Gastric Acid-Protective and Intestinal Targeted Nanogels Enable Anti-Bacterial Activity of Cefquinome
Abstract
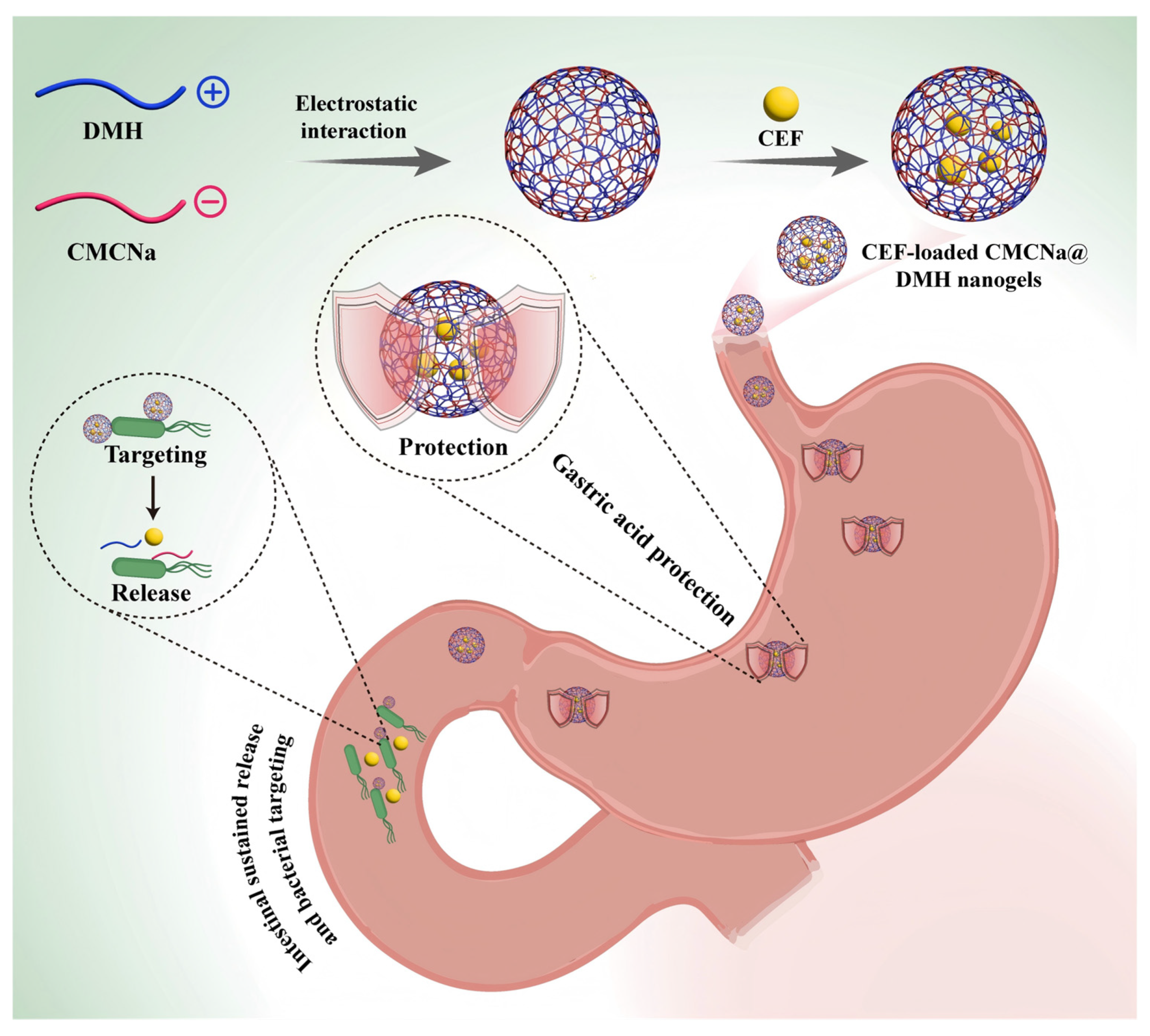
1. Introduction
2. Results and Discussion
2.1. Formulation Optimization
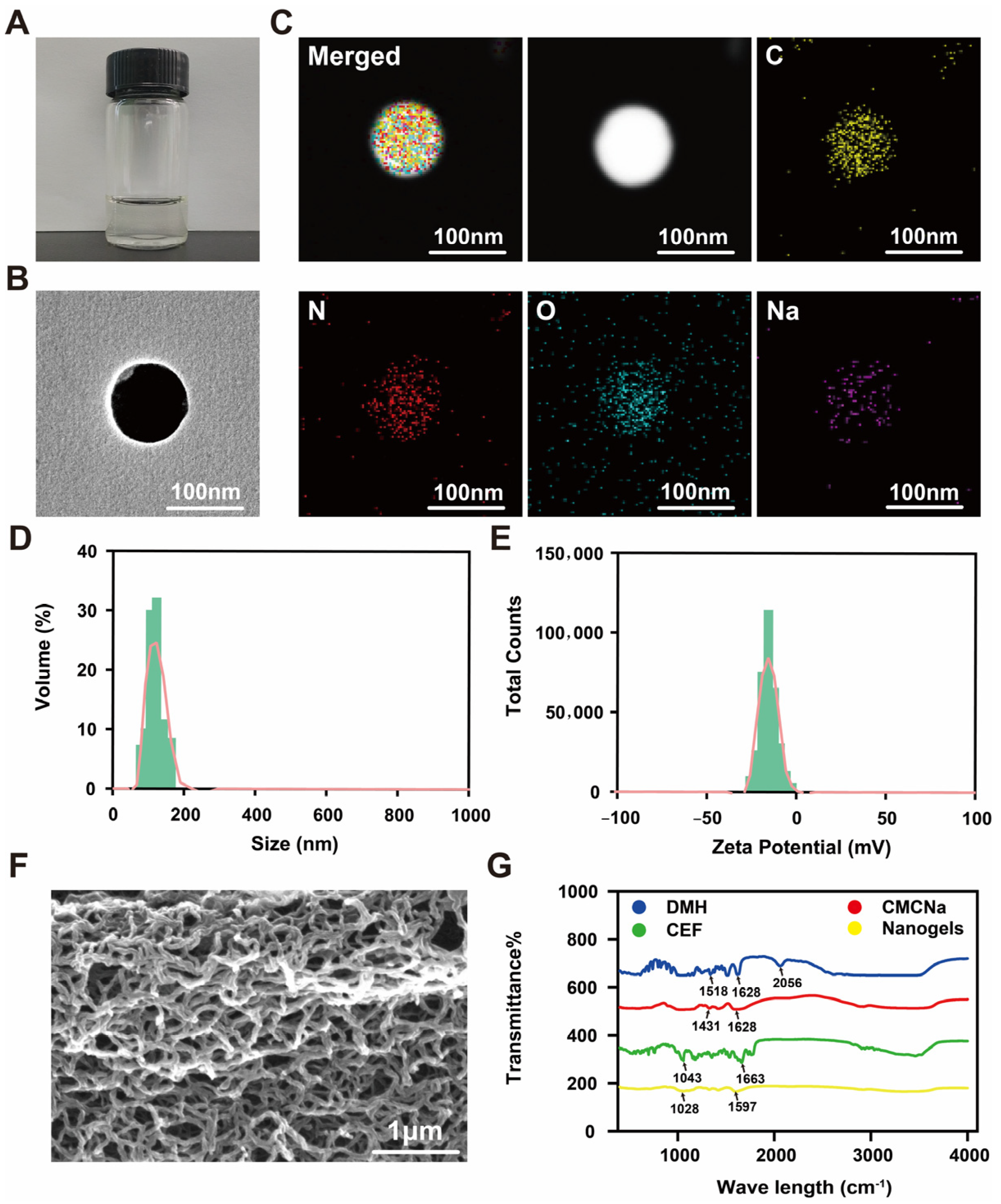
2.2. Characterization
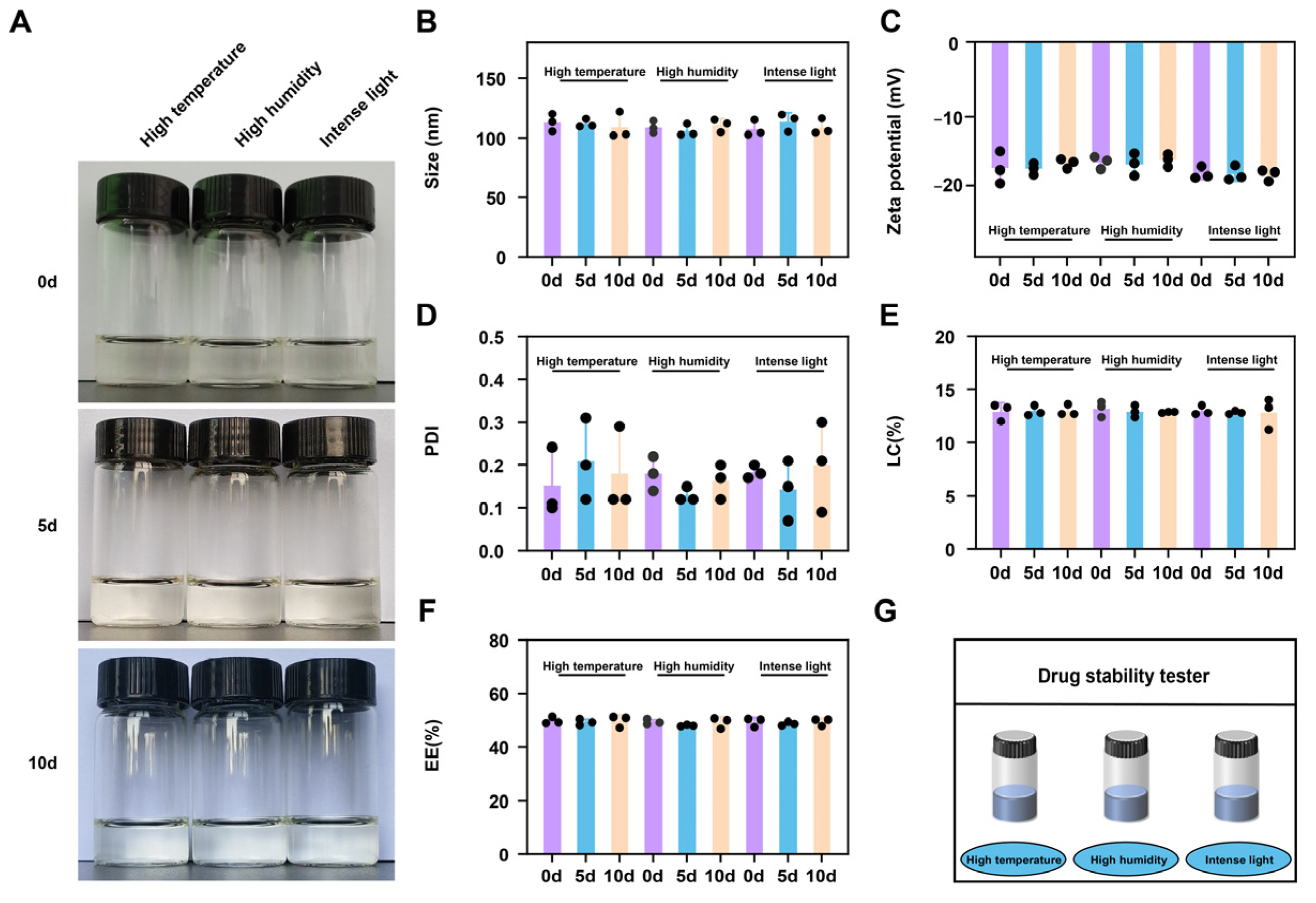
2.3. Stability
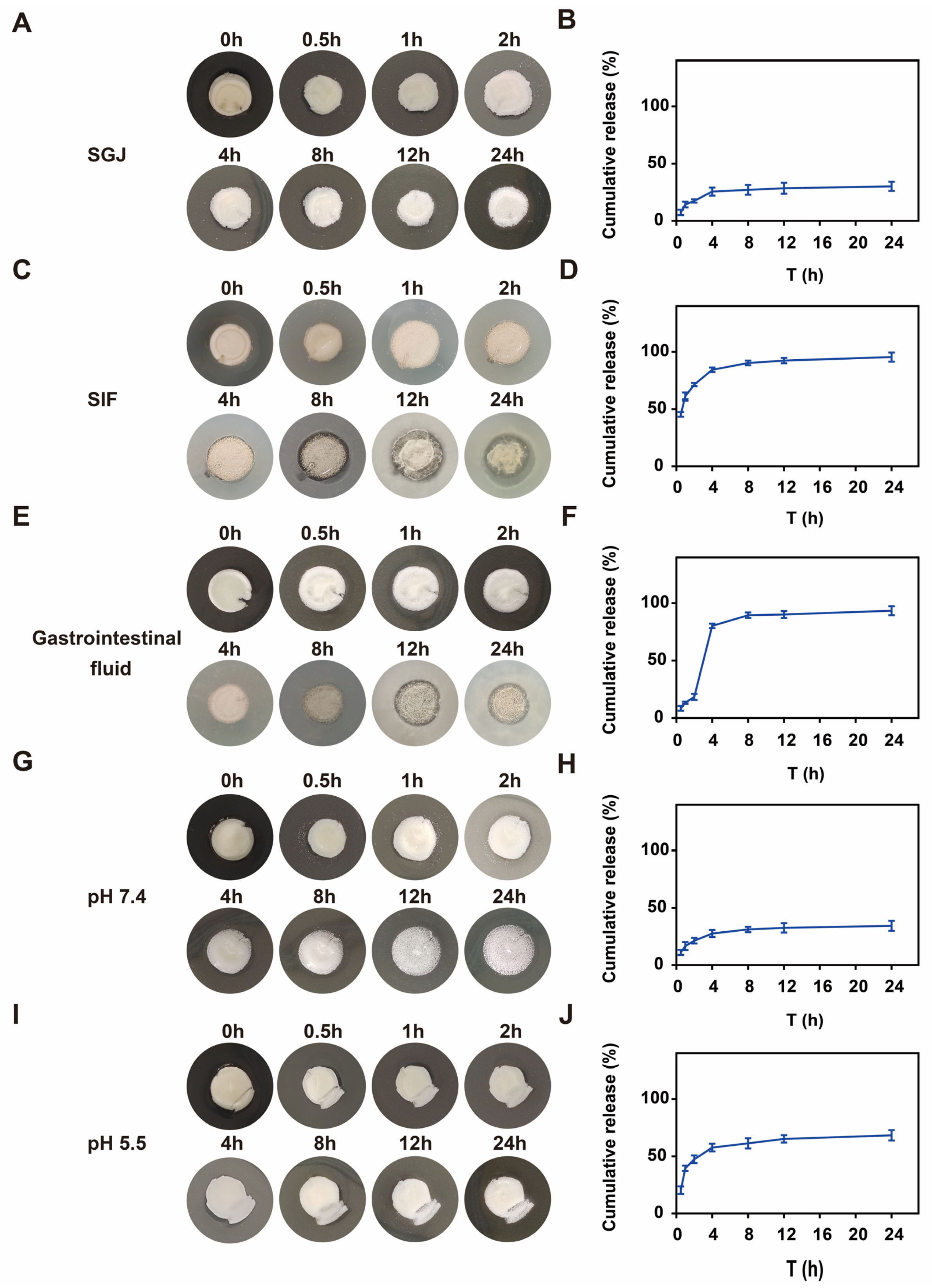
2.4. Gastrointestinal Fluid-Responsive Performances
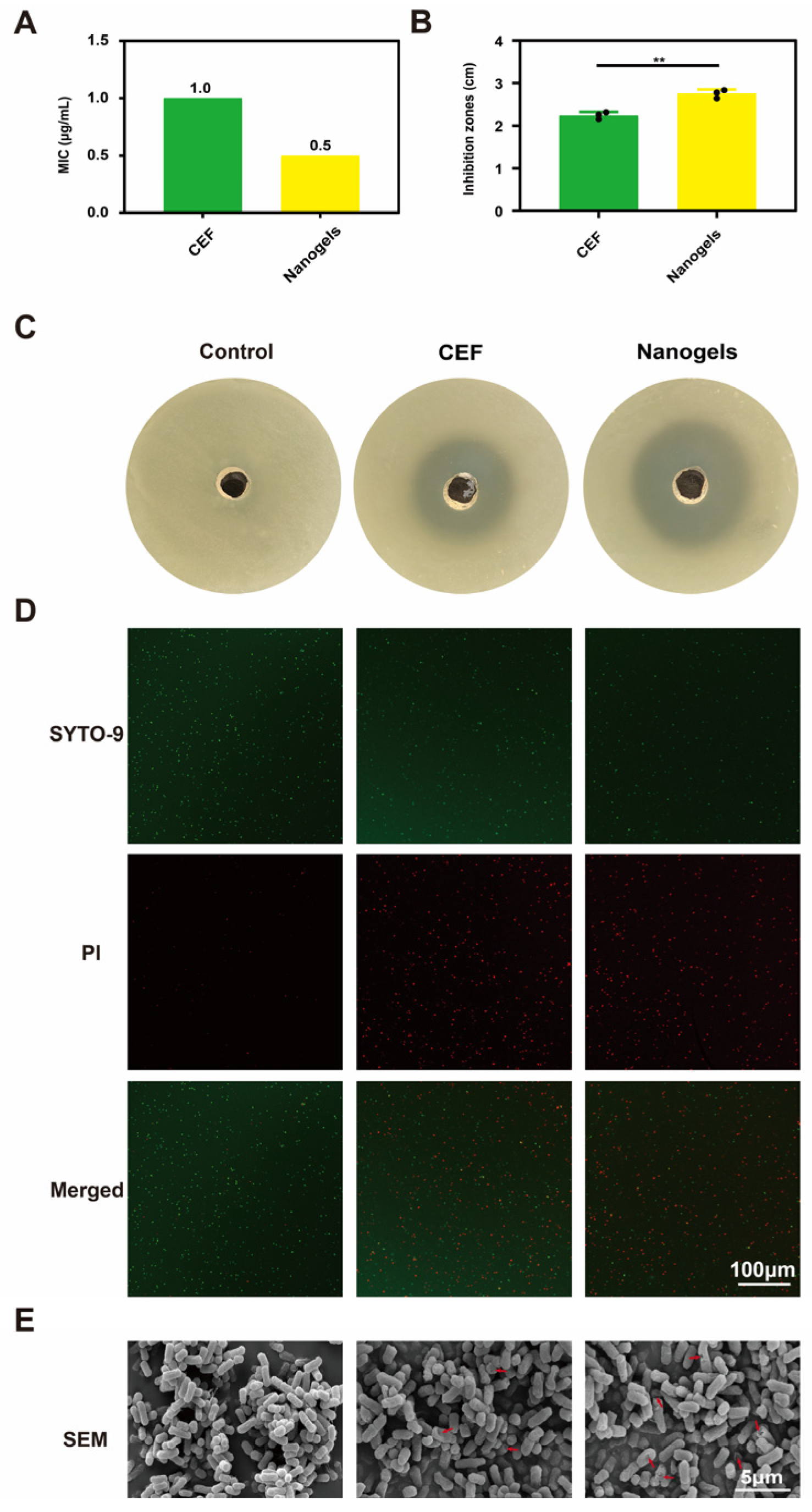
2.5. In Vitro Antibacterial Activity
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Formulation of CEF-Loaded CMCNa@DMH Nanogels
4.3. Box–Behnken Response Surface Analysis
4.4. Physicochemical Characterization
4.5. Gastrointestinal Fluid-Responsive Release
4.6. Stability Evaluation
4.7. In Vitro Antibacterial Activity Studies
4.7.1. Broth Macrodilution Method
4.7.2. Bacterial Inhibition Zones
4.7.3. Analysis of Live/Dead Bacterial Staining
