± 0.03 ab | 0.84 ± 0.05 ab | 0.87 ± 0.05 ab | 0.89 ± 0.07 ab | 0.85 ± 0.07 ab | 0.87 ± 0.03 ab | 0.87 ± 0.11 ab | 0.84 ± 0.09 ab | 0.85 ± 0.07 ab | 0.97 ± 0.09 a |
| Sucrose +*@ | 4.65 ± 0.04 a | 1.58 ± 0.14 e | 4.18 ± 0.03 a | 4.41 ± 0.30 a | 2.69 ± 0.41 cd | 4.48 ± 0.14 a | 4.42 ± 0.16 a | 3.41 ± 0.04 b | 3.29 ± 0.36 bc | 4.66 ± 0.33 a | 2.56 ± 0.06 d | 3.22 ± 0.48 bc |
| Glucose +*@ | 0.78 ± 0.04 c | 0.49 ± 0.04 c | 0.84 ± 0.04 c | 21.55 ± 1.75 a | 17.81 ± 2.61 b | 22.27 ± 0.14 a | 22.30 ± 0.74 a | 21.3 ± 0.26 a | 20.00 ± 1.48 ab | 22.14 ± 1.57 a | 19.59 ± 0.55 ab | 19.7 ± 1.35 ab |
| Fructose +* | 0.44 ± 0.05 b | 0.54 ± 0.07 ab | 0.67 ± 0.06 ab | 0.53 ± 0.10 ab | 0.58 ± 0.09 ab | 0.55 ± 0.07 ab | 0.53 ± 0.10 ab | 0.77 ± 0.17 a | 0.79 ± 0.26 a | 0.59 ± 0.10 ab | 0.61 ± 0.05 ab | 0.71 ± 0.15 ab |
| Total Sugars +*@ | 10.24 ± 0.05 d | 7.14 ± 0.29 d | 10.31 ± 0.21 d | 30.65 ± 2.37 ab | 25.29 ± 3.51 c | 31.69 ± 0.27 a | 31.36 ± 1.10 ab | 29.98 ± 0.44 ab | 28.38 ± 2.44 abc | 31.63 ± 2.30 a | 27.18 ± 0.65 bc | 28.21 ± 2.21 abc |
| Organic acids (g/L) | ||||||||||||
| Citric acid +*@ | 4.89 ± 0.13 a | 4.15 ± 0.28 abc | 4.65 ± 0.18 ab | 4.64 ± 0.45 ab | 4.03 ± 0.19 bc | 4.74 ± 0.13 ab | 3.83 ± 0.43 cd | 3.58 ± 0.12 cd | 3.20 ± 0.67 d | 4.10 ± 0.3 bc | 3.67 ± 0.15 cd | 3.77 ± 0.11 cd |
| α-Ketoglutaric acid +*@ | 0.00 ± 0.00 d | 0.10 ± 0.01 c | 0.11 ± 0.01 c | 0.00 ± 0.00 d | 0.11 ± 0.02 c | 0.11 ± 0.01 c | 0.00 ± 0.00 d | 0.12 ± 0.01 bc | 0.14 ± 0.03 ab | 0.00 ± 0.00 d | 0.13 ± 0.01 abc | 0.15 ± 0.02 a |
| Malic acid * | 0.38 ± 0.03 ab | 0.34 ± 0.07 ab | 0.32 ± 0.03 b | 0.36 ± 0.04 ab | 0.38 ± 0.12 ab | 0.36 ± 0.02 ab | 0.37 ± 0.06 ab | 0.46 ± 0.08 ab | 0.41 ± 0.16 ab | 0.53 ± 0.07 a | 0.43 ± 0.09 ab | 0.42 ± 0.08 ab |
| Pyruvic acid +*@ | 0.00 ± 0.00 d | 0.09 ± 0.01 bc | 0.07 ± 0.02 c | 0.00 ± 0.00 d | 0.08 ± 0.02 bc | 0.06 ± 0.02 c | 0.00 ± 0.00 d | 0.08 ± 0.01 bc | 0.08 ± 0.02 bc | 0.00 ± 0.00 d | 0.1 ± 0.01 ab | 0.12 ± 0.02 a |
| Succinic acid +@ | 1.35 ± 0.05 b | 0.30 ± 0.03 e | 0.61 ± 0.03 cd | 1.27 ± 0.16 b | 0.34 ± 0.05 e | 0.66 ± 0.05 c | 1.37 ± 0.23 b | 0.43 ± 0.11 cde | 0.36 ± 0.10 e | 1.61 ± 0.07 a | 0.36 ± 0.04 e | 0.40 ± 0.05 de |
| Lactic acid +*@ | 0.00 ± 0.00 e | 2.03 ± 0.06 b | 0.64 ± 0.10 d | 0.00 ± 0.00 e | 2.22 ± 0.11 b | 0.58 ± 0.03 d | 0.00 ± 0.00 e | 1.37 ± 0.26 c | 1.54 ± 0.28 c | 0.00 ± 0.00 e | 2.88 ± 0.21 a | 2.48 ± 0.5 ab |
| Acetic acid +*@ | 0.00 ± 0.00 c | 1.66 ± 0.11 b | 0.54 ± 0.10 c | 0.00 ± 0.00 c | 1.74 ± 0.13 b | 0.49 ± 0.09 c | 0.00 ± 0.00 c | 1.32 ± 0.22 b | 1.66 ± 0.35 b | 0.00 ± 0.00 c | 2.66 ± 0.25 a | 2.57 ± 0.61 a |
| Total acids +*@ | 6.60 ± 0.16 cd | 8.63 ± 0.19 ab | 6.90 ± 0.24 cd | 6.25 ± 0.64 cd | 8.85 ± 0.41 ab | 6.93 ± 0.19 cd | 5.55 ± 0.69 d | 7.28 ± 0.37 bc | 7.36 ± 1.57 bc | 6.22 ± 0.43 cd | 10.18 ± 0.38 a | 9.87 ± 1.17 a |
| Acid molar ratio (A/L)+*@ | 0.00 ± 0.00 d | 1.23 ± 0.07 bc | 1.27 ± 0.21 bc | 0.00 ± 0.00 d | 1.18 ± 0.10 c | 1.26 ± 0.20 bc | 0.00 ± 0.00 d | 1.45 ± 0.08 ab | 1.61 ± 0.08 a | 0.00 ± 0.00 d | 1.39 ± 0.07 abc | 1.55 ± 0.06 a |
a–g Statistical analysis using an ANOVA at 95% confidence interval with Tukey’s post hoc test across eight treatments if the interaction effect is significant. + Depicts a significant (p < 0.05) bifidobacterial effect. * Depicts a significant (p < 0.05) supplementation effect. @ Depicts a significant (p < 0.05) interaction effect. ‡ T 0 h, data taken from Tindjau et al. (2023) [19], as they were part of the same experiment. CN = unsupplemented control, G = glucose supplementation, GC = Glucose and cysteine supplementation, GCY = Glucose, cysteine, and yeast extract supplementation.
