Polydopamine Nanocomposite Hydrogel for Drug Slow-Release in Bone Defect Repair: A Review of Research Advances
Abstract
1. Introduction
2. Application and Function of PDA in Bone Defect Repair
2.1. Anti-Inflammation
2.2. Promoting Cell Adhesion and Proliferation
2.3. Promoting Osteogenesis and Angiogenesis
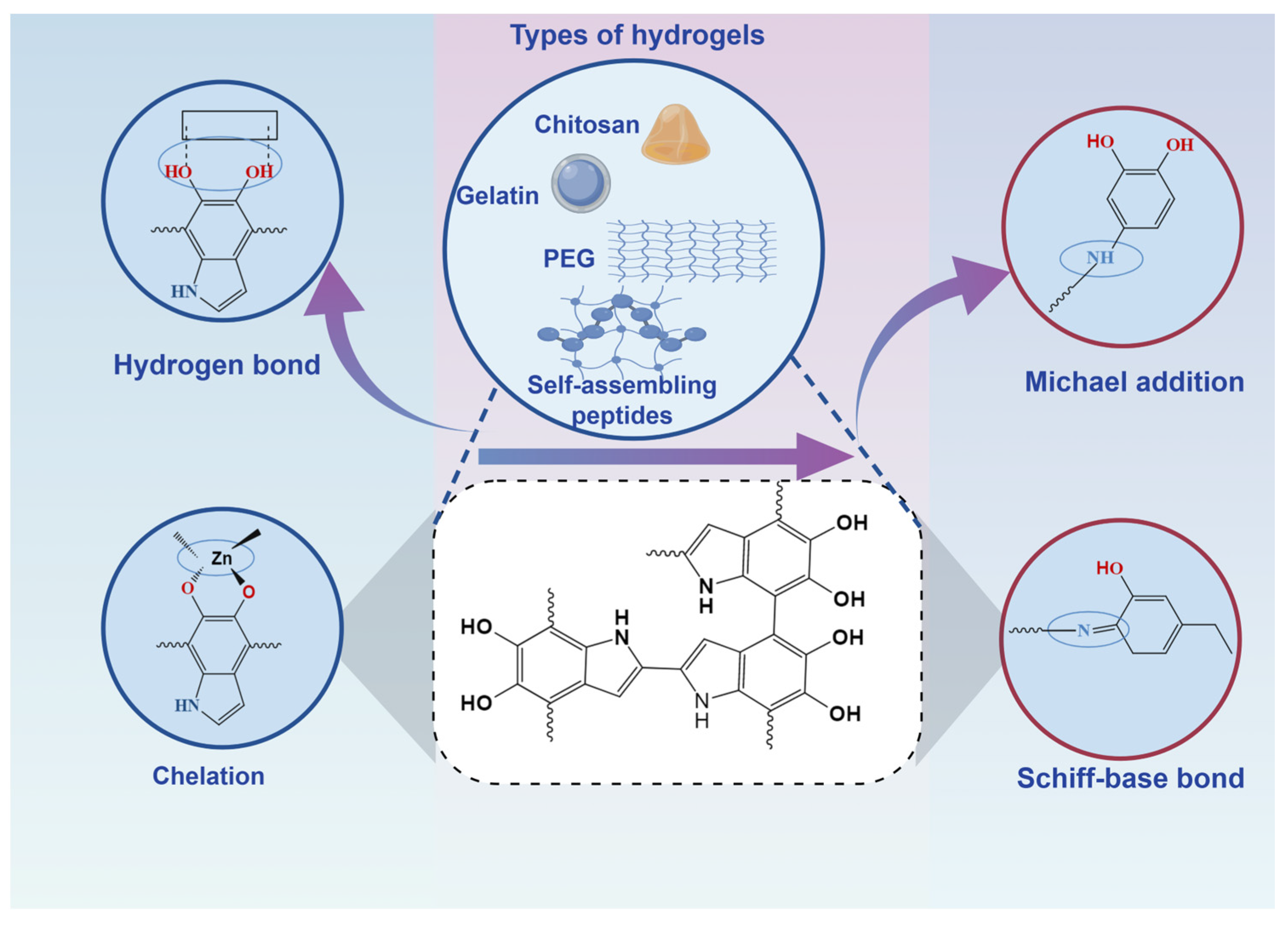
3. Advances in the Application of PDA-Hydrogel Based Drug Slow Release System in the Repair of Bone Defects
3.1. Composite Nanohydrogels of Natural Materials with Polydopamine Nanostructures
3.1.1. Chitosan-Based PDA Hydrogel
3.1.2. Alginate-Based PDA Hydrogel
3.1.3. Gelatin-Based PDA Hydrogel
3.2. Composite Nanohydrogels of Synthetic Materials with Polydopamine Nanostructures
3.2.1. Nanohydrogels Based on Frequently Studied Synthetic Materials
3.2.2. Nanohydrogels Based on Self-Assembling Peptides
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, S.; Sun, X.; Su, C.; Xue, Y.; Song, X.; Deng, R. Macrophages—Bone marrow mesenchymal stem cells crosstalk in bone healing. Front. Cell Dev. Biol. 2023, 11, 1193765. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.B.; Lin, T. Modifying MSC Phenotype to Facilitate Bone Healing: Biological Approaches. Front. Bioeng. Biotechnol. 2020, 8, 641. [Google Scholar] [CrossRef] [PubMed]
- Habibovic, P. Strategic Directions in Osteoinduction and Biomimetics. Tissue Eng. Part A 2017, 23, 1295–1296. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone Tissue Engineering: Recent Advances and Challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed]
- Kawecki, F.; Clafshenkel, W.P.; Fortin, M.; Auger, F.A.; Fradette, J. Biomimetic Tissue-Engineered Bone Substitutes for Maxillofacial and Craniofacial Repair: The Potential of Cell Sheet Technologies. Adv. Healthc. Mater. 2018, 7, e1700919. [Google Scholar] [CrossRef]
- Subbiah, R.; Lin, E.Y.; Athirasala, A.; Romanowicz, G.E.; Lin, A.S.P.; Califano, J.V.; Guldberg, R.E.; Bertassoni, L.E. Engineering of an osteoinductive and growth factor-free injectable bone-like microgel for bone regeneration. Adv. Healthc. Mater. 2023, 12, e2200976. [Google Scholar] [CrossRef]
- Zeng, Y.; Hoque, J.; Varghese, S. Biomaterial-assisted local and systemic delivery of bioactive agents for bone repair. Acta Biomater. 2019, 93, 152–168. [Google Scholar] [CrossRef]
- Chen, J.; Ashames, A.; Buabeid, M.A.; Fahelelbom, K.M.; Ijaz, M.; Murtaza, G. Nanocomposites drug delivery systems for the healing of bone fractures. Int. J. Pharm. 2020, 585, 119477. [Google Scholar] [CrossRef]
- Wang, Y.; Newman, M.R.; Benoit, D.S. Development of controlled drug delivery systems for bone fracture-targeted therapeutic delivery: A review. Eur. J. Pharm. Biopharm. 2018, 127, 223–236. [Google Scholar] [CrossRef]
- Bai, X.; Gao, M.; Syed, S.; Zhuang, J.; Xu, X.; Zhang, X.-Q. Bioactive hydrogels for bone regeneration. Bioact. Mater. 2018, 3, 401–417. [Google Scholar] [CrossRef]
- Shi, W.; Jiang, Y.; Wu, T.; Zhang, Y.; Li, T. Advancements in drug-loaded hydrogel systems for bone defect repair. Regen. Ther. 2023, 25, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, S.; Chen, W.; Hu, Y.; Geng, Z.; Su, J. Bone/cartilage targeted hydrogel: Strategies and applications. Bioact. Mater. 2022, 23, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Tang, J.; Hu, L.; Feng, Y.; Li, H.; Yin, C.; Tang, F. Experimental anti-tumor effect of emodin in suspension—In situ hydrogels formed with self-assembling peptide. Drug Deliv. 2021, 28, 1810–1821. [Google Scholar] [CrossRef] [PubMed]
- Raeisi, A.; Farjadian, F. Commercial hydrogel product for drug delivery based on route of administration. Front. Chem. 2024, 12, 1336717. [Google Scholar] [CrossRef]
- Farjadian, F.; Mirkiani, S.; Ghasemiyeh, P.; Kafshboran, H.R.; Mehdi-Alamdarlou, S.; Raeisi, A.; Esfandiarinejad, R.; Soleymani, S.; Goshtasbi, G.; Firouzabadi, N.; et al. Smart nanogels as promising platform for delivery of drug, gene, and vaccine; therapeutic applications and active targeting mechanism. Eur. Polym. J. 2024, 219, 113400. [Google Scholar] [CrossRef]
- Dhand, A.P.; Galarraga, J.H.; Burdick, J.A. Enhancing Biopolymer Hydrogel Functionality through Interpenetrating Networks. Trends Biotechnol. 2021, 39, 519–538. [Google Scholar] [CrossRef]
- Zheng, F.; Yang, X.; Li, J.; Tian, Z.; Xiao, B.; Yi, S.; Duan, L. Coordination with zirconium: A facile approach to improve the mechanical properties and thermostability of gelatin hydrogel. Int. J. Biol. Macromol. 2022, 205, 595–603. [Google Scholar] [CrossRef]
- Chen, M.; Le, D.Q.; Baatrup, A.; Nygaard, J.V.; Hein, S.; Bjerre, L.; Kassem, M.; Zou, X.; Bünger, C. Self-assembled composite matrix in a hierarchical 3-D scaffold for bone tissue engineering. Acta Biomater. 2011, 7, 2244–2255. [Google Scholar] [CrossRef]
- Wu, L.; Chen, G.; Li, Z. Layered Rare-Earth Hydroxide/Polyacrylamide Nanocomposite Hydrogels with Highly Tunable Photoluminescence. Small 2017, 13, 1604070. [Google Scholar] [CrossRef]
- Bedhiafi, T.; Idoudi, S.; Alhams, A.A.; Fernandes, Q.; Iqbal, H.; Basineni, R.; Uddin, S.; Dermime, S.; Merhi, M.; Billa, N. Applications of polydopaminic nanomaterials in mucosal drug delivery. J. Control. Release 2023, 353, 842–849. [Google Scholar] [CrossRef]
- Hong, S.; Na, Y.S.; Choi, S.; Song, I.T.; Kim, W.Y.; Lee, H. Non-Covalent Self-Assembly and Covalent Polymerization Co-Contribute to Polydopamine Formation. Adv. Funct. Mater. 2012, 22, 4711–4717. [Google Scholar] [CrossRef]
- Sarkari, S.; Khajehmohammadi, M.; Davari, N.; Li, D.; Yu, B. The effects of process parameters on polydopamine coatings employed in tissue engineering applications. Front. Bioeng. Biotechnol. 2022, 10, 1005413. [Google Scholar] [CrossRef]
- Hong, D.; Lee, H.; Kim, B.J.; Park, T.; Choi, J.Y.; Park, M.; Lee, J.; Cho, H.; Hong, S.-P.; Yang, S.H.; et al. A degradable polydopamine coating based on disulfide-exchange reaction. Nanoscale 2015, 7, 20149–20154. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, C.; Xu, J.; Wang, K.; Lu, X.; Zhang, H.; Qu, S.; Zhen, G.; Ren, F. Bioadhesive Microporous Architectures by Self-Assembling Polydopamine Microcapsules for Biomedical Applications. Chem. Mater. 2015, 27, 848–856. [Google Scholar] [CrossRef]
- Ku, S.H.; Ryu, J.; Hong, S.K.; Lee, H.; Park, C.B. General functionalization route for cell adhesion on non-wetting surfaces. Biomaterials 2010, 31, 2535–2541. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Zhang, M.; Zeng, Q.; Pan, W.; Huang, Y.; Qian, Y.; Dong, W.; Qi, X.; Shen, J. Mussel-inspired agarose hydrogel scaffolds for skin tissue engineering. Bioact. Mater. 2021, 6, 579–588. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Cheng, X.; Wang, J.; Pan, Y.; Zhang, S.; Jian, X. PDA-BPs integrated mussel-inspired multifunctional hydrogel coating on PPENK implants for anti-tumor therapy, antibacterial infection and bone regeneration. Bioact. Mater. 2023, 27, 546–559. [Google Scholar] [CrossRef]
- Li, Z.; Lin, H.; Shi, S.; Su, K.; Zheng, G.; Gao, S.; Zeng, X.; Ning, H.; Yu, M.; Li, X.; et al. Controlled and Sequential Delivery of Stromal Derived Factor-1 α (SDF-1α) and Magnesium Ions from Bifunctional Hydrogel for Bone Regeneration. Polymers 2022, 14, 2872. [Google Scholar] [CrossRef]
- Hathout, R.M.; Metwally, A.A.; El-Ahmady, S.H.; Metwally, E.S.; Ghonim, N.A.; Bayoumy, S.A.; Erfan, T.; Ashraf, R.; Fadel, M.; El-Kholy, A.I.; et al. Dual stimuli-responsive polypyrrole nanoparticles for anticancer therapy. J. Drug Deliv. Sci. Technol. 2018, 47, 176–180. [Google Scholar] [CrossRef]
