Development and Characterization of Cannabidiol Gummy Using 3D Printing
Abstract
1. Introduction
2. Results and Discussion
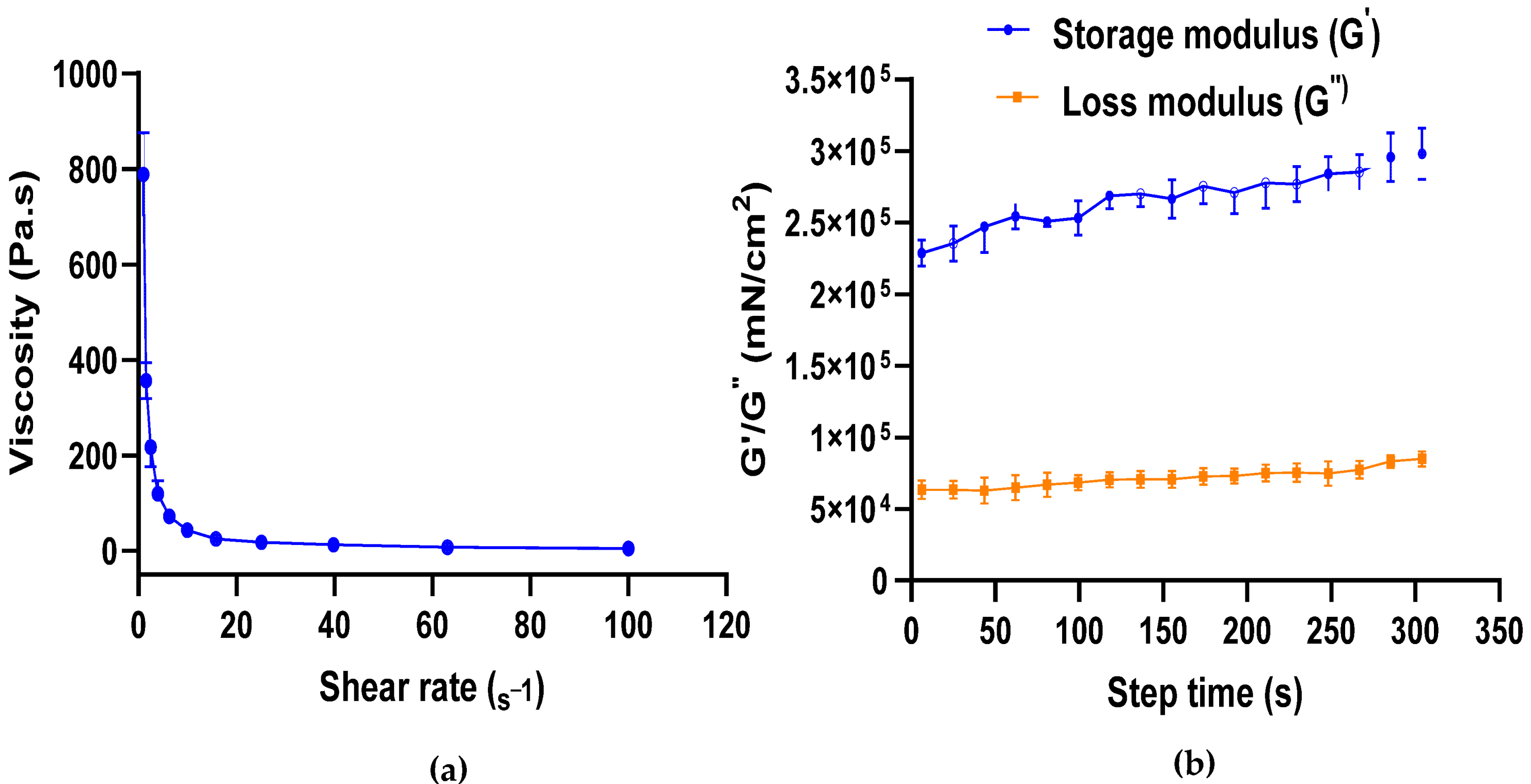
2.1. Formulation of CBD Gel and Its Rheological Studies
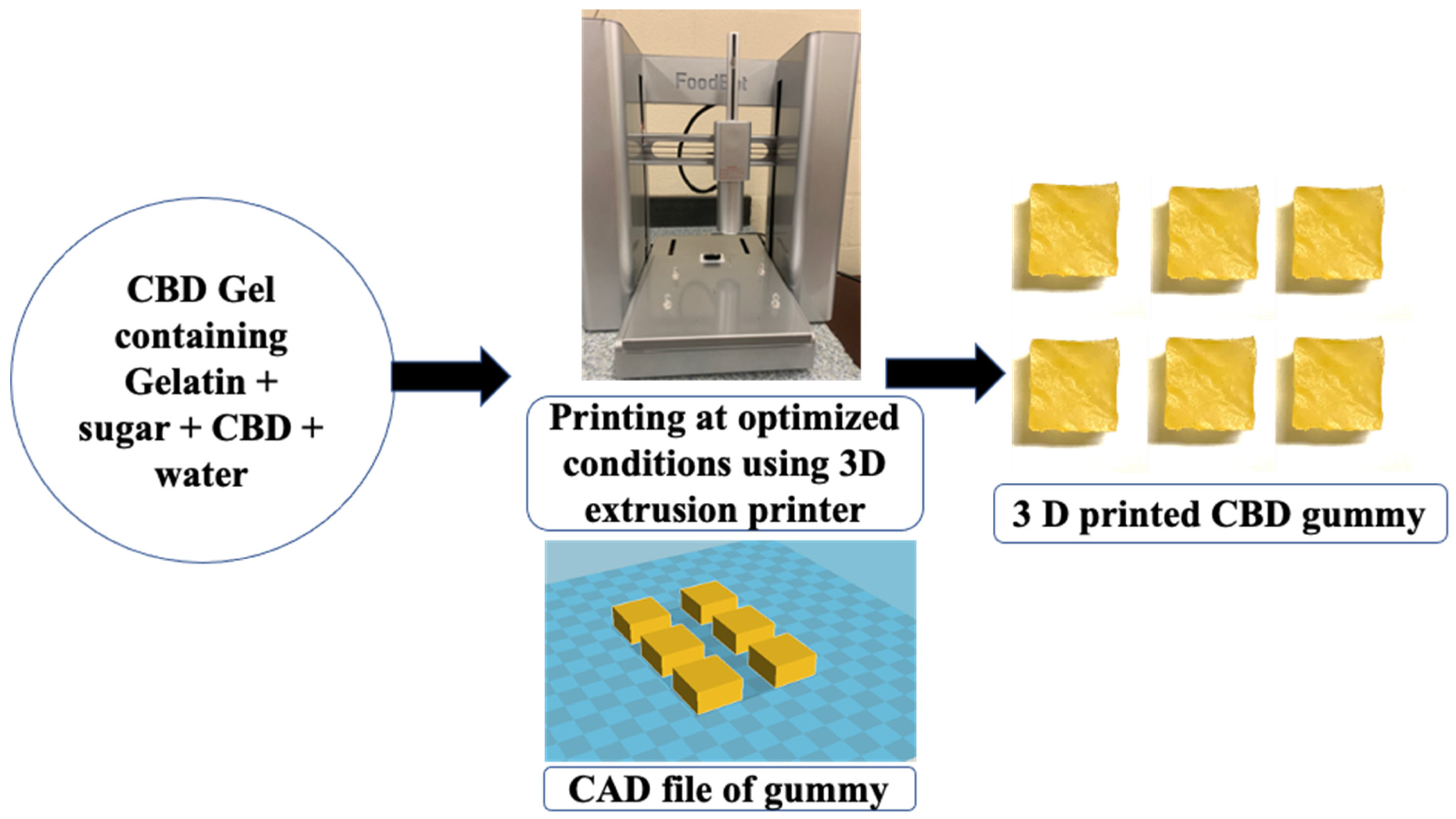
2.2. 3D Printing of CBD Gummy and Characterization Using Texture Analyzer
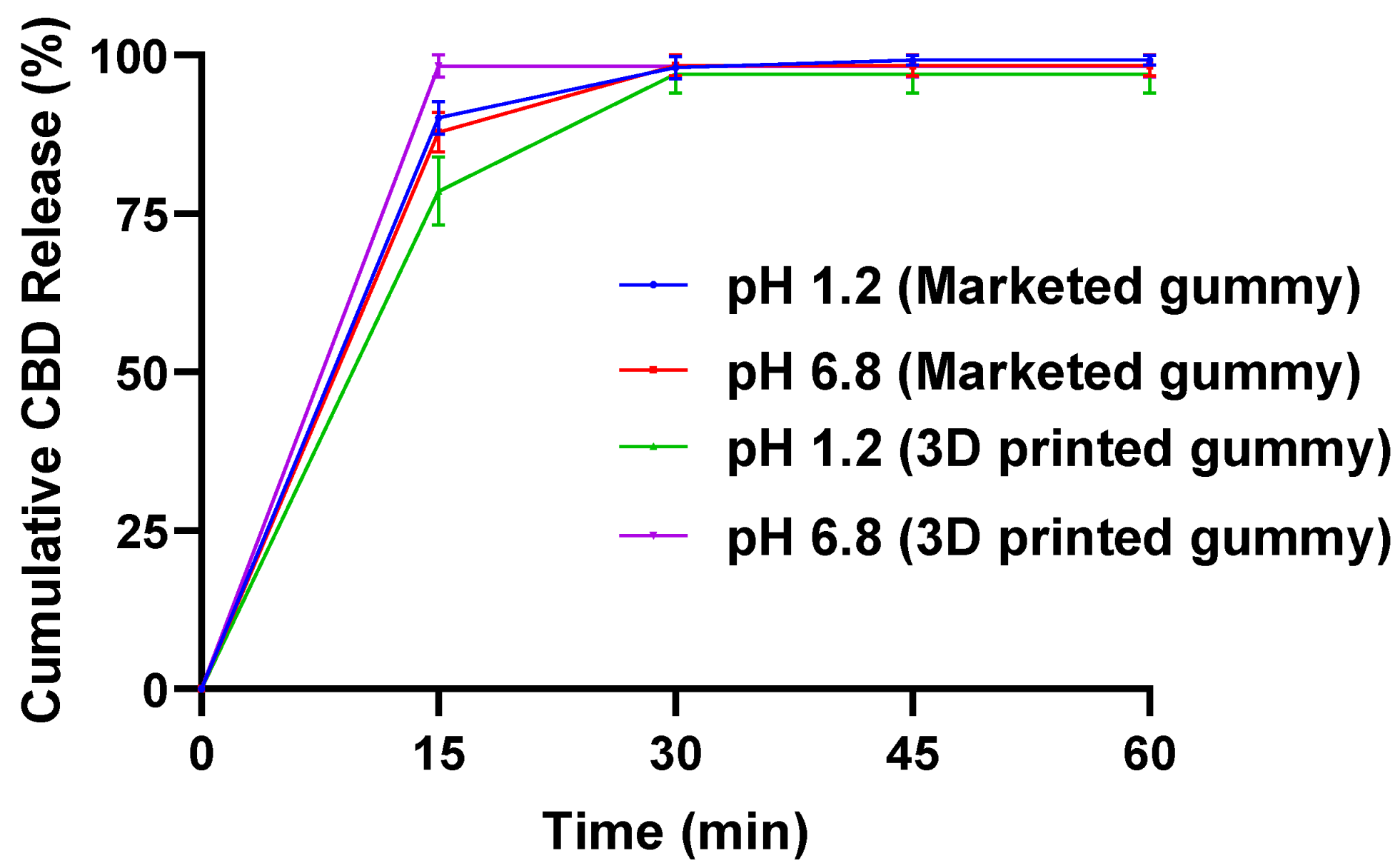
2.3. In Vitro Dissolution Study
2.4. In Vitro Dissolution Study
2.5. Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Formulation of CBD Gel
4.2.2. Rheological Study of CBD Gel
4.2.3. Three-Dimensional Printing of CBD Gummy
4.2.4. Characterization of the Gummy Using Texture Analyzer
4.2.5. HPLC Analysis
4.2.6. Drug Content Study
4.2.7. In Vitro Dissolution Study
4.2.8. Stability Study
4.2.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CBD | Cannabidiol |
| HPLC | High-pressure liquid chromatography |
| P-GDF | Polymeric gummy drug formulation |
| HPMC | Hydroxy propyl methyl cellulose |
References
- Martino, R.; Foley, N.; Bhogal, S.; Diamant, N.; Speechley, M.; Teasell, R. Dysphagia after stroke: Incidence, diagnosis, and pulmonary complications. Stroke 2005, 36, 2756–2763. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, S.; Tulunay-Ugur, O.E. Dysphagia in the older patient. Otolaryngol. Clin. N. Am. 2018, 51, 769–777. [Google Scholar] [CrossRef]
- Thiyagalingam, S.; Kulinski, A.E.; Thorsteinsdottir, B.; Shindelar, K.L.; Takahashi, P.Y. Dysphagia in older adults. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2021; pp. 488–497. [Google Scholar]
- Eisenstadt, E.S. Dysphagia and aspiration pneumonia in older adults. J. Am. Assoc. Nurse Pract. 2010, 22, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Cichero, J.A.; Lam, P.; Steele, C.M.; Hanson, B.; Chen, J.; Dantas, R.O.; Duivestein, J.; Kayashita, J.; Lecko, C.; Murray, J. Development of international terminology and definitions for texture-modified foods and thickened fluids used in dysphagia management: The IDDSI framework. Dysphagia 2017, 32, 293–314. [Google Scholar] [CrossRef] [PubMed]