3.3. Free Amino Acid Changes
Figure 2 illustrates the predicted amino acid metabolism pathway based on Lee & O’Sullivan (2010) [11] and the Kyoto Encyclopedia of Genes and Genomes (KEGG). Table S1 details the changes in free amino acids before and after supplementation and fermentation. The data reveals that GC and GCY supplementation led to a non-significant change in the total amino acid content except for strain Bl-04 in GCY soy whey, which showed a significant decrease. CN and G supplemented soy whey had significant decreases in the total amino acid only after fermentation with Bl-04. The minimal nutrients in CN soy whey and lack of cysteine in CN and G samples were not optimal for bifidobacterial growth. However, Engevik et al. (2021) [32] reported that amino acids can be used as a nutrient source for B. dentium, which could explain the significant reduction in total free amino acids [32]. The majority of amino acids showed a significant decrease with the exception of cystathionine in G soy whey fermented with Bl-04, although specific changes varied with individual amino acids (Figure 2).
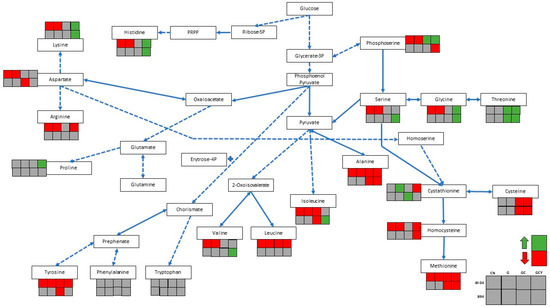
Figure 2.
Predicted amino acid metabolism pathway in bifidobacteria [11]. Solid arrows refer to single step reactions, while dotted arrows are multi-step reactions.  = concentration reduced after fermentation,
= concentration reduced after fermentation,  = concentration increased after fermentation. CN = control, G = glucose supplemented, GC = glucose and cysteine supplemented, GCY = glucose, cysteine, and yeast extract supplemented. See Table S1 for detailed free amino acid composition of soy whey.
= concentration increased after fermentation. CN = control, G = glucose supplemented, GC = glucose and cysteine supplemented, GCY = glucose, cysteine, and yeast extract supplemented. See Table S1 for detailed free amino acid composition of soy whey.
 = concentration reduced after fermentation,
= concentration reduced after fermentation,  = concentration increased after fermentation. CN = control, G = glucose supplemented, GC = glucose and cysteine supplemented, GCY = glucose, cysteine, and yeast extract supplemented. See Table S1 for detailed free amino acid composition of soy whey.
= concentration increased after fermentation. CN = control, G = glucose supplemented, GC = glucose and cysteine supplemented, GCY = glucose, cysteine, and yeast extract supplemented. See Table S1 for detailed free amino acid composition of soy whey.
Amino acid biosynthetic pathways in bifidobacteria have been proposed by Lee & O’Sullivan. (2010) [11]. The bifidobacterial genes involved in the biosynthesis of all 20 amino acids have been predicted, however some genes may not be functional in certain media. Amino acids are used for basic metabolic processes such as enzyme and cell membrane syntheses [33]. The CN and G media are lacking in free amino acids compared to GC and GCY soy whey, which resulted in a significant decrease in total free amino acids. Amino acids such as lysine, serine and alanine are key amino acids in the murein (peptidoglycan of cell wall) of B. lactis subsp. animalis, which would have led to a decrease in its concentration through fermentation [33]. The surplus amino acids present in GCY soy whey could possibly be converted from one to another depending on the needs of the bacteria. The increase in threonine content in GC and GCY could be due to the synthesis from glycerate-3-phosphate through glycolysis, which can be converted to phosphoserine and ultimately, threonine [11]. The threonine supplement was shown to have a synergistic effect with cysteine in the improvement of growth and acid production by bifidobacteria in soymilk [34].
Sulfur-containing amino acids such as methionine significantly decreased for all the treatments in both bifidobacterial fermentations, while cysteine only reduced in GC and GCY supplements. Homocysteine, on the other hand, was significantly reduced in the CN, G, and GCY supplements but not in the GC supplement in both fermentations. Finally, cystathionine had a mixed trend after fermentation. Sulfur-containing amino acids such as cysteine and methionine play an important role as sources of organic sulfur due to bifidobacteria being a sulfur auxotroph [25]. Cysteine can be irreversibly converted to cystathionine through a sulfur-metabolizing pathway in bifidobacteria. Cysteine is first converted to cystathionine with cystathionine γ-lyase, next to homocysteine with cystathionine β-lyase, and finally to methionine with methionine synthase [21]. The capability of strain Bl-04 to grow in media without cysteine supplementation was suggested by Wada et al. (2021) [24] due to the possible existence of a reverse transsulfuration pathway where methionine can be utilized by bifidobacteria to form cysteine [24].