- Ni, X.; Gao, Y.; Zhang, X.; Lei, Y.; Sun, G.; You, B. An eco-friendly smart self-healing coating with NIR and pH dual-responsive superhydrophobic properties based on biomimetic stimuli-responsive mesoporous polydopamine microspheres. Chem. Eng. J. 2021, 406, 126725. [Google Scholar] [CrossRef]
- Zhong, Z.; Fang, C.; He, S.; Zhang, T.; Liu, S.; Zhang, Y.; Wang, Q.; Ding, X.; Zhou, W.; Wang, X. Sequential Release Platform of Heparin and Urokinase with Dual Physical (NIR-II and Bubbles) Assistance for Deep Venous Thrombosis. ACS Biomater. Sci. Eng. 2020, 6, 6790–6799. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Wang, X.; Hou, Y.; Hong, X.; Gong, T.; Zhang, Z.; Sun, X. Rational design of Polymeric Hybrid Micelles to Overcome Lymphatic and Intracellular Delivery Barriers in Cancer Immunotherapy. Theranostics 2017, 7, 4383–4398. [Google Scholar] [CrossRef]
- Xie, Y.; Zheng, Y.; Fan, J.; Wang, Y.; Yue, L.; Zhang, N. Novel Electronic–Ionic Hybrid Conductive Composites for Multifunctional Flexible Bioelectrode Based on in Situ Synthesis of Poly(dopamine) on Bacterial Cellulose. ACS Appl. Mater. Interfaces 2018, 10, 22692–22702. [Google Scholar] [CrossRef]
- Wang, L.; Han, L.; Xue, P.; Hu, X.; Wong, S.-W.; Deng, M.; Tseng, H.C.; Huang, B.-W.; Ko, C.-C. Dopamine suppresses osteoclast differentiation via cAMP/PKA/CREB pathway. Cell. Signal. 2021, 78, 109847. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Tao, B.; He, Y.; Mu, C.; Liu, G.; Zhang, J.; Liao, Q.; Liu, P.; Cai, K. Remote eradication of biofilm on titanium implant via near-infrared light triggered photothermal/photodynamic therapy strategy. Biomaterials 2019, 223, 119479. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; Li, W.; Yu, B.; Li, B.; Jordan, R.; Jia, X.; Zhou, F. Mussel-Inspired Two-Dimensional Freestanding Alkyl-Polydopamine Janus Nanosheets. Angew. Chem. Int. Ed. Engl. 2019, 58, 12018–12022. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Brust, T.F.; Lee, H.J.; Lee, S.C.; Watts, V.J.; Yeo, Y. Polydopamine-Based Simple and Versatile Surface Modification of Polymeric Nano Drug Carriers. ACS Nano 2014, 8, 3347–3356. [Google Scholar] [CrossRef]
- Zhu, M.; Shi, Y.; Shan, Y.; Guo, J.; Song, X.; Wu, Y.; Wu, M.; Lu, Y.; Chen, W.; Xu, X.; et al. Recent developments in mesoporous polydopamine-derived nanoplatforms for cancer theranostics. J. Nanobiotechnol. 2021, 19, 387. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.-Y.; Lin, Y.-J.; Enriquez, E.; Peng, X.-F.; Turng, L.-S. Highly Stretchable and Biocompatible Strain Sensors Based on Mussel-Inspired Super-Adhesive Self-Healing Hydrogels for Human Motion Monitoring. ACS Appl. Mater. Interfaces 2018, 10, 20897–20909. [Google Scholar] [CrossRef]
- Rossi, F.; Kurashina, Y.; Onoe, H. Can nanoparticles enhance drug-delivery performance of hydrogels? Nanomedicine 2023, 18, 653–657. [Google Scholar] [CrossRef]
- Mohammadpour, R.; Dobrovolskaia, M.A.; Cheney, D.L.; Greish, K.F.; Ghandehari, H. Subchronic and chronic toxicity evaluation of inorganic nanoparticles for delivery applications. Adv. Drug Deliv. Rev. 2019, 144, 112–132. [Google Scholar] [CrossRef] [PubMed]
- Paris, J.L.; Baeza, A.; Vallet-Regí, M. Overcoming the stability, toxicity, and biodegradation challenges of tumor stimuli-responsive inorganic nanoparticles for delivery of cancer therapeutics. Expert Opin. Drug Deliv. 2019, 16, 1095–1112. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, X.; Wang, X.; Qi, M.; Pan, J.; Wang, Q. One-Step Nanosurface Self-Assembly of d-Peptides Renders Bubble-Free Ultrasound Theranostics. Nano Lett. 2019, 19, 2251–2258. [Google Scholar] [CrossRef]
- Zhu, J.; Feng, C.; Zhang, W.; Zhong, M.; Tang, W.; Wang, Z.; Shi, H.; Yin, Z.; Shi, J.; Huang, Y.; et al. Activation of dopamine receptor D1 promotes osteogenic differentiation and reduces glucocorticoid-induced bone loss by upregulating the ERK1/2 signaling pathway. Mol. Med. 2022, 28, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, D.; Wang, X.; He, Y.; Luan, W.; Qi, F.; Ding, J. Polydopamine-mediated covalent functionalization of collagen on a titanium alloy to promote biocompatibility with soft tissues. J. Mater. Chem. B 2019, 7, 2019–2031. [Google Scholar] [CrossRef]
- Kim, H.; Lee, Y.H.; Kim, N.K.; Kang, I.K. Immobilization of Collagen on the Surface of a PEEK Implant with Monolayer Nanopores. Polymers 2022, 14, 1633. [Google Scholar] [CrossRef]
- Xue, X.; Hu, Y.; Wang, S.; Chen, X.; Jiang, Y.; Su, J. Fabrication of physical and chemical crosslinked hydrogels for bone tissue engineering. Bioact. Mater. 2021, 12, 327–339. [Google Scholar] [CrossRef]
- Lu, G.; Xu, Y.; Liu, Q.; Chen, M.; Sun, H.; Wang, P.; Wang, Y.; Li, X.; Hui, X.; Luo, E.; et al. An instantly fixable and self-adaptive scaffold for skull regeneration by autologous stem cell recruitment and angiogenesis. Nat. Commun. 2022, 13, 2499. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, H.; Miron, R.J.; Zhang, Y. Modulating macrophage polarization on titanium implant surface by poly(dopamine)-assisted immobilization of IL4. Clin. Implant. Dent. Relat. Res. 2019, 21, 977–986. [Google Scholar] [CrossRef]
- Su, J.; Du, Z.; Xiao, L.; Wei, F.; Yang, Y.; Li, M.; Qiu, Y.; Liu, J.; Chen, J.; Xiao, Y. Graphene oxide coated Titanium Surfaces with Osteoimmunomodulatory Role to Enhance Osteogenesis. Mater. Sci. Eng. C 2020, 113, 110983. [Google Scholar] [CrossRef]
- Zhang, D.; Zheng, H.; Geng, K.; Shen, J.; Feng, X.; Xu, P.; Duan, Y.; Li, Y.; Wu, R.; Gou, Z.; et al. Large fuzzy biodegradable polyester microspheres with dopamine deposition enhance cell adhesion and bone regeneration in vivo. Biomaterials 2021, 272, 120783. [Google Scholar] [CrossRef] [PubMed]
- Agilan, P.; Saranya, K.; Rajendran, N. Bio-inspired polydopamine incorporated titania nanotube arrays for biomedical applications. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127489. [Google Scholar] [CrossRef]
- Qiaoxia, L.; Yujie, Z.; Meng, Y.; Yizhu, C.; Yan, W.; Yinchun, H.; Xiaojie, L.; Weiyi, C.; Di, H. Hydroxyapatite/tannic acid composite coating formation based on Ti modified by TiO2 nanotubes. Colloids Surf. B Biointerfaces 2020, 196, 111304. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xu, Y.; Zhu, M.; Gu, Y.; Zhang, W.; Shao, H.; Wang, Y.; Ping, Z.; Hu, X.; Wang, L.; et al. Inhibition of titanium-particle-induced inflammatory osteolysis after local administration of dopamine and suppression of osteoclastogenesis via D2-like receptor signaling pathway. Biomaterials 2016, 80, 1–10. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Yin, X.; Sun, Y.; Weng, J.; Zhou, J.; Feng, B. Inflammatory responses to micro/nano-structured titanium surfaces with silver nanoparticles in vitro. J. Mater. Chem. B 2019, 7, 3546–3559. [Google Scholar] [CrossRef]
- Feng, P.; Liu, M.; Peng, S.; Bin, S.; Zhao, Z.; Shuai, C. Polydopamine modified polycaprolactone powder for fabrication bone scaffold owing intrinsic bioactivity. J. Mater. Res. Technol. 2021, 15, 3375–3385. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, H.; Zhao, B.; Shen, R.; Liu, D.; Hong, J.; Wei, H.; Xi, P.; Chen, F.; Bai, D. MC3T3-E1 preosteoblast cell-mediated mineralization of hydroxyapatite by poly-dopamine-functionalized graphene oxide. J. Bioact. Compat. Polym. 2015, 30, 289–301. [Google Scholar] [CrossRef]
- Chen, J.; Mei, M.L.; Li, Q.-L.; Chu, C.-H. Mussel-inspired silver-nanoparticle coating on porous titanium surfaces to promote mineralization. RSC Adv. 2016, 6, 104025–104035. [Google Scholar] [CrossRef]