4.7.4. Morphological Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Liu, N.; Gao, Y.; Liu, J.; Huang, X.; Zhang, Q.; Li, Y.; Zhao, J.; Wang, J.; Zhao, G. Surveillance and Reduction Control of Escherichia coli and Diarrheagenic E. coli During the Pig Slaughtering Process in China. Front. Vet. Sci. 2021, 8, 735076. [Google Scholar] [CrossRef]
- Van Den Honert, M.S.; Gouws, P.A.; Hoffman, L.C. Escherichia coli Antibiotic Resistance Patterns from Co-Grazing and Non-Co-Grazing Livestock and Wildlife Species from Two Farms in the Western Cape, South Africa. Antibiotics 2021, 10, 618. [Google Scholar] [CrossRef]
- Ribeiro, J.; Silva, V.; Monteiro, A.; Vieira-Pinto, M.; Igrejas, G.; Reis, F.S.; Barros, L.; Poeta, P. Antibiotic Resistance among Gastrointestinal Bacteria in Broilers: A Review Focused on Enterococcus spp. and Escherichia coli. Antibiotics 2023, 13, 1362. [Google Scholar] [CrossRef] [PubMed]
- Thuthikkadu Indhuprakash, S.; Karthikeyan, M.; Gopal, G.; Ambi, S.V.; Sekaran, S.; Palaniappan, B.; Diraviyam, T. Antibody therapy against antibiotic-resistant diarrheagenic Escherichia coli: A systematic review. Immunotherapy 2021, 13, 1305–1320. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Chen, Y.; Liu, C.; He, X.; Zheng, Y.; Chen, X.; Wang, Y.; Chen, W.; Wu, Y.; Shen, Y.; et al. Cefquinome Sulfate Oily Nanosuspension Designed for Improving its Bioavailability in the Treatment of Veterinary Infections. Int. J. Nanomed. 2022, 17, 2535–2553. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yu, N.; Tang, Y.; Liu, C.; Zhang, Y.; Chen, X.; Wu, H.; Li, X.; Liu, Y. Pharmacokinetics and relative bioavailability study of two cefquinome sulfate intramammary infusions in cow milk. Front. Vet. Sci. 2024, 11, 1384076. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Dai, W.; Lu, Z.; Lei, Z.; Yang, B.; He, B.; Zhou, H.; Cao, J. Preparation and evaluation of cefquinome-loaded gelatin microspheres and the pharmacokinetics in pigs. J. Vet. Pharmacol. Ther. 2018, 41, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, S.; Dou, H.; Wu, W.; Liu, Q.; Zhang, L.; Shen, Y.; Shu, G.; Yuan, Z.; Lin, J.; et al. Preparation of cefquinome sulfate cationic proliposome and evaluation of its efficacy on Staphylococcus aureus biofilm. Colloids Surf. B Biointerfaces 2019, 182, 110323. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, P.; Yin, C.; Li, Y.; Liao, Z.; Yang, C.; Liu, H.; Wang, W.; Fan, C.; Sun, D.; et al. Antibiotic-Derived Carbon-Nanodot-Decorated Hydrogel for Reactive Oxygen Species-Enhanced Anti-Infection Through Biofilm Damage. Adv. Funct. Mater. 2023, 33, 2300341. [Google Scholar] [CrossRef]
- Luo, Y.; Cui, L.; Zou, L.; Zhao, Y.; Chen, L.; Guan, Y.; Zhang, Y. Mechanically strong and on-demand dissoluble chitosan hydrogels for wound dressing applications. Carbohydr. Polym. 2022, 294, 119774. [Google Scholar] [CrossRef]
- Wu, J.; Wang, W.; Shen, J.; Zhou, N.; Li, Y.; Tang, B.; Zhang, M. A Thermosensitive Hydrogel with Efficient NIR Photothermal Conversion as Injectable Wound Dressing for Accelerating Skin Wound Healing. Adv. Funct. Mater. 2024, 34, 2312374. [Google Scholar] [CrossRef]
- López-Iglesias, C.; Markovina, A.; Nirmalananthan-Budau, N.; Resch-Genger, U.; Klinger, D. Optically monitoring the microenvironment of a hydrophobic cargo in amphiphilic nanogels: Influence of network composition on loading and release. Nanoscale 2024, 16, 9525–9535. [Google Scholar] [CrossRef]
- Knipe, J.M.; Strong, L.E.; Peppas, N.A. Enzyme- and pH-Responsive Microencapsulated Nanogels for Oral Delivery of siRNA to Induce TNF-α Knockdown in the Intestine. Biomacromolecules 2016, 17, 788–797. [Google Scholar] [CrossRef]
- Luo, W.; Zhang, M.; Jiang, Y.; Ma, G.; Liu, J.; Dawood, A.S.; Xie, S.; Algharib, S.A. Manipulated Slow Release of Florfenicol Hydrogels for Effective Treatment of Anti-Intestinal Bacterial Infections. Int. J. Nanomed. 2025, 20, 541–555. [Google Scholar] [CrossRef]
- Luo, W.; Liu, J.; Zhang, M.; Jiang, Y.; Sun, B.; Xie, S.; Sobhy Dawood, A.; Attia Algharib, S.; Gao, X. Florfenicol core-shell composite nanogels as oral administration for efficient treatment of bacterial enteritis. Int. J. Pharm. 2024, 662, 124499. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Cai, K.; Zhao, J.; Liu, C.; Li, H.; Tan, P.; Li, Y.; Li, D.; Ma, X. Mannosylated MOF Encapsulated in Lactobacillus Biofilm for Dual-Targeting Intervention Against Mammalian Escherichia coli Infections. Adv. Mater. 2025, 202503056. [Google Scholar] [CrossRef] [PubMed]
- Asadi, K.; Azarpira, N.; Heidari, R.; Hamidi, M.; Yousefzadeh-Chabok, S.; Nemati, M.M.; Ommati, M.M.; Amini, A.; Gholami, A. Trinitroglycerin-loaded chitosan nanogels accelerate angiogenesis in wound healing process. Int. J. Biol. Macromol. 2024, 278 Pt 3, 134937. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zuo, R.; Song, X.; Gong, J.; Wang, J.; Lin, M.; Yang, F.; Cheng, X.; Gao, X.; Peng, L.; et al. Optimization of Maduramicin Ammonium-Loaded Nanostructured Lipid Carriers Using Box-Behnken Design for Enhanced Anticoccidial Effect against Eimeria tenella in Broiler Chickens. Pharmaceutics 2022, 14, 1330. [Google Scholar] [CrossRef] [PubMed]
- Asfour, M.H.; Kassem, A.A.; Salama, A. Topical nanostructured lipid carriers/inorganic sunscreen combination for alleviation of all-trans retinoic acid-induced