3.4. Isoflavone and Antioxidant Capacity Changes
Isoflavone changes are presented in Table 2. Fermentation by both B94 and Bl-04 led to a significant decrease in total glucosides (22–60%) whilst increasing their respective aglycones (50–300%). CN and G samples fermented with Bl-04 led to a significant decrease in glucosides and a significant increase in total aglycones. Both strains also led to a decreased concentration of isoflavone glucosides while increasing the isoflavone aglycones in GC and GCY. The difference in β-glucosidase activity between strain B94 and Bl-04 can be seen in the fermented GCY samples, where B94 samples had almost twice the amount of aglycones compared to Bl-04 samples. The supplementation had a significant effect on total glucosides, but not total aglycones, with interactive effects being significant for both variables (strain and supplement). The supplementation of yeast extract has been shown to stimulate the production of β-glucosidase in B. longum, which could explain the significant effect in the reduction of glucosides due to supplementation [27]. The increase in isoflavone aglycones is of significance during the development of a functional product. The presence of the glucoside group in isoflavones has been reported to reduce its bioactivity, as aglycones are more lipid soluble and are hence able to penetrate intestinal villi resulting in improved bioavailability and bioactivity [35].
The antioxidant capacities of fermented and unfermented soy whey was determined using the DPPH scavenging assay and ORAC assay. As outlined in Table 2, an increase in aglycone concentration led to an increase in the DPPH scavenging activity (by 2–3-fold). However, the same cannot be said about the ORAC assay. An increase in isoflavone aglycones does not always correspond to an increase in the ORAC antioxidant capacity. The fermentation of soy whey by non-Saccharomyces yeast was seen to result in significant changes in the DPPH antioxidant capacity [5]. These warrant a wider range of assays to precisely determine the antioxidant capacity of a fermented soy beverage. While in vitro antioxidant assays are widely employed, their physiological effects may not be reflected. Ex vivo or in vivo experiments can be evaluated to determine its antioxidant capacities [36].
3.5. Vitamin B12 Changes
The vitamin B12 results are presented in Table 2. GC, and GCY supplements led to the production of vitamin B12 by both B. lactis strains. Strain Bl-04 produced 3.01 and 4.56 µg/L of vitamin B12 in GC and GCY soy whey, respectively. Strain B94 produced 2.06 and 2.06 µg/L of vitamin B12 in GC and GCY soy whey, respectively. Strain Bl-04 could grow in CN and G soy, however this did not lead to the production of vitamin B12, which leads us to believe that a reducing environment may be necessary for its production. Two distinct pathways of B12 production exist in bacteria, namely aerobic and anaerobic. The aerobic pathway is differentiated with oxygen being a requirement in corrin ring biosynthesis and cobalt chelation happening late in the pathway. The anaerobic pathway is differentiated through early cobalt chelation to precorrin-2 [13]. Facultatively anaerobic bacteria, such as Bacillus megaterium and Propionibacterium freundenreichii, are the industrial producers of vitamin B12. Interestingly, both microbes were able to produce vitamin B12 when grown in aerobic and anaerobic conditions [13,37]. However, this is not the case for B. lactis Bl-04. Strain Bl-04 was able to grow in soy whey supplemented with and without cysteine (a reducing agent). Vitamin B12 was only detected in fermented soy whey supplemented with cysteine, which leads us to believe that the production of vitamin B12 by B. lactis can only happen in strictly anaerobic conditions.

Table 2.
Isoflavone, antioxidant capacity, and vitamin B12 of soy whey before and after B. lactis Bl-04 or B94 fermentation.
Table 2.
Isoflavone, antioxidant capacity, and vitamin B12 of soy whey before and after B. lactis Bl-04 or B94 fermentation.
| CN | G | GC | GCY | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T 0 h ‡ | T 48 h Bl-04 | T 48 h B94 | T 0 h ‡ | T 48 h Bl-04 | T 48 h B94 | T 0 h ‡ | T 48 h Bl-04 | T 48 h B94 | T 0 h ‡ | T 48 h Bl-04 | T 48 h B94 | |
| Isoflavones (mg/L) | ||||||||||||
| Daidzin +*@ | 34.37 ± 1.57 a | 16.80 ± 0.83 de | 27.2 ± 4.27 abcd | 33.02 ± 1.93 ab | 21.44 ± 6.47 bcde | 27.12 ± 1.7 abcd | 29.57 ± 6.03 abc | 19.95 ± 6.95 cde | 24.47 ± 0.10 abcde | 23.29 ± 10.28 abcde | 22.77 ± 4.04 abcde | 14.00 ± 2.23 e |
| Glycitin | 7.00± 0.56 a | 5.67 ± 0.20 a | 6.22 ± 0.99 a | 7.03 ± 0.47 a | 5.27 ± 1.63 a | 6.81 ± 0.21 a | 5.92 ± 1.31 a | 5.66 ± 1.57 a | 6.93 ± 0.08 a | 4.57 ± 1.99 a | 5.73 ± 0.90 a | 5.94 ± 0.36 a |