- Wu, M.; Wang, T.; Wang, Y.; Wang, H. Ultrafast bone-like apatite formation on bioactive tricalcium silicate cement using mussel-inspired polydopamine. Ceram. Int. 2019, 45, 3033–3043. [Google Scholar] [CrossRef]
- Lin, H.; Fu, Y.; Gao, Y.; Mo, A. Integrated design of a mussel-inspired hydrogel biofilm composite structure to guide bone regeneration. Macromol. Mater. Eng. 2020, 305, 2000064. [Google Scholar] [CrossRef]
- Chien, C.; Liu, T.; Kuo, W.; Wang, M.; Tsai, W. Dopamine-assisted immobilization of hydroxyapatite nanoparticles and RGD peptides to improve the osteoconductivity of titanium. J. Biomed. Mater. Res. Part A 2013, 101, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Bachir, A.I.; Zareno, J.; Moissoglu, K.; Plow, E.F.; Gratton, E.; Horwitz, A.R. Integrin-associated complexes form hierarchically with variable stoichiometry in nascent adhesions. Curr. Biol. 2014, 24, 1845–1853. [Google Scholar] [CrossRef]
- Yuan, Z.; Huang, S.; Lan, S.; Xiong, H.; Tao, B.; Ding, Y.; Liu, Y.; Liu, P.; Cai, K. Surface engineering of titanium implants with enzyme-triggered antibacterial properties and enhanced osseointegration in vivo. J. Mater. Chem. B 2018, 6, 8090–8104. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.-M.; Mao, C.; Liu, W.; Liu, Y.-H.; Ren, Y.; Xu, L.; Xu, J.-Z.; Zhao, B.; Gul, R.M.; Li, Z.-M. Nanotopographical polymeric surface with mussel-inspired decoration to enhance osteoblast differentiation. Appl. Surf. Sci. 2019, 481, 987–993. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, T.; Bei, H.P.; Zhao, Y.; Zhao, X. Sculpting bio-inspired surface textures: An adhesive janus periosteum. Adv. Funct. Mater. 2021, 31, 2104636. [Google Scholar] [CrossRef]
- Hanami, K.; Nakano, K.; Saito, K.; Okada, Y.; Yamaoka, K.; Kubo, S.; Kondo, M.; Tanaka, Y. Dopamine D2-like receptor signaling suppresses human osteoclastogenesis. Bone 2013, 56, 1–8. [Google Scholar] [CrossRef]
- Chiang, T.-I.; Lane, H.-Y.; Lin, C.-H. D2 dopamine receptor gene (DRD2) Taq1A (rs1800497) affects bone density. Sci. Rep. 2020, 10, 13236. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Q.; Jeong, H.-W.; Han, D.; Fabian, J.; Drexler, H.C.; Stehling, M.; Schöler, H.R.; Adams, R.H. Dopamine signaling regulates hematopoietic stem and progenitor cell function. Blood 2021, 138, 2051–2065. [Google Scholar] [CrossRef]
- Wang, C.-X.; Ge, X.-Y.; Wang, M.-Y.; Ma, T.; Zhang, Y.; Lin, Y. Dopamine D1 receptor-mediated activation of the ERK signaling pathway is involved in the osteogenic differentiation of bone mesenchymal stem cells. Stem Cell Res. Ther. 2020, 11, 12. [Google Scholar] [CrossRef]
- Taylor, E.L.; Weaver, S.R.; Lorang, I.M.; Arnold, K.M.; Bradley, E.W.; de Velasco, E.M.F.; Wickman, K.; Westendorf, J.J. GIRK3 deletion facilitates kappa opioid signaling in chondrocytes, delays vascularization and promotes bone lengthening in mice. Bone 2022, 159, 116391. [Google Scholar] [CrossRef]
- Wang, J.; Cui, Y.; Zhang, B.; Sun, S.; Xu, H.; Yao, M.; Wu, D.; Wang, Y. Polydopamine-Modified functional materials promote bone regeneration. Mater. Des. 2024, 238, 112655. [Google Scholar] [CrossRef]
- Gao, B.; Chen, L.; Zhao, Y.; Yan, X.; Wang, X.; Zhou, C.; Shi, Y.; Xue, W. Methods to prepare dopamine/polydopamine modified alginate hydrogels and their special improved properties for drug delivery. Eur. Polym. J. 2019, 110, 192–201. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.-Y.; Napiwocki, B.N.; Peng, X.-F.; Turng, L.-S. Mussel-inspired electroactive chitosan/graphene oxide composite hydrogel with rapid self-healing and recovery behavior for tissue engineering. Carbon 2017, 125, 557–570. [Google Scholar] [CrossRef]
- Di, X.; Hang, C.; Xu, Y.; Ma, Q.; Li, F.; Sun, P.; Wu, G. Bioinspired tough, conductive hydrogels with thermally reversible adhesiveness based on nanoclay confined NIPAM polymerization and a dopamine modified polypeptide. Mater. Chem. Front. 2020, 4, 189–196. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Wang, X.; Wang, Y.; Zhang, Q.; Cheng, Y. A Polydopamine Nanoparticle-Knotted Poly(ethylene glycol) Hydrogel for On-Demand Drug Delivery and Chemo-photothermal Therapy. Chem. Mater. 2017, 29, 1370–1376. [Google Scholar] [CrossRef]
- Han, L.; Li, P.; Tang, P.; Wang, X.; Zhou, T.; Wang, K.; Ren, F.; Guo, T.; Lu, X. Mussel-inspired cryogels for promoting wound regeneration through photobiostimulation, modulating inflammatory responses and suppressing bacterial invasion. Nanoscale 2019, 11, 15846–15861. [Google Scholar] [CrossRef]
- Liu, Y.; Xi, Y.; Zhao, J.; Zhao, J.; Li, J.; Huang, G.; Li, J.; Fang, F.; Gu, L.; Wang, S. Preparation of therapeutic-laden konjac hydrogel for tumor combination therapy. Chem. Eng. J. 2019, 375, 122048. [Google Scholar] [CrossRef]
- Zeng, J.; Shi, D.; Gu, Y.; Kaneko, T.; Zhang, L.; Zhang, H.; Kaneko, D.; Chen, M. Injectable and Near-Infrared-Responsive Hydrogels Encapsulating Dopamine-Stabilized Gold Nanorods with Long Photothermal Activity Controlled for Tumor Therapy. Biomacromolecules 2019, 20, 3375–3384. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Gao, F.; Xu, Z.; Dai, F.; Liu, W. An Injectable Supramolecular Polymer Nanocomposite Hydrogel for Prevention of Breast Cancer Recurrence with Theranostic and Mammoplastic Functions. Adv. Funct. Mater. 2018, 28, 1801000. [Google Scholar] [CrossRef]
- Song, J.; Hu, H.; Jian, C.; Wu, K.; Chen, X. New Generation of Gold Nanoshell-Coated Esophageal Stent: Preparation and Biomedical Applications. ACS Appl. Mater. Interfaces 2016, 8, 27523–27529. [Google Scholar] [CrossRef]
- Han, L.; Liu, K.; Wang, M.; Wang, K.; Fang, L.; Chen, H.; Zhou, J.; Lu, X. Mussel-Inspired Adhesive and Conductive Hydrogel with Long-Lasting Moisture and Extreme Temperature Tolerance. Adv. Funct. Mater. 2018, 28, 1704195. [Google Scholar] [CrossRef]
- Wan, Z.; Dong, Q.; Guo, X.; Bai, X.; Zhang, X.; Zhang, P.; Liu, Y.; Lv, L.; Zhou, Y. A dual-responsive polydopamine-modified hydroxybutyl chitosan hydrogel for sequential regulation of bone regeneration. Carbohydr. Polym. 2022, 297, 120027. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, K.; Darabi, M.A.; Yuan, Q.; Xing, M. Mussel-inspired alginate gel promoting the osteogenic differentiation of mesenchymal stem cells and anti-infection. Mater. Sci. Eng. C 2016, 69, 496–504. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Y.; Zhang, X.; Ma, J.; Wang, M. Double-crosslinked bifunctional hydrogels with encapsulated anti-cancer drug for bone tumor cell ablation and bone tissue regeneration. Colloids Surf. B Biointerfaces 2022, 213, 112364. [Google Scholar] [CrossRef]
- Gan, D.; Wang, Z.; Xie, C.; Wang, X.; Xing, W.; Ge, X.; Yuan, H.; Wang, K.; Tan, H.; Lu, X. Mussel-Inspired Tough Hydrogel with In Situ Nanohydroxyapatite Mineralization for Osteochondral Defect Repair. Adv. Healthc. Mater. 2019, 12, e2203040. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, X.; Tan, B.; Shan, Y.; Zhao, X.; Liao, J. Near-infrared light control of GelMA/PMMA/PDA hydrogel with mild photothermal therapy for skull regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2022, 133, 112641. [Google Scholar] [CrossRef] [PubMed]
- Dashtimoghadam, E.; Fahimipour, F.; Tongas, N.; Tayebi, L. Microfluidic fabrication of microcarriers with sequential delivery of VEGF and BMP-2 for bone regeneration. Sci. Rep. 2020, 10, 11764. [Google Scholar] [CrossRef]