- Persons, O. Pharmacological management of persistent pain in older persons. J. Am. Geriatr. Soc. 2009, 57, 1331–1346. [Google Scholar]
- Stubbs, B.; Schofield, P.; Binnekade, T.; Patchay, S.; Sepehry, A.; Eggermont, L. Pain is associated with recurrent falls in community-dwelling older adults: Evidence from a systematic review and meta-analysis. Pain. Med. 2014, 15, 1115–1128. [Google Scholar] [CrossRef]
- Gambardella, V.; Tarazona, N.; Cejalvo, J.M.; Lombardi, P.; Huerta, M.; Roselló, S.; Fleitas, T.; Roda, D.; Cervantes, A. Personalized medicine: Recent progress in cancer therapy. Cancers 2020, 12, 1009. [Google Scholar] [CrossRef]
- Tarahi, M.; Tahmouzi, S.; Kianiani, M.R.; Ezzati, S.; Hedayati, S.; Niakousari, M. Current Innovations in the Development of Functional Gummy Candies. Foods 2023, 13, 76. [Google Scholar] [CrossRef]
- Kawamoto, S.; Tanaka, S.; Miura, M.; Kashiwagura, Y.; Kamiya, C.; Hakamata, A.; Odagiri, K.; Inui, N.; Watanabe, H.; Namiki, N. Palatability of aripiprazole gummies prepared from commercially available products: Pharmaceutical formulation for improving patient adherence. Chem. Pharm. Bull. 2023, 71, 441–446. [Google Scholar] [CrossRef]
- Tanaka, S.; Kawamoto, S.; Kashiwagura, Y.; Hakamata, A.; Odagiri, K.; Okura, T.; Inui, N.; Watanabe, H.; Namiki, N.; Uchida, S. Improved Palatability of Gummy Drugs of Epinastine Hydrochloride Using Organoleptic Taste-Masking Methods. BPB Rep. 2023, 6, 184–188. [Google Scholar] [CrossRef]
- Grétarsdóttir, K.G. Development of Vitamin D Gummy Supplements and Their Shelf-Life. Ph.D. Thesis, University of Iceland, Reykjavik, Iceland, 2019. [Google Scholar]
- Goetz, L.H.; Schork, N.J. Personalized medicine: Motivation, challenges, and progress. Fertil. Steril. 2018, 109, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Krzyszczyk, P.; Acevedo, A.; Davidoff, E.J.; Timmins, L.M.; Marrero-Berrios, I.; Patel, M.; White, C.; Lowe, C.; Sherba, J.J.; Hartmanshenn, C. The growing role of precision and personalized medicine for cancer treatment. Technology 2018, 6, 79–100. [Google Scholar] [CrossRef]