| Genistin +* | 44.80 ± 1.81 a | 11.69 ± 1.34 de | 22.22 ± 3.51 cde | 42.78 ± 2.28 ab | 17.18 ± 5.37 de | 19.97 ± 0.84 cde | 37.91 ± 7.55 ab | 16.01 ± 5.58 de | 23.92 ± 0.70 cd | 30.47 ± 13.64 bc | 16.55 ± 2.03 de | 9.66 ± 2.08 e |
| Total glucosides +*@ | 86.15 ± 3.91 a | 34.15 ± 2.18 de | 55.63 ± 8.74 cde | 82.81 ± 4.63 ab | 43.88 ± 13.42 de | 53.89 ± 1.55 cde | 73.38 ± 14.86 abc | 41.62 ± 13.97 de | 55.31 ± 0.73 cde | 58.32 ± 25.89 bcd | 45.04 ± 6.95 de | 29.59 ± 4.19 e |
| Daidzein +@ | 7.61 ± 0.56 de | 13.71 ± 0.96 ab | 7.64 ± 1.15 de | 7.03 ± 0.55 de | 8.72 ± 2.94 cde | 9.72 ± 0.36 bcd | 5.81 ± 1.29 de | 9.59 ± 2.73 bcd | 12.55 ± 0.93 abc | 4.80 ± 2.03 e | 8.67 ± 1.32 cde | 16.47 ± 2.64 a |
| Glycitein +@ | 2.12 ± 0.34 abcd | 2.31 ± 0.18 ab | 1.83 ± 0.13 bcd | 2.04 ± 0.09 abcd | 1.85 ± 0.18 bcd | 1.95 ± 0.07 bcd | 1.72 ± 0.29 cd | 1.88 ± 0.27 bcd | 2.23 ± 0.13 abc | 1.64 ± 0.25 d | 1.89 ± 0.21 bcd | 2.50 ± 0.24 a |
| Genistein +*@ | 3.36 ± 0.18 d | 11.21 ± 1.02 ab | 7.25 ± 1.07 c | 3.30 ± 0.23 d | 7.79 ± 2.53 bc | 9.53 ± 0.33 bc | 2.51 ± 0.46 d | 8.34 ± 2.09 bc | 11.59 ± 1.43 ab | 2.31 ± 1.03 d | 8.91 ± 1.76 bc | 14.82 ± 3.02 a |
| Total aglycones +@ | 13.08 ± 0.93 def | 27.22 ± 2.1 ab | 16.71 ± 2.33 def | 12.37 ± 0.75 ef | 18.34 ± 5.59 cde | 21.19 ± 0.39 bcd | 10.03 ± 1.98 f | 19.8 ± 4.95 bcde | 26.35 ± 2.46 abc | 8.73 ± 3.29 f | 19.46 ± 3.26 bcde | 33.79 ± 5.88 a |
| Total isoflavones + | 99.23 ± 4.28 a | 61.36 ± 4.22 c | 72.33 ± 10.92 abc | 95.18 ± 5.17 ab | 62.22 ± 18.89 c | 75.08 ± 1.79 abc | 83.41 ± 16.81 abc | 61.42 ± 18.11 c | 81.65 ± 3.12 abc | 67.04 ± 29.17 abc | 64.49 ± 10.2 bc | 63.38 ± 1.71 bc |
| ORAC (Trolox equivalent, mg/mL) +*@ | 0.38 ± 0.12 de | 0.65 ± 0.11 bcd | 0.33 ± 0.08 e | 0.48 ± 0.09 bcde | 0.55 ± 0.06 bcde | 0.35 ± 0.13 de | 0.55 ± 0.09 cde | 1.09 ± 0.14 a | 0.70 ± 0.10 bc | 0.73 ± 0.18 bc | 0.77 ± 0.10 ab | 0.67 ± 0.05 bcd |
| DPPH (Trolox equivalent, mg/mL) +*@ | 0.004 ± 0.01 e | 0.03 ± 0.01 bcd | 0.02 ± 0.01 bcde | 0.01 ± 0.00 de | 0.03 ± 0.01 bcd | 0.02 ± 0.01 bcde | 0.02 ± 0.00 cde | 0.08 ± 0.01 a | 0.06 ± 0.00 bc | 0.01 ± 0.00 de | 0.07 ± 0.01 b | 0.06 ± 0.02 bc |
| Vitamin B12 (µg/L) +*@ | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 3.01 ± 0.73 b | 2.06 ± 0.26 c | 0.00 ± 0.00 d | 4.56 ± 0.49 a | 2.06 ± 0.16 c |
a–f Statistical analysis using an ANOVA at 95% confidence interval with Tukey’s post hoc test performed across eight treatments if the interaction effect is significant. + Depicts a significant (p < 0.05) bifidobacterial effect. * Depicts a significant (p < 0.05) supplementation effect. @ Depicts a significant (p < 0.05) interaction effect. ‡ T 0 h, data taken from Tindjau et al. (2023) [19], as they were part of the same experiment. CN = unsupplemented control, G = glucose supplementation, GC = Glucose and cysteine supplementation, GCY = Glucose, cysteine, and yeast extract supplementation.
3.6. Free Mineral Changes
The free mineral changes are summarized in Figure 3. Cobalt showed a significant decrease in CN, G, and GCY for Bl-04 with a non-significant decrease in GC. On the other hand, B94 showed a significant decrease in the GC and GCY samples. Copper concentration decreased significantly in all samples after fermentation (Figure 3). Copper is an essential element that plays a role in ATP production, DNA integrity, and antioxidant response [38]. Copper is most commonly internalized in bacterial cells through the copper uptake porter from the extracellular region [39], which can explain the decrease after fermentation. Cobalt is an essential cofactor in ATP production and a part of vitamin B12 synthesis, which leads to a decrease after fermentation [40]. Iron, on the other hand, exhibited an opposite trend where fermented samples showed a significant increase in concentration by 0.03, 0.01, 0.1, and 0.12 mg/L in CN, G, GC, and GCY soy whey fermented with Bl-04 and 0.09, 0.09 mg/L in GC and GCY soy whey fermented with B94. Soy whey has been shown to contain 22.4 mg/L of phytic acid [5]. The breakdown of this antinutritional phytic acid could have led to an increase in free iron content. Iron deficiency has been linked to plant-based diets [10], and fermented soy whey could act as an avenue for plant-based diet adopters to increase their iron intake.
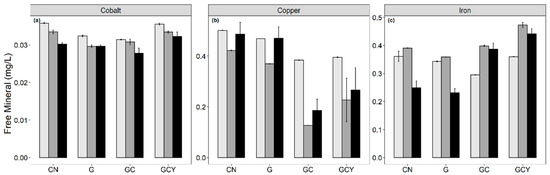
Figure 3.
Free mineral composition of unfermented ( ) and B. lactis (
) and B. lactis ( ) Bl-04 or B94 fermented (■) soy whey with different supplements, where CN = control, G = glucose supplemented, GC = glucose and cysteine supplemented, GCY = glucose, cysteine, and yeast extract supplemented: (a) cobalt, (b) copper, (c) iron. Error bars represent mean ± SD. Refer to Supplementary Table S2 for the detailed statistical interpretation and mineral composition of soy whey.