- Li, X.; Pang, Y.; Guan, L.; Li, L.; Zhu, Y.; Whittaker, A.K.; Yang, B.; Zhu, S.; Lin, Q. Mussel-inspired antimicrobial hydrogel with cellulose nanocrystals/tannic acid modified silver nanoparticles for enhanced calvarial bone regeneration. Int. J. Biol. Macromol. 2024, 270 Pt 2, 132419. [Google Scholar] [CrossRef]
- Han, Z.; Wang, F.; Xiong, W.; Meng, C.; Yao, Y.; Cui, W.; Zhang, M. Precise Cell Type Electrical Stimulation Therapy Via Force-electric Hydrogel Microspheres for Cartilage Healing. Adv. Mater. 2024, 37, e2414555. [Google Scholar] [CrossRef]
- Sun, H.; Shang, Y.; Guo, J.; Maihemuti, A.; Shen, S.; Shi, Y.; Liu, H.; Che, J.; Jiang, Q. Artificial Periosteum with Oriented Surface Nanotopography and High Tissue Adherent Property. ACS Appl. Mater. Interfaces 2023, 15, 45549–45560. [Google Scholar] [CrossRef]
- Chen, S.; Tan, S.; Zheng, L.; Wang, M. Multilayered Shape-Morphing Scaffolds with a Hierarchical Structure for Uterine Tissue Regeneration. ACS Appl. Mater. Interfaces 2024, 16, 6772–6788. [Google Scholar] [CrossRef]
- Li, H.; Zhao, T.; Yuan, Z.; Gao, T.; Yang, Y.; Li, R.; Tian, Q.; Tang, P.; Guo, Q.; Zhang, L. Cartilage lacuna-biomimetic hydrogel microspheres endowed with integrated biological signal boost endogenous articular cartilage regeneration. Bioact. Mater. 2024, 41, 61–82. [Google Scholar] [CrossRef]
- Wu, M.; Liu, H.; Li, D.; Zhu, Y.; Wu, P.; Chen, Z.; Chen, F.; Chen, Y.; Deng, Z.; Cai, L. Smart-Responsive Multifunctional Therapeutic System for Improved Regenerative Microenvironment and Accelerated Bone Regeneration via Mild Photothermal Therapy. Adv. Sci. 2024, 11, e2304641. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhang, Y.; Zhao, Y.; Chu, L.; Meng, X.; Ye, L.; Li, X.; Wang, Z.; Wu, P. Photoactivated Hydrogel Therapeutic System with MXene-Based Nanoarchitectonics Potentiates Endogenous Bone Repair Through Reshaping the Osteo-Vascularization Network. Small 2024, 20, e2403003. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Li, Y.; Qin, Y.; Huang, Z.; Wu, Y.; Sun, L.; Wang, C.; Wang, W.; Feng, G.; Qi, Y. In Situ Deposition of Drug and Gene Nanoparticles on a Patterned Supramolecular Hydrogel to Construct a Directionally Osteochondral Plug. Nano-Micro Lett. 2023, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Jo, J.-I.; Kanda, R.; Nishiura, A.; Hashimoto, Y.; Matsumoto, N. Bioactive Polyetheretherketone with Gelatin Hydrogel Leads to Sustained Release of Bone Morphogenetic Protein-2 and Promotes Osteogenic Differentiation. Int. J. Mol. Sci. 2023, 24, 12741. [Google Scholar] [CrossRef]
- Li, M.; Wu, H.; Gao, K.; Wang, Y.; Hu, J.; Guo, Z.; Hu, R.; Zhang, M.; Pang, X.; Guo, M.; et al. Smart Implantable Hydrogel for Large Segmental Bone Regeneration. Adv. Healthc. Mater. 2024, 13, e2402916. [Google Scholar] [CrossRef]
- Wu, M.; Chen, F.; Liu, H.; Wu, P.; Yang, Z.; Zhang, Z.; Su, J.; Cai, L.; Zhang, Y. Bioinspired sandwich-like hybrid surface functionalized scaffold capable of regulating osteogenesis, angiogenesis, and osteoclastogenesis for robust bone regeneration. Mater. Today Bio 2022, 17, 100458. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; Sun, Y.; Tan, X.; Wang, C.; Wang, Z.; Ye, L. Multiscale design of stiffening and ROS scavenging hydrogels for the augmentation of mandibular bone regeneration. Bioact. Mater. 2022, 20, 111–125. [Google Scholar] [CrossRef]
- Wu, M.; Liu, H.; Zhu, Y.; Wu, P.; Chen, Y.; Deng, Z.; Zhu, X.; Cai, L. Bioinspired soft-hard combined system with mild photothermal therapeutic activity promotes diabetic bone defect healing via synergetic effects of immune activation and angiogenesis. Theranostics 2024, 14, 4014–4057. [Google Scholar] [CrossRef]
- Shi, W.; Gao, Y.; Wu, Y.; Sun, J.; Xu, B.; Lu, X.; Wang, Q. A multifunctional polydopamine/genipin/alendronate nanoparticle licences fibrin hydrogels osteoinductive and immunomodulatory potencies for repairing bone defects. Int. J. Biol. Macromol. 2023, 249, 126072. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Yang, L.; Li, W.; Chen, K.; Shang, L.; Bai, Y.; Zhao, Y. Mussel-inspired multi-bioactive microsphere scaffolds for bone defect photothermal therapy. Mater. Today Bio 2024, 29, 101363. [Google Scholar] [CrossRef]
- Wu, M.; Liu, H.; Zhu, Y.; Chen, F.; Chen, Z.; Guo, L.; Wu, P.; Li, G.; Zhang, C.; Wei, R.; et al. Mild Photothermal-Stimulation Based on Injectable and Photocurable Hydrogels Orchestrates Immunomodulation and Osteogenesis for High-Performance Bone Regeneration. Small 2023, 19, e2300111. [Google Scholar] [CrossRef]
- Huang, L.; Wu, T.; Sun, J.; Lin, X.; Peng, Y.; Zhang, R.; Gao, Y.; Xu, S.; Sun, Y.; Zhou, Y.; et al. Biocompatible chitin-based Janus hydrogel membranes for periodontal repair. Acta Biomater. 2024, 190, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, G.; Xu, K.; Wang, L.; Yu, L.; Xing, M.M.; Qiu, X. Mussel-inspired dual-functional PEG hydrogel inducing mineralization and inhibiting infection in maxillary bone reconstruction. Mater. Sci. Eng. C 2018, 90, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Shen, L.; Liu, H.-F.; Zou, X.-H.; Zhao, J.; Huang, Y.; Zhu, Y.-F.; Li, Z.-Y.; Xu, C.; Luo, L.-H.; et al. The marriage of immunomodulatory, angiogenic, and osteogenic capabilities in a piezoelectric hydrogel tissue engineering scaffold for military medicine. Mil. Med. Res. 2023, 10, 35. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Shen, Y.-F.; Ho, C.-C.; Yu, J.; Wu, Y.-H.A.; Wang, K.; Shih, C.-T.; Shie, M.-Y. Osteogenic and angiogenic potentials of the cell-laden hydrogel/mussel-inspired calcium silicate complex hierarchical porous scaffold fabricated by 3D bioprinting. Mater. Sci. Eng. C 2018, 91, 679–687. [Google Scholar] [CrossRef]
- Liu, C.; Wu, J.; Gan, D.; Li, Z.; Shen, J.; Tang, P.; Luo, S.; Li, P.; Lu, X.; Zheng, W. The characteristics of mussel-inspired nHA/OSA injectable hydrogel and repaired bone defect in rabbit. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 1814–1825. [Google Scholar] [CrossRef]
- Pang, Y.; Guan, L.; Zhu, Y.; Niu, R.; Zhu, S.; Lin, Q. Gallic acid-grafted chitosan antibacterial hydrogel incorporated with polydopamine-modified hydroxyapatite for enhancing bone healing. Front. Bioeng. Biotechnol. 2023, 11, 1162202. [Google Scholar] [CrossRef]
- Kwack, K.H.; Ji, J.Y.; Park, B.; Heo, J.S. Fucoidan (Undaria pinnatifida)/Polydopamine Composite-Modified Surface Promotes Osteogenic Potential of Periodontal Ligament Stem Cells. Mar. Drugs 2022, 20, 181. [Google Scholar] [CrossRef]
- Deng, H.; Shu, X.; Wang, Y.; Zhang, J.; Yin, Y.; Wu, F.; He, J. Matrix Stiffness Regulated Endoplasmic Reticulum Stress-mediated Apoptosis of Osteosarcoma Cell through Ras Signal Cascades. Cell Biochem. Biophys. 2023, 81, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Xiong, Y.; Wang, X.; Yu, T.; Zhou, C. Synthesis and characterization of multifunctional organic-inorganic composite hydrogel formed with tissue-adhesive property and inhibiting infection. Mater. Sci. Eng. C 2021, 118, 111532. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Lyu, Z.; Hu, F.; Yang, L.; Yang, K.; Chen, M.; Wang, Y. A chondroitin sulphate hydrogel with sustained release of SDF-1α for extensive cartilage defect repair through induction of cell homing and promotion of chondrogenesis. J. Mater. Chem. B 2024, 12, 8672–8687. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, J.; Yan, J.; Guo, Z.; Zhang, F. Magnetic microspheres mimicking certain functions of macrophages: Towards precise antibacterial potency for bone defect healing. Mater. Today Bio 2023, 20, 100651. [Google Scholar] [CrossRef]