- Zajicek, A.; Fossler, M.J.; Barrett, J.S.; Worthington, J.H.; Ternik, R.; Charkoftaki, G.; Lum, S.; Breitkreutz, J.; Baltezor, M.; Macheras, P. A report from the pediatric formulations task force: Perspectives on the state of child-friendly oral dosage forms. AAPS J. 2013, 15, 1072–1081. [Google Scholar] [CrossRef]
- Jîtcă, C.-M.; Jîtcă, G.; Ősz, B.-E.; Pușcaș, A.; Imre, S. Stability of Oral Liquid Dosage Forms in Pediatric Cardiology: A Prerequisite for Patient’s Safety—A Narrative Review. Pharmaceutics 2023, 15, 1306. [Google Scholar] [CrossRef] [PubMed]
- Levin, J.B.; Krivenko, A.; Howland, M.; Schlachet, R.; Sajatovic, M. Medication adherence in patients with bipolar disorder: A comprehensive review. CNS Drugs 2016, 30, 819–835. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Tian, Y.; Zhang, E.; Gao, X.; Zhang, H.; Liu, N.; Han, X.; Sun, Y.; Wang, Z.; Zheng, A. Semisolid extrusion 3D printing of propranolol hydrochloride gummy chewable tablets: An innovative approach to prepare personalized medicine for pediatrics. AAPS PharmSciTech 2022, 23, 166. [Google Scholar] [CrossRef]
- Carvalho, M.; Almeida, I.F. The role of pharmaceutical compounding in promoting medication adherence. Pharmaceuticals 2022, 15, 1091. [Google Scholar] [CrossRef] [PubMed]
- Germain, I. The Nutritional Challenges in Dysphagia: Not Only a Matter of Nutrients. In Dysphagia-New Advances; IntechOpen: London, UK, 2022. [Google Scholar]
- Ikebuchi, K.; Matsuda, Y.; Takeda, M.; Takeda, M.; Abe, T.; Tominaga, K.; Yano, S.; Isomura, M.; Nabika, T.; Kanno, T. Relationship between masticatory function and bone mineral density in community-dwelling elderly: A cross-sectional study. Healthcare 2021, 9, 845. [Google Scholar] [CrossRef]
- Garg, T.; Goyal, A.K. Medicated chewing gum: Patient compliance oral drug delivery system. Drug Deliv. Lett. 2014, 4, 72–78. [Google Scholar] [CrossRef]
- Bagde, A.; Dev, S.; Sriram, L.M.K.; Spencer, S.D.; Kalvala, A.; Nathani, A.; Salau, O.; Mosley-Kellum, K.; Dalvaigari, H.; Rajaraman, S. Biphasic burst and sustained transdermal delivery in vivo using an AI-optimized 3D-printed MN patch. Int. J. Pharm. 2023, 636, 122647. [Google Scholar] [CrossRef]