) Bl-04 or B94 fermented (■) soy whey with different supplements, where CN = control, G = glucose supplemented, GC = glucose and cysteine supplemented, GCY = glucose, cysteine, and yeast extract supplemented: (a) cobalt, (b) copper, (c) iron. Error bars represent mean ± SD. Refer to Supplementary Table S2 for the detailed statistical interpretation and mineral composition of soy whey.
 ) and B. lactis (
) and B. lactis ( ) Bl-04 or B94 fermented (■) soy whey with different supplements, where CN = control, G = glucose supplemented, GC = glucose and cysteine supplemented, GCY = glucose, cysteine, and yeast extract supplemented: (a) cobalt, (b) copper, (c) iron. Error bars represent mean ± SD. Refer to Supplementary Table S2 for the detailed statistical interpretation and mineral composition of soy whey.
) Bl-04 or B94 fermented (■) soy whey with different supplements, where CN = control, G = glucose supplemented, GC = glucose and cysteine supplemented, GCY = glucose, cysteine, and yeast extract supplemented: (a) cobalt, (b) copper, (c) iron. Error bars represent mean ± SD. Refer to Supplementary Table S2 for the detailed statistical interpretation and mineral composition of soy whey.
3.7. Free Volatile Changes
The changes in soy volatiles, short-chain fatty acids, volatile sulfur-containing compounds, and 2,3-butanedione contents are shown in Figure 4. Tables S3 and S4 contain the complete list of volatiles. Overall, endogenous aldehydes such as hexanal and heptanal were metabolized to low levels, while their respective alcohols and acids contents increased. Short-chain fatty acids such as acetic, butyric, and isovaleric acids were produced. 2,3-Butanedione was produced independent of supplementation, while hydrogen sulfide and thiophene-3-methyl were only produced in the GC and GCY samples, suggesting their cysteine origin. Overall, the fermentation of non-supplemented and supplemented soy whey with Bl-04 or B94 led to a higher total volatile peak area.
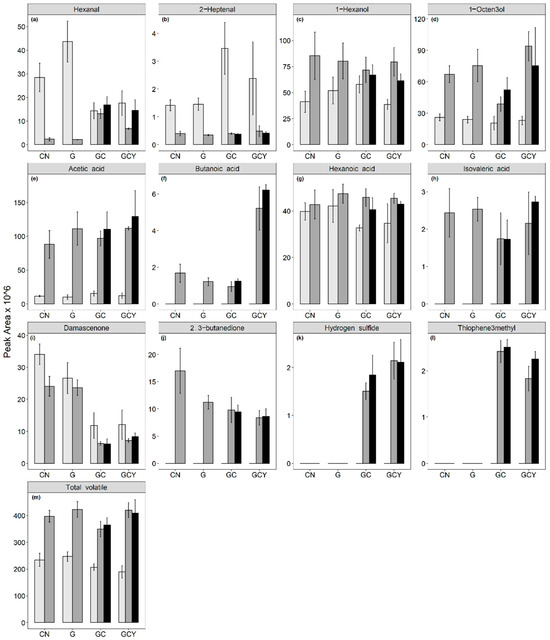
Figure 4.
Free volatile composition of unfermented ( ) and B. lactis (
) and B. lactis ( ) Bl-04 or B94 fermented (■) soy whey with different supplements, where CN = control, G = glucose supplemented, GC = glucose and cysteine supplemented, GCY = glucose, cysteine, and yeast extract supplemented: (a) hexanal, (b) 2-heptenal, (c) 1-hexanol, (d) 1-octen-3-ol, (e) acetic acid, (f) butanoic acid, (g) hexanoic acid, (h) isovaleric acid, (i) damascenone, (j) 2,3-butanedione, (k) hydrogen sulfide, (l) thiophene-3-methyl, (m) total volatile. Error bars represent mean ± SD. Refer to Supplementary Table S3 for the detailed volatile composition of soy whey and statistical interpretation.
) Bl-04 or B94 fermented (■) soy whey with different supplements, where CN = control, G = glucose supplemented, GC = glucose and cysteine supplemented, GCY = glucose, cysteine, and yeast extract supplemented: (a) hexanal, (b) 2-heptenal, (c) 1-hexanol, (d) 1-octen-3-ol, (e) acetic acid, (f) butanoic acid, (g) hexanoic acid, (h) isovaleric acid, (i) damascenone, (j) 2,3-butanedione, (k) hydrogen sulfide, (l) thiophene-3-methyl, (m) total volatile. Error bars represent mean ± SD. Refer to Supplementary Table S3 for the detailed volatile composition of soy whey and statistical interpretation.
 ) and B. lactis (
) and B. lactis ( ) Bl-04 or B94 fermented (■) soy whey with different supplements, where CN = control, G = glucose supplemented, GC = glucose and cysteine supplemented, GCY = glucose, cysteine, and yeast extract supplemented: (a) hexanal, (b) 2-heptenal, (c) 1-hexanol, (d) 1-octen-3-ol, (e) acetic acid, (f) butanoic acid, (g) hexanoic acid, (h) isovaleric acid, (i) damascenone, (j) 2,3-butanedione, (k) hydrogen sulfide, (l) thiophene-3-methyl, (m) total volatile. Error bars represent mean ± SD. Refer to Supplementary Table S3 for the detailed volatile composition of soy whey and statistical interpretation.
) Bl-04 or B94 fermented (■) soy whey with different supplements, where CN = control, G = glucose supplemented, GC = glucose and cysteine supplemented, GCY = glucose, cysteine, and yeast extract supplemented: (a) hexanal, (b) 2-heptenal, (c) 1-hexanol, (d) 1-octen-3-ol, (e) acetic acid, (f) butanoic acid, (g) hexanoic acid, (h) isovaleric acid, (i) damascenone, (j) 2,3-butanedione, (k) hydrogen sulfide, (l) thiophene-3-methyl, (m) total volatile. Error bars represent mean ± SD. Refer to Supplementary Table S3 for the detailed volatile composition of soy whey and statistical interpretation.