- Li, S.; Jia, C.; Han, H.; Yang, Y.; Xiaowen, Y.; Chen, Z. Characterization and biocompatibility of a bilayer PEEK-based scaffold for guiding bone regeneration. BMC Oral Health 2024, 24, 1138. [Google Scholar] [CrossRef]
- Liu, H.; Li, K.; Yi, D.; Ding, Y.; Gao, Y.; Zheng, X. Deferoxamine-Loaded Chitosan-Based Hydrogel on Bone Implants Showing Enhanced Bond Strength and Pro-Angiogenic Effects. J. Funct. Biomater. 2024, 15, 112. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Wang, J.; Ye, C.; Lin, J.; Ran, J.; Jia, Z.; Gong, J.; Zhang, Y.; Xiang, J.; Lu, X.; et al. Polyphenol-mediated redox-active hydrogel with H2S gaseous-bioelectric coupling for periodontal bone healing in diabetes. Nat. Commun. 2024, 15, 9071. [Google Scholar] [CrossRef]
- Im, S.; Choe, G.; Seok, J.M.; Yeo, S.J.; Lee, J.H.; Kim, W.D.; Lee, J.Y.; Park, S.A. An osteogenic bioink composed of alginate, cellulose nanofibrils, and polydopamine nanoparticles for 3D bioprinting and bone tissue engineering. Int. J. Biol. Macromol. 2022, 205, 520–529. [Google Scholar] [CrossRef]
- Ma, W.; Yang, M.; Wu, C.; Wang, S.; Du, M. Bioinspired self-healing injectable nanocomposite hydrogels based on oxidized dextran and gelatin for growth-factor-free bone regeneration. Int. J. Biol. Macromol. 2023, 251, 126145. [Google Scholar] [CrossRef]
- Luo, S.; Wu, J.; Jia, Z.; Tang, P.; Sheng, J.; Xie, C.; Liu, C.; Gan, D.; Hu, D.; Zheng, W.; et al. An Injectable, Bifunctional Hydrogel with Photothermal Effects for Tumor Therapy and Bone Regeneration. Macromol. Biosci. 2019, 19, e1900047. [Google Scholar] [CrossRef]
- Jung, A.; Makkar, P.; Amirian, J.; Lee, B.-T. A novel hybrid multichannel biphasic calcium phosphate granule-based composite scaffold for cartilage tissue regeneration. J. Biomater. Appl. 2018, 32, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Douglas, T.; Wlodarczyk, M.; Pamula, E.; Declercq, H.; de Mulder, E.; Bucko, M.; Balcaen, L.; Vanhaecke, F.; Cornelissen, R.; Dubruel, P.; et al. Enzymatic mineralization of gellan gum hydrogel for bone tissue-engineering applications and its enhancement by polydopamine. J. Tissue Eng. Regen. Med. 2014, 8, 906–918. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, X.; Yang, T.; Wang, X.; Li, T.; Sun, M.; Jiao, K.; Jia, W.; Yang, Y.; Yan, Y.; et al. Hyaluronic acid methacrylate/Pluronic F127 hydrogel enhanced with spermidine-modified mesoporous polydopamine nanoparticles for efficient synergistic periodontitis treatment. Int. J. Biol. Macromol. 2024, 281 Pt 1, 136085. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tang, S.; Luo, Z.; Liu, K.; Luo, Y.; Wen, W.; Ding, S.; Li, L.; Liu, M.; Zhou, C.; et al. Chitin whisker/chitosan liquid crystal hydrogel assisted scaffolds with bone-like ECM microenvironment for bone regeneration. Carbohydr. Polym. 2024, 332, 121927. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Chen, H.; Cheng, S.; Wu, C.; Wang, L.; Du, M. Gelatin hydrogel reinforced with mussel-inspired polydopamine-functionalized nanohydroxyapatite for bone regeneration. Int. J. Biol. Macromol. 2023, 240, 124287. [Google Scholar] [CrossRef]
- Li, M.; Wei, F.; Yin, X.; Xiao, L.; Yang, L.; Su, J.; Weng, J.; Feng, B.; Xiao, Y.; Zhou, Y. Synergistic regulation of osteoimmune microenvironment by IL-4 and RGD to accelerate osteogenesis. Mater. Sci. Eng. C 2020, 109, 110508. [Google Scholar] [CrossRef]
- Yan, L.; Zhou, T.; Ni, R.; Jia, Z.; Jiang, Y.; Guo, T.; Wang, K.; Chen, X.; Han, L.; Lu, X. Adhesive Gelatin-Catechol Complex Reinforced Poly(Acrylic Acid) Hydrogel with Enhanced Toughness and Cell Affinity for Cartilage Regeneration. ACS Appl. Bio Mater. 2020, 5, 4366–4377. [Google Scholar] [CrossRef]
- Zhang, F.-X.; Liu, P.; Ding, W.; Meng, Q.-B.; Su, D.-H.; Zhang, Q.-C.; Lian, R.-X.; Yu, B.-Q.; Zhao, M.-D.; Dong, J.; et al. Injectable Mussel-Inspired highly adhesive hydrogel with exosomes for endogenous cell recruitment and cartilage defect regeneration. Biomaterials 2021, 278, 121169. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, S.; Weng, Z.; Zhang, W.; Wan, X.; Cui, T.; Ye, J.; Liao, L.; Wang, X. Jelly-Inspired Injectable Guided Tissue Regeneration Strategy with Shape Auto-Matched and Dual-Light-Defined Antibacterial/Osteogenic Pattern Switch Properties. ACS Appl. Mater. Interfaces 2020, 12, 54497–54506. [Google Scholar] [CrossRef]
- Ren, S.; Tang, X.; Liu, L.; Meng, F.; Yang, X.; Li, N.; Zhang, Z.; Aimaijiang, M.; Liu, M.; Liu, X.; et al. Reinforced Blood-Derived Protein Hydrogels Enable Dual-Level Regulation of Bio-Physiochemical Microenvironments for Personalized Bone Regeneration with Remarkable Enhanced Efficacy. Nano Lett. 2022, 22, 3904–3913. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Li, B.; Zheng, Y.; Han, Y.; Chen, D.-F.; Yeung, K.W.K.; Cui, Z.; Liang, Y.; Li, Z.; et al. Near-Infrared Light Triggered Phototherapy and Immunotherapy for Elimination of Methicillin-Resistant Staphylococcus aureus Biofilm Infection on Bone Implant. ACS Nano 2020, 14, 8157–8170. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, L.; Wei, C.; Ren, S.; Liu, X.; Wang, X.; Song, J.; Li, Y.; Wang, Z.; Qiao, S.; et al. Immunomodulatory Blood-Derived Hybrid Hydrogels as Multichannel Microenvironment Modulators for Augmented Bone Regeneration. ACS Appl. Mater. Interfaces 2022, 14, 53523–53534. [Google Scholar] [CrossRef]
- Yin, J.; Han, Q.; Zhang, J.; Liu, Y.; Gan, X.; Xie, K.; Xie, L.; Deng, Y. MXene-Based Hydrogels Endow Polyetheretherketone with Effective Osteogenicity and Combined Treatment of Osteosarcoma and Bacterial Infection. ACS Appl. Mater. Interfaces 2020, 12, 45891–45903. [Google Scholar] [CrossRef]
- Zheng, L.; Li, D.; Wang, W.; Zhang, Q.; Zhou, X.; Liu, D.; Zhang, J.; You, Z.; Zhang, J.; He, C. Bilayered Scaffold Prepared from a Kartogenin-Loaded Hydrogel and BMP-2-Derived Peptide-Loaded Porous Nanofibrous Scaffold for Osteochondral Defect Repair. ACS Biomater. Sci. Eng. 2019, 5, 4564–4573. [Google Scholar] [CrossRef]
- Abie, N.; Ünlü, C.; Pinho, A.R.; Gomes, M.C.; Remmler, T.; Herb, M.; Grumme, D.; Tabesh, E.; Shahbazi, M.-A.; Mathur, S.; et al. Designing of a Multifunctional 3D-Printed Biomimetic Theragenerative Aerogel Scaffold via Mussel-Inspired Chemistry: Bioactive Glass Nanofiber-Incorporated Self-Assembled Silk Fibroin with Antibacterial, Antiosteosarcoma, and Osteoinductive Properties. ACS Appl. Mater. Interfaces 2024, 16, 22809–22827. [Google Scholar] [CrossRef]
- Zhao, J.; Qiu, P.; Wang, Y.; Wang, Y.; Zhou, J.; Zhang, B.; Zhang, L.; Gou, D. Chitosan-based hydrogel wound dressing: From mechanism to applications, a review. Int. J. Biol. Macromol. 2023, 244, 125250. [Google Scholar] [CrossRef]
- Sarmah, D.; Rather, M.A.; Sarkar, A.; Mandal, M.; Sankaranarayanan, K.; Karak, N. Self-cross-linked starch/chitosan hydrogel as a biocompatible vehicle for controlled release of drug. Int. J. Biol. Macromol. 2023, 237, 124206. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Liu, D.; Wang, M.; Yu, C.; Han, Z.; Xu, M.; Yue, W.; Nie, G. β-Alanine enhancing the crosslink of chitosan/poly-(γ-glutamic acid) hydrogel for a potential alkaline-adapted wound dressing. Int. J. Biol. Macromol. 2023, 231, 123157. [Google Scholar] [CrossRef] [PubMed]
- Mu, M.; Li, X.; Tong, A.; Guo, G. Multi-functional chitosan-based smart hydrogels mediated biomedical application. Expert Opin. Drug Deliv. 2019, 16, 239–250. [Google Scholar] [CrossRef]