- Bagde, A.; Mosley-Kellum, K.; Spencer, S.; Singh, M. 3D DLP-printed cannabinoid microneedles patch and its pharmacokinetic evaluation in rats. J. Pharm. Pharmacol. 2024, 76, 616–626. [Google Scholar] [CrossRef]
- Mannoor, M.S.; Jiang, Z.; James, T.; Kong, Y.L.; Malatesta, K.A.; Soboyejo, W.O.; Verma, N.; Gracias, D.H.; McAlpine, M.C. 3D printed bionic ears. Nano Lett. 2013, 13, 2634–2639. [Google Scholar] [CrossRef]
- Serrano, D.R.; Kara, A.; Yuste, I.; Luciano, F.C.; Ongoren, B.; Anaya, B.J.; Molina, G.; Diez, L.; Ramirez, B.I.; Ramirez, I.O. 3D printing technologies in personalized medicine, nanomedicines, and biopharmaceuticals. Pharmaceutics 2023, 15, 313. [Google Scholar] [CrossRef] [PubMed]
- Gültekin, H.E.; Tort, S.; Tuğcu-Demiröz, F.; Acartürk, F. 3D printed extended release tablets for once daily use: An in vitro and in vivo evaluation study for a personalized solid dosage form. Int. J. Pharm. 2021, 596, 120222. [Google Scholar] [CrossRef] [PubMed]
- Ongoren, B.; Yuste, I.; Anaya, B.J.; Luciano, F.C.; Sánchez-Guirales, S.A.; Kara, A.; Serrano, D.R. 3D printing in personalized medicine. In Fundamentals and Future Trends of 3D Printing in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2025; pp. 101–126. [Google Scholar]
- Andriotis, E.G.; Paraskevopoulou, A.; Fatouros, D.G.; Zhang, H.; Ritzoulis, C. Design of Aerated Oleogel–Hydrogel Mixtures for 3D Printing of Personalized Cannabis Edibles. Gels 2024, 10, 654. [Google Scholar] [CrossRef]
- Vaz, V.M.; Kumar, L. 3D printing as a promising tool in personalized medicine. Aaps Pharmscitech 2021, 22, 49. [Google Scholar] [CrossRef] [PubMed]
- Robles-Martinez, P.; Xu, X.; Trenfield, S.J.; Awad, A.; Goyanes, A.; Telford, R.; Basit, A.W.; Gaisford, S. 3D printing of a multi-layered polypill containing six drugs using a novel stereolithographic method. Pharmaceutics 2019, 11, 274. [Google Scholar] [CrossRef]