Hydrogen sulfide produced during the fermentation of the cysteine-supplemented soy whey by B. lactis is the main byproduct of the cysteine degradation pathway [41]. The presence of hydrogen sulfide resulted in the cysteine-supplemented samples having a rotten egg smell, which could significantly reduce consumer acceptance of fermented soy whey. On the other hand, 2,3-butanedione is commonly associated as the main aroma of butter and commonly found in the volatile analysis of cheese and wine. The production of 2,3-butanedione in fermented samples is due to 2-acetolactate or acetoin oxidation [42]. The production of 2,3-Butanedione has been reported in lactic acid bacteria through a pathway involving the conversion of citric acid [43]. The main volatile SCFA produced by B. lactis is acetic acid with butanoic and isovaleric acid being produced minimally. Acetic acid production in B. lactis is known to be through the bifidus pathway, while butanoic and isovaleric acid production could be from the metabolism of amino acids [31,44]. SCFAs play an important role in the treatment of inflammatory bowel diseases [30] and could improve the functionality of B. lactis fermented soy whey. The acetic acid results from Section 3.2 are observed in our GC analyses, where acetic acid content was significantly higher in fermented samples compared to unfermented. The reduction of soy endogenous aldehydes (i.e., hexanal and heptanal) led to a product with less “beany” aromas. An increase in hexanoic acid and hexanol was also observed in correspondence to the reduction in the C6 aldehyde. This can be explained by a reduction or oxidation reaction creating the respective alcohol or acid.
A PCA of 11 selected volatiles in both fermented and unfermented samples (Figure 5) was performed. The two PCs accumulated 78.26% variance, with PC1 accounting for 63.83% and PC2 with 14.43%. There was a clear separation between the fermented and unfermented samples. In the fermented samples, there was a distinct separation, particularly between samples supplemented with and without cysteine. The separation between the fermented and unfermented samples was driven by the amount of endogenous soy aldehydes (hexanal and heptenal), acids (butanoic, isovaleric and acetic), and 2,3-butanedione. Conversely, the separation of the fermented GC and GCY samples was driven by the production of hydrogen sulfide and thiophene-3-methyl.
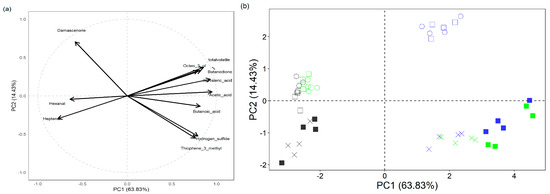
Figure 5.
PCA plots of volatile components in fermented soy whey: (a) loading plot and (b) score plot. ○ CN = control, □ G = glucose supplementation, X GC = glucose and cysteine supplementation, ■ GCY = glucose, cysteine, and yeast extract supplementation.  = B94 fermented samples,
= B94 fermented samples, = Bl-04 fermented samples,
= Bl-04 fermented samples,  = unfermented samples.
= unfermented samples.
 = B94 fermented samples,
= B94 fermented samples, = Bl-04 fermented samples,
= Bl-04 fermented samples,  = unfermented samples.
= unfermented samples.
4. Conclusions
The present study demonstrates the possibility of utilizing B. lactis to develop a functional beverage or ingredient by upcycling soy whey. B. lactis Bl-04 exhibited excellent growth independent of supplementation, while cysteine supplementation is necessary for B94 growth. In addition to growth, vitamin B12 was only synthesized in the presence of cysteine. Iron was improved in fermented soy whey irrespective of supplementation. Yeast extract greatly improved the acetic acid content in soy whey, which being a short-chain fatty acid could bring additional bioactivity. However, cysteine supplementation led to the production of volatile sulfur compounds, resulting in an unpleasant aroma. The increase in iron, vitamin B12, and amino acids from cysteine supplementation could open possibilities of developing a beverage or functional ingredient to supplement dietary choices that may lack these nutrients. Future work could establish the possibility of utilizing Bifidobacterium fermentation to upcycle side-stream products including aquafaba and cheese whey.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation9121024/s1. Figure S1: Changes of (a) cell count, and (b) pH of B. lactis Bl-04 or B94 fermented soy whey stored for 4 weeks at ambient (25 °C) or chilled (4 °C) temperature. Blue line represents 48-h fermentation at 37 °C. ○ CN = control, □ G = glucose supplemented, X GC = glucose and cysteine supplemented, ■ GCY = glucose, cysteine, and yeast extract supplemented; Table S1. Free amino acids of soy whey before and after B. lactis Bl-04 or B94 fermentation; Table S2. Two-way ANOVA on soy whey free minerals before and after B. lactis Bl-04 or B94 fermentation; Table S3. Two-way ANOVA on soy whey volatiles before and after B. lactis Bl-04 or B94 fermentation; Table S4. Relative peak area of soy whey volatiles before and after B. lactis Bl-04 or B94 fermentation.