- Gou, D.; Qiu, P.; Hong, F.; Wang, Y.; Ren, P.; Cheng, X.; Wang, L.; Liu, T.; Liu, J.; Zhao, J. Polydopamine modified multifunctional carboxymethyl chitosan/pectin hydrogel loaded with recombinant human epidermal growth factor for diabetic wound healing. Int. J. Biol. Macromol. 2024, 274, 132917. [Google Scholar] [CrossRef]
- Oh, D.X.; Hwang, D.S. A biomimetic chitosan composite with improved mechanical properties in wet conditions. Biotechnol. Prog. 2013, 29, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hassanzadeh, P.; Miyake, T.; Jin, J.; Rolandi, M. Squid beak inspired water processable chitosan composites with tunable mechanical properties. J. Mater. Chem. B 2016, 4, 2273–2279. [Google Scholar] [CrossRef]
- Ryu, J.H.; Jo, S.; Koh, M.; Lee, H. Bio-Inspired, Water-Soluble to Insoluble Self-Conversion for Flexible, Biocompatible, Transparent, Catecholamine Polysaccharide Thin Films. Adv. Funct. Mater. 2014, 24, 7709–7716. [Google Scholar] [CrossRef]
- Edson, J.A.; Ingato, D.; Wu, S.; Lee, B.; Kwon, Y.J. Aqueous-Soluble, Acid-Transforming Chitosan for Efficient and Stimuli-Responsive Gene Silencing. Biomacromolecules 2018, 19, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Ryu, J.H.; Lee, D.Y.; Lee, H. Bio-inspired catechol conjugation converts water-insoluble chitosan into a highly water-soluble, adhesive chitosan derivative for hydrogels and LbL assembly. Biomater. Sci. 2013, 1, 783–790. [Google Scholar] [CrossRef]
- Qiao, H.; Sun, M.; Su, Z.; Xie, Y.; Chen, M.; Zong, L.; Gao, Y.; Li, H.; Qi, J.; Zhao, Q.; et al. Kidney-specific drug delivery system for renal fibrosis based on coordination-driven assembly of catechol-derived chitosan. Biomaterials 2014, 35, 7157–7171. [Google Scholar] [CrossRef]
- Sakono, N.; Nakamura, K.; Ohshima, T.; Hayakawa, R.; Sakono, M. Tyrosinase-mediated Peptide Conjugation with Chitosan-coated Gold Nanoparticles. Anal. Sci. 2018, 35, 79–83. [Google Scholar] [CrossRef]
- Samyn, P. A platform for functionalization of cellulose, chitin/chitosan, alginate with polydopamine: A review on fundamentals and technical applications. Int. J. Biol. Macromol. 2021, 178, 71–93. [Google Scholar] [CrossRef]
- Rahnama, H.; Khorasani, S.N.; Aminoroaya, A.; Molavian, M.R.; Allafchian, A.; Khalili, S. Facile preparation of chitosan-dopamine-inulin aldehyde hydrogel for drug delivery application. Int. J. Biol. Macromol. 2021, 185, 716–724. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, X. Alginate hydrogel dressings for advanced wound management. Int. J. Biol. Macromol. 2020, 162, 1414–1428. [Google Scholar] [CrossRef]
- Rastogi, P.; Kandasubramanian, B. Review of alginate-based hydrogel bioprinting for application in tissue engineering. Biofabrication 2019, 11, 042001. [Google Scholar] [CrossRef]
- Nagakura, T.; Hirata, H.; Tsujii, M.; Sugimoto, T.; Miyamoto, K.; Horiuchi, T.; Nagao, M.; Nakashima, T.; Uchida, A. Effect of viscous injectable pure alginate sol on cultured fibroblasts. Plast. Reconstr. Surg. 2005, 116, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Schulz, A.; Gepp, M.M.; Stracke, F.; von Briesen, H.; Neubauer, J.C.; Zimmermann, H. Tyramine-conjugated alginate hydrogels as a platform for bioactive scaffolds. J. Biomed. Mater. Res. Part A 2019, 107, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Yuan, N.; Shao, K.; Huang, S.; Chen, C. Chitosan, alginate, hyaluronic acid and other novel multifunctional hydrogel dressings for wound healing: A review. Int. J. Biol. Macromol. 2023, 240, 124321. [Google Scholar] [CrossRef]
- Raus, R.A.; Nawawi, W.M.F.W.; Nasaruddin, R.R. Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci. 2021, 16, 280–306. [Google Scholar] [CrossRef]
- Lan, L.; Ping, J.; Li, H.; Wang, C.; Li, G.; Song, J.; Ying, Y. Skin-Inspired All-Natural Biogel for Bioadhesive Interface. Adv. Mater. 2024, 36, e2401151. [Google Scholar] [CrossRef]
- Ye, Z.; Mao, Z. Research Progress on the Regulation of Structure and Antioxidant Properties of Polydopamine-Based Nano-materials and Their Biomedical Applications. Mater. China 2022, 41, 679–688. [Google Scholar] [CrossRef]
- Kashi, M.; Nazarpak, M.H.; Nourmohammadi, J.; Moztarzadeh, F. Study the effect of different concentrations of polydopamine as a secure and bioactive crosslinker on dual crosslinking of oxidized alginate and gelatin wound dressings. Int. J. Biol. Macromol. 2024, 277 Pt 3, 134199. [Google Scholar] [CrossRef]
- Rezk, A.I.; Obiweluozor, F.O.; Choukrani, G.; Park, C.H.; Kim, C.S. Drug release and kinetic models of anticancer drug (BTZ) from a pH-responsive alginate polydopamine hydrogel: Towards cancer chemotherapy. Int. J. Biol. Macromol. 2019, 141, 388–400. [Google Scholar] [CrossRef]
- Liang, M.; He, C.; Dai, J.; Ren, P.; Fu, Y.; Wang, F.; Ge, X.; Zhang, T.; Lu, Z. A high-strength double network polydopamine nanocomposite hydrogel for adhesion under seawater. J. Mater. Chem. B 2020, 8, 8232–8241. [Google Scholar] [CrossRef]
- Xu, J.; Wang, G.; Wu, Y.; Ren, X.; Gao, G. Ultrastretchable Wearable Strain and Pressure Sensors Based on Adhesive, Tough, and Self-healing Hydrogels for Human Motion Monitoring. ACS Appl. Mater. Interfaces 2019, 11, 25613–25623. [Google Scholar] [CrossRef] [PubMed]
- Montazerian, H.; Baidya, A.; Haghniaz, R.; Davoodi, E.; Ahadian, S.; Annabi, N.; Khademhosseini, A.; Weiss, P.S. Stretchable and Bioadhesive Gelatin Methacryloyl-Based Hydrogels Enabled by in Situ Dopamine Polymerization. ACS Appl. Mater. Interfaces 2021, 13, 40290–40301. [Google Scholar] [CrossRef] [PubMed]
- Wu, E.; Huang, L.; Shen, Y.; Wei, Z.; Li, Y.; Wang, J.; Chen, Z. Application of gelatin-based composites in bone tissue engineering. Heliyon 2024, 10, e36258. [Google Scholar] [CrossRef]
- Afewerki, S.; Sheikhi, A.; Kannan, S.; Ahadian, S.; Khademhosseini, A. Gelatin-polysaccharide composite scaffolds for 3D cell culture and tissue engineering: Towards natural therapeutics. Bioeng. Transl. Med. 2018, 4, 96–115. [Google Scholar] [CrossRef]
- Pei, X.; Zhang, H.; Zhou, Y.; Zhou, L.; Fu, J. Stretchable, self-healing and tissue-adhesive zwitterionic hydrogels as strain sensors for wireless monitoring of organ motions. Mater. Horizons 2020, 7, 1872–1882. [Google Scholar] [CrossRef]
- Lee, H.A.; Park, E.; Lee, H. Polydopamine and Its Derivative Surface Chemistry in Material Science: A Focused Review for Studies at KAIST. Adv. Mater. 2020, 32, e1907505. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Song, J.; Wang, S.; Liu, W. PVA-Based Hydrogels: Promising Candidates for Articular Cartilage Repair. Macromol. Biosci. 2021, 21, 2100147. [Google Scholar] [CrossRef]
- Sun, S.; Cui, Y.; Yuan, B.; Dou, M.; Wang, G.; Xu, H.; Wang, J.; Yin, W.; Wu, D.; Peng, C. Drug delivery systems based on polyethylene glycol hydrogels for enhanced bone regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1117647. [Google Scholar] [CrossRef]
- Washington, M.A.; Balmert, S.C.; Fedorchak, M.V.; Little, S.R.; Watkins, S.C.; Meyer, T.Y. Monomer sequence in PLGA microparticles: Effects on acidic microclimates and in vivo inflammatory response. Acta Biomater. 2018, 65, 259–271. [Google Scholar] [CrossRef]
- Wu, Z.; Yuan, K.; Zhang, Q.; Guo, J.J.; Yang, H.; Zhou, F. Antioxidant PDA-PEG nanoparticles alleviate early osteoarthritis by inhibiting osteoclastogenesis and angiogenesis in subchondral bone. J. Nanobiotechnol. 2022, 20, 479. [Google Scholar] [CrossRef]