photosensitivity: Box-Behnken design optimization, in vitro and in vivo evaluation. Eur. J. Pharm. Sci. 2019, 134, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Long, T.; Tan, W.; Tian, X.; Tang, Z.; Hu, K.; Ge, L.; Mu, C.; Li, X.; Xu, Y.; Zhao, L.; et al. Gelatin/alginate-based microspheres with sphere-in-capsule structure for spatiotemporal manipulative drug release in gastrointestinal tract. Int. J. Biol. Macromol. 2023, 226, 485–495. [Google Scholar] [CrossRef] [PubMed]
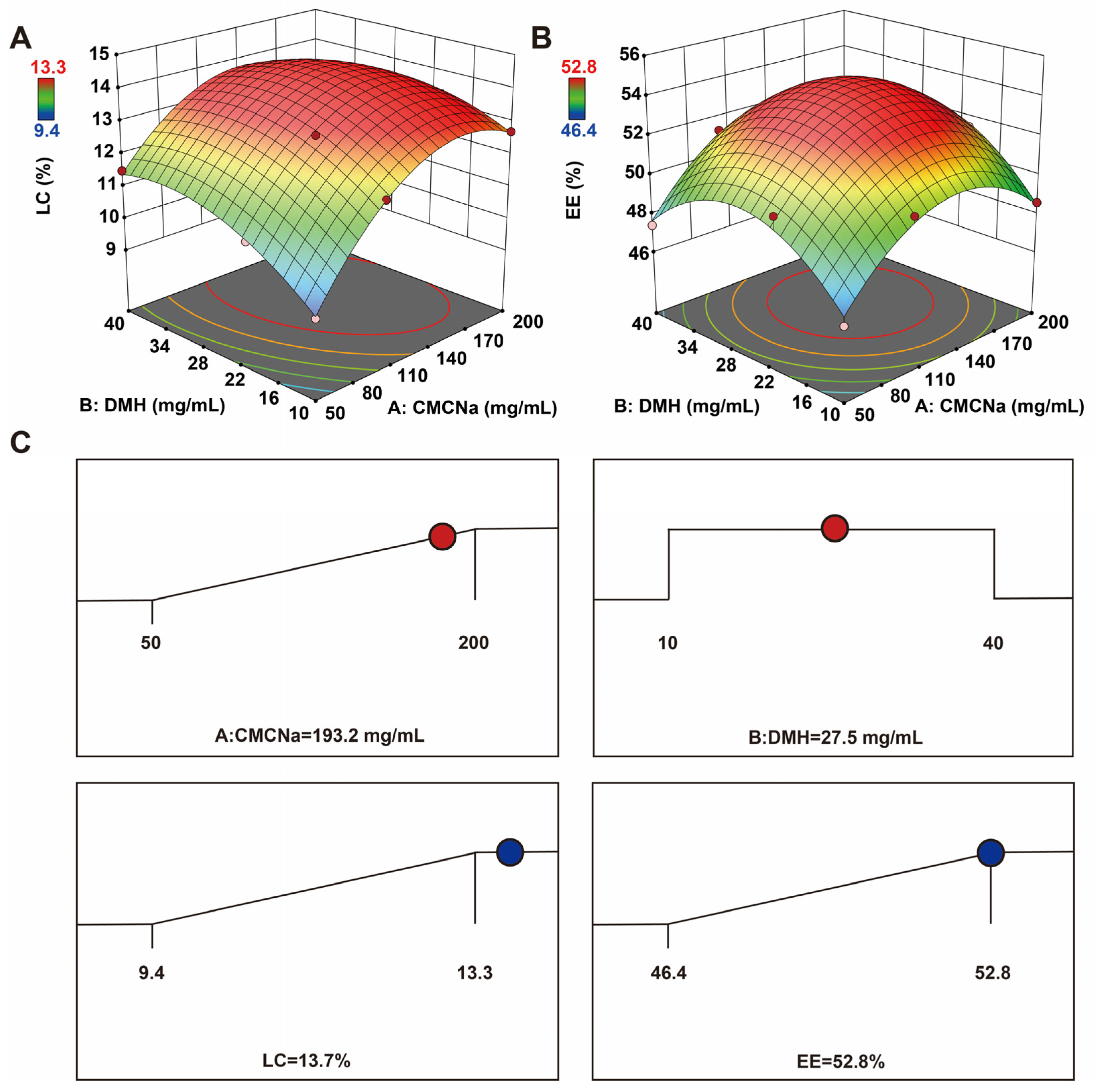
| Run | CMCNa (mg/mL) | DMH (mg/mL) | LC (%) | EE (%) |
|---|---|---|---|---|
| 1 | 50 | 10 | 9.4 | 46.4 |
| 2 | 200 | 10 | 12.7 | 48.6 |
| 3 | 50 | 40 | 11.5 | 47.4 |
| 4 | 200 | 40 | 12.8 | 50.5 |
| 5 | 50 | 20 | 10.8 | 50.3 |
| 6 | 200 | 20 | 13.3 | 51.5 |
| 7 | 100 | 10 | 12.0 | 50.3 |
| 8 | 100 | 40 | 13.1 | 51.3 |
| 9 | 100 | 20 | 13.2 | 52.8 |
| 10 | 100 | 20 | 13.2 | 52.8 |
| 11 | 100 | 20 | 13.2 | 52.8 |
| 12 | 100 | 20 | 13.2 | 52.8 |