Author Contributions
Conceptualization, R.T., J.-Y.C. and S.-Q.L.; Data curation, R.T.; Formal analysis, R.T.; Funding acquisition, S.-Q.L.; Investigation, J.-Y.C. and S.-Q.L.; Methodology, R.T. and J.-Y.C.; Supervision, J.-Y.C. and S.-Q.L.; Visualization, R.T.; Writing—original draft, R.T.; Writing—review & editing, J.-Y.C. and S.-Q.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Acknowledgments
The authors thank Liu Yunjiao and Lu Yuyun for technical advice.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tso, R.; Forde, C.G. Unintended consequences: Nutritional impact and potential pitfalls of switching from animal- to plant-based foods. Nutrients 2021, 13, 2527. [Google Scholar] [CrossRef] [PubMed]
- Alcorta, A.; Porta, A.; Tárrega, A.; Alvarez, M.D.; Pilar Vaquero, M. Foods for plant-based diets: Challenges and innovations. Foods 2021, 10, 293. [Google Scholar] [CrossRef] [PubMed]
- Bakaloudi, D.R.; Halloran, A.; Rippin, H.L.; Oikonomidou, A.C.; Dardavesis, T.I.; Williams, J.; Wickramasinghe, K.; Breda, J.; Chourdakis, M. Intake and adequacy of the vegan diet. A systematic review of the evidence. Clin. Nutr. 2021, 40, 3503–3521. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Ekperigin, M.M.; Akindahunsi, A.A. Coagulants modulate the antioxidant properties & hypocholesterolemic effect of tofu (curdled soymilk). Nutr. Health 2007, 18, 369–381. [Google Scholar] [PubMed]
- Chua, J.Y.; Lu, Y.; Liu, S.Q. Evaluation of five commercial non-Saccharomyces yeasts in fermentation of soy (tofu) whey into an alcoholic beverage. Food Microbiol. 2018, 76, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Chua, J.Y.; Liu, S.Q. Soy whey: More than just wastewater from tofu and soy protein isolate industry. Trends Food Sci. Technol. 2019, 91, 24–32. [Google Scholar] [CrossRef]
- Tu, C.; Tang, S.; Azi, F.; Hu, W.; Dong, M. Use of kombucha consortium to transform soy whey into a novel functional beverage. J. Funct. Foods 2019, 52, 81–89. [Google Scholar] [CrossRef]
- Tu, C.; Azi, F.; Huang, J.; Xu, X.; Xing, G.; Dong, M. Quality and metagenomic evaluation of a novel functional beverage produced from soy whey using water kefir grains. LWT 2019, 113, 108258. [Google Scholar] [CrossRef]
- Tangyu, M.; Muller, J.; Bolten, C.J.; Wittmann, C. Fermentation of plant-based milk alternatives for improved flavour and nutritional value. Appl. Microbiol. Biotechnol. 2019, 103, 9263–9275. [Google Scholar] [CrossRef]
- Craig, W.J.; Mangels, A.R.; Fresán, U.; Marsh, K.; Miles, F.L.; Saunders, A.V.; Haddad, E.H.; Heskey, C.E.; Johnston, P.; Larson-Meyer, E.; et al. The safe and effective use of plant-based diets with guidelines for health professionals. Nutrients 2021, 13, 4144. [Google Scholar] [CrossRef]
- Lee, J.-H.; O’Sullivan, D.J. Genomic Insights into Bifidobacteria. Microbiol. Mol. Biol. Rev. 2010, 74, 378–416. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, Y.; Morishita, T.; Mutai, M. Comparative studies on synthesis of water-soluble vitamins among human species of bifidobacteria. Agric. Biol. Chem. 1985, 49, 13–19. [Google Scholar]
- Moore, S.J.; Warren, M.J. The anaerobic biosynthesis of vitamin B12. Biochem. Soc. Trans. 2012, 40, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Rocha, C.G.; Gronenberg, L.S.; Mack, M.; Commichau, F.M.; Genee, H.J. Microbial cell factories for the sustainable manufacturing of B vitamins. Curr. Opin. Biotechnol. 2019, 56, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Picard, C.; Fioramonti, J.; Francois, A.; Robinson, T.; Neant, F.; Matuchansky, C. Review article: Bifidobacteria as probiotic agents—Physiological effects and clinical benefits. Aliment. Pharmacol. Ther. 2005, 22, 495–512. [Google Scholar] [CrossRef] [PubMed]
- Meance, S.; Cayuela, C.; Turchet, P.; Raimondi, A.; Lucas, C.; Antoine, J.M. A fermented milk with a Bifidobacterium probiotic strain DN-173 010 shortened oro-fecal gut transit time in elderly. Microb. Ecol. Health Dis. 2001, 13, 217–222. [Google Scholar] [CrossRef]
- Mättö, J.; Alakomi, H.L.; Vaari, A.; Virkajärvi, I.; Saarela, M. Influence of processing conditions on Bifidobacterium animalis subsp. lactis functionality with a special focus on acid tolerance and factors affecting it. Int. Dairy J. 2006, 16, 1029–1037. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Tindjau, R.; Chua, J.Y.; Liu, S.Q. Growth and metabolic behavior of probiotic Bifidobacterium longum subsp. longum in minimally supplemented soy (tofu) whey. Future Foods 2023, 8, 100272. [Google Scholar] [CrossRef]
- Rozada, R.; Vázquez, J.A.; Charalampopoulos, D.; Thomas, K.; Pandiella, S.S. Effect of storage temperature and media composition on the survivability of Bifidobacterium breve NCIMB 702257 in a malt hydrolisate. Int. J. Food Microbiol. 2009, 133, 14–21. [Google Scholar] [CrossRef]
- Schöpping, M.; Gaspar, P.; Neves, A.R.; Franzén, C.J.; Zeidan, A.A. Identifying the essential nutritional requirements of the probiotic bacteria Bifidobacterium animalis and Bifidobacterium longum through genome-scale modeling. npj Syst. Biol. Appl. 2021, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Daneshi, M.; Ehsani, M.R.; Razavi, S.H.; Labbafi, M. Effect of refrigerated storage on the probiotic survival and sensory properties of milk/carrot juice mix drink. Electron. J. Biotechnol. 2013, 16, 5. [Google Scholar] [CrossRef]