- Khaled, S.A.; Alexander, M.R.; Irvine, D.J.; Wildman, R.D.; Wallace, M.J.; Sharpe, S.; Yoo, J.; Roberts, C.J. Extrusion 3D printing of paracetamol tablets from a single formulation with tunable release profiles through control of tablet geometry. Aaps Pharmscitech 2018, 19, 3403–3413. [Google Scholar] [CrossRef]
- Prasad, L.K.; Smyth, H. 3D Printing technologies for drug delivery: A review. Drug Dev. Ind. Pharm. 2016, 42, 1019–1031. [Google Scholar] [CrossRef]
- Sadia, M.; Isreb, A.; Abbadi, I.; Isreb, M.; Aziz, D.; Selo, A.; Timmins, P.; Alhnan, M.A. From ‘fixed dose combinations’ to ‘a dynamic dose combiner’: 3D printed bi-layer antihypertensive tablets. Eur. J. Pharm. Sci. 2018, 123, 484–494. [Google Scholar] [CrossRef]
- Mosley-Kellum, K.; Bagde, A.; Spencer, S.; Dev, S.; Singh, M. Development of 3D DLP Printed Sustained Release Ibuprofen Tablets and Their Pharmacokinetic Evaluation in Rats. AAPS PharmSciTech 2023, 24, 88. [Google Scholar] [CrossRef] [PubMed]
- Herrada-Manchón, H.; Rodríguez-González, D.; Fernández, M.A.; Suñé-Pou, M.; Pérez-Lozano, P.; García-Montoya, E.; Aguilar, E. 3D printed gummies: Personalized drug dosage in a safe and appealing way. Int. J. Pharm. 2020, 587, 119687. [Google Scholar] [CrossRef]
- Tagami, T.; Ito, E.; Kida, R.; Hirose, K.; Noda, T.; Ozeki, T. 3D printing of gummy drug formulations composed of gelatin and an HPMC-based hydrogel for pediatric use. Int. J. Pharm. 2021, 594, 120118. [Google Scholar] [CrossRef]
- Ganatra, P.; Jyothish, L.; Mahankal, V.; Sawant, T.; Dandekar, P.; Jain, R. Drug-loaded vegan gummies for personalized dosing of simethicone: A feasibility study of semi-solid extrusion-based 3D printing of pectin-based low-calorie drug gummies. Int. J. Pharm. 2024, 651, 123777. [Google Scholar] [CrossRef]
- Zhou, L.; Meng, F.-B.; Li, Y.-C.; Shi, X.-D.; Yang, Y.-W.; Wang, M. Effect of peach gum polysaccharide on the rheological and 3D printing properties of gelatin-based functional gummy candy. Int. J. Biol. Macromol. 2023, 253, 127186. [Google Scholar] [CrossRef] [PubMed]