- Liu, Y.; Hong, H.; Xiao, Y.; Kwok, M.L.; Liu, H.; Tian, X.Y.; Choi, C.H.J. Dopamine Receptor-Mediated Binding and Cellular Uptake of Polydopamine-Coated Nanoparticles. ACS Nano 2021, 15, 13871–13890. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zheng, C.; Webster, T.J. Self-assembled peptide nanomaterials for biomedical applications: Promises and pitfalls. Int. J. Nanomed. 2016, 12, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Qiang, W.; Li, W.; Li, X.; Chen, X.; Xu, D. Bioinspired polydopamine nanospheres: A superquencher for fluorescence sensing of biomolecules. Chem. Sci. 2014, 5, 3018–3024. [Google Scholar] [CrossRef]
- Hing, K.A. Bone repair in the twenty–first century: Biology, chemistry or engineering? Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2004, 362, 2821–2850. [Google Scholar] [CrossRef]
- Raeburn, J.; Cardoso, A.Z.; Adams, D.J. The importance of the self-assembly process to control mechanical properties of low molecular weight hydrogels. Chem. Soc. Rev. 2013, 42, 5143–5156. [Google Scholar] [CrossRef]
- Liyanage, W.; Ardoña, H.A.M.; Mao, H.-Q.; Tovar, J.D. Cross-Linking Approaches to Tuning the Mechanical Properties of Peptide π-Electron Hydrogels. Bioconjug. Chem. 2016, 28, 751–759. [Google Scholar] [CrossRef]
- Maslovskis, A.; Guilbaud, J.-B.; Grillo, I.; Hodson, N.; Miller, A.F.; Saiani, A. Self-assembling peptide/thermoresponsive polymer composite hydrogels: Effect of peptide–polymer interactions on hydrogel properties. Langmuir 2014, 30, 10471–10480. [Google Scholar] [CrossRef]
- Adhikari, B.; Nanda, J.; Banerjee, A. Pyrene-containing peptide-based fluorescent organogels: Inclusion of graphene into the organogel. Chemistry 2011, 17, 11488–11496. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Zhang, X.; Miao, X.; Yang, Z.; Xu, H. γ-Ray-responsive supramolecular hydrogel based on a diselenide-containing polymer and a peptide. Angew. Chem. Int. Ed. Engl. 2013, 52, 6233–6237. [Google Scholar] [CrossRef]
- Fichman, G.; Schneider, J.P. Dopamine Self-Polymerization as a Simple and Powerful Tool to Modulate the Viscoelastic Mechanical Properties of Peptide-Based Gels. Molecules 2021, 26, 1363. [Google Scholar] [CrossRef]
- Falcone, N.; Andoy, N.M.O.; Sullan, R.M.A.; Kraatz, H.-B. Peptide-Polydopamine Nanocomposite Hydrogel for a Laser-Controlled Hydrophobic Drug Delivery. ACS Appl. Bio Mater. 2021, 4, 6652–6657. [Google Scholar] [CrossRef] [PubMed]





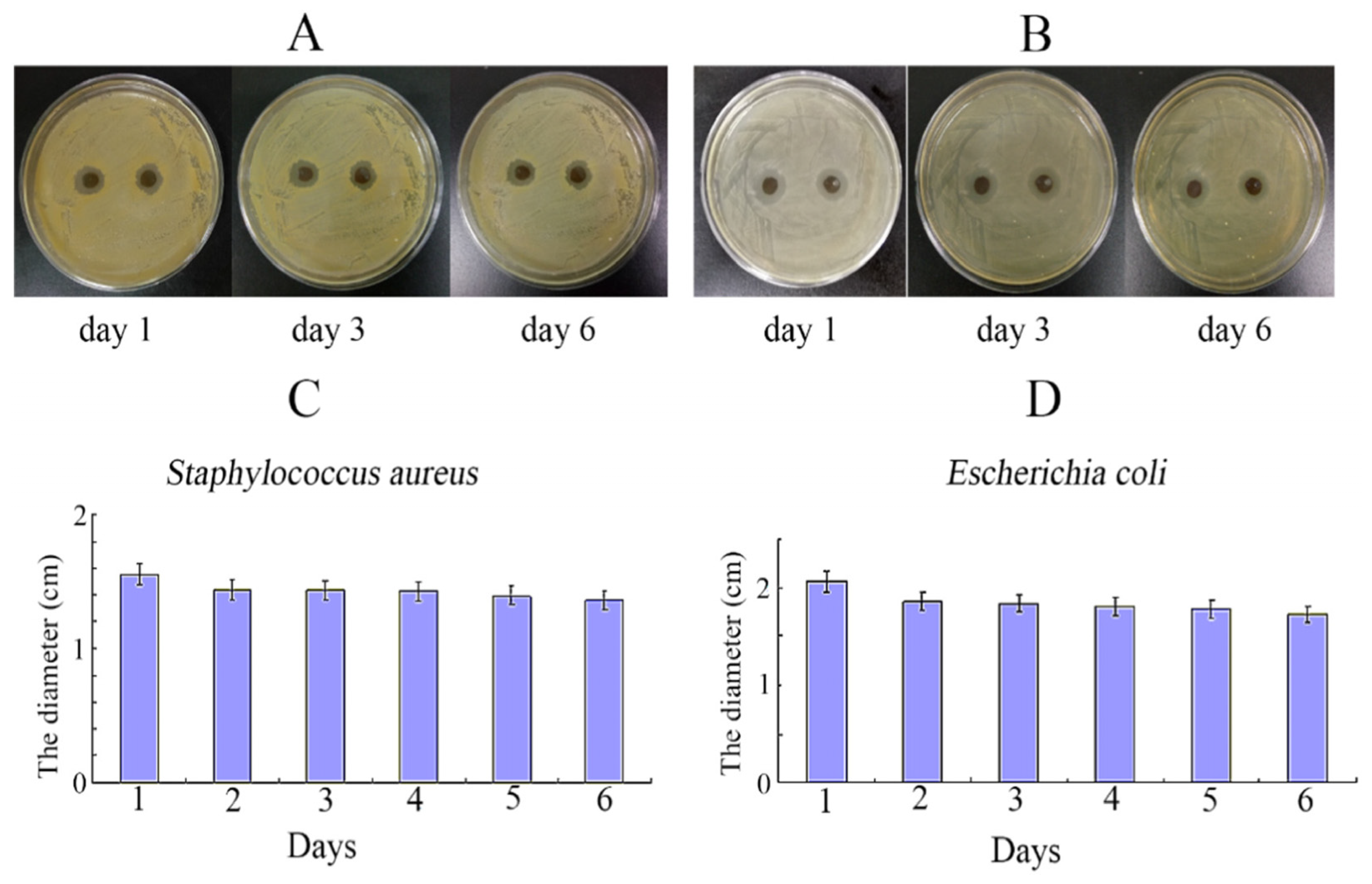

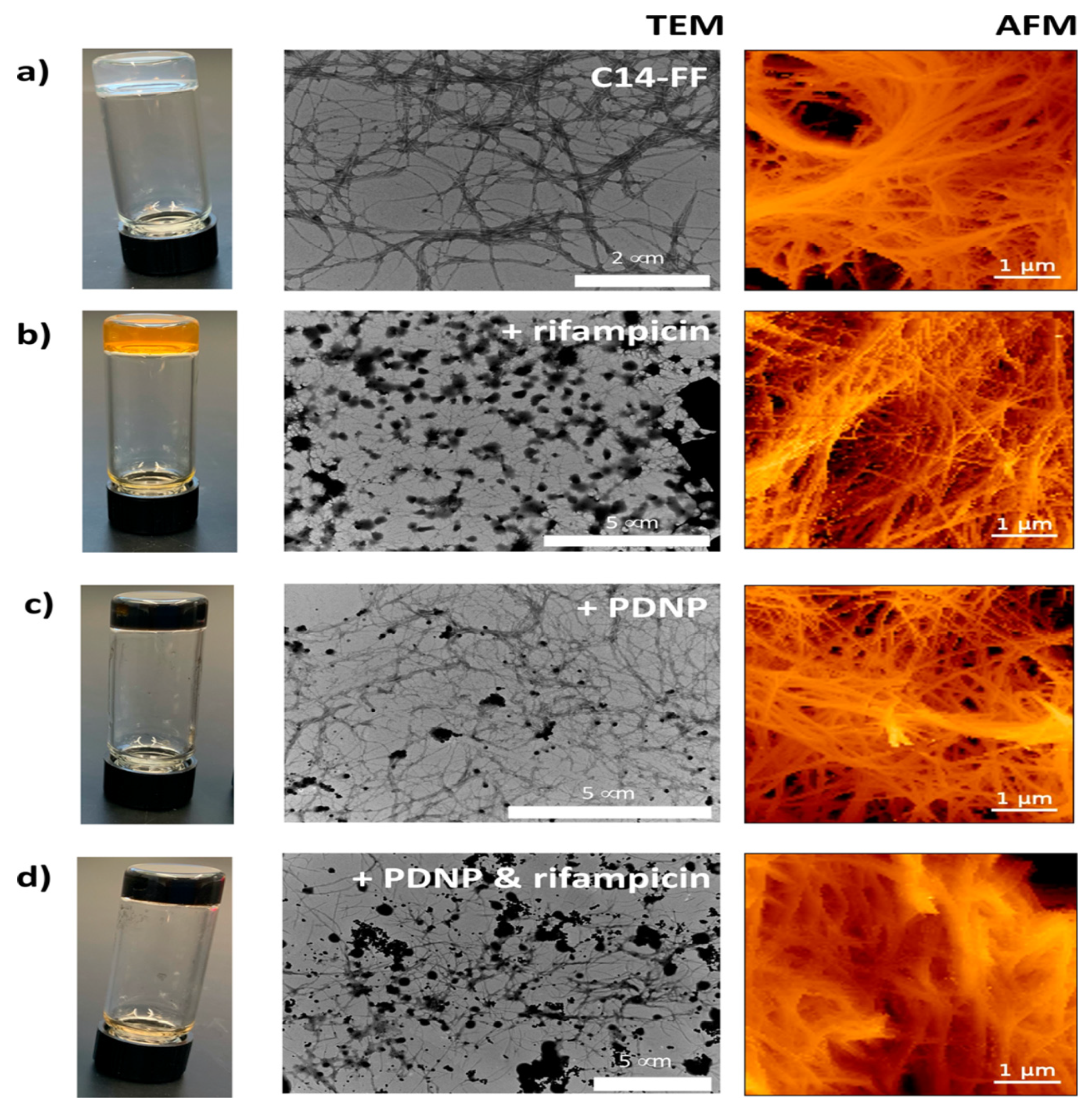

| Type of Composite Hydrogel | Types of Cells/Bacteria | Type of Animal Model | Drugs/Growth Factors/Others | Advantages | Disadvantages | Reference |
|---|---|---|---|---|---|---|
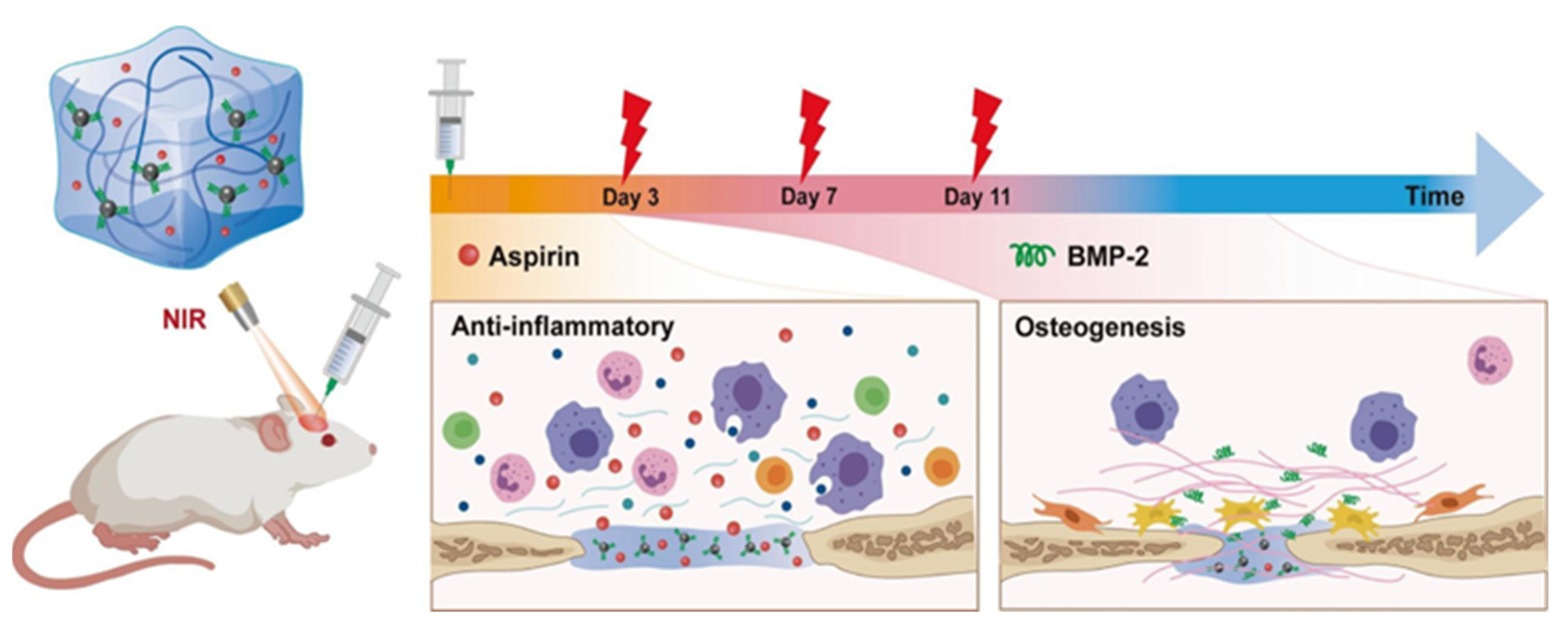
| PDA/Hydroxybutyl chitosan hydrogel | hBMMSCs | SD rats | Aspirin | Dual responsive properties | Complex processing | Wan et al. [82] |
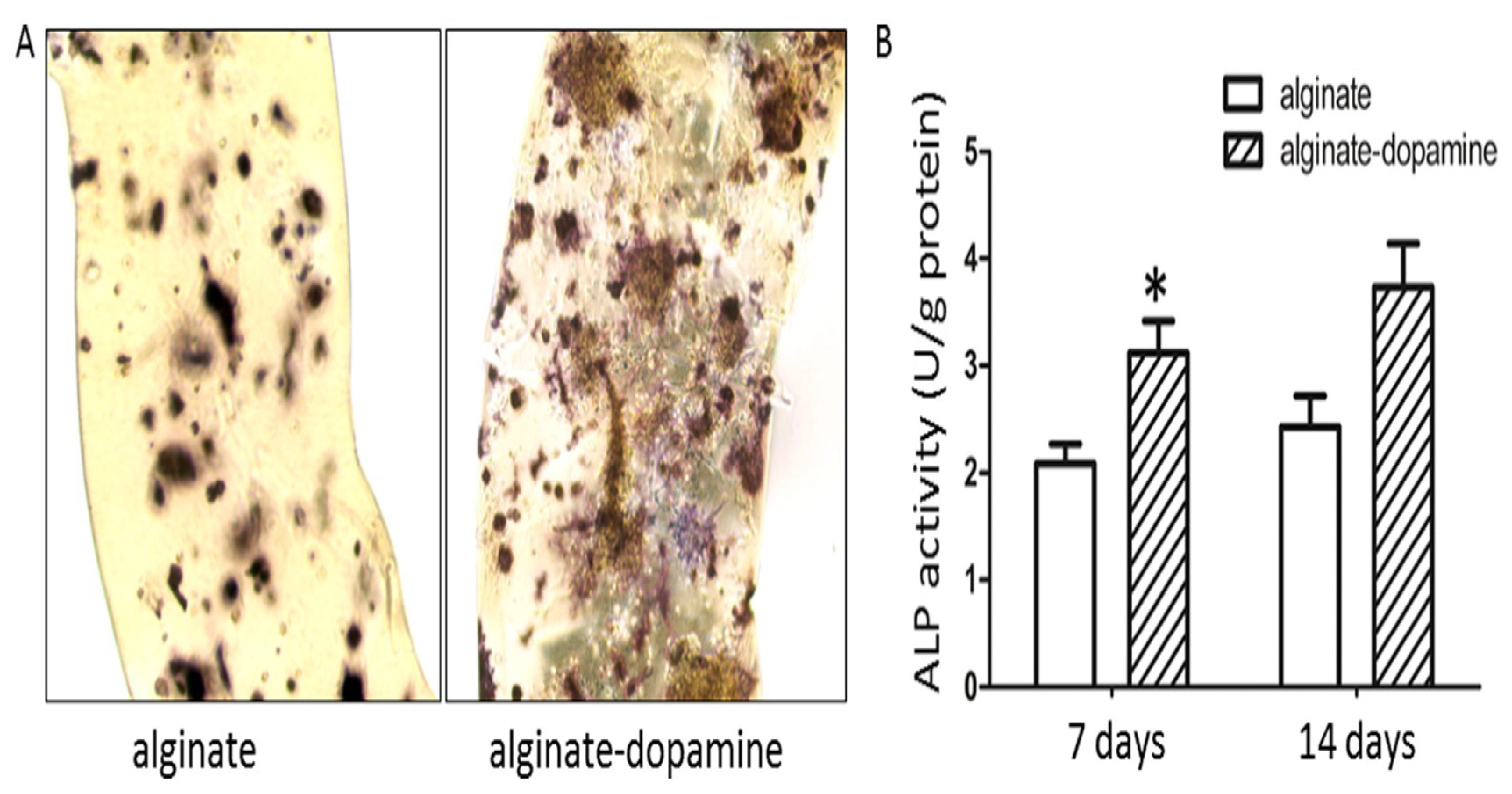
| PDA/alginate hydrogel | BMSCs | / | Silver Nanoparticles | Antibacterial/anti-infective properties | Insufficient mechanical strength | Zhang et al. [83] |
| PDA/Alginate-allylated double-cross-linked hydrogels | MG63 rBMSCs | / | DOX | Fast kinetic response/High UV cross-linking conversion efficiency/Excellent mechanical properties | Insufficient stability of drug release | Chen et al. [84] |
| GelMA-PDA/HA hydrogel | BMSCs | New Zealand white rabbits | BMP-2 TGF-β3 | Excellent osteochondral repair | Reducing degradation speed | Gan et al. [85] |
| GelMA/PMMA/PDA hydrogel | BMSCs | BALB/c rats | / | Good biocompatibility/degradation properties | Local overheating | Wu et al. [86] |
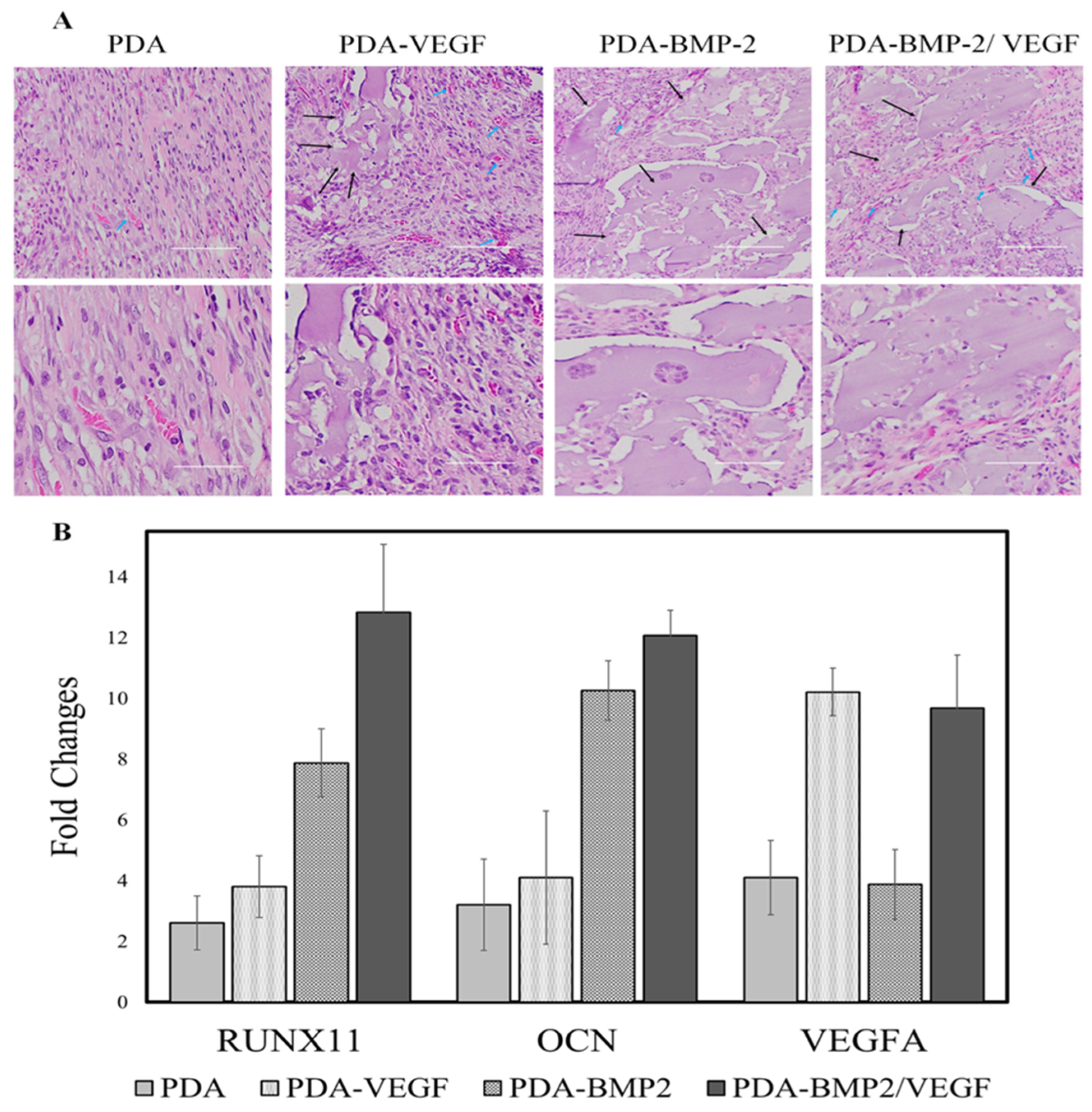
| PLGA-PDA-/Alginate-RGD hydrogel | BMSCs HUVECs HELA MC3T3-E1 | Fischer 344 rats SD rats | BMP-2 VEGF BP Doxorubicin Hydrochloride | Precise and timely drug release | Complex processing | Dashtimoghaamet al. [87] |
| Polyvinyl alcohol (PVA)/PDA@HAP hydrogel | BMSCs | SD rats | Silver NanoparticlesHAP | Long-lasting antimicrobial effect | Induction of cytotoxicity by silver nanoparticles | Li et al. [88] |
| PDA/Hyaluronic acid methacrylate hydrogel MPs | BMSCs | SD rats Rabbits | Barium titanate nanoparticles Stem cell recruitment peptides | Precise electrical stimulation | Poor stability | Han et al. [89] |
| PDA hydrogel | hBMSCs | SD rats | CNT PLA | High mechanical strength | Large manufacturing scale | Sun et al. [90] |
| PDA@E2/HA/GelMA/Gel hydrogel | BMSCs | / | Employing estradiol BMSCs | Adaptation of complex defects | Degradation rate/trigger conditions need to be optimized | Chen et al. [91] |
| MSN@pDA/Chitosan (CS) hydrogel | SMSCs | SD rats Rabbits | TGF-β3 IGF-I PDGF-BB | Activation of endogenous cartilage repair | Insufficient stability | Li et al. [92] |
| PDA/Gelatin methacrylate/sodium alginate methacrylate (GA) hybrid hydrogel | HUVECs MC3T3-E1 | SD rats | DFO PO43− | Improving the microenvironment of bone regeneration | Insufficient biocompatibility/photothermal efficiency | Wu et al. [93] |
| PDA/Hydroxypropyl chitosan/Gelatin (HG) hydrogel | HUVECs MC3T3-E1 | BALB/c rats | aFGF Ti3C2Tx Mxene nanosheets | Accelerating critical bone defect healing | Inadequate biosecurity/limited demand for light control devices | Wu et al. [94] |
| KGN@PDA/UPy hydrogel | BMSCs | Rabbits | KGN miRNA@CaP NPs | Stable mechanical properties/strong self-healing ability | Complex forming process/insufficient transfection efficiency | Kang et al. [95] |
| PDA/ PEEK/Gelatin hydrogel | hMSCs | / | BMP-2 | Enhanced osteogenic differentiation./Improved bio-inertness of the material | Limited drug loading | Zhang et al. [96] |
| PDA/Fpolyacrylamide/Silk fibroin hydrogel | BMSCs HUVECs | SD rats | BMP-2DFO | Excellent interfacial adhesion/structural toughness/mechanical stiffness. | Need for multiple photothermal interventions, large implants to trigger foreign body reactions | Li et al. [97] |
| BML@β-TCP/PDA carboxymethyl chitosan hydrogel | MC3T3-E1 | SD rats | BML-284 | Multipurpose bone repair | High preparation costs | Wu et al. [98] |
| GPEGD/PDA hydrogel | MC3T3-E1 | SD rats | BMP-2 Heparin | Excellent mechanical properties/biocompatibility | Limited cell infiltration/Insufficient stability of ROS scavengers | Wu et al. [99] |
| PDA@zeolitic imidazolate framework-8/Soft matrix hydrogel | MC3T3-E1 RAW264.7 HUVECs | SD rats | Zn2+ | Good structural stability/mechanical support | The complex diabetic microenvironment impairs the therapeutic effect | Wu et al. [100] |
| PDA/Al/GP/Fibrin hydrogels | EMSCs | SD rats | Al GP | Dual functions of osteoinduction and immunomodulation | Alendronate affects the balance of bone remodeling | Shi et al. [101] |
| Methacrylated silk fibroin (SFMA)/PDA hydrogel | HUVECs BMSCs | SD rats | MAP | Exhibits good biocompatibility/physicochemical properties | Uneven distribution of photothermal agent/local overheating | Ma et al. [102] |
| Alginate methacrylate/Alginate/PDA hydrogel | MC3T3-E1 | SD rats | Ti3C2 MXene nanosheets | Good biocompatibility/osteogenic activity/immune-regulatory functions | Phototherapy produces free radicals that damage cells | Wu et al. [103] |
| PDA/Polysaccharide chitin hydrogel | MC3T3-E1 BMSCs | Wistar male rats | Cu2+ Nano HAP | High biocompatibility/Significant osteogenic activity. | Insufficient interfacial bonding strength | Huang et al. [104] |
| PDA/Polyethylene hydrogel (PEG) | MC3T3-E1 | SD rats | AgNPs | Mineralization/anti-infection dual function | Reducing the elasticity of hydrogel | Xu et al. [105] |