- Marco, M.L.; Sanders, M.E.; Gänzle, M.; Arrieta, M.C.; Cotter, P.D.; De Vuyst, L.; Hill, C.; Holzapfel, W.; Lebeer, S.; Merenstein, D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Wada, M.; Fukiya, S.; Suzuki, A.; Matsumoto, N.; Matsuo, M.; Yokota, A. Methionine utilization by bifidobacteria: Possible existence of a reverse transsulfuration pathway. Biosci. Microbiota Food Health 2021, 40, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, C.; Duranti, S.; Milani, C.; Mancabelli, L.; Lugli, G.A.; Turroni, F.; Mangifesta, M.; Viappiani, A.; Ossiprandi, M.C.; van Sinderen, D.; et al. Exploring amino acid auxotrophy in Bifidobacterium bifidum PRL2010. Front. Microbiol. 2015, 6, 1331. [Google Scholar] [CrossRef] [PubMed]
- Ejby, M.; Fredslund, F.; Andersen, J.M.; Žagar, A.V.; Henriksen, J.R.; Andersen, T.L.; Svensson, B.; Slotboom, D.J.; Hachem, M.A. An atp binding cassette transporter mediates the uptake of α-(1,6)-linked dietary oligosaccharides in bifidobacterium and correlates with competitive growth on these substrates. J. Biol. Chem. 2016, 291, 20220–20231. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.R.; Youn, S.Y.; Ji, G.E.; Park, M.S. Production of α- and β-galactosidases from Bifidobacterium longum subsp. longum RD47. J. Microbiol. Biotechnol. 2014, 24, 675–682. [Google Scholar] [CrossRef]
- Andersen, J.M.; Barrangou, R.; Hachem, M.A.; Lahtinen, S.J.; Goh, Y.J.; Svensson, B.; Klaenhammer, T.R. Transcriptional analysis of oligosaccharide utilization by Bifidobacterium lactis Bl-04. BMC Genom. 2013, 14, 312. [Google Scholar] [CrossRef]
- Van Der Meulen, R.; Adriany, T.; Verbrugghe, K.; De Vuyst, L. Kinetic analysis of bifidobacterial metabolism reveals a minor role for succinic acid in the regeneration of NAD+ through its growth-associated production. Appl. Environ. Microbiol. 2006, 72, 5204–5210. [Google Scholar] [CrossRef]
- Deleu, S.; Machiels, K.; Raes, J.; Verbeke, K.; Vermeire, S. Short chain fatty acids and its producing organisms: An overlooked therapy for IBD? EBioMedicine 2021, 66, 103293. [Google Scholar] [CrossRef]
- Usta-Gorgun, B.; Yilmaz-Ersan, L. Short-chain fatty acids production by Bifidobacterium species in the presence of salep. Electron. J. Biotechnol. 2020, 47, 29–35. [Google Scholar] [CrossRef]
- Engevik, M.A.; Danhof, H.A.; Hall, A.; Engevik, K.A.; Horvath, T.D.; Haidacher, S.J.; Hoch, K.M.; Endres, B.T.; Bajaj, M.; Garey, K.W.; et al. The metabolic profile of Bifidobacterium dentium reflects its status as a human gut commensal. BMC Microbiol. 2021, 21, 154. [Google Scholar] [CrossRef] [PubMed]
- Biavati, B.; Mattarelli, P. Bifidobacterium. In Bergey’s Manual of Systematics of Archaea and Bacteria; Wiley: Hoboken, NJ, USA, 2015; pp. 1–57. [Google Scholar]
- Kamaly, K.M. Bifidobacteria fermentation of soybean milk. Food Res. Int. 1997, 30, 675–682. [Google Scholar] [CrossRef]
- Hsiao, Y.H.; Ho, C.T.; Pan, M.H. Bioavailability and health benefits of major isoflavone aglycones and their metabolites. J. Funct. Foods. 2020, 74, 104164. [Google Scholar] [CrossRef]
- Pasqualetti, V.; Locato, V.; Fanali, C.; Mulinacci, N.; Cimini, S.; Morgia, A.M.; Pasqua, G.; De Gara, L. Comparison between in vitro chemical and ex vivo biological assays to evaluate antioxidant capacity of botanical extracts. Antioxidants 2021, 10, 1136. [Google Scholar] [CrossRef] [PubMed]
- Piwowarek, K.; Lipińska, E.; Hać-Szymańczuk, E.; Kieliszek, M.; Ścibisz, I. Propionibacterium spp.—Source of propionic acid, vitamin B12, and other metabolites important for the industry. Appl. Microbiol. Biotechnol. 2018, 102, 515–538. [Google Scholar] [CrossRef] [PubMed]
- Pajarillo, E.A.B.; Lee, E.; Kang, D.K. Trace metals and animal health: Interplay of the gut microbiota with iron, manganese, zinc, and copper. Anim. Nutr. 2021, 7, 750–761. [Google Scholar] [CrossRef]
- Andrei, A.; Öztürk, Y.; Khalfaoui-Hassani, B.; Rauch, J.; Marckmann, D.; Trasnea, P.I.; Daldal, F.; Koch, H.-G. Cu homeostasis in bacteria: The ins and outs. Membranes 2020, 10, 242. [Google Scholar] [CrossRef]
- Chamlagain, B.; Sugito, T.A.; Deptula, P.; Edelmann, M.; Kariluoto, S.; Varmanen, P.; Piironen, V. In situ production of active vitamin B12 in cereal matrices using Propionibacterium freudenreichii. Food Sci. Nutr. 2018, 6, 67–76. [Google Scholar] [CrossRef]
- Richardson, A.J.; McKain, N.; Wallace, R.J. Ammonia production by human faecal bacteria, and the enumeration, isolation and characterization of bacteria capable of growth on peptides and amino acids. BMC Microbiol. 2013, 13, 6. [Google Scholar] [CrossRef]
- Martin, F.; Cachon, R.; Pernin, K.; De Coninck, J.; Gervais, P.; Guichard, E.; Cayot, N. Effect of oxidoreduction potential on aroma biosynthesis by lactic acid bacteria in nonfat yogurt. J. Dairy Sci. 2011, 94, 614–622. [Google Scholar] [CrossRef]
- Le Bars, D.; Yvon, M. Formation of diacetyl and acetoin by Lactococcus lactis via aspartate catabolism. J. Appl. Microbiol. 2008, 104, 171–177. [Google Scholar] [CrossRef]
- Elsden, S.R.; Hilton, M.G. Volatile acid production from threonine, valine, leucine and isoleucine by clostridia. Arch. Microbiol. 1978, 117, 165–172. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).