- Desu, P.K.; Maddiboyina, B.; Vanitha, K.; Rao Gudhanti, S.N.; Anusha, R.; Jhawat, V. 3D printing technology in pharmaceutical dosage forms: Advantages and challenges. Curr. Drug Targets 2021, 22, 1901–1914. [Google Scholar] [CrossRef] [PubMed]
- Pitzanti, G.; Mathew, E.; Andrews, G.P.; Jones, D.S.; Lamprou, D.A. 3D Printing: An appealing technology for the manufacturing of solid oral dosage forms. J. Pharm. Pharmacol. 2022, 74, 1427–1449. [Google Scholar] [CrossRef] [PubMed]
- Boehnke, K.F.; Scott, J.R.; Litinas, E.; Sisley, S.; Williams, D.A.; Clauw, D.J. Pills to pot: Observational analyses of cannabis substitution among medical cannabis users with chronic pain. J. Pain 2019, 20, 830–841. [Google Scholar] [CrossRef]
- Russo, E.B. Cannabinoids in the management of difficult to treat pain. Ther. Clin. Risk Manag. 2008, 4, 245–259. [Google Scholar] [CrossRef]
- Kalvala, A.K.; Bagde, A.; Arthur, P.; Kulkarni, T.; Bhattacharya, S.; Surapaneni, S.; Patel, N.K.; Nimma, R.; Gebeyehu, A.; Kommineni, N. Cannabidiol-loaded extracellular vesicles from human umbilical cord mesenchymal stem cells alleviate paclitaxel-induced peripheral neuropathy. Pharmaceutics 2023, 15, 554. [Google Scholar] [CrossRef]
- Salau, O.; Bagde, A.; Kalvala, A.; Singh, M. Enhancement of transdermal permeation of cannabinoids and their pharmacodynamic evaluation in rats. Int. J. Pharm. 2022, 624, 122016. [Google Scholar] [CrossRef]
- Liang, E.; Wang, Z.; Li, X.; Wang, S.; Han, X.; Chen, D.; Zheng, A. 3D Printing Technology Based on Versatile Gelatin-Carrageenan Gel System for Drug Formulations. Pharmaceutics 2023, 15, 1218. [Google Scholar] [CrossRef] [PubMed]
- Raeder, V.; Boura, I.; Leta, V.; Jenner, P.; Reichmann, H.; Trenkwalder, C.; Klingelhoefer, L.; Chaudhuri, K.R. Rotigotine transdermal patch for motor and non-motor Parkinson’s disease: A review of 12 years’ clinical experience. CNS Drugs 2021, 35, 215–231. [Google Scholar] [CrossRef]
- Marfil, P.H.; Anhê, A.C.; Telis, V.R. Texture and microstructure of gelatin/corn starch-based gummy confections. Food Biophys. 2012, 7, 236–243. [Google Scholar] [CrossRef]