| PDA/Chitosan/Gelatin hydrogel | HUVECs MC3T3-E1 RAW 264.7 | SD rats | Hydroxyapatite BaTiO3 NPs | Excellent immunomodulation/angiogenesis/osteogenesis/suitable for combat wound repair | Complex material processing | Wu et al. [106] |
| PDACS/PCL/Hydrogel | HUVECs WJMSCs | / | HUVECs WJMSCs | Synergized to promote osteogenesis/vascularization | Calcium silicate degradation products affect pH/limited microstructure control | Chen et al. [107] |
| Oxidized sodium alginate (OSA)/Gelatin (Gel)/PDA-nHA hydrogel | BMSCs | Japanese big-ear white rabbits | nHA | Injectable/easy to operate | Long-term stability of nano-hydroxyapatite (nHA) in vivo is insufficient | Liu et al. [108] |
| CGH/PDA@HAP hydrogel | BMSCs | SD rats | Gallic acid Hydroxyapatite | Enhanced antibacterial and osteogenic synergy | Generation of acidic degradation products | Pang et al. [109] |
| Characterization of the fucoidan/PDA hydrogel | PDLSCs | / | / | Enhanced osteogenic potential | Quality control of fucoidan sulfate was low | Kwack et al. [110] |
| Polyacrylamide/PDA hydrogel | MG-63 | / | / | Matrix stiffness targets osteosarcoma cell apoptosis | Stiffness parameters need to be highly precise/difficult to adjust in clinical application | Deng et al. [111] |
| Xanthan gum-PDA hydrogel | BMSCs | SD rats | SDF-1α Mg2+ | Excellent injectability/mechanical properties | High SDF-1α inactivation | Li et al. [28] |
| OSA-GelDA@ACP/DA/Ag hydrogel | / | / | ACP DA Ag+ | Composite hydrogel combines tissue adhesion and anti-infection functions | The hydrogel flexibility/bond strength decreased | Zhong et al. [112] |
| PDA/Chondroitin sulfate hydrogel | rBMSCs | New Zealand rabbits | SDF-1α | Sustained-release SDF-1α | Lack of control over release kinetics | Wu et al. [113] |
| ALG/GelAGE-PDA@DOX hydrogels | rBMSCs MG 63 | / | Sr2+ DOX | Synergistic effect of chemotherapy and photothermal therapy (PTT) | Degree of cross-linking affects the stability of drug release | Chen et al. [84] |
| PDA/GMS/Osteogenic hydrogel | P. gingivalis | SD rats | Amino antibacterial nanoparticle Magnetic nanoparticles | Precision antimicrobial therapy | Magnetic field conditioning devices limit clinical applications | Zhou et al. [114] |
| PDA/Nano-hydroxyapatite (nHAP) hydrogel | MC3T3-E1 | / | PEEK Aspirin | Good biocompatibility/compressive strength/modulus | Poor cell adhesion to the inert surface of PEEK | Li et al. [115] |
| CS/PDA hydrogel | HUVECs | / | DFO | Enhanced bond strength/angiogenic effect | Short half-life of deferoxamine/frequent injections required | Liu et al. [116] |
| BNP-PEDOT-PSF-AG hydrogel | PDLSCs | SD rats | Bovine serum albumin nanoparticles Hydrogen sulfide | Promoting alveolar bone regeneration/reversing inflammatory microenvironment under diabetic conditions | Difficulty in controlling the release of H2S gas | Fang et al. [117] |
| Alginate/TOCNF/PDA hydrogel | MC3T3-E1 | / | TOCNFs PDANPs | High osteogenic activity | Low structural fidelity after printing | Im et al. [118] |
| GO-PHA-CPs hydrogel | MC3T3-E1 | SD rats | CPs | Exhibits excellent injectability/adhesion/antioxidant activity/osteoinductive properties | Limited self-repair capacity/degradation rate mismatch with bone formation rate | Ma et al. [119] |
| DA-nano-hydroxyapatite hydrogel | 4T1 BMSCs | BALB/c mice | DDP | Synergistic photothermal anti-tumor/bone regeneration capabilities | Photothermal agents are potentially toxic | Luo et al. [120] |
| MCG-HG-PLGA-PD-B hydrogel | ATDC5 MC3T3-E1 | / | BMP-7 | Promoting Structural Bionicity in Cartilage Regeneration | Insufficient scaffold porosity connectivity | Jung et al. [121] |
| Gellan gum/PDA hydrogel | MC3T3-E1 | / | ALP | Polydopamine enhances the efficiency of mineralization | Increased material brittleness/complex preparation process | Douglas et al. [122] |
| PF-127/HAMA/M@S (PH/M@S) hydrogel | rBMSCs HUVEC RAW264.7 | Mice | M@S NPs | Cost-effective/easy to synthesize/possesses multiple therapeutic capabilities | Nanoparticles prone to leakage | Liu et al. [123] |
| PDA/LC hydrogel | BMSCs E. coli S. aureus | SD rats | / | Excellent osteogenic activity/angiogenic capacity/antimicrobial effects | Chitosan causes allergic reactions/complex preparation | Li et al. [124] |
| Gel-PHA hydrogel | MC3T3-E1 | SD rats | nHA | Enhanced mechanical and osteogenic properties of gelatin hydrogels | Reduced hydrogel elasticity | Ma et al. [125] |
| T/DOP-IL 4/CG-RGD hydrogel | BMSCs | / | IL-4 RGD peptide | IL-4 and RGD synergistically regulate the osteoimmune microenvironment | IL-4 short half-life/RGD overexpression | Li et al. [126] |
| PDA/Gel-PAA hydrogel | BMSCs | New Zealand rabbits | TGF-β3 | Enhances the toughness and cell affinity of PAA hydrogels | Catechol oxidative cross-linking is irreversible/affects controllability of degradation | Yan et al. [127] |
| AD/CS/RSF/EXO hydrogel | BMSCs | SD rats | Exosomes | Excellent mechanical properties/biodegradability/biocompatibility/the ability | Low efficiency in exosome extraction and loading | Zhang et al. [128] |
| CTP-SA/TiO2@PDA hydrogel | HUVEC BMSCs Staphylococcus aureus Escherichia coli Streptococcus mutans | SD rats | Cu2O TiO2 NPs | Enhanced antimicrobial activity | Insufficient mechanical properties | Xu et al. [129] |
| PDA@SiO2-PRF hydrogel | BMSCs | SD rats | PRF | Multi-level regulation of microenvironment | Proteins are prone to degradation/short shelf life | Ren et al. [130] |
| Chitosan/ Polydopamine/NO-PVA hydrogel | MRSA MC3T3-E1 | SD rats | Ti-RP/PCP/RSNO NO | With combined photothermal/immunotherapy | Photothermal effect damages surrounding healthy tissue | Li et al. [131] |
| PnP-iPRF hydrogel | BMSCs RAW 264.7 | Rats | i-PRF | Multiple pathways regulate the microenvironment | Immunogenic risk has not been completely ruled out | Li et al. [132] |
| SP@MX/GelMA hydrogel | MG-63 MC3T3-E1 | Kunming mice | Tobramycin | Significantly enhances the initial adhesion and proliferation of cells | MXene nanosheets trigger inflammation | Yin et al. [133] |
| Gelatin-Silkfibroin-Oxidized dextran/PLLA-PLGA-PCL/PDA hydrogel | BMSCs | SD rats | Kartogenin P24 peptides | Excellent cell compatibility/Dual-layer scaffolds synergistically repair osteochondral defects | Weak interfacial bonding strength between the two layers | Zheng et al. [134] |
| SFO-TA-BGNF-PDA hydrogel | MG-63 | / | Bioactive glass | Integrated antimicrobial activity/antiosteosarcoma properties/osteoinduction of multiple functions | Aerogel has low mechanical strength and is not suitable for load-bearing applications | Abie et al. [135] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Tang, J.; Guo, W.; Dong, X.; Cao, K.; Tang, F. Polydopamine Nanocomposite Hydrogel for Drug Slow-Release in Bone Defect Repair: A Review of Research Advances. Gels 2025, 11, 190. https://doi.org/10.3390/gels11030190
Li X, Tang J, Guo W, Dong X, Cao K, Tang F. Polydopamine Nanocomposite Hydrogel for Drug Slow-Release in Bone Defect Repair: A Review of Research Advances. Gels. 2025; 11(3):190. https://doi.org/10.3390/gels11030190
Chicago/Turabian StyleLi, Xiaoman, Jianhua Tang, Weiwei Guo, Xuan Dong, Kaisen Cao, and Fushan Tang. 2025. "Polydopamine Nanocomposite Hydrogel for Drug Slow-Release in Bone Defect Repair: A Review of Research Advances" Gels 11, no. 3: 190. https://doi.org/10.3390/gels11030190
APA StyleLi, X., Tang, J., Guo, W., Dong, X., Cao, K., & Tang, F. (2025). Polydopamine Nanocomposite Hydrogel for Drug Slow-Release in Bone Defect Repair: A Review of Research Advances. Gels, 11(3), 190. https://doi.org/10.3390/gels11030190