| 13 | 100 | 20 | 13.2 | 52.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wang, T.; Han, S.; Liu, J.; Zhang, X.; Zhou, Z.; Dawood, A.S.; Luo, W. Gastric Acid-Protective and Intestinal Targeted Nanogels Enable Anti-Bacterial Activity of Cefquinome. Gels 2025, 11, 503. https://doi.org/10.3390/gels11070503
Li X, Wang T, Han S, Liu J, Zhang X, Zhou Z, Dawood AS, Luo W. Gastric Acid-Protective and Intestinal Targeted Nanogels Enable Anti-Bacterial Activity of Cefquinome. Gels. 2025; 11(7):503. https://doi.org/10.3390/gels11070503
Chicago/Turabian StyleLi, Xianqiang, Tianhui Wang, Shuo Han, Jinhuan Liu, Xiuping Zhang, Zhiqiang Zhou, Ali Sobhy Dawood, and Wanhe Luo. 2025. "Gastric Acid-Protective and Intestinal Targeted Nanogels Enable Anti-Bacterial Activity of Cefquinome" Gels 11, no. 7: 503. https://doi.org/10.3390/gels11070503
APA StyleLi, X., Wang, T., Han, S., Liu, J., Zhang, X., Zhou, Z., Dawood, A. S., & Luo, W. (2025). Gastric Acid-Protective and Intestinal Targeted Nanogels Enable Anti-Bacterial Activity of Cefquinome. Gels, 11(7), 503. https://doi.org/10.3390/gels11070503