- DeMars, L.L.; Ziegler, G.R. Texture and structure of gelatin/pectin-based gummy confections. Food Hydrocoll. 2001, 15, 643–653. [Google Scholar] [CrossRef]
- Kurt, A.; Bursa, K.; Toker, O.S. Gummy candies production with natural sugar source: Effect of molasses types and gelatin ratios. Food Sci. Technol. Int. 2022, 28, 118–127. [Google Scholar] [CrossRef]
- Ge, H.; Wu, Y.; Woshnak, L.L.; Mitmesser, S.H. Effects of hydrocolloids, acids and nutrients on gelatin network in gummies. Food Hydrocoll. 2021, 113, 106549. [Google Scholar] [CrossRef]
- Le, H.; Wang, X.; Wei, Y.; Zhao, Y.; Zhang, J.; Zhang, L. Making polyol gummies by 3D printing: Effect of polyols on 3D printing characteristics. Foods 2022, 11, 874. [Google Scholar] [CrossRef]
- Gebeyehu, A.; Surapaneni, S.K.; Huang, J.; Mondal, A.; Wang, V.Z.; Haruna, N.F.; Bagde, A.; Arthur, P.; Kutlehria, S.; Patel, N. Polysaccharide hydrogel based 3D printed tumor models for chemotherapeutic drug screening. Sci. Rep. 2021, 11, 372. [Google Scholar] [CrossRef]
- Čižauskaitė, U.; Jakubaitytė, G.; Žitkevičius, V.; Kasparavičienė, G. Natural ingredients-based gummy bear composition designed according to texture analysis and sensory evaluation in vivo. Molecules 2019, 24, 1442. [Google Scholar] [CrossRef]
- Kean, E.A.; Adeleke, O.A. A child-friendly anti-infective gummy formulation: Design, physicochemical, micromechanical, and taste sensory evaluation. Drug Deliv. Transl. Res. 2024, 14, 1319–1337. [Google Scholar] [CrossRef] [PubMed]
- Vojvodić Cebin, A.; Bunić, M.; Mandura Jarić, A.; Šeremet, D.; Komes, D. Physicochemical and sensory stability evaluation of gummy candies fortified with mountain germander extract and prebiotics. Polymers 2024, 16, 259. [Google Scholar] [CrossRef] [PubMed]
- Fraguas-Sánchez, A.I.; Fernández-Carballido, A.; Martin-Sabroso, C.; Torres-Suárez, A.I. Stability characteristics of cannabidiol for the design of pharmacological, biochemical and pharmaceutical studies. J. Chromatogr. B 2020, 1150, 122188. [Google Scholar] [CrossRef] [PubMed]
- Kosović, E.; Sýkora, D.; Kuchař, M. Stability study of cannabidiol in the form of solid powder and sunflower oil solution. Pharmaceutics 2021, 13, 412. [Google Scholar] [CrossRef]
- Aare, M.; Bagde, A.; Nathani, A.; Rishi, A.K.; Singh, M. Enhanced oral bioavailability and in vitro evaluation of cannabidiol camel milk-derived exosome formulation in resistant MDA-MB-231 and MDA-MB-468 breast cancer cells. Int. J. Pharm. 2024, 663, 124375. [Google Scholar] [CrossRef]
- Bagde, A.; Patel, K.; Mondal, A.; Kutlehria, S.; Chowdhury, N.; Gebeyehu, A.; Patel, N.; Kumar, N.; Singh, M. Combination of UVB absorbing titanium dioxide and quercetin nanogel for skin cancer chemoprevention. AAPS PharmSciTech 2019, 20, 240. [Google Scholar] [CrossRef]
- Saingam, W.; Sakunpak, A. Development and validation of reverse phase high performance liquid chromatography method for the determination of delta-9-tetrahydrocannabinol and cannabidiol in oromucosal spray from cannabis extract. Rev. Bras. De Farmacogn. 2018, 28, 669–672. [Google Scholar] [CrossRef]
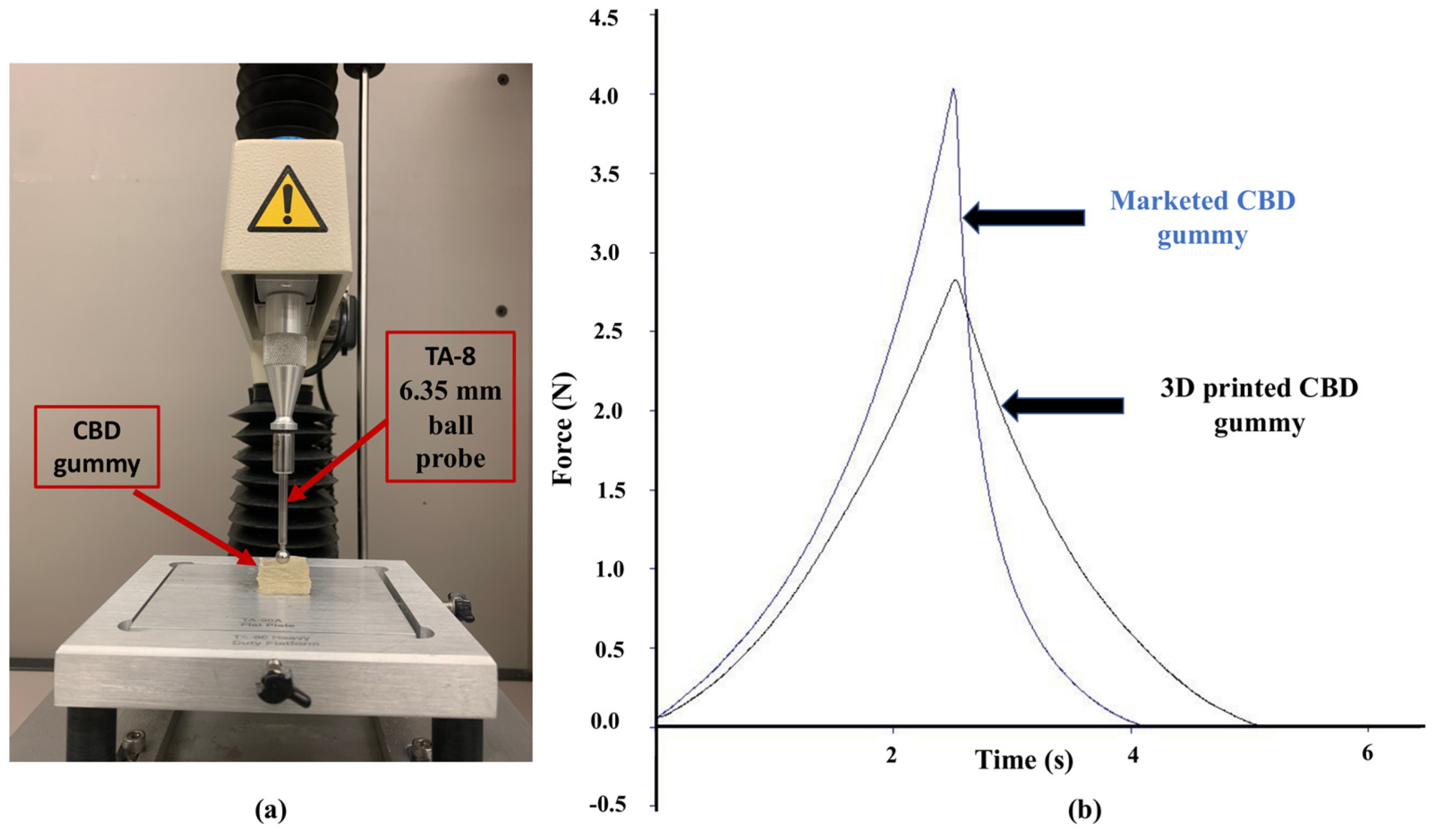
| Gummy | Firmness (N) | Toughness (N.s) | Resilience (%) | Elastic Recovery (%) | Tackiness (N) |
|---|---|---|---|---|---|
| 3D-printed | 2.82 ± 0.15 | 2.84 ± 0.18 | 91.00 ± 1.90 | 98.58 ± 1.20 | −0.0052 ± 0.0004 |
| Marketed | 4.03 ± 0.10 | 3.51 ± 0.14 | 36.60 ± 3.60 | 66.60 ± 0.40 | −0.0033 ± 0.0003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagde, A.; Messiha, M.; Singh, M. Development and Characterization of Cannabidiol Gummy Using 3D Printing. Gels 2025, 11, 189. https://doi.org/10.3390/gels11030189
Bagde A, Messiha M, Singh M. Development and Characterization of Cannabidiol Gummy Using 3D Printing. Gels. 2025; 11(3):189. https://doi.org/10.3390/gels11030189
Chicago/Turabian StyleBagde, Arvind, Mina Messiha, and Mandip Singh. 2025. "Development and Characterization of Cannabidiol Gummy Using 3D Printing" Gels 11, no. 3: 189. https://doi.org/10.3390/gels11030189
APA StyleBagde, A., Messiha, M., & Singh, M. (2025). Development and Characterization of Cannabidiol Gummy Using 3D Printing. Gels, 11(3), 189. https://doi.org/10.3390/gels11030